Abstract
Background
The risk of esophageal adenocarcinoma (EAC) in non-dysplastic Barrett’s esophagus (NDBE) is considered to be approximately 0.3% per year or even lower, according to population-based studies. Data from countries with low EAC incidence are scarce. Our principal aim was to determine the incidence of high-grade dysplasia (HGD) and EAC in NDBE. Our secondary aims were to identify the predictors of progression and to calculate the incidence of HGD/EAC, by using the calculation method for surveillance time in population-based studies.
Materials and methods
A cohort of NDBE patients was prospectively followed up. Cases of HGD and EAC (study end points) diagnosed during the first year of follow-up were considered as prevalent. Only cases with an endoscopic surveillance time > 1 year were included in our analysis.
Results
We enrolled 331 patients (251 men) in the surveillance program. Their median age was 59 years (interquartile range (IQR): 47–67 years). Their median NDBE length was 3 cm (IQR: 2–4 cm). Of these patients, 80 died during the follow-up (one from EAC) and two were lost to follow-up. After 2284 patient-years of endoscopic follow-up (median surveillance time, 5 years (IQR: 2–10 years)), we found that five cases of HGD and two cases of EAC were diagnosed. The incidence of HGD/EAC was 3.1 cases per 1000 patient-years (95% CI: 1.3–6.0) and that of EAC was 0.9 (95% CI: 0.2–2.9). The incidence of HGD/EAC in short segments (≤ 3 cm) was 0.7 cases per 1000 patient-years (95% CI: 0.3–3.4). The sole variable that we found associated with progression was NDBE length. If the total surveillance time was considered (3537 patient-years), the incidence of HGD and EAC was only slight lower.
Conclusions
The incidence of HGD and EAC was very low in NDBE. Therefore, current surveillance guidelines must be reassessed, at least for short-segment BE.
Keywords: Adenocarcinoma, Barrett’s esophagus, cancer risk, dysplasia, segment length, surveillance
Introduction
Barrett’s esophagus (BE) results from the metaplastic replacement of the normal squamous lining of the distal esophagus by a columnar epithelium. BE is associated with gastroesophageal reflux and is the only known precursor of esophageal adenocarcinoma (EAC).
The estimated cancer risk in BE has decreased significantly in the last 20 years. Its incidence until the year 2000 was estimated to be approximately 1% per year, with reports ranging from 0 to 2%.1,2 In the year 2000, Shaheen et al.3 showed a risk overestimation due to publication bias; and a 0.5% risk for BE was proposed. Recently, population-based studies that show a lower risk of cancer in BE were published,4,5 reporting cancer incidence rates as low as 0.12% per year.5
Since 2007, there were five meta-analyses6–10 that were published that show the EAC incidence rates in BE ranging from 0.33% to 0.7%. Although these incidence rates were calculated excluding incident cases of high-grade dysplasia (HGD) or EAC, only the meta-analysis by Desai et al.10 excludes studies with patients with any grade of dysplasia at enrollment.
Most of the studies on BE cancer risk are from the US, Northern Europe and the UK, where the incidence of EAC is high. The risk of EAC in Southern Europe, where EAC is less prevalent, is not well known; and all studies, except one from Italy,11 include only a small number of patients.
Endoscopic and population-based studies use different methodologies for surveillance time calculation. In endoscopic studies, it is calculated from the index (enrollment into the cohort) to the last performed upper gastrointestinal (GI) endoscopy.12 In population-based studies, it is calculated as the time elapsed from BE diagnosis to HGD or EAC diagnosis, death, or the end of the study period, whichever came first4,5,13; however, the impact of these different study methodologies on the magnitude of the calculated cancer risk was not evaluated.
Thus, the principal aim of the present study was to calculate the risks of EAC, HGD and HGD/EAC in a cohort of Portuguese patients with non-dysplastic Barrett’s esophagus (NDBE), whom were prospectively enrolled in a surveillance program. Our secondary aims were as follows:
To identify predictors of progression of BE to HGD and EAC; and
To calculate the incidence of EAC, HGD and HGD/EAC in this cohort, by using the calculation method for surveillance time in population-based studies.
Methods and materials
Our BE surveillance program was a single-center program of the Instituto Português de Oncologia de Lisboa (IPOL) [Portuguese Cancer Institute of Lisbon] for patients whom were referred from South Portugal, which started in 1995 and was approved by the IPOL Institutional Review Board (IRB). All the endoscopies were performed by dedicated endoscopists: The great majority of them were performed by one study author (ADP). Experienced gastrointestinal pathologists from the IPOL Pathology Department read all the biopsies: The great majority were read by another study author (PC). All of the patients provided informed consent for inclusion into the program and written consent to undergo endoscopy. The IRB also approved the present study in July 2011, and a waiver of consent was given.
The inclusion criteria were as follows: The presence of columnar-lined esophagus and the presence of intestinal metaplasia (IM) (defined by the presence of goblet cells) in the biopsy samples from at least one endoscopy. If the IM was not detected in the first upper GI endoscopy, a second endoscopy was performed 1 year later. If still no IM was observed, the patient was discharged from surveillance. Biopsies were performed according to the Seattle protocol. All of the biopsy samples were submitted to the pathology laboratory in separate bottles. In 1998, the surveillance protocol was established according to the guidelines of the American College of Gastroenterology. These involve performing an endoscopy 1 year after the initial endoscopy, and every 3 years thereafter, in patients whom tested negative for dysplasia. If indefinite or low-grade dysplasia were diagnosed, patients were given intensive proton pump inhibitors (PPIs) and endoscopy was repeated at 3- to 6-month intervals, until dysplasia had either regressed or stabilized. If EAC or HGD was diagnosed and confirmed by another independent experienced gastrointestinal pathologist, the patients were referred for surgical or endoscopic resection.
We recorded the following information regarding the patients with BE: Demographic characteristics (age at diagnosis, gender and ethnicity), endoscopic data (date of the procedure; length of BE in centimeters; classification into short (≤3 cm) and long segments (>3 cm), and according to the Prague classification system for BE,14 for those patients with endoscopies performed after 2006; and the presence of hiatal hernia) and histological diagnosis (including the presence or absence of IM; dysplasia grade (namely negative, indefinite, low grade, or high grade); and the presence of EAC). We also recorded the use of PPIs (at baseline and at each surveillance endoscopy), anti-reflux surgery, and histories of smoking and alcohol consumption (at baseline and whenever any changes occurred).
For the purposes of this study, only those patients with BE of a minimum length of 1 cm, 1 year of surveillance, and at least one surveillance endoscopy were considered. The index endoscopy was defined as the first endoscopy performed in our hospital that met the diagnostic criteria for BE, independently if it was performed before or after the start of the surveillance program. In patients previously diagnosed with BE in other institutions, the first endoscopy performed in the context of the surveillance program was considered as the index.
Surveillance time was considered as the time (in years) elapsed from the index endoscopy to the last endoscopy performed with biopsies. If a patient returned with a diagnosis of EAC or HGD after being discharged from the endoscopic surveillance (due to age, severe comorbidities, refusal to maintain surveillance, or failure to comply with two consecutively scheduled endoscopies), then the case was not considered as diagnosed in the context of the surveillance program. To calculate the surveillance time to achieve our secondary aim, we considered the time elapsed from the index endoscopy to death, esophageal EAC or HGD diagnosis; or the study period end of 30 June 2013; whichever occurred first.
All cases of EAC and HGD diagnosed during the first year of surveillance were considered as prevalent and excluded from the analysis. Patients were considered lost to follow-up if at the end of the study period (30 June 2013), their status could not be assessed. This information was obtained from the Portuguese National Health Service Users Registry. If the information was considered insufficient, the patients or their family members were contacted by phone or letter, in order to obtain the missing information. As Portuguese legislation does not allow the obtaining of information on the cause of death from the death certificates, we obtained the information on death from esophageal or any other cancers of all the patients whom died during the study period, from the South Regional Portuguese Cancer Registry.
Statistical analyses
As all continuous variables had a non-normal distribution, we used non-parametric tests to compare the means or distribution of two samples. Association between variables was tested by using the chi-square or Fisher exact test. Logistic regression was performed for the variables that met statistical significance, and the odds ratio (OR) was calculated. We used the Kaplan-Meier test to estimate the probability of progression (or remaining free of progression) to HGD/EAC and we used the log-rank test to compare the equality of progression distributions between two or more groups. Pairwise log-rank comparisons were conducted to determine which groups had different survival distributions. If more than two groups were considered, the level of statistical significance was adjusted, to compensate for making multiple comparisons. Statistical analyses was conducted by using IBM SPSS Statistics version 21 (IBM, Armonk, New York, USA).
Results
Patients
The characteristics of the patients in our cohort are summarized in Table 1. Briefly, our cohort was composed of 331 patients with BE (251 were male gender), whose median age at diagnosis was 59 years (interquartile range (IQR): 47–67 years). Nine patients had been previously diagnosed with BE and surveilled for a mean of 2 years in others hospitals. The median age at the end of the follow-up period was 65 years (IQR: 55–75 years). Two patients (0.6% of the cohort) were lost to follow-up. The median BE length was 3 cm (IQR: 2–4 cm), with 39.6% of the segments classified as long. Endoscopic follow-up accounted for 2284 patient-years, with a median of 5 years (IQR: 2–10 years).
Table 1.
Characteristics of the 331 patients with Barrett’s esophagus.
| Gender | |
|---|---|
| Male, n (%) | 251 (75.8) |
| Female, n (%) | 80 (24.2) |
| Race | |
| Caucasians/non-Caucasians | 330/1 |
| Age at diagnosis of Barrett’s esophagus | |
| Median | 59 |
| IQR | 47–67 |
| Age at end of endoscopic follow-up | |
| Median | 65 |
| IQR | 55–75 |
| Age at end of total follow-up | |
| Median | 69 |
| IQR | 58–78 |
| Length of Barrett’s esophagus (I) | |
| Median | 3 |
| IQR | 2–4 |
| Range (cm) | 1–16 |
| Length of Barrett’s esophagus (II) | |
| Short segments/long segments | 208/123 |
| Praga’s classification | |
| n | 258 |
| Circular (C) – Median (IQR) | 1 (0–3) |
| Maximum (M) – Median (IQR) | 3 (2–4) |
| Hiatal hernia | |
| Yes/no, n (%) | 258 (78)/73 (22) |
| Tobacco use | |
| Current or former smoker/nonsmoker, n (%) | 86 (26)/245 (74) |
| Alcohol | |
| Current or former user/never, n (%) | 118 (36)/213 (64) |
| Anti-reflux surgery | |
| n (%) | 35 (10.6) |
| Status at the end of the study | |
| Alive, n (%) | 231(75.8) |
| Dead, n (%) | 80 (24.2) |
| Lost to follow-up, n (%) | 2 (0.6) |
| Age of death, median (IQR) | 79.5 (73.3–84) |
| Follow-up years, median (IQR) | 10 (5–14) |
| Deaths from esophageal adenocarcinoma | |
| n | 1 |
| % cohort | 0.3 |
| % deaths | 1.25 |
IQR: Interquartile range
Incidence of EAC in the cohort
None of the patients in the cohort had prevalent EAC. During endoscopic follow-up, two patients were diagnosed with EAC, and both were staged as pT1N0 by their surgical resection specimens (Table 2). The median age at EAC diagnosis was 59 years. Cancer was diagnosed at a median follow-up of 9 years (95% CI: 4 – 9). The incidence of EAC was 0.9 (95% CI: 0.2 – 2.9) per 1000 patient-years of follow-up (Table 3). The annual risk of cancer was 0.09% (95% CI: 0.02 – 2.9).
Table 2.
Follow-up and incident cases of EAC and HGD in the cohort
| Endoscopic follow-up | |
|---|---|
| Surveillance endoscopies | |
| n | 932 |
| Median | 3 |
| IQR | 2–5 |
| Person-years at risk | 2284 |
| Follow-up | |
| Median (years) | 5 |
| IQR | 2–10 |
| LGD cases in the first year endoscopy | |
| n | 4 |
| LGD cases after first year endoscopy | |
| n | 8 |
| Incident cases of EAC/ HGD | |
| n | 7 |
| Incident cases of EAC | |
| n | 2 |
| Age at diagnosis, median years | 59 |
| Follow-up until diagnosis, median years | 9 |
| Incident cases of HGD | |
| n | 5 |
| Age at diagnosis 0, median years | 57 |
| Follow-up until diagnosis, median years | 6 |
BE: Barrett’s esophagus; EAC: esophageal adenocarcinoma; HGD: high-grade dysplasia; IQR: interquartile range; LGD: low-grade dysplasia
Table 3.
Incidence of EAC and of HGD in the cohort.
| Variable | n | Endoscopic surveillance |
|||
|---|---|---|---|---|---|
| Person-years at risk, n | Events in study cohort, n | Incidence rate/1000 Person-years | |||
| Adenocarcinoma | |||||
| Total cohort | 331 | 2284 | 2 | 0.9 (0.2–2.9) | |
| Gender | Male | 251 | 1725 | 2 | 1.2 (1.9–3.8) |
| Female | 80 | 559 | 0 | – | |
| Length | Short | 208 | 1457 | 0 | – |
| Long | 123 | 827 | 2 | 2.4 (0.4–8.0) | |
| Length (cm) | ≤ 3 cm | 208 | 1457 | 0 | – |
| >3 cm, but ≤ 6 cm | 90 | 577 | 1 | 1.7 (0.1–8.6) | |
| > 6 cm | 33 | 250 | 1 | 4.0 (0.2–19.8) | |
| High-grade dysplasia | |||||
| Total cohort | 331 | 2284 | 5 | 2.2 (0.8–4.9) | |
| Gender | Male | 251 | 1725 | 5 | 2.9 (1.1–6.4) |
| Female | 80 | 559 | 0 | – | |
| Length | Short | 208 | 1457 | 1 | 0.7 (0.03–3.4) |
| Long | 123 | 827 | 4 | 4.8 (1.5–11.7) | |
| Length (cm) | ≤ 3 cm | 208 | 1457 | 1 | 0.7 (0.03–3.4) |
| > 3 cm, but ≤ 6 cm | 90 | 577 | 2 | 3.5 (0.6–11.5) | |
| >6 cm | 33 | 250 | 2 | 8.0 (1.3–26.4) | |
EAC: esophageal adenocarcinoma; HGD: high-grade dysplasia
Incidence of HGD in the cohort
Five cases of incident HGD (that was confirmed in surgical or endoscopic resection specimens, in all cases) were observed during endoscopic follow-up. The incidence of HGD in the endoscopic follow-up was 2.2 (95% CI: 0.8 – 4.9) per 1000 patient-years of follow-up. The annual risk of HGD was 0.22% (95% CI: 0.08 – 0.49).
Incidence of HGD or EAC in the cohort
When the cases of HGD and EAC were considered together, we had seven cases that were diagnosed during endoscopic follow-up, corresponding to an incidence of 3.1 (95% CI: 1.3 – 6.0) per 1000 patient-years. The annual risk of HGD or EAC was 0.31% (95% CI: 0.13 – 0.60).
The patients who had progressed to HGD/EAC had significantly longer BE segments (median 5.5 cm versus 3.0 cm) than the patients who did not (Mann-Whitney test, p = 0.03). We observed that there was an association between BE length and progression to HGD/EAC, with the long-segment cases having a high probability of progression (Fisher exact test, p < 0.005). The association between length and progression to HGD/EAC was confirmed by logistic regression (OR 1.46; 95%CI: 1.18 – 1.80). There was a 46% increase in the risk of HGD/EAC for every 1-cm increase in the BE length (p < 0.005).
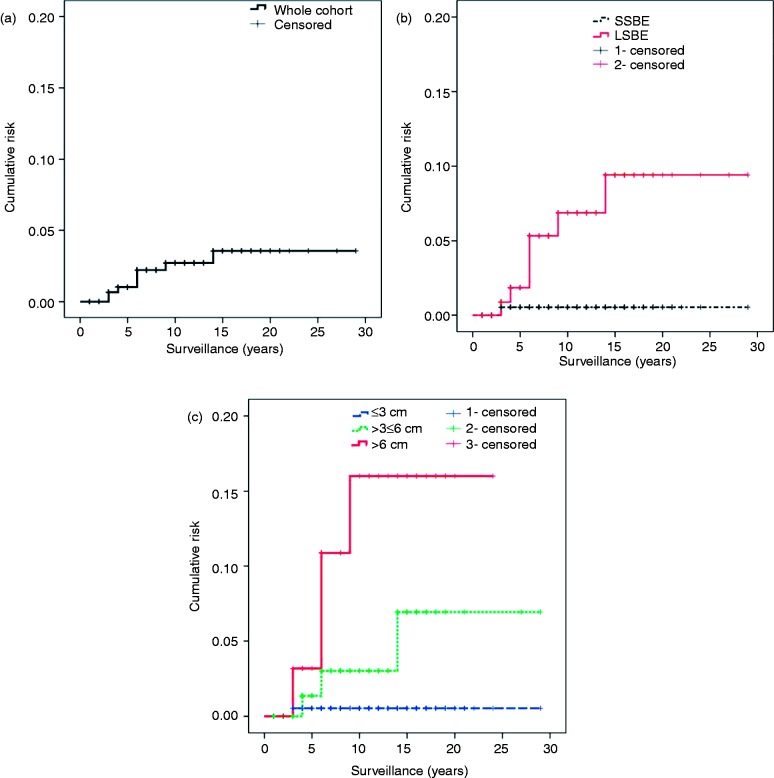
The observed incidence during endoscopic follow-up was 0.7 and 7.3 per 1000 patient-years, in the SSBE and long-segment BE, respectively (p < 0.005). If three classes of length were considered (subdividing the long segments into ≤ 6-cm and > 6-cm segments), the observed incidence of HGD/EAC was progressively higher. The incidence in those segments > 6 cm long was >15-fold that observed in the segments ≤ 3 cm long (Table 4). In our Kaplan-Meier analysis (Figure 1), the cumulative incidence of HGD/EAC significantly differed between the short and long segments (p < 0.009). Also, the cumulative incidence of HGD and EAC significantly differed between segments ≤ 3 cm, > 3 but ≤ 6 cm, and > 6 cm (p < 0.01); however, in the log-rank pairwise comparison test, the significant difference in cumulative incidence distribution was only observed in segments ≤ 3 cm and > 6 cm (p < 0.02).
Table 4.
Incidence of HGD/EAC in the cohort
| Variable | n | Endoscopic surveillance |
|||
|---|---|---|---|---|---|
| Person-year at risk, n | Events in study cohort, n | Incidence rate/1000 Person-Yr (95% CI) | |||
| Total cohort | 331 | 2284 | 7 | 3.1 (1.3–6.0) | |
| Gender | Male | 251 | 1725 | 7 | 4.1 (1.8–8.0) |
| Female | 80 | 559 | 0 | – | |
| Length (cm) | ≤ 3 cm | 208 | 1457 | 1 | 0.7 (0.3–3.4) |
| >3 and ≤ 6 cm | 90 | 577 | 3 | 5.2 (1.3–14.2) | |
| > 6 cm | 33 | 250 | 3 | 12.0 (3.1–27.8) | |
| Length | Short | 208 | 1457 | 1 | 0.7 (0.3–3.4) |
| Long | 123 | 827 | 6 | 7.3 (2.9–15.1) | |
Figure 1.
Kaplan-Meier curves with cumulative risk of developing high-grade dysplasia or adenocarcinoma for patients with non-dysplastic Barrett’s esophagus. (a) Whole cohort; (b) Patients with SSBE and LSBE; and (c) Patients with Barrett’s esophagus length of ≤ 3 cm, > 3 but ≤ 6 cm, and > 6 cm.
cm: centimeters; LSBE: long-segment Barrett’s esophagus; SSBE: short-segment Barrett’s esophagus
Besides length, no other variables (gender, age, tobacco smoking, alcohol, diagnosis of low-grade dysplasia at 1 year or at any time of the follow-up, and medical or surgical treatment) were associated with neoplastic progression in our BE cohort.
Incidence and follow-up of EAC and HGD, based on the surveillance time calculated using the same method used in population-based studies
When we used a population-based methodology, as described in the Methods section, the follow-up of the cohort accounted for 3537 patient-years at risk (Table 5). In our cohort, there were 238 patients that were followed up beyond the endoscopic surveillance time, accounting for 1253 patient-years of non-endoscopic follow-up (median 3 years; IQR: 1 – 10 years). During this time, only one patient was diagnosed with EAC (Stage IV), 8 years after being discharged from endoscopic follow-up because of a failure to comply with two scheduled endoscopies and refusal to set a new appointment. When we considered only the patients with ≥2 years of non-endoscopic follow-up, the incidence of EAC was 0.8 (95% CI: 0.04 – 4.2) per 1000 patient-years.
Table 5.
Incidence of HGD/EAC in the cohort considering surveillance time calculation methodology used by population-based studies
| Variable | n | Total Surveillance |
|||
|---|---|---|---|---|---|
| Person-years at risk, n | Events in study cohort, n | Incidence rate/1000 Person-yrs (95% CI) | |||
| Total cohort | 331 | 3537 | 8 | 2.2 (1.0–4.3) | |
| Gender | Male | 251 | 2605 | 8 | 3.1 (1.4–5.8) |
| Female | 80 | 932 | 0 | – | |
| Length (cm) | ≤ 3 cm | 208 | 2304 | 1 | 0.4 (0.2–2.4) |
| > 3 and ≤ 6 cm | 90 | 859 | 3 | 3.5 (0.9–9.5) | |
| > 6 cm | 33 | 374 | 4 | 10.7 (4.0–28.5) | |
| Length | Short | 208 | 2304 | 1 | 0.4 (0.2–2.4) |
| Long | 123 | 1233 | 7 | 5.7 (2.5–11.2) | |
Although the number of events, such as HGD, substantially differed between the two periods, the incidence analysis of HGD/EAC revealed that there was no significant difference between the intervention (endoscopic follow-up) and non-intervention (non-endoscopic follow-up). The HGD/EAC (n = 8) incidence in the entire cohort based on the time of total follow-up, calculated by using the same method used in population-based studies, and was 2.2 (95% CI: 1.0 – 4.3) per 1000 patient-years. With this methodology, we found that BE length was again the sole variable that was associated with progression to HGD/EAC, with similar ORs of 1.46 (95% CI: 1.20 – 1.80) and differences to those observed for endoscopic surveillance.
Patient outcomes
Within the cohort, 80 patients (24%) died during the study period, but only one (0.3%) death was caused by EAC. This translated into an annual mortality rate from EAC of 0.3 per 1000 person-years (95% CI: 0.01 – 1.3), or 0.03% per annum. The patients who died were significantly older at the time of their diagnosis (median age 68 years versus 54 years; p < 0.0005) and also at the end of the follow-up period (median age 79.5 years versus 65 years; p < 0.0005), and they had shorter endoscopic follow-up periods (median follow-up of 3 years versus 6 years; p < 0.005) than those who were still alive at the end of the study. The total follow-up (endoscopic plus non-endoscopic) period did not differ between the patients who died and those who survived until the final surveillance follow-up (median follow-up of 10 years versus 11 years; p = 0.06). The patients who died after being discharged from endoscopic surveillance (n = 69) were significantly older than those who died during their endoscopic surveillance period (78 years versus 68 years; p < 0.0005).
Discussion
In the present study, we calculated the incidence of HGD, EAC and HGD/EAC in a cohort of 331 patients with NDBE. We observed a low incidence of the different endpoints, that is, 0.09, 0.22 and 0.31 per 1000 patient-years for EAC, HGD and HGD/EAC, respectively.
The reported risk of EAC associated to BE has significantly decreased in the past 2 decades. In the five meta-analyses6–10 published since 2000, the annual risk of EAC ranged from 0.33% to 0.7%. Two of these meta-analyses7,9 reported a risk of EAC or HGD of approximately 1%. Desai et al.,10 in their meta-analysis, reported the lowest cancer incidence and they claimed that the higher incidences observed in the previous four meta-analyses were due to methodological errors, namely the inclusion of dysplastic cases, prevalent cancers and duplicated reports.15
Since the last meta-analysis, there were five reports on cancer incidence in NDBE that were published,5,11,16–18 with the observed annual risk ranging from 0.12% to 0.25%. Four of these reports5,11,17,18 indicated that the risk of HGD/EAC ranged from 0.26% to 0.85%. The lowest cancer and EAC/HGD incidence (0.12% and 0.26%, respectively) were reported by Hvid-Jensen et al.5 in a population-based cohort of more than 11,000 Danish patients with BE.
Population-based studies tend to be associated with a low incidence of esophageal cancer. Despite the strengths of these studies, some limitations are evident.19,20 As they generally used pathology databases, some of the cases might have been misdiagnosed as BE, and this might have lowered the observed risk. It must also be noted that these studies did not provide information about the use of ablative therapies in the cohort, and this might have biased the results.
Our results are below the lowest risk threshold in all but one of the recent studies (population based or not), despite coming from a referral center for BE. Recently, a study that used data from a BE registry of >1000 patients highlighted a greater risk of neoplastic progression than in population-based registries.18 Patients with dysplastic BE were carefully excluded from our cohort, and the cases of prevalent cancers were also excluded from our analysis. This may explain in part our results, but other possible factors must be considered.
Two-thirds of our study cohort had SSBE. This has been associated with a lower risk of cancer. In the meta-analysis by Desai et al.,10 16 out of 57 studies provided information about the incidence of EAC in SSBE. For 967 patients and 4456 patient-years of follow-up (< 10% of the total follow-up in the meta-analyses), they observed a cancer incidence of 1.9 cases per 1000 patient-years (versus 3.3 for the overall population). We must consider the possibility that the high prevalence (63%) of SSBE in our cohort could be responsible for the observed low incidence of neoplastic progression; however, a recent study from the US by Anaparthy et al.21 that included 1175 patients, of whom 61% had SSBE, showed an incidence of EAC of 0.26%, and of EAC and HGD of 0.67%. In 721 patients with SSBE, the incidence rates were 0.1% and 0.31%, respectively. Thus, for a cohort with a prevalence of SSBE, mean BE length (3.6 cm), and mean follow-up (5.5 years) that were similar to those of our cohort, the observed cancer incidence in SSBE was comparable to that observed in our entire cohort. Therefore, other reasons besides SSBE prevalence must be identified to explain the observed low cancer risk.
In 2009, the incidence of esophageal cancer in the southern region of Portugal, where the patients in the cohort lived, was 4.5 cases per 100.000 inhabitants (population at risk: 4 million and cases (n): 262)22; however, only about one-quarter of the cancer cases was EAC. The low incidence of EAC may be related both to a low prevalence of BE, or to BE with a very low cancer risk. Our results showed that the Portuguese population with BE has a very low risk of progression to HGD or EAC.
The accepted risk factors of BE progression to EAC include: A long BE length, male gender and central obesity.23 Age, smoking, acid suppression therapy and the length of hiatal hernia have also been considered as predictors of BE progression.24 Although progression to HGD or EAC was observed in male-gendered patients only, we observed no significant differences concerning gender. In our cohort, the length of BE was associated with HGD/EAC risk, which was 10-fold higher in the LSBE than in SSBE. As an increased cancer risk in very long BE segments is reported,25 we also analyzed the HGD/EAC risk in patients with long segments, ≤ or > 6 cm long. Although the risk was 2-fold higher in patients with the longer segments, this difference was not statistically significant.
Of the 80 patients who died during the study period, only one died from an EAC-related cause. In the meta-analysis by Sikkema et al.,9 deaths from EAC occurred in 1.4% of the patients (range: 0 – 2.6%), accounting for 7.4% of the observed deaths (range: 0 – 16.7%) and representing 57% of the incident cancers (range: 0 – 100%).
Recently, three publications analyzed the question of mortality in BE patients.26–28 Schouten et al.,26 in a population-based cohort of 605 patients, reported 2.1% mortality related to EAC. Caygill et al.,27 in their 3 decades of experience at a single center, reported 3.9% mortality from EAC in 1175 patients. Solaymani-Dodaran et al.28 reported that mortality from EAC occurred in 0.5% of a cohort of 8448 patients and this accounted for 4.5% of the mortality.
In our cohort, death from esophageal cancer occurred in one-third of incidental cancers. The reasons for this low mortality rate are unknown. Two recent studies show that participation in an adequately performed surveillance program reduces mortality from EAC29 and that EAC is detected at an earlier stage during BE surveillance.30 In our cohort, most of the patients who progressed were diagnosed with HGD or superficial EAC. A recent meta-analysis shows that PPI therapy is associated with a significantly decreased risk of progression to EAC or HGD.31 The patients in our cohort had been receiving PPI for a long time.
When all types of follow-up (endoscopic and non-endoscopic) were considered, the observed risk of progression to EAC and HGD/EAC was similar to that observed only during endoscopic surveillance.
Surveillance intervals proposed by the guidelines are weak recommendations that are based on low-quality evidence.32 The American College of Gastroenterology guidelines33 suggest endoscopic surveillance of NDBE every 3 years, while the American Gastroenterology Association32 suggests intervals of 3–5 years. The British Society of Gastroenterology34 stratifies the intervals according to the BE length, suggesting 2–3 years for the long segments and a 3–5 year interval for SSBE. The very low HGD/EAC risk that was observed in the SSBE patients of our cohort strongly suggest that intervals of at least 5 years seem safe and adequate for these patients, in countries with low EAC. Increasing the surveillance intervals in SSBE will decrease the burden of endoscopic surveillance in BE. If these results are replicated in studies from other low-EAC incidence countries, the indication for surveillance in this setting must be challenged.
The strengths of this study of a prospective cohort of BE patients include the following:
A well-defined diagnostic criterion for BE for inclusion in the cohort;
The exclusion of patients with any degree of dysplasia from the index endoscopy or before;
The long-term follow-up, both endoscopic and total; and
The very small number of patients lost to follow-up.
Meanwhile, its limitations include the following:
The relatively small size of the cohort, which was associated with an unexpected very low number of events; and
The inaccessible information on the cause of death, besides cancer-related causes.
In conclusion, the EAC, HGD and HGD/EAC incidence in our cohort of Portuguese patients with NDBE was very low. Therefore, current international surveillance guidelines use in countries with a low EAC incidence must probably be reassessed, principally those for SSBE.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Cooper BT, Barbezat GO. Barrett's oesophagus: A clinical study of 52 patients. Q J Med 1987; 62: 97–108. [PubMed] [Google Scholar]
- 2.Hameeteman W, Tytgat GN, Houthoff HJ, et al. Barrett's esophagus: Development of dysplasia and adenocarcinoma. Gastroenterology 1989; 96: 1249–1256. [DOI] [PubMed] [Google Scholar]
- 3.Shaheen NJ, Crosby MA, Bozymski EM, et al. Is there publication bias in the reporting of cancer risk in Barrett's esophagus? Gastroenterology 2000; 119: 333–338. [DOI] [PubMed] [Google Scholar]
- 4.De Jonge PJ, Van Blankenstein M, Looman CW, et al. Risk of malignant progression in patients with Barrett's oesophagus: A Dutch nationwide cohort study. Gut 2010; 59: 1030–1036. [DOI] [PubMed] [Google Scholar]
- 5.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011; 365: 1375–1383. [DOI] [PubMed] [Google Scholar]
- 6.Thomas T, Abrams KR, De Caestecker JS, et al. Meta analysis: Cancer risk in Barrett's oesophagus. Aliment Pharmacol Ther 2007; 26: 1465–1477. [DOI] [PubMed] [Google Scholar]
- 7.Yousef F, Cardwell C, Cantwell MM, et al. The incidence of esophageal cancer and high-grade dysplasia in Barrett's esophagus: A systematic review and meta-analysis. Am J Epidemiol 2008; 168: 237–249. [DOI] [PubMed] [Google Scholar]
- 8.Wani S, Puli SR, Shaheen NJ, et al. Esophageal adenocarcinoma in Barrett's esophagus after endoscopic ablative therapy: A meta-analysis and systematic review. Am J Gastroenterol 2009; 104: 502–513. [DOI] [PubMed] [Google Scholar]
- 9.Sikkema M, De Jonge PJ, Steyerberg EW, et al. Risk of esophageal adenocarcinoma and mortality in patients with Barrett's esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2010; 8: 235–244. [DOI] [PubMed] [Google Scholar]
- 10.Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett's oesophagus: A meta-analysis. Gut 2012; 61: 970–976. [DOI] [PubMed] [Google Scholar]
- 11.Rugge M, Zaninotto G, Parente P, et al. Barrett's esophagus and adenocarcinoma risk: The experience of the North-Eastern Italian Registry (EBRA). Ann Surg 2012; 256: 788–795. [DOI] [PubMed] [Google Scholar]
- 12.Wani S, Falk G, Hall M, et al. Patients with nondysplastic Barrett's esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2011; 9: 220–227. [DOI] [PubMed] [Google Scholar]
- 13.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett's esophagus patients: Results from a large population-based study. J Natl Cancer Inst 2011; 103: 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: The Prague C & M criteria. Gastroenterology 2006; 131: 1392–1399. [DOI] [PubMed] [Google Scholar]
- 15.Desai TK, Samala N. The incidence of esophageal adenocarcinoma among patients with nondysplastic Barrett's esophagus has been overestimated. Clin Gastroenterol Hepatol 2011; 9: 363–365. [DOI] [PubMed] [Google Scholar]
- 16.Cooper S, Menon S, Nightingale P, et al. Risk factors for the development of oesophageal adenocarcinoma in Barrett's oesophagus: A UK primary care retrospective nested case-control study. Unit Europ Gastroenterol J 2014; 2: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shakhatreh MH, Duan Z, Kramer J, et al. The incidence of esophageal adenocarcinoma in a national veterans cohort with Barrett's esophagus. Am J Gastroenterol 2014; 109: 1862–1869. [DOI] [PubMed] [Google Scholar]
- 18.Picardo SL, O'Brien MP, Feighery R, et al. A Barrett's esophagus registry of over 1000 patients from a specialist center highlights greater risk of progression than population-based registries and high risk of low-grade dysplasia. Dis Esophagus 2015; 28: 121–126. [DOI] [PubMed] [Google Scholar]
- 19.Corley DA. Understanding cancer incidence in Barrett's esophagus: Light at the end of the tunnel. J Natl Cancer Inst 2011; 103: 994–995. [DOI] [PubMed] [Google Scholar]
- 20.Wani S. Population-based estimates of cancer and mortality in Barrett's esophagus: Implications for the future. Clin Gastroenterol Hepatol 2011; 9: 723–724. [DOI] [PubMed] [Google Scholar]
- 21.Anaparthy R, Gaddam S, Kanakadandi V, et al. Association between length of Barrett's esophagus and risk of high-grade dysplasia or adenocarcinoma in patients without dysplasia. Clin Gastroenterol Hepatol 2013; 11: 1430–1436. [DOI] [PubMed] [Google Scholar]
- 22.Registo Oncologico Regional (ROR) Sul. Incidência, sobrevivivência e mortalidade por cancro na região sul de Portugal - ISM 2008 | 2009 [Incidence, survival and mortality (ISM) from cancer in the southern region of Portugal: ISM 2008/2009]. Lisbon: ROR Sul, 2014.
- 23.Bennett C, Moayyedi P, Corley DA, et al. BOB CAT: A large-scale review and Delphi Consensus for management of Barrett's esophagus with no dysplasia, indefinite for, or low-grade dysplasia. Am J Gastroenterol 2015; 110: 662–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad GA, Bansal A, Sharma P, et al. Predictors of progression in Barrett's esophagus: Current knowledge and future directions. Am J Gastroenterol 2010; 105: 1490–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikkema M, Looman CW, Steyerberg EW, et al. Predictors for neoplastic progression in patients with Barrett's esophagus: A prospective cohort study. Am J Gastroenterol 2011; 106: 1231–1238. [DOI] [PubMed] [Google Scholar]
- 26.Schouten LJ, Steevens J, Huysentruyt CJ, et al. Total cancer incidence and overall mortality are not increased among patients with Barrett's esophagus. Clin Gastroenterol Hepatol 2011; 9: 754–761. [DOI] [PubMed] [Google Scholar]
- 27.Caygill CP, Royston C, Charlett A, et al. Mortality in Barrett's esophagus: Three decades of experience at a single center. Endoscopy 2012; 44: 892–898. [DOI] [PubMed] [Google Scholar]
- 28.Solaymani-Dodaran M, Card TR, West J. Cause-specific mortality of people with Barrett's esophagus compared with the general population: A population-based cohort study. Gastroenterology 2013; 144: 1375–1383. [DOI] [PubMed] [Google Scholar]
- 29.Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett's esophagus and mortality from esophageal adenocarcinoma: A population-based cohort study. Am J Gastroenterol 2014; 109: 1215–1222. [DOI] [PubMed] [Google Scholar]
- 30.Kastelein F, Van Olphen SH, Steyerberg EW, et al. Impact of surveillance for Barrett's oesophagus on tumour stage and survival of patients with neoplastic progression. Gut . Epub ahead of print 22 April 2015. DOI: 10.1136/gutjnl-2014-308802. [DOI] [PubMed] [Google Scholar]
- 31.Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: A systematic review and meta-analysis. Gut 2014; 63: 1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 2011; 140: 1084–1091. [DOI] [PubMed] [Google Scholar]
- 33.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol 2008; 103: 788–797. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald RC, Di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014; 63: 7–42. [DOI] [PubMed] [Google Scholar]