Abstract
Background and aim
This prospective randomized study was designed to assess the efficacy of 10-day and 14-day rifabutin-based triple therapy as a third- or fourth-line rescue therapy.
Methods
Patients who failed first- and second-line eradication therapy were enrolled. H. pylori was isolated from gastric biopsy specimens and the rpoB mutation status, a factor of resistance to rifamycins, and minimum inhibitory concentrations (MICs) of rifabutin and amoxicillin were determined. Enrolled patients were randomly assigned to receive 10-day or 14-day eradication therapy with esomeprazole (20 mg, 4 times a day (q.i.d.)), amoxicillin (500 mg, q.i.d.), and rifabutin (300 mg, once a day (q.d.s.)). Poor compliance was defined as intake of <80% of study drugs. Successful H. pylori eradication was confirmed using a [13C] urea breath test or a stool antigen test, 12 weeks after the end of therapy.
Results
Twelve patients were assigned to the 10-day group, and 17, to the 14-day group. Intention-to-treat and per-protocol analyses of eradication rates were 83.3% and 81.8% for the 10-day group and 94.1% and 91.7% for the 14-day group, respectively. All patients with rpoB mutation-positive strains (n = 3) showed successful eradication, irrespective of the regimen received. Therapy was stopped due to adverse events in 8.3% and 29.3% of patients in the 10-day and 14-day groups, respectively.
Conclusion
Both the 10-day and 14-day therapies were effective as rescue regimens. In particular, the 14-day therapy resulted in successful eradication in over 90% of patients, but the 10-day treatment may be enough to obtain a successful eradication rate, considering the tolerability of therapy.
Keywords: Rifabutin, rpoB, amoxicillin
Introduction
Helicobacter pylori infection is associated with peptic ulcers, gastric mucosa-associated lymphoid tissue lymphomas, and gastric cancer. Studies have shown that H. pylori eradication is effective for the treatment of these gastrointestinal diseases.1–3 The first-line regimen for the treatment of H. pylori infection in Japan is triple therapy with a proton pump inhibitor (PPI), amoxicillin, and clarithromycin, whereas the second-line regimen consists of a PPI, amoxicillin, and metronidazole.4,5 However, 2–3% of patients with H. pylori strains that are resistant to both first- and second-line regimens require third-line eradication therapy.6,7 In addition, we previously showed that eradication could be achieved in about 80% of patients with seven-day sitafloxacin-based triple therapy as a third-line regimen.8 Nevertheless, H. pylori may persist in some cases even with these eradication strategies. Rifabutin-based therapy has been reported as a rescue treatment in these patients.9–13 The target of all rifamycins, including rifabutin, is DNA-directed RNA polymerase, mostly β subunit encoded by the rpoB gene.14 There are no previous reports correlating the efficacy of rifabutin-containing treatment and the status of rpoB mutation.15–17 Although a meta-analysis showed that the eradication rate of 10-day therapy including rifabutin was superior to that of seven-day therapy with second-line treatment,18 the efficacy of a 10-day regimen including rifabutin as third- or fourth-line therapy has not been determined. Accordingly, we assessed the efficacy and safety of 10-day and 14-day rifabutin-based triple therapies as third-line or fourth-line regimens for H. pylori eradication and analyzed the correlation between the status of rpoB mutation and successful eradication.
Materials and methods
Study population
This study was a prospective, randomized, open-label study conducted at the Keio University Hospital (Tokyo, Japan). The study was approved by the research ethics committee of the Keio University School of Medicine (No. 20130195; 29 July 2013) and registered with the University Hospital Medical Information (UMIN) Clinical Trials Registry (UMIN000011963, http://www.umin.ac.jp/ctr/). Patients in whom eradication treatment with both first- (PPI, amoxicillin, and clarithromycin) and second-line (PPI, amoxicillin, and metronidazole) triple therapies failed were enrolled after obtaining written informed consent. Among them were also included patients who failed eradication even with sitafloxacin-based third-line treatment.
Study design
H. pylori isolates were obtained from gastric biopsy specimens before the start of treatment. The minimum inhibitory concentrations (MICs) of amoxicillin and rifabutin against H. pylori isolates and the rpoB mutation status of the H. pylori strains were determined using previously described methods.15,19 After stratification by the MICs of rifabutin (cut-off point, 0.25 µg/ml), randomization was performed by blind drawing of random cards contained in sealed envelopes. Then, patients were randomized to either the 10-day group (20 mg esomeprazole, 4 times a day (q.i.d.) 500 mg amoxicillin, q.i.d.; and 300 mg rifabutin, q.d.s.) or the 14-day group (20 mg esomeprazole, q.i.d.; 500 mg amoxicillin, q.i.d.; and 300 mg rifabutin, once a day (q.d.s.)).
Twelve weeks after the end of eradication therapy, successful eradication was confirmed using a [13C] urea breath test (UBT) or the H. pylori stool antigen test (HpSA).20 The cut-off value for a negative UBT was <2.5‰.21
Susceptibility of H. pylori to antimicrobial agents
The cut-off points for antimicrobial resistance of H. pylori were defined as 0.06 µg/ml for amoxicillin and 0.25 µg/ml for rifabutin, in accordance with previous reports.8,19
Outcomes
The main outcome measure was the overall eradication rate with the 10-day or 14-day regimen. The secondary outcome measures were the frequencies of adverse events and the eradication rates associated with rpoB mutation-positive or rpoB mutation-negative H. pylori strains with the 10-day or 14-day regimen. Associated factors for eradication success were also assessed.
Treatment compliance and adverse events
Patients were interviewed for adverse events at the end of treatment. Blood samples were collected before treatment and at the end of treatment. Treatment compliance was assessed by counting all leftover tablets at the end of the treatment period. Poor compliance was defined as intake of <80% of study drugs. We set the discontinuance criteria of administration as the appearance of a wide range of rashes, any ophthalmic symptoms, any psychological symptoms, a high fever, or other serious adverse events.
Statistical analysis
Comparison of the patients’ demographic characteristics, eradication rates, frequency of adverse events, and correlation between rpoB mutation status and the MICs of rifabutin were conducted with the Fisher's exact test and the Student's t-test, as appropriate. Statistical analyses were performed using SPSS 22 for Windows (SPSS Inc., Chicago, IL, USA). Data are expressed as the mean ± standard deviation.
Sample size and study conduct
According to the previous reports of third-line rifabutin based therapies,18 the eradication rates were almost 60–80%. Thus, assuming an eradication rate of 60.0% in the 10-day group and 80.0% in the 14-day group in our study, an α-error of 0.05, and a power of 0.80, we determined that 164 participants (82 patients in each group) were needed for this study. We estimated that the proportion of rpoB mutation-positive strains would be 0.2–2%. Therefore, over 170 participants were to be recruited to participate in this study.
Results
Patient characteristics
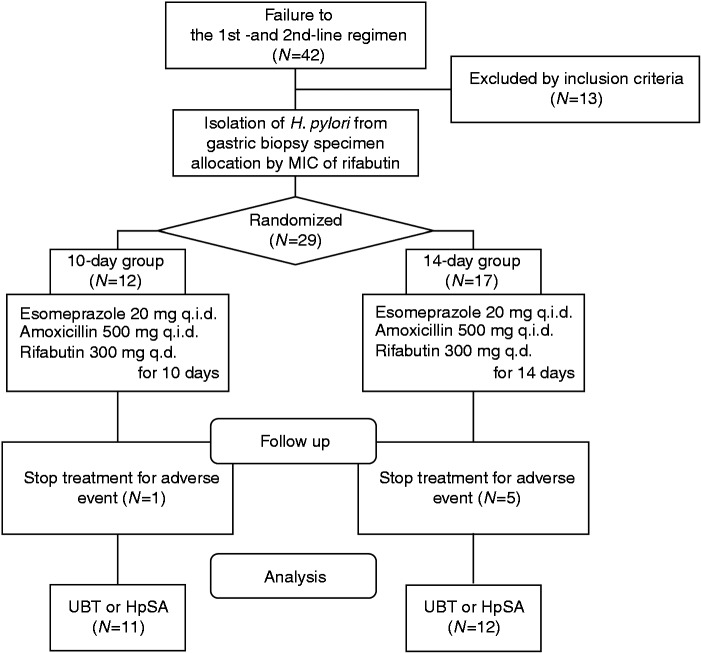
Although all adverse events were not serious, we have independently decided to prematurely terminate patient enrollment given the relatively high percentage of patients who could not complete the regimen because of adverse events in the 14-day group (29.4%). Overall, a total of 42 patients were enrolled in this study (Figure 1). Of these, 13 were excluded before randomization due to a loss to follow-up (n = 2), discontinued treatment before randomization (n = 4), or H. pylori not detectable by culture (n = 7). After stratification according to the MICs of rifabutin, 29 patients (12 men and 17 women; mean age, 48.0 ± 11.1 years) were randomly assigned to the 10-day group (12 patients) and 14-day group (17 patients) (intention-to-treat (ITT) patients). One patient with a rifabutin resistant strain (MICs ≥ 0.25) was assigned to the 14-day group. Of the 12 patients in the 10-day group, one was excluded from the per protocol (PP) analysis due to treatment withdrawal for adverse events. Of the 17 patients in the 14-day group, five were excluded from the PP analysis due to treatment withdrawal for adverse events.
Figure 1.
Flow diagram of this study.
HpSA: Helicobacter pylori stool antigen test; MIC: minimum inhibitory concentration; UBT, urea breath test.
No significant differences in demographic characteristics were observed between the two groups among the ITT patients (Table 1). RpoB-positive H. pylori strains were detected in one (7.1%) and two (11.8%) patients in the 10-day and 14-day groups, respectively. The rpoB mutation was detected only at positions L525 or V538 (Table 2). One patient in the 14-day group had a rifabutin-resistant H. pylori strain (Table 2). Two patients in the 14-day group had been previously diagnosed with peptic ulcers. A total of nine and 11 patients in the 10-day and 14-day groups, respectively, who had already received eradication treatment with sitafloxacin-based third-line regimens were treated as fourth-line eradication (Table 1).
Table 1.
Patients’ demographics.
| 10-day (n = 12) | 14-day (n = 17) | p-value | |
|---|---|---|---|
| Mean age, mean ± SD, years | 50.3 ± 13.9 | 46.5 ± 8.8 | 0.38a |
| Gender, n (%) | 0.25b | ||
| Male | 3 (25.0) | 9 (52.9) | |
| Female | 9 (75.0) | 8 (47.1) | |
| Smokers, n (%) | 1 (8.3) | 3 (17.6) | 0.62b |
| Alcohol drinkers, n (%) | 4 (33.3) | 8 (47.1) | 0.70b |
| BMI, mean ± SD, kg/m2 | 21.6 ± 2.5 | 22.7 ± 4.7 | 0.46a |
| Fourth eradication, n (%) | 9 (75.0) | 11 (64.7) | 0.55b |
| Dyspepsia, n (%) | 6 (50.0) | 9 (52.9) | 1.00b |
| Peptic ulcer, n (%) | 0 (0.0) | 2 (11.8) | 0.50b |
| H. pylori status | |||
| Presence of mutation in rpoB, n (%) | 1 (7.1) | 2 (11.8) | 1.00b |
| Resistance to rifabutin (MIC ≥ 0.25 µg/ml), n (%) | 0 (0.0) | 1 (5.9) | 1.00a |
| Rifabutin MIC, mean ± SD, µg/ml | 0.00 ± 0.01 | 0.06 ± 0.24 | 0.35a |
| Resistance to amoxicillin (MIC ≥ 0.06 µg/ml), n (%) | 8 (66.7) | 8 (47.1) | 0.45a |
| Amoxicillin MIC, mean ± SD, µg/ml | 0.24 ± 0.56 | 0.07 ± 0.08 | 0.31b |
BMI: body mass index; H. pylori: Helicobacter pylori; MIC: minimum inhibitory concentration; SD: standard deviation.
Alcohol drinkers were defined as people who consumed at least one drink of alcohol per month.
Student’s t-test: bFisher’s exact test.
Table 2.
Outcomes of patients with rpoB mutation-positive Helicobacter pylori.
| Eradication group | MICs of rifabutin (µg/ml) | Amino acid change | Eradication result | |
|---|---|---|---|---|
| Patient #1 | 10-day | 0.03 | Val 538 Ile | Success |
| Patient #2 | 14-day | 1 | Leu 525 Ile | Success |
| Patient #3 | 14-day | 0 | Val 538 Ile | Success |
H. pylori: Helicobacter pylori; MIC: minimum inhibitory concentration.
Correlation between rpoB mutation status and MICs of rifabutin
There was a significant association between rpoB mutation status and the MICs of rifabutin (p < 0.01). The mean MIC of the rpoB-negative strains was 0.00 µg/ml (95% confidence interval (CI), 0.00–0.01 µg/ml), and that of the rpoB-positive strains was 0.06 µg/ml (95% CI, 0.00–0.30 µg/ml). Two V538-mutated strains were sensitive to rifabutin, whereas the L525 mutated strain was resistant to rifabutin (Table 2). All strains without rpoB mutation were sensitive to rifabutin.
Eradication rates
In the ITT analysis, eradication rates of 83.3% (10/12; 95% CI, 58.6–100%) and 94.1% (16/17; 95% CI, 81.7–100%) were noted in the 10-day and 14-day groups, respectively. In the PP analysis, eradication rates of 81.8% (9/11; 95% CI, 54.6–100%) and 91.7% (11/12; 95% CI, 73.3–100%) were noted in the 10-day and 14-day groups, respectively. One patient in the 10-day group and five patients in the 14-day group, who had stopped treatment due to adverse events, finally achieved eradication.
All patients in whom H. pylori could not be eradicated were treated with a fourth-line regimen (Table 3). The eradication rates in the 10-day and 14-day fourth-line treatment groups were 77.8% (7/9; 95% CI, 43.9–100%) and 90.9% (10/11; 95% CI, 70.7–100%). On the other hand, three patients in the 10-day group and six patients in the 14-day group achieved complete eradication after third-line treatment.
Table 3.
Associated factors for the eradication success in each regimen
| Eradicated | Not-eradicated | p-value | |
|---|---|---|---|
| 10-day regimen | (n = 10) | (n = 2) | |
| H. pylori status | |||
| Presence of mutation in rpoB, n (%) | 1 (10.0) | 0 (0) | 1a |
| Rifabutin MIC, mean ± SD, µg/ml | 0.00 ± 0.01 | 0.00 ± 0.00 | 0.68b |
| Amoxicillin MIC, mean ± SD, µg/ml | 0.29 ± 0.61 | 0.00 ± 0.00 | 0.53b |
| Mean age, mean ± SD, years | 51.10 ± 14.46 | 46.00 ± 14.14 | 0.66b |
| Gender, n (%) | 1a | ||
| Male | 3 (30.0) | 0 (0) | |
| Female | 7 (70.0) | 2 (100) | |
| Smokers, n (%) | 2 (20.0) | 1 (50.0) | 0.45a |
| Alcohol drinkers, n (%) | 4 (40.0) | 0 (0) | 0.51a |
| BMI, mean ± SD, kg/m2 | 22.25 ± 2.52 | 20.96 ± 0.00 | 0.50b |
| Fourth eradication, n (%) | 7 (70) | 2 (100) | 1a |
| Dyspepsia, n (%) | 5 (50.0) | 1 (50.0) | 1a |
| Peptic ulcer, n (%) | 0 (0) | 0 (0) | 1a |
| 14-day regimen | (n = 16) | (n = 1) | |
| H. pylori status | |||
| Presence of mutation in rpoB, n (%) | 2 (12.5) | 0 (0) | 1a |
| Rifabutin MIC, mean ± SD, µg/ml | 0.06 ± 0.25 | 0.00 ± 0.00 | 0.89b |
| Amoxicillin MIC, mean ± SD, µg/ml | 0.07 ± 0.08 | 0.06 ± 0.00 | 0.81b |
| Mean age, mean ± SD, years | 45.69 ± 8.44 | 59 ± 0.00 | 0.15b |
| Gender, n (%) | 1a | ||
| Male | 8 (50.0) | 1 (100) | |
| Female | 8 (50.0) | 0 (0) | |
| Smokers, n (%) | 1 (6.3) | 0 (0) | 1a |
| Alcohol drinkers, n (%) | 8 (50.0) | 0 (0) | 1a |
| BMI, mean ± SD, kg/m2 | 23.20 ± 4.76 | 24.54 ± 0.00 | 0.79b |
| Fourth eradication, n (%) | 10 (62.5) | 1 (100) | 1a |
| Dyspepsia, n (%) | 9 (56.3) | 0 (0) | 0.47a |
| Peptic ulcer, n (%) | 2 (12.5) | 0 (0) | 1a |
BMI: body mass index; H. pylori: Helicobacter pylori; MIC: minimum inhibitory concentration; SD: standard deviation.
Alcohol drinkers were defined as people who consumed at least one drink of alcohol per month.
Fisher’s exact test; bStudent’s t-test.
Efficacy comparison by baseline antibiotic sensitivity and rpoB mutation status
Three of all eradication failure strains in our study were sensitive to rifabutin. On the other hand, all of the rpoB mutated strains, including a rifabutin-resistant strain, were successfully eradicated (Table 2).
Of the amoxicillin-resistant strains, 60.0% (6/10) and 43.8% (7/16) were eradicated in 10-day and 14-day regimen, respectively. Two eradication failure strains treated with the 10-day regimen were sensitive to amoxicillin (Table 3). One eradication failure strain treated with the 14-day regimen was resistant to amoxicillin (MIC = 0.06). There were no significant relationships between successful eradication and MICs of amoxicillin in both groups (Table 3).
Safety assessment
The ratios of adverse events were 75.0% in the 10-day group and 94.1% in the 14-day group (p = 0.28) (Table 4). Diarrhea was more frequent in the 14-day group than the 10-day group (29.4% vs 0%, p = 0.06). Although one patient in both groups developed leukopenia (1800/mm3 and 2400/mm3), these patients recovered after one week of treatment. One and five patients withdrew from treatment due to adverse events in the 10-day and 14-day groups, respectively. After treatment was discontinued, the patients recovered from their symptoms. Other reported adverse events were mild and tolerable.
Table 4.
Comparison of adverse events
| 10-day (n = 12) | 14-day (n = 17) | p-valuea | |
|---|---|---|---|
| Adverse event | |||
| Total, n (%) | 9 (75.0) | 16 (94.1) | 0.28 |
| Stop treatment, n (%) | 1(8.3) | 5 (29.4) | 0.35 |
| Fever, n (%) | 2 (16.6) | 6 (35.3) | 0.41 |
| Diarrhea, n (%) | 0 | 5 (29.4) | 0.06 |
| Headache, n (%) | 3 (25.0) | 3 (17.7) | 0.67 |
| Liver dysfunction, n (%) | 2 (16.6) | 3 (17.7) | 1 |
| Soft stool, n (%) | 2 (16.6) | 2 (11.8) | 1 |
| Urine discoloration, n (%) | 1 (8.3) | 3 (17.7) | 0.62 |
| Rash, n (%) | 1 (8.3) | 2 (11.8) | 1 |
| Leukopenia, n (%) | 1 (8.3) | 1 (5.9) | 1 |
| Stomatitis, n (%) | 1 (8.3) | 0 | 0.41 |
| Dysgeusia, n (%) | 1 (8.3) | 0 | 0.41 |
| Vertigo, n (%) | 0 | 1 (5.9) | 1 |
| Fatigue, n (%) | 0 | 1 (5.9) | 1 |
| Photophobia, n (%) | 0 | 1 (5.9) | 1 |
Fisher’s exact test.
Discussion
The present study, to our knowledge, is the first report analyzing the correlations among rpoB mutation status, the MICs of rifabutin, and successful H. pylori eradication with 10-day or 14-day rifabutin-based regimens. Interestingly, all patients with rpoB mutated strains, including a rifabutin-resistant strain treated with the 14-day regimen, showed eradication (Table 2). Two of the V538 mutated strains were sensitive to rifabutin. These data are compatible with our previous reports that only strains with a V538I mutation showed low MICs for rifabutin, whereas all other types of mutation induce resistance to rifabutin.19 Thus, it is likely that the 10-day and 14-day rifabutin-based regimens are effective for rifabutin-sensitive strains even in the presence of the V538 mutation. Wang et al. reported that the L525I point mutation induced strong resistance to rifampicin.22 In our study, the strain harboring the L525I mutation showed resistance to rifabutin. The fact that the rifabutin-resistant strain was eradicated with a 14-day regimen as a fourth-line therapy suggested that there are two possibilities for successful eradication: first, the high dose of PPI may enforce the activity of amoxicillin and rifabutin, and second, rifabutin and amoxicillin may exhibit a synergistic effect on antimicrobial activity. Lim et al. performed a randomized study in which two groups of patients were treated with seven-day regimens with amoxicillin, rifabutin, and either 60 mg or 120 mg lansoprazole. Eradication rates of the 120 mg lansoprazole group were significantly higher (96.3%) than those of the 60 mg lansoprazole group (78.1%).23 This study suggested that high doses of PPI might enforce the activity of amoxicillin and/or rifabutin. Fiorini et al. also pointed out that a high dose of PPI might increase the intra-gastric concentration of rifabutin by decreasing gastric volume.24 Goh et al. reported that the eradication rate of high-dose rabeprazole-amoxicillin dual therapy (amoxicillin 3000 mg, rabeprazole 60 mg for two weeks) was 71.4% as rescue therapy.25 Attumi et al. performed the eradication rate of high-dose dexlansoprazole and amoxicillin dual therapy (amoxicillin 2000 mg, dexlansoprazole 240 mg for two weeks) was 53.8%.26 The eradication rates of our 10 day and 14 day rifabutin, amoxicillin and PPI regimens were clearly superior to previous high-dose PPI-amoxicillin, thus it is probable that rifabutin contributes an additive effect or synergistic effect in our rifabutin, amoxicillin and PPI regimens. However, a synergistic effect between amoxicillin and rifabutin is not well known. Further study is expected to verify the effectiveness of a combination of amoxicillin and rifabutin for the eradication of H. pylori.
In this study, 10-day and 14-day regimens resulted in successful H. pylori eradication in over 80% and 90% of cases, respectively, which is a higher level than that achieved in previous reports of rifabutin-based third-line or fourth-line therapies. Gisbert and Calvet showed by meta-analysis that the mean H. pylori eradication rates of rifabutin-based third-line and fourth-line line therapies were 66% (95% CI, 55–77%) and 70% (95% CI, 60–79%), respectively.18 It is reasonable to suppose that the high H. pylori eradication rates observed in our study result from a more appropriate dosage and duration of rifabutin, amoxicillin, and PPI (esomeprazole) treatment. With respect to rifabutin dosage, Perri et al. performed a randomized study in which two groups of patients were treated with 10-day regimens with pantoprazole, amoxicillin, and either 150 mg or 300 mg rifabutin.27 The H. pylori eradication rates in the 300 mg rifabutin group were significantly higher (86.6%) than those in the 150 mg rifabutin group (66.6%). Regarding PPI dosage, rifabutin therapies with high dose PPI achieved more effective responses than normal PPI dosing, as described above. Instead, a meta-analysis evaluating the duration of treatment showed that 10-day rifabutin-based treatments were superior to seven-day rifabutin-based treatments.18 In this study, the eradication rate of both the 10-day and the 14-day group was over 80% in the ITT patients. Eradication was achieved in all patients who discontinued treatment (mean dosing period of patients who discontinued treatment = 8.4 days; 95% CI, 6.1–10.7 days). Considering these results, the 10-day treatment would be sufficient to obtain a successful eradication rate.
In this study, all patients in whom eradication failed were treated with fourth-line treatment, as in a previous study.28 All strains in patients with treatment failure were rifabutin-sensitive and two eradication failure strains treated with the 10-day regimen were sensitive to amoxicillin, thus other factors may have decreased rifabutin efficacy, given the high number of failed previous therapies.
Six patients stopped treatment due to adverse events in this study (one patient in the 10-day group, five patients in the 14-day group). The major reasons for stopping treatment were fever (one patient in the 10-day group, three patients in the 14-day group) and diarrhea (three patients in the 14-day group). Diarrhea is a common adverse event as for most eradication therapies.1,2,8 In this study, diarrhea was more frequent in the 14-day group than in the 10-day treatment group (29.4% vs 0%, p = 0.059). On the other hand, fever is not as common an adverse event as for other non-rifabutin based eradication therapies.18 In this study, fever occurred in two patients in the 10-day group (16.6%) and in six patients in the 14-day group (35.3%). Totally, two patients (6.9%) developed leukopenia (one patient in the 10-day group, one patient in the 14-day group). In some cases, myelotoxicity was reported in H. pylori treatment with rifabutin including therapies, and its rate was 1.5–3.0%.18
Our results must be interpreted within a limitation of the study. The sample size of this study was not large. Since a higher proportion of patients at fourth eradication regimen existed in the 10-day group in respect to the 14-day group (75% vs 65%), the overall successful eradication rate of the 14-day group might be overestimated. Furthermore, the number of rpoB-mutated strains and high rifabutin MIC strains was smaller. The relationship between successful eradication and rpoB mutation or rifabutin MIC could not be sufficiently determined. However, we decided that it was better to prematurely terminate this study due to the high percentage of patients in the 14-day group who could not complete the regimen because of adverse events.
In conclusion, both the 10-day and 14-day therapies were effective as rescue regimens. In particular, the 14-day therapy resulted in successful eradication in over 90% of patients and overcame even a rifabutin-resistant strain. On the other hand, it is possible that a 10-day treatment is enough to obtain a successful eradication rate, considering the tolerability of therapy.
Acknowledgement
Rifabutin for drug sensitivity testing was kindly provided by Pfizer Co. Ltd.
Funding
This work was supported by a Grant-in-Aid for Young Scientists (B) (26860527, to JM), a Grant-in-Aid for Scientific Research B (25293178, to HS), and a Grant-in-Aid for Challenging Exploratory Research (26670065, to HS) from the Japan Society for the Promotion of Science (JSPS), MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S1411003, to HS), the Princess Takamatsu Cancer Research grants (to HS), a grant from the Smoking Research Foundation (to HS), and Keio Gijuku Academic Development Funds (to JM and to HS).
Conflict of interest
During the last two years, HS received scholarship funds for the research from Astellas Pharm Inc., Astra-Zeneca K.K., Otsuka Pharmaceutical Co. Ltd, Takeda Pharmaceutical Co. Ltd, and Zeria Pharmaceutical Co. Ltd and received service honoraria from Astellas Pharm Inc., Astra-Zeneca K.K., Otsuka Pharmaceutical Co. Ltd, Takeda Pharmaceutical Co. Ltd, and Zeria Pharmaceutical Co. Ltd. TK received scholarship funds for the research from Astellas Pharm Inc., Astra-Zeneca K.K., Otsuka Pharmaceutical Co. Ltd, Takeda Pharmaceutical Co. Ltd, Eisai Pharmaceutical Co. Ltd, Zeria Pharmaceutical Co. Ltd, Tanabe Mitsubishi Pharmaceutical Co. Ltd, JIMRO Co. Ltd, Kyorin Pharmaceutical Co. Ltd, and received service honoraria from Astellas Pharm Inc., Eisai Pharmaceutical Co. Ltd, JIMRO Co. Ltd., Tanabe Mitsubishi Pharmaceutical Co. Ltd, Otsuka Pharmaceutical Co. Ltd, Takeda Pharmaceutical Co. Ltd, Miyarisan Pharmaceutical Co. Ltd, and Zeria Pharmaceutical Co. Ltd. The other authors have declared no conflict of interest.
References
- 1.Miwa H, Sakaki N, Sugano K, et al. Recurrent peptic ulcers in patients following successful Helicobacter pylori eradication: A multicenter study of 4940 patients. Helicobacter 2004; 9: 9–16. [DOI] [PubMed] [Google Scholar]
- 2.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet 2008; 372: 392–397. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura S, Sugiyama T, Matsumoto T, et al. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: A multicentre cohort follow-up study of 420 patients in Japan. Gut 2012; 61: 507–513. [DOI] [PubMed] [Google Scholar]
- 4.Asaka M, Sugiyama T, Kato M, et al. A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter 2001; 6: 254–261. [DOI] [PubMed] [Google Scholar]
- 5.Murakami K, Sato R, Okimoto T, et al. Efficacy of triple therapy comprising rabeprazole, amoxicillin and metronidazole for second-line Helicobacter pylori eradication in Japan, and the influence of metronidazole resistance. Aliment Pharmacol Ther 2003; 17: 119–123. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki M, Ogasawara N, Utsumi K, et al. Changes in 12-year first-line eradication rate of Helicobacter pylori based on triple therapy with proton pump inhibitor, amoxicillin and clarithromycin. J Clin Biochem Nutr 2010; 47: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asaoka D, Nagahara A, Matsuhisa T, et al. Trends of second-line eradication therapy for Helicobacter pylori in Japan: A multicenter study in the Tokyo metropolitan area. Helicobacter 2013; 18: 468–472. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzaki J, Suzuki H, Nishizawa T, et al. Efficacy of sitafloxacin-based rescue therapy for Helicobacter pylori after failures of first- and second-line therapies. Antimicrob Agents Chemother 2012; 56: 1643–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Elios MM, Silvestri E, Emmi G, et al. Helicobacter pylori: Usefulness of an empirical fourth-line rifabutin-based regimen. Expert Rev Gastroenterol Hepatol 2012; 6: 437–439. [DOI] [PubMed] [Google Scholar]
- 10.Borody TJ, Pang G, Wettstein AR, et al. Efficacy and safety of rifabutin-containing ‘rescue therapy' for resistant Helicobacter pylori infection. Aliment Pharmacol Ther 2006; 23: 481–488. [DOI] [PubMed] [Google Scholar]
- 11.Gisbert JP, Gisbert JL, Marcos S, et al. Empirical rescue therapy after Helicobacter pylori treatment failure: A 10-year single-centre study of 500 patients. Aliment Pharmacol Ther 2008; 27: 346–354. [DOI] [PubMed] [Google Scholar]
- 12.Gisbert JP, Calvet X, Bujanda L, et al. ‘Rescue' therapy with rifabutin after multiple Helicobacter pylori treatment failures. Helicobacter 2003; 8: 90–94. [DOI] [PubMed] [Google Scholar]
- 13.van Zanten SV, Desai S, Best L, et al. Rescue therapy using a rifabutin-based regimen is effective for cure of Helicobacter pylori infection. Can J Gastroenterol 2010; 24: 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heep M, Beck D, Bayerdorffer E, et al. Rifampin and rifabutin resistance mechanism in Helicobacter pylori. Antimicrob Agents Chemother 1999; 43: 1497–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishizawa T, Suzuki H, Matsuzaki J, et al. Helicobacter pylori resistance to rifabutin in the last 7 years. Antimicrob Agents Chemother 2011; 55: 5374–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glocker E, Bogdan C, Kist M. Characterization of rifampicin-resistant clinical Helicobacter pylori isolates from Germany. J Antimicrob Chemother 2007; 59: 874–879. [DOI] [PubMed] [Google Scholar]
- 17.De Francesco V, Giorgio F, Hassan C, et al. Worldwide H. pylori antibiotic resistance: A systematic review. J Gastrointestin Liver Dis 2010; 19: 409–414. [PubMed] [Google Scholar]
- 18.Gisbert JP, Calvet X. Review article: Rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther 2012; 35: 209–221. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki S, Suzuki H, Nishizawa T, et al. Past rifampicin dosing determines rifabutin resistance of Helicobacter pylori. Digestion 2009; 79: 1–4. [DOI] [PubMed] [Google Scholar]
- 20.Vaira D, Malfertheiner P, Megraud F, et al. Diagnosis of Helicobacter pylori infection with a new non-invasive antigen-based assay. HpSA European study group. Lancet 1999; 354: 30–33. [DOI] [PubMed] [Google Scholar]
- 21.Kato M, Asaka M, Ohara S, et al. Clinical studies of 13C-urea breath test in Japan. J Gastroenterol 1998; 33: S36–S39. [PubMed] [Google Scholar]
- 22.Wang G, Wilson TJ, Jiang Q, Taylor DE. Spontaneous mutations that confer antibiotic resistance in Helicobacter pylori. Antimicrob Agents Chemother 2001; 45: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim HC, Lee YJ, An B, et al. Rifabutin-based high-dose proton-pump inhibitor and amoxicillin triple regimen as the rescue treatment for Helicobacter pylori. Helicobacter 2014; 19: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiorini G, Vakil N, Zullo A, et al. Culture-based selection therapy for patients who did not respond to previous treatment for Helicobacter pylori infection. Clin Gastroenterol Hepatol 2013; 11: 507–510. [DOI] [PubMed] [Google Scholar]
- 25.Goh KL, Manikam J, Qua CS. High-dose rabeprazole-amoxicillin dual therapy and rabeprazole triple therapy with amoxicillin and levofloxacin for 2 weeks as first and second line rescue therapies for Helicobacter pylori treatment failures. Aliment Pharmacol Ther 2012; 35: 1097–1102. [DOI] [PubMed] [Google Scholar]
- 26.Attumi TA, Graham DY. High-dose extended-release lansoprazole (dexlansoprazole) and amoxicillin dual therapy for Helicobacter pylori infections. Helicobacter 2014; 19: 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perri F, Festa V, Clemente R, et al. Randomized study of two “rescue” therapies for Helicobacter pylori-infected patients after failure of standard triple therapies. Am J Gastroenterol 2001; 96: 58–62. [DOI] [PubMed] [Google Scholar]
- 28.Van der Poorten D, Katelaris PH. The effectiveness of rifabutin triple therapy for patients with difficult-to-eradicate Helicobacter pylori in clinical practice. Aliment Pharmacol Ther 2007; 26: 1537–1542. [DOI] [PubMed] [Google Scholar]