Abstract
Background
A medical device containing the film-forming agent reticulated protein and a prebiotic mixture of vegetable oligo- and polysaccharides has been developed, recently receiving European approval as MED class III for the treatment of chronic/functional or recidivant diarrhoea due to different causes including irritable bowel syndrome (IBS). In the present paper, we evaluate a protein preparation containing these components in comparison with placebo in adult patients with diarrhoea-predominant IBS.
Methods
In a randomised, placebo-controlled, double-blind, parallel group, multicentre clinical trial, patients were randomly assigned to receive the combination of oligo- and polysaccharides and reticulated protein and placebo (four oral tablets/day for 56 days). Demographic, clinical and quality of life characteristics and presence and intensity of abdominal pain and flatulence (seven-point Likert scale) were assessed at three study visits (baseline and at 28 and 56 days). Stool emissions were recorded on the diary card using the seven-point Bristol Stool Scale.
Results
A total of 128 patients were randomised to receive either tablets containing the combination (n = 63) or placebo (n = 65). Treatment with oligo- and polysaccharides and reticulated protein was safe and well tolerated. A significant improvement in symptoms across the study was observed in patients treated with oligo- and polysaccharides and reticulated protein between visit 2 and visit 3 in abdominal pain (p = 0.0167) and flatulence (p = 0.0373). We also detected a statistically significant increase in the quality of life of patients receiving the active treatment from baseline to visit 3 (p < 0.0001).
Conclusions
Treatment with oligo- and polysaccharides and reticulated protein is safe, improving IBS symptoms and quality of life of patients with diarrhoea-predominant IBS.
Keywords: Irritable bowel syndrome, reticulated protein, vegetable oligo-saccharides, vegetable polysaccharides, stools, efficacy, mucosal protectors, abdominal pain, flatulence, quality of life
Introduction
Irritable bowel syndrome (IBS) is the most frequently diagnosed functional gastrointestinal disorder in primary and secondary care (up to 50% of all office visits to gastroenterology clinics).1–4 It is characterised by abdominal discomfort, pain and changes in bowel habits, in the absence of an organic cause.1,2 Discomfort or abdominal pain relieved by defecation, associated with changes in stool form, is considered a typical clinical manifestation in IBS.3,5–9 IBS is a problematic disorder resulting in impaired quality of life (QoL) and high health care costs and high resource utilisation,1–3,10 remaining a clinical challenge in the 21st century.3
The pathogenesis of IBS, not yet completely understood, is complex and multifactorial and includes physiological, emotional, cognitive and behavioural pathways (biopsychosocial model), a number of which implicate a role for the gastrointestinal microbiota.1–3,11 Due to the heterogeneity of IBS, there is no standard or definitive treatment and the chronic use of drugs should be minimised as much as possible or even avoided.3 Symptoms can be controlled by non-pharmacologic management during variable time periods and eliminating some exacerbating factors such as certain drugs, stressor conditions or changes in dietary habits.3 In fact, the development of new effective treatments to control the symptoms represents a huge challenge currently.4,12
In this regard, since non-pharmacological options including film-forming agents and prebiotics could have a role in IBS, a medical device containing the film-forming agent reticulated protein and a prebiotic mixture of vegetable oligo- and polysaccharides has been developed, recently receiving European approval as MED class III for the treatment of chronic/functional or recidivant diarrhoea due to different causes, including IBS.
This combination represents a new non-pharmacological option for the treatment of IBS based on the combination of a mucosal protector, with film-forming properties to control diarrhoea,13,14 and the prebiotic mixture of vegetable oligo- and polysaccharides, which could have a role in the microbiota’s composition, especially by increasing bifidobacteria, which can be regarded as a marker of intestinal health.15,16 The present randomised, placebo-controlled, double-blind, parallel group, multicentre clinical trial was performed to evaluate the safety and efficacy of tablets containing oligo- and polysaccharides and reticulated protein, in comparison with placebo in adult patients with diarrhoea-predominant IBS.
Methods
The study protocol was approved by the Ethics Committee of Human Experimentation in Romania and procedures were in accordance with the ethical standards laid down in the Declaration of Helsinki, as revised in the year 2000. Written informed consent was obtained from all participants. Patients were recruited in different Romanian private offices of general practitioners (with the participation of 15 centres from Bucharest, Galati, Craiova and Gluj) in the context of their routine clinical practice.
Study population and study design
This randomised, placebo-controlled, double-blind, parallel group, multicentre clinical trial was performed to evaluate the safety and efficacy of the active treatment (tablets containing a mixture of vegetable oligo- and polysaccharides: 750 mg; reticulated protein, 250 mg; and the excipients corscarmellose sodium, 133 mg and magnesium stearate, 17 mg), in comparison with placebo (tablets containing corn starch, corscarmellose sodium and magnesium stearate) in adult patients with diarrhoea-predominant IBS, with good general health status (normal physical and analytical conditions). Potential participants were excluded if they had organic gastrointestinal diseases.
The mixture of oligo- and polysaccharides is manufactured by Beneo orafti, GmbH (Germany) by the extraction from vegetable roots (chicory).
The reticulated protein is manufactured by Laboratorios Argenol, SA (Spain) by the mixture of tannins and protein (gelatin).
The dose used in this clinical study is based on the results of previous preclinical studies performed by the company.
Diarrhoea-predominant IBS was diagnosed following Rome III criteria,2,17 according to which recurrent abdominal pain or discomfort had to be present at least three days/month in the last three months, associated with two or more points: improvement with defecation, onset associated with a change in frequency of stools, onset associated with a change in form (appearance) of stool. In diarrhoea-predominant IBS, the stool pattern includes loose (mushy) or watery stools (Bristol scale 6–7)18,19 ≥ 25% and hard or lumpy stool <25% in the absence of antidiarrhoeal or laxative use.2,17,20
Participants’ allocation to treatment group was determined by a computer-generated randomisation list, which was stratified by centre. Double-blinding procedures were applied during the whole study period.
Patients were randomly assigned to receive the combination (Novintethical Pharma, SA) and placebo at a ratio of 1:1 in the form of oral tablets at a posology of four tablets/day (two before breakfast and two before dinner) during a period of eight weeks (56 days).
The active and placebo tablets were manufactured by direct compression, following the processes of dispensing, mixing and packaging.
After intake of tablets, the active substances of the product, a mixture of vegetable oligo- and polysaccharides and reticulated protein, are not absorbed in the gastrointestinal tract. Their effect is local on the intestinal mucosa.
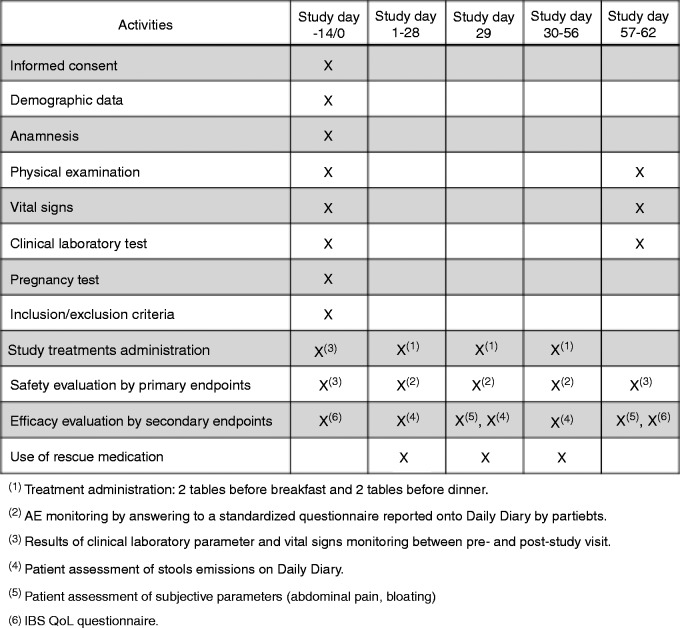
The study chronogram and workflow are shown in Figure 1(a) and (b), respectively. During the first enrolment visit, patients from the two groups were given a 56-day treatment (with the first dose being administered at the time of recruitment) and were instructed to complete a patient diary card to daily register the time of treatment intake, the severity and frequency of adverse events (AEs), the number and type of stool emissions and the use of rescue medication for IBS symptoms. At this visit, demographic and clinical characteristics were also recorded.
Figure 1.
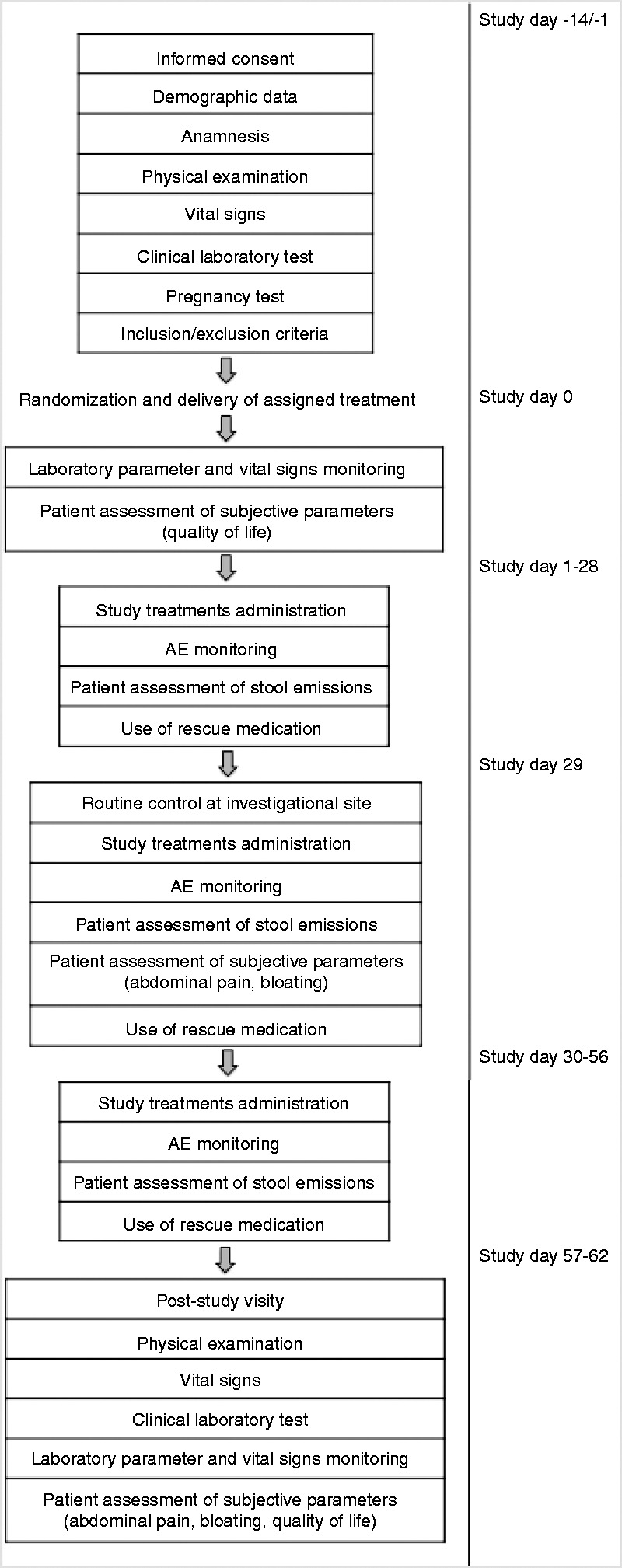
Study chronogram (a) and study workflow (b). AE: adverse event; IBS: irritable bowel syndrome; QoL: quality of life.
During visit 1 and visit 3 (day 56), patients were interviewed using the IBS QoL questionnaire21 and physical examination and blood sampling for haematological and biochemical analysis were performed (Figure 1(a)).
During visits 1, 2 (day 28) and 3, patients measured the presence and intensity of abdominal pain and flatulence on a seven-point Likert scale (7 = very much better, 6 = much better, 5 = somewhat better, 4 = same, 3 = somewhat worse, 2 = much worse, 1 = very much worse) (Figure 1(a)).
Stool emissions (including number of emissions/day) were recorded on the diary card and the consistency of each stool was assessed using the seven-point Bristol Stool Scale.18,19
At visit 3, patients returned the completed diary card and the remaining medication and data were transferred to the case report form. Adherence of treatment was assessed by calculation of the percentage of patients who took all the recommended medication during the 56-day period.
The IBS QoL questionnaire is a 34-item measure constructed specifically to assess the subjective well-being of patients with IBS, including eight dimensions (dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual dysfunction, and relationships). Each item is scored on a five-point scale (1 = not at all, 5 = a great deal). The summed total score is transformed to a zero to 100 scale ranging from zero (maximum QoL) to 100 (minimum).21 In the version we used, higher scores on the IBS QoL indicated poorer QoL. This version has been used to assess QoL of IBS patients in other published studies.22
Primary variables
The primary safety variable was the prevalence of AEs in both groups of patients. The frequency, intensity and relationship with the studied product were recorded during the three study visits, together with significant changes in vital signs and analytical parameters.
The primary efficacy variable was the rate of clinical remission at four and eight weeks, defined as the disappearance of diarrhoea, i.e. two or less nonwatery stools emissions per day (stool of type 5 or less on the Bristol scale).18,19
Statistical analysis
Sample size (n = 130, n = 65 in each group) was calculated to have an 80% of power to detect, with 95% probability, a non-inferiority margin difference between the group proportions of 0.1100.
The comparison between the active and the placebo group on the clinical remission was performed using Fisher’s exact test and by constructing a 95% confidence interval (CI) around the difference between the clinical remission rates showed by the active group and placebo group.
Non-parametric analyses were carried out on the abdominal pain and flatulence parameters to test the presence of any significant difference between the improvement rates of the treatment group vs placebo group. An independent sample t-test was used to compare the changes from baseline on the IBS QoL summed scores of the two groups.
The difference between the frequency of AEs occurrence was assessed using Fisher’s exact test and by constructing a 95% CI around the difference between the mean frequency of AEs occurrence in both groups.
In all cases, p < 0.05 was considered significant. According to CPMP/ICH/363/96 and CPMP/EWP/482/99 guidelines, both two-sided (at 95% significance level) and one-sided tests (at 97.5% significance level) were performed. Statistical analyses were performed using IBM SPSS 19 for Windows.
Results
A total of 130 Caucasian patients were recruited (n = 65 in each group) and 128 patients were randomised to receive active treatment (n = 63) and placebo (n = 65), two patients being excluded.
Demographic characteristics were homogeneous in both groups (Table 1), with more women than men in the whole sample (69.35% vs 30.65%) and in both groups and with a mean age of 48.29 ± 13.95 years in the active group and 47.72 ± 14.21 years in the placebo group (Table 1).
Table 1.
Baseline characteristics, anthropometric data, analytical parameters and concomitant and previous diseases
| Statistic variable | Active group | Placebo group | |
|---|---|---|---|
| Gender (F/M) | n (%) | 43 (68.25)/20 (31.75) | 45 (69.23)/20 (30.77) |
| Age (years) | Mean (SD) | 48.29 (13.95) | 47.72 (14.21) |
| Weight (kg) | Mean (SD) | 69.91 (13.48) | 72.27 (13.70) |
| Height (cm) | Mean (SD) | 166.00 (7.34) | 167 (8.92) |
| Temperature (℃) | Mean (SD) | 36.50 (0.41) | 36.49 (0.30) |
| Systolic blood pressure (mmHg) | Mean (SD) | 124.1 (15.16) | 124.9 (15.99) |
| Diastolic blood pressure (mmHg) | Mean (SD) | 76.77 (9.85) | 76.58 (10.51) |
| Heart rate (beats/min) | Mean (SD) | 71.6 (7.68) | 71.8 (8.61) |
| Analytical parameters | Mean (SD) | ||
| Haemoglobin (g/dl) | 13.68 (1.31) | 13.80 (1.42) | |
| Glucose (mg/dl) | 96.66 (26.93) | 95.33 (17.07) | |
| Creatinine (mg/dl) | 0.87 (0.18) | 0.86 (0.22) | |
| AST (U/l) | 24.48 (10) | 22.40 (7.71) | |
| ALT (U/l) | 28.26 (13.89) | 25.75 (14.01) | |
| Gamma-GT (U/l) | 32.66 (25.46) | 32.20 (20.62) | |
| Alkaline phosphatase (U/l) | 115.9 (62.56) | 113.70 (55.75) | |
| Erythrocyte sedimentation rate (mm/h) | 13.13 (10.20) | 16.67 (17.06) | |
| Concomitant and previous diseases | n (%) | ||
| Cardiovascular diseases | 7 (11.11) | 6 (9.23) | |
| Endocrine diseases | 4 (6.35) | 2 (3.07) | |
| Gastrointestinal diseases | 3 (4.76) | 7 (10.67) | |
| Hepatic diseases | 2 (3.17) | 3 (4.61) | |
| Musculoskeletal diseases | 1 (1.59) | 2 (3.07) | |
| Dermatological diseases | 1 (1.59) | – | |
| Respiratory diseases | 1 (1.59) | – | |
| Urinary disorders | 1 (1.59) | 2 (3.07) | |
| Arteriosclerosis | – | 1 (1.54) |
F: female; M:male; AST: aspartate aminotransferase; ALT: alanine aminotransferase; Gamma-GT: gamma-glutamyltransferase.
Parameters recorded at baseline, including vital signs and analytical analyses, were comparable in both groups (Table 1). Similar profiles of concomitant and previous diseases were recorded in both groups, with similar percentages of patients having previous and underlying diseases (72.13% in the active group vs 76.56% in placebo) (Table 1).
Concomitant medications taken during the study period were similar in both groups (Table 2).
Table 2.
Concomitant medications taken during the study period
| Group | Medication (active substance) | Duration of treatment during the study period |
|---|---|---|
| Active group | Metoprolol/enalapril | 56 days |
| Sucralfate | 1 day | |
| Oral combined contraceptives | 56 days | |
| Perindopril/indapamide | 56 days | |
| Amlopidine/metoprolol/gingko biloba/diosmine | 56 days | |
| Atenolol/glycazide/insulin | 56 days | |
| Pantoprazole | 29 days | |
| Placebo group | Levothyroxine/indapamide/enalapril | 56 days |
| Omeprazol/ramipril | 56 days | |
| Metoprolol/enalapril | 56 days | |
| Oral combined contraceptives | 56 days | |
| Perindropil/metoprolol/leflunomide/AAS/metformine | 56 days | |
| Metoprolol/quinapril/fenofibrate/esomeprazol/nicergoline/AAS |
AAS: acetylsalicylic acid.
Results of adherence showed that all 128 patients included in the analysis were 100% complaint with the study treatment.
Regarding the primary study variable, i.e. safety assessment, in the whole sample, a total of 15 and nine AEs were reported at visits 2 and 3, respectively. At visit 2, nine AEs (14.75% of patients) were reported in the active group and six (10.00%) in the placebo group, without statistically significant differences in AE prevalence between both groups (p = 0.1533). At visit 3, the occurrence of AEs decreased, with four events (6.67%) in the active group and five (8.47%) in the placebo group, with statistically significant differences between both groups (p = 0.0361).
In both groups, main reported AEs included gastrointestinal events such as abdominal and stomach ache, difficulties evacuating, and bloating. In the active group headache was also reported, while in the placebo group, insomnia, agitation and itching were also observed. All of them were of mild or moderate intensity and no serious AEs were reported during the whole study period. Only gastrointestinal events were considered as possibly related to the study treatment.
Active treatment did not produce relevant changes in clinical signs, without statistically significant differences between baseline and visit 3 values.
Likewise, no statistically significant changes were observed in the analytical parameters after active treatment.
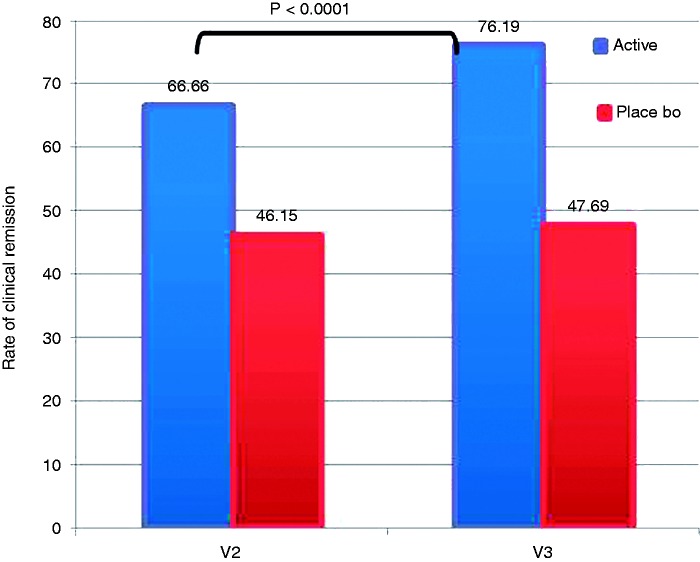
Regarding the primary efficacy variable, i.e., the rate of clinical remission at four and eight weeks, defined as the disappearance of diarrhoea, i.e. two or less nonwatery stools emissions per day (stool of type 5 or less on the Bristol scale), we observed a statistically significant increase in the rate of clinical remissions across the study in the active group (also higher than in the placebo group) (66.66% vs 46.15% at visit 2 and 76.19% vs 47.69% at visit 3, respectively) (p < 0.0001 among visits, Kruskal-Wallis test) (Figure 2).
Figure 2.
Rate of clinical remission during the study period (defined as the disappearance of diarrhoea, i.e. two or less nonwatery stools emissions per day (stool of type 5 or less on the Bristol scale).
In patients treated with oligo- and polysaccharides and reticulated protein, the mean (±SD) number of stools emitted at days 1, 28 and 56 was 2.91 (±1.63), 2.43 (±1.41) and 2.25 (±1.47), respectively. Patients treated with oligo- and polysaccharides and reticulated protein during 28 or 56 days reduced the number of stools emissions compared to patients treated with placebo during the same period of time, in a statistically significant manner (Student t test; p = 0.031 at day 28 and p = 0.001 at day 56). The mean reduction of stool emissions was 0.53 (95% CI: 0.05 to 1.02 stool emissions) at day 28 and 0.84 (95% CI: 0.34 to 1.33 stool emissions) at day 56. A higher reduction of stool emissions was observed at longer periods of treatment with oligo- and polysaccharides and reticulated protein: 28 days of treatment reduced the stool emissions in a mean average of 0.48 (95% CI: 0.17 to 0.78 stool emissions) compared to day 1 (p = 0.02; paired t test), whereas 56 days of treatment reduced the stool emissions a mean average of 0.656 (95% CI: 0.29 to 1.02 stool emissions) compared to day 1 (p = 0.01).
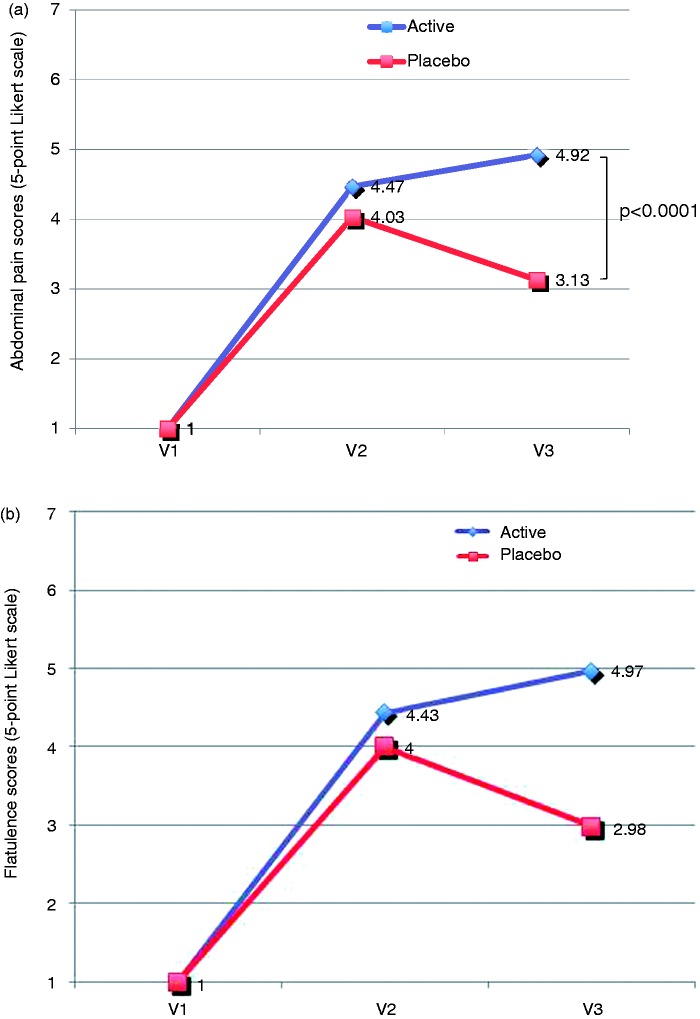
In the whole sample, we reported an improvement in the intensity of abdominal pain and flatulence measured on a seven-point Likert scale, with significant increases in the percentages of patients feeling very much better from visit 2 to visit 3 (8.80% vs 26.23% for abdominal pain and 8.94% vs 26.23% for flatulence). In the active group, we also detected a significant improvement in symptoms across the study, with statistically significant differences between visit 2 (day 28) and visit 3 (day 56) in the abdominal pain (p = 0.0167, visit 2 vs visit 3, Mann Whitney test) and flatulence assessment (p = 0.0373, visit 2 vs visit 3) (Figures 3(a) and (b)).
Figure 3.
Evolution of abdominal pain and flatulence according to a seven-point Likert scale. (a) Evolution of abdominal pain in active and placebo groups from baseline to visit 3 based on a seven-point Likert scale (7 = very much better, 6 = much better, 5 = somewhat better, 4 = same, 3 = somewhat worse, 2 = much worse, 1 = very much worse). (b) Evolution of flatulence in active and placebo groups from baseline to visit 3 based on a seven-point Likert scale (7 = very much better, 6 = much better, 5 = somewhat better, 4 = same, 3 = somewhat worse, 2 = much worse, 1 = very much worse).
At the end of the study, the percentage of patients with abdominal pain was significantly lower in the active group than in the placebo group (0.6% and 58.4% of patients, respectively) (p < 0.05). The mean average of abdominal pain score was 1.79 (95% CI: 1.38 to 2.12 score), this score being higher in patients treated with reticulated protein and oligo- and polysaccharides, with a mean (±SD) score of 4.92 (±0.86), than in patients treated with placebo, with a mean (±SD) score of 3.13 (±1.36) (Figure 3(a)). The mean difference was statistically significant (p < 0.0001).
Regarding flatulence, treatment with oligo- and polysaccharides and reticulated protein produced a mean reduction of 0.43 (95% CI: 0.13 to 0.728) in the score reported by the patients at the end of the study, this reduction being statistically significant (paired t test, p = 0.005). At the end of the study, the mean average flatulence score was 1.98 (95% CI: 1.58 to 2.38 score), significantly higher in patients treated with oligo- and polysaccharides and reticulated protein than in patients treated with placebo (p < 0.0001) (Figure 3(b)). At the end of the study, the percentage of patients with flatulence was significantly lower than the percentage in the active group than in patients treated with placebo (0.8% and 63.1% of patients, respectively) (Fisher’s exact test, p < 0.05).
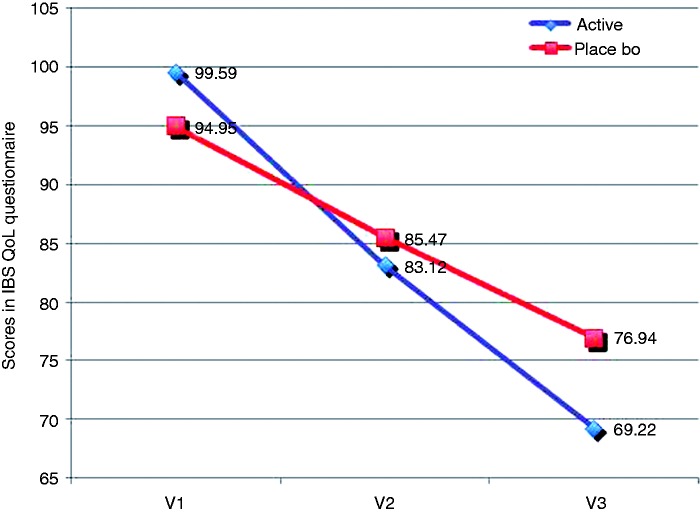
Regarding the punctuation in the IBS QoL questionnaire, we detected a statistically significant increase in the QoL of patients receiving oligo- and polysaccharides and reticulated protein from baseline to visit 3 (99.59 ± 23.17 at baseline to 69.22 ± 24.79 at visit 3, p < 0.0001, Mann Whitney test) (Figure 4), while a lower improvement in QoL was detected in the placebo group (94.95 ± 25.62 at baseline to 76.94 ± 27.12 at visit 3, p < 0.0001, unpaired t test) (Figure 4). In the comparison of scores obtained between groups, we detected statistically a significant higher improvement in the active group than in the placebo group (p = 0.0053, Mann-Whitney U test).
Figure 4.
Scores obtained in the IBS QoL questionnaire from baseline to visit 3. The IBS QoL questionnaire is a 34-item measure constructed specifically to assess the subjective well-being of patients with IBS, including eight dimensions (dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual dysfunction, and relationships). Each item is scored on a five-point scale (1 = not at all, 5 = a great deal). To facilitate score interpretation, the summed total score is transformed to a zero to 100 scale ranging from zero (maximum QoL) to 100 (minimum QoL). IBS: irritable bowel syndrome; QoL: quality of life.
Finally, treatment compliance was high in the whole sample (100% at visit 2 and 99.16% at visit 3).
Discussion
Nowadays, in a context in which IBS is described as multifactorial with a strong psychosocial component, in which many different drugs have been proposed but with debatable real benefits,1,12 the use of non-pharmacological strategies, such as prebiotics and/or film-forming agents is receiving increasing attention.4,16,23–27
In our study, for the first time, we have demonstrated that the combination of a mixture of oligo- and polysaccharides and a reticulated protein is a safe and feasible treatment to reduce main symptoms and to improve QoL in patients with IBS. Both ingredients are not absorbed, their effect local on the intestinal mucosa. We consider that, since IBS is a multifactorial disease in which dietary habits and gastrointestinal microbiota have a role, we consider that the favourable effects observed are due to the synergistic action of both components, acting on the intestinal mucosa (reticulated protein) and increasing bifidobacteria counts (mixture of oligo- and polysaccharides). These two effects have synergistically produced the observed decrease of IBS symptoms, such as diarrhoea, abdominal pain and flatulence, and the increase of QoL.
These results support the use of the combination vs the use of the components alone (reticulated protein and oligo- and polysaccharides), as reported in previous studies where no clear benefits were observed with these or similar compounds.15,28,29
In a multicentre, randomised, double-blind, placebo-controlled parallel group study consisting of a two-week trial in 96 patients with IBS, although symptoms worsened in patients with IBS at the onset of treatment with 20 g fructo-oligosaccharides/day, continuous treatment for 12 weeks resulted in no worsening of symptoms.29
Better results were obtained in another comparative, randomised, double-blind study in which participants with IBS symptoms were randomised to receive either 5 g of short-chain fructo-oligosaccharides or placebo daily in divided doses. Following six weeks of supplementation, a statistically significant decrease in symptom scores was observed in the group taking fructo-oligosaccharides compared to placebo.15,28
In our study, the use of a combination of a prebiotic and a film-forming agent can provide synergistic effects to regulate the two main conditions of IBS, visceral hypersensitivity (which leads to abdominal discomfort or pain) and gastrointestinal motor disturbances (leading to diarrhoea or constipation),17 by influencing the gut microbiota balance (mixture) and the micro-inflammation state of the intestine (protein) present in patients with IBS.
These inulin-type prebiotics are oligo- or polysaccharide long chains comprised primarily of linked fructose molecules that are considered to be bifidogenic (stimulating the growth of bifidobacteria and the Lactobacilli species), thus promoting specific changes in the composition of the gastrointestinal microbiota.15,30–32
Since the role of the gastrointestinal microbiota in the pathogenesis of IBS, and in particular the relative lower numbers of bifidobacteria in diarrhoea-predominant IBS, has been demonstrated,2,4 the results of our study support the use of the mixture in IBS at the studied low doses (1.2 g/day). Our results are in line with other studies,2,33 indicating that the dose of the prebiotic is important in determining the clinical benefit in IBS, with some evidence that higher doses may have a negative impact on symptoms.2
Our results support the previous knowledge according to which a less diverse and unstable community of bacteria is present in IBS and that manipulation of microbiota can influence the key symptoms, including abdominal pain and bowel habit and other prominent features of IBS.4
In addition, the favourable results obtained in our study should also be attributed to the effect of the reticulated protein belonging to a new class of agents, which may be defined as ‘film-forming agents’ or ‘mucosal protectors’ (also including gelatin tannate, gelatin or xyloglucan) that are able to form a protective mucoadhesive film in the intestine, reducing inflammation of the wall13,23 (Bueno et al., in preparation). In previous in vitro and in vivo studies, we have demonstrated that this type of protein, linked to tannins' molecules, are able to prevent gut leakiness and subsequent inflammation by creating a consistent biobarrier in the intestine13,14 (Bueno et al., in preparation).
We consider that the reticulated protein, acting as an intestinal film-forming and protective agent, can also contribute to the reduction of characteristic symptoms in patients with diarrhoea-predominant IBS, thus leading to an improvement of QoL during the treatment.
Since in diarrhoea-predominant IBS an altered intestinal barrier is present and it is associated with immune activation and clinical symptoms,34 the use of mucosal protectors constitutes a new alternative for a more efficient control of symptoms in diarrhoea-predominant IBS.
Of note, for all efficacy variables, we obtained better outcomes at the end of the treatment after 56 days of treatment, than at visit 2 after 28 days. We consider that these results support the long-term use of the product to produce those intestinal changes necessary to decrease IBS symptoms and to increase QoL of patients.
Overall, the combination of oligo- and polysaccharides and reticulated protein is a safe and feasible option, able to reduce IBS symptoms and increase QoL in patients, thus supporting the inclusion of oligo- and polysaccharides and reticulated protein for the management of IBS in current clinical practice.
Acknowledgements
We acknowledge CEBIS International SRL (Bucharest, Romania) for the performance and statistical analysis of the study. EudraCT number: 2012-002915-24.
Funding
This work was supported by Novintethical, SA, Lugano, Switzerland.
Conflict of interest
Núria Piqué received honoraria from Novintethical, SA, to write this article. The other authors have nothing to declare.
References
- 1.Bellini M, Gambaccini D, Stasi C, et al. Irritable bowel syndrome: A disease still searching for pathogenesis, diagnosis and therapy. World J Gastroenterol 2014; 20: 8807–8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelan K. Mechanisms and effectiveness of prebiotics in modifying the gastrointestinal microbiota for the management of digestive disorders. Proc Nutr Soc 2013; 72: 288–298. [DOI] [PubMed] [Google Scholar]
- 3.Soares RL. Irritable bowel syndrome: A clinical review. World J Gastroenterol 2014; 20: 12144–12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy PJ, Cryan JF, Dinan TG, et al. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World J Gastroenterol 2014; 20: 14105–14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minocha A, Johnson WD, Abell TL, et al. Prevalence, sociodemography, and quality of life of older versus younger patients with irritable bowel syndrome: A population-based study. Dig Dis Sci 2006; 51: 446–453. [DOI] [PubMed] [Google Scholar]
- 6.Quigley EM, Bytzer P, Jones R, et al. Irritable bowel syndrome: The burden and unmet needs in Europe. Dig Liver Dis 2006; 38: 717–723. [DOI] [PubMed] [Google Scholar]
- 7.Saito YA, Locke GR, Talley NJ, et al. A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. Am J Gastroenterol 2000; 95: 2816–2824. [DOI] [PubMed] [Google Scholar]
- 8.Longstreth GF, Yao JF. Irritable bowel syndrome and surgery: A multivariable analysis. Gastroenterology 2004; 126: 1665–1673. [DOI] [PubMed] [Google Scholar]
- 9.Boyce PM, Koloski NA, Talley NJ. Irritable bowel syndrome according to varying diagnostic criteria: Are the new Rome II criteria unnecessarily restrictive for research and practice? Am J Gastroenterol 2000; 95: 3176–3183. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal N, Spiegel BM. The effect of irritable bowel syndrome on health—related quality of life and health care expenditures. Gastroenterol Clin North Am 2011; 40: 11–19. [DOI] [PubMed] [Google Scholar]
- 11.Parkes GC, Brostoff J, Whelan K, et al. Gastrointestinal microbiota in irritable bowel syndrome: Their role in its pathogenesis and treatment. Am J Gastroenterol 2008; 103: 1557–1567. [DOI] [PubMed] [Google Scholar]
- 12.De Ponti F. Drug development for the irritable bowel syndrome: Current challenges and future perspectives. Front Pharmacol 2013; 4: 7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esteban Carretero J, Durbán Reguera F, López-Argüeta Alvarez S, et al. A comparative analysis of response to vs. ORS + gelatin tannate pediatric patients with acute diarrhea [article in English and Spanish]. Rev Esp Enferm Dig 2009; 101: 41–48. [DOI] [PubMed] [Google Scholar]
- 14.Allegrini A, Costantini M. Gelatine tannate for the treatment of acute diarrhoea in adults. J Gastroint Dig Syst 2012; 2: 3–3. [Google Scholar]
- 15.Kelly G. Inulin-type prebiotics: A review. (Part 2). Altern Med Rev 2009; 14: 36–55. [PubMed] [Google Scholar]
- 16.Roberfroid M, Gibson GR, Hoyles L, et al. Prebiotic effects: Metabolic and health benefits. Br J Nutr 2010; 104(Suppl 2): S1–S63. [DOI] [PubMed] [Google Scholar]
- 17.Saha L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol 2014; 20: 6759–6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaton KW, O’Donnell LJ. An office guide to whole-gut transit time. Patients’ recollection of their stool form. J Clin Gastroenterol 1994; 19: 28–30. [DOI] [PubMed] [Google Scholar]
- 19.Palsson OS, Baggish J, Whitehead WE. Episodic nature of symptoms in irritable bowel syndrome. Am J Gastroenterol 2014; 109: 1450–1460. [DOI] [PubMed] [Google Scholar]
- 20.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 21.Patrick DL, Drossman DA, Frederick IO, et al. Quality of life in persons with irritable bowel syndrome: Development and validation of a new measure. Dig Dis Sci 1998; 43: 400–411. [DOI] [PubMed] [Google Scholar]
- 22.Asadi M, Deh Cheshmeh MG, Mahmoodi M, et al. Relationship between student QoL with irritable bowel syndrome and related factors at Ahvaz Jundishapur University of Medical Sciences. Jundishapur J Chronic Dis Care 2015; 4: e26624–e26624. [Google Scholar]
- 23.Rahimi R, Abdollahi M. Herbal medicines for the management of irritable bowel syndrome: A comprehensive review. World J Gastroenterol 2012; 18: 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scaldaferri F, Pizzoferrato M, Gerardi V, et al. The gut barrier: New acquisitions and therapeutic approaches. J Clin Gastroenterol 2012; 46(Suppl): S12–S17. [DOI] [PubMed] [Google Scholar]
- 25.Condratovici CP, Llop X, Piqué N, et al. Xyloglucan and gelatine for the treatment of acute gastroenteritis in children: Results of a randomized, controlled, open-label, parallel group, multicentre, national clinical trial. UEG week 2015. Barcelona, Spain, 24–28 October 2015.
- 26.Gnessi L, Llop X and Piqué N. Xyloglucan for the treatment of acute diarrhea: Results of a randomized, controlled, open-label, parallel group, multicentre, national clinical trial. UEG week 2015. Barcelona, Spain, 24–28 October 2015. [DOI] [PMC free article] [PubMed]
- 27.Gnessi L, Piqué N and Llop X. Gélatine-xyloglucan est efficace et bien toléré dans le traitement des diarrhées aiguës. Résultats d’un essai clinique multicentrique en groupes parallèles, randomisé, ouvert et contrôlé. Journées Francophones d’Hépato-gastroentérologie et d’Oncologie Digestive (JFHOD). Paris, France, 19–22 March 2015, communication no. P-276, p.221.
- 28.Paineau D, Payen F, Panserieu S, et al. The effects of regular consumption of short-chain fructo-oligosaccharides on digestive comfort of subjects with minor functional bowel disorders. Br J Nutr 2008; 99: 311–318. [DOI] [PubMed] [Google Scholar]
- 29.Olesen M, Gudmand-Hoyer E. Efficacy, safety, and tolerability of fructooligosaccharides in the treatment of irritable bowel syndrome. Am J Clin Nutr 2000; 72: 1570–1575. [DOI] [PubMed] [Google Scholar]
- 30.Bakker-Zierikzee AM, Alles MS, Knol J, et al. Effects of infant formula containing a mixture of galacto- and fructo-oligosaccharides or viable Bifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br J Nutr 2005; 94: 783–790. [DOI] [PubMed] [Google Scholar]
- 31.Boehm G, Lidestri M, Casetta P, et al. Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecal bifidobacteria in preterm infants. Arch Dis Child Fetal Neonatal Ed 2002; 86: F178–F181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moro G, Arslanoglu S, Stahl B, et al. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child 2006; 91: 814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whelan K. Probiotics and prebiotics in the management of irritable bowel syndrome: A review of recent clinical trials and systematic reviews. Curr Opin Clin Nutr Metab Care 2011; 14: 581–587. [DOI] [PubMed] [Google Scholar]
- 34.Vicario M, González-Castro AM, Martínez C, et al. Increased humoral immunity in the jejunum of diarrhoea-predominant irritable bowel syndrome associated with clinical manifestations. Gut 2015; 64: 1379–1388. [DOI] [PubMed] [Google Scholar]