Abstract
The Nobel Prize winning discovery of nitric oxide (NO) in 1986 was the starting point for a new innovation in drug discovery. NO acting as a mediator at different physiological systems is believed to be involved in many physiological and pathological conditions through the formation of the second messenger cyclic guanosine monophosphate (cGMP). cGMP-dependent vasodilation effect of NO is important in regulating pulmonary and systemic pressures, maintaining penis erection, preventing atherosclerosis, preventing platelet aggregation, and protecting and controlling cardiac functions. The main enzyme involved in the termination of cGMP effects is phosphodiesterase enzyme 5 (PDE-5), which is overexpressed in ventricular hypertrophy and heart failure. A milestone in drug discovery was the selective inhibitors of PDE-5 that developed to be a multibillion dollar blockbuster in drug market. PDE-5 inhibitors are approved for the treatment of erectile dysfunctions (EDs), pulmonary hypertension, and benign prostatic hypertrophy. They are also under clinical trials for their cardiac protection against damage induced by ischemia or heart failure. This review article is an update about the pharmacotherapeutics of PDE-5 inhibitors and the majestic history that led to their discovery. The information reported in this review was obtained from the electronic sources of different databases such as PubMed Central, Google Scholar, and Scopus. Keywords used for search included cGMP (mechanisms and functions), EDs (drugs used), nitric oxide, and PDE-5 inhibitors (clinical applications). A total of 165 articles were studied, of which 45 articles were referred to in this review.
Keywords: Cyclic guanosine monophosphate, Nitric oxide, Phosphodiesterase enzyme 5 inhibitors
Introduction
It was a mystery that acetylcholine (ACh) can contract all smooth muscles of the body except those of the blood vessels. It was not until 1980 when Furchgott and Zawadzki discovered that the relaxation of blood vessels by ACh requires the presence of endothelial cells, and that ACh, acting on muscarinic receptors of these cells, stimulates the release of substance(s) that cause relaxation of the vascular smooth muscle.[1] Furchgott named these substances as the endothelium-derived relaxing factor(s), which was proposed by Furchgott and Ignarro independently in 1986 to be nitric oxide (NO).[2] NO was found to mediate its biological effects by activating guanylyl cyclase (GC) and increasing cyclic guanosine monophosphate (cGMP) synthesis which, in turn, activates certain proteins resulting in different actions including smooth muscle relaxation, cardiac protection, neuronal plasticity, endothelial permeability, and gene transcription. cGMP actions were found to be terminated by phosphodiesterase enzyme 5 (PDE-5).[3] Although nonselective PDE inhibitors (such as theophylline) have been in the therapeutic use for over 80 years, many important selective PDE-5 inhibitors have been introduced for the treatment of a wide range of diseases in the past 10 years. This review is an attempt to discuss the pharmacological basis of the NO-cGMP system and briefly describes the clinical applications of drugs that inhibit PDE-5.
Literature Search
Literature search of the electronic sources of different databases such as PubMed Central, Google Scholar, and Scopus were used. Keywords used for search include but not limited to cyclic guanosine monophosphate (mechanisms and functions), erectile dysfunctions (EDs) (drugs used), nitric oxide, and phosphodiesterase enzyme 5 inhibitors (clinical applications). One hundred and sixty-five articles were studied, out of which 45 articles were referred to in this review.
Physiology and the Pathophysiology of the Nitric Oxide-Cyclic Guanosine Monophosphate System
NO is synthesized by the oxidation of L-arginine catalyzed by NO synthases (NOSs) that utilizes NADPH and O2 as substrates[4] [Figure 1]. Three isoforms of NOS have been discovered: Neuronal NOS (nNOS or NOS 1), inducible NOS (iNOS or NOS 2), and finally endothelial NOS (eNOS or NOS 3).[4]
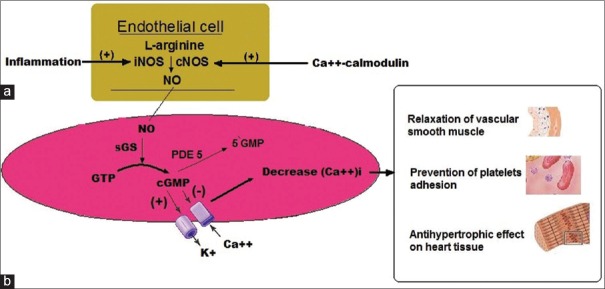
Figure 1.
(a) In the endothelial cell, nitric oxide is produced from L-arginine by the enzymatic action of nitric oxide synthase. (b) In the cell, nitric oxide will activate soluble guanylyl cyclase converting guanosine triphosphate to cyclic guanosine monophosphate. Cyclic guanosine monophosphate will activate cyclic guanosine monophosphate-dependent protein kinase, which leads to a decrease in intracellular calcium (Ca++)I (see text for details). The actions of cyclic guanosine monophosphate are terminated by hydrolysis by phosphodiesterase enzyme 5
NO mediates its biological effects by activating GC and increasing cGMP synthesis from guanosine triphosphate (GTP) [Figure 1]. There are two forms of GC, soluble GC and membrane-bound particulate GCs.[5] The formed cGMP activates certain cGMP-dependent protein kinase (cGK), which, in turn, activates certain proteins resulting in different actions including smooth muscle relaxation, cardiac protection, neuronal plasticity, endothelial permeability, and gene transcription.[5]
The cGMP generated by GC is hydrolyzed by the members of the cyclic nucleotide PDEs, a group of enzymes that control the rate of hydrolysis of cyclic adenosine monophosphate (cAMP) and cGMP. Eleven different PDE families (PDE 1–11) have been described,[6] some PDEs are selective for the hydrolysis of cAMP (PDE 4, 7, and 8) or cGMP (PDE 5, 6, and 9). Others can hydrolyze both cAMP and cGMP (PDE 1, 2, 3, 10, and 11).[6] Defects in the PDEs functions had been implicated in many pathological conditions including inflammations, cardiovascular diseases, neurodegeneration, and cancer.[7] On the other hand, selective PDEs inhibitors were developed for the treatment of different diseases including ED, respiratory diseases, and benign prostatic hypertrophy (vide infra). The following will provide a brief description of the most important pharmacological effects related to the therapeutic uses of NO-cGMP system.
Relaxation of vascular smooth muscle
cGMP is a powerful vasodilator with a short half-life of a few seconds. The formed cGK causes a decrease in intracellular calcium, which ensures that myosin light chain kinase can no longer phosphorylate myosin molecule and thereby stopping the cross-bridge cycle leading to the relaxation of the smooth muscles of the blood vessels [Figure 1].[7] The decrease of intracellular calcium can be achieved by different mechanisms, including but not limited to, (a) an increase in the open probability of large-conductance Ca++-activated K+ channels, resulting in hyperpolarization of the membrane and closing of voltage-dependent Ca++ channels thereby reducing Ca++ influx or (b) alternatively an activation of the sarcoplasmic/endoplasmic reticulum Ca++-ATPase, promoting Ca++ reuptake into the sarcoplasmic/endoplasmic reticulum, decreasing intracellular calcium.[7]
Because of its powerful vasodilatory effect, NO has been suggested to be one of the most important metabolic modulators of blood pressure (BP), tonically restraining BP by at least 30 mmHg in healthy young adults.[8] Genetically engineered mice with deletion of the eNOS gene have been found to have an increased arterial BP, illustrating a role of NO in the control of the cardiovascular homeostasis.[9] Furthermore, it was shown that other kinases may affect the activity of eNOS, where the endothelial cells of endothelium-specific liver kinase B1 (LKB1) knockout (LKB1endo−/−) mice exhibited reduced eNOS activity and AMP kinase (a downstream enzyme of LKB1) compared with wild-type cells. These knockout (LKB1endo−/−) mice exhibited hypertension, cardiac hypertrophy, and impaired endothelium-dependent relaxation. These findings indicate that LKB1 regulates eNOS activity affecting endothelial function and BP.[10]
Moreover, the vasodilating effect of NO is the main vasoactive NANC (nonadrenergic and noncholinergic) mediator of penile erection through the relaxation of corpus cavernosum smooth muscle.[11] Impaired NO bioactivity is believed to be one of the major pathogenic mechanisms of ED (vide infra).
Cardiac functions
Mammalian myocardium has been found to express both eNOS and nNOS.[12] eNOS-derived NO via cGMP-dependent signaling has been reported to increase the left ventricular (LV) compliance, attenuate beta-adrenergic inotropy and enhance parasympathetic/muscarinic responses, and mediate the negative inotropic response to β3 adrenoreceptor stimulation.[12] Moreover, NOS has the ability to alter intracellular Ca++ handling and the myofilament response to Ca++, thereby impacting the systolic and diastolic performance of the myocardium.[13] Findings from experiments using NO donors and NOS inhibition or gene deletion clearly implicate dysfunctional NOS as a critical contributor to many cardiovascular disease states including heart failure, diabetic cardiomyopathy, ischemia/reperfusion injury, and atrial fibrillation.[13] On the other hand, high output iNOS isoform may also be expressed in pathological conditions.[14] The regulation of the cardiac functions by endogenous NO has been suggested to be dependent on the distinct subcellular locations of nNOS and eNOS, through vascular-dependent and vascular-independent effects.[14]
Moreover, several reports have provided experimental evidences that cGMP degradation by PDE-5 has functional importance in cardiomyocytes, especially when they are exposed to overload and oxidative stress. In animal models, pressure overload resulted in increased myocardial PDE-5 levels, where superoxide dismutase blunted the increase in PDE-5 and protected against LV hypertrophy and congestive heart failure, suggesting that oxidative stress increases PDE-5 expression leading to hypertrophy.[15] Furthermore, stimulation of myocyte hypertrophy by phenylephrine was blunted by PDE-5 gene silencing in a PKG-dependent manner. These data confirm the role of PDE-5 in cardiomyocyte hypertrophy modulation.[16] Similar results were reported in humans where ventricular PDE-5 expression was increased in patients with advanced heart failure.[17] These results led to a tremendous interest in identifying new clinical uses of PDE-5 inhibitors in various ailments of cardiovascular diseases.
Blood platelets aggregation
The platelet anti-aggregatory effects of cAMP and cGMP are well documented. In experiments published in 1990, Radomski et al.[18] have shown that human platelets contain NOS, which was activated when platelets were stimulated. The consequent generation of NO modulated platelet reactivity by increasing cGMP, which led to the phosphorylation of substrate proteins by PKGI, preventing platelet aggregation.[18] Radomski et al. suggested that the activity of this pathway in platelets plays an important physiological, pathophysiological, and therapeutic significance. On the other hand, prostacyclin produced its anti-aggregatory effect through the formation of cAMP which phosphorylated substrate proteins by PKA.[19] PKA and PKGI phosphorylate, a broad panel of substrate proteins, will result in the inactivation of small G-proteins of the Ras and Rho families, inhibition of the release of Ca++ from intracellular stores, and modulation of actin cytoskeleton dynamics.[19] This anti-thrombotic effect is believed to be one of the vascular-dependent effects of NO in regulating and protecting cardiac functions.[14] Moreover, defects in platelet cyclic nucleotide signaling have been suggested to play a role in common diseases such as ischemic heart disease, heart failure, and diabetes, where reduced sensitivity of platelets to the inhibitory effects of NO contributes to platelet hyper-reactivity.[20]
Approved Clinical Applications of PDE-5 Inhibitors
Despite the many advances that have been made in the basic biological sciences in the past four decades, few new innovated groups of drugs have been introduced into the medical practice. One of these groups is selective PDE inhibitors. Although nonselective PDE inhibitors (such as theophylline) have been in the therapeutic use for over 80 years, many important selective PDE-5 inhibitors have been introduced for the treatment of a wide range of diseases in the past 10 years.
The first selective PDE-5 inhibitor introduced in the early 80s of the last century was zaprinast, which was proved to have moderate bronchodilator effects in exercise-induced asthma.[21] Later, zaprinast was shown to produce relaxation of the smooth muscles of the rat aorta and to enhance NO-induced relaxation of isolated corpus cavernosum.[22] This initiated a rapid progress in the development of selective PDE-5 inhibitors that culminated in the discovery of sildenafil, which was introduced initially for the treatment of angina pectoris and proved later to be effective in the treatment of several clinical conditions. The following are descriptions of the approved clinical uses of PDE-5 inhibitors.
Erectile dysfunctions
Ignarro et al. in 1990 reported that NO (through cGMP-dependent action) is responsible for the relaxation of rabbit corpus cavernosum smooth muscle induced by electrical field stimulation and proposed that NO production could potentially mediate penile erection.[23] The same relaxation under the same condition was reported in human corpus cavernosum.[24] Later, many researches had identified NO as the primary biochemical mediating erectile function, and impairment of NO release or action is a major pathogenic mechanism of organic ED.[25] ED is defined as the consistent inability to attain or maintain a penile erection of sufficient quality to permit satisfactory sexual intercourse.[25] The penile erection reported as a side effect of sildenafil during its phase I clinical trial for the treatment of angina had led to the proposal that PDE-5 inhibitors may be useful to treat ED.[26] After several clinical trials, sildenafil was the first PDE-5 inhibitors approved in 1998 by the American Food and Drug Administration (FDA) for the treatment of EDs. Sildenafil became a wonder drug for millions of patients and because of the huge market demand, many new PDE-5 inhibitors were introduced including tadalafil, vardenafil, udenafil, avanafil, and mirodenafil.
All PDE-5 inhibitors are equally effective and safe for the treatment of ED; however, they differ in their side effects, potency, and duration of action. Sildenafil-induced blue vision changes were reported to be due to the inhibition of PDE-6 in the retina. Tadalafil, on the other hand, is far less active against PDE-6 isoenzyme than either sildenafil or vardenafil.[27] In terms of potency, tadalafil was reported to be the most effective agent, followed by vardenafil and most preferred by patients and physicians.[28] The preference of patients and their physicians to tadalafil might have to do with its long duration of action (half-life time 17.5 h compared to 4 h for sildenafil) which allows the patient more freely to choose the timing and setting of the sexual intercourse.
The efficacy of PDE-5 inhibitors therapy, based on measures of the International Index of Erectile Function (IIEF) compared with placebo, ranges from 65% to 75% for successful intercourse and from 80% to 85% for significantly improved erections.[29] This efficacy has been demonstrated in a broad population, including men with hypertension, diabetes mellitus, postradical prostatectomy; psychogenic, organic, or mixed causes of ED, and those with mild-to-severe ED.[29]
PDE-5 inhibitors are prescribed either as “on-demand” dosing taken an hour before sexual intercourse or continuous “once-daily” dosing. The on-demand PDE-5 inhibitors were reported to be inadequate to restore a “normal” sexual life in 40–50% of the patients with ED. On the other hand, outcomes generally improved with repeated daily dosing, especially in the group of patients that initially do not respond.[30]
Side effects of PDE-5 inhibitors include headache, dyspepsia, back pain, myalgia (muscle aches), flushing, and stuffy or runny nose. These side effects are usually mild in nature and the discontinuation rate because of these side effects are always low.[30] However, contraindications for the use of PDE-5 inhibitors include history of unstable angina pectoris or myocardial infarction, some arrhythmias, and poorly controlled hypertension.[31] The concomitant use of PDE-5 inhibitors with nitrites and nitrates is contraindicated because of the severe hypotension that results from the combination.[31] PDE-5 inhibitors should not be given in combination with other drugs which inhibit or induce the liver enzyme CYP3A4, including HIV protease inhibitors, ketoconazole, itraconazole, erythromycin, and grapefruit juice because of the potential pharmacokinetic interactions.[32] Concurrent administration of sildenafil and warfarin may increase the risk of bleeding. Drugs prolonging QTc interval should never be administered together with vardenafil for the potential risk of further increase in QTc.[33]
In summary, the discovery of long-acting PDE-5 inhibitors such as tadalafil and the daily dose regimen has undoubtedly proven to be highly effective in the management of ED from a variety of causes.
Benign prostatic hyperplasia
Benign prostatic hyperplasia (BPH) involves hyperplasia of prostatic stromal and epithelial cells that may partially or completely obstruct urine flow through the urethra. Clinically, BPH leads to voiding dysfunction, which is most often referred to as lower urinary tract symptoms (LUTSs). Men who present with symptomatic BPH and LUTS are at an increased risk for sexual dysfunction, including ED and ejaculatory dysfunction.[34]
The main groups that are used for the treatment of LUTS associated with BPH are α1 adrenergic receptor antagonists (e.g., tamsulosin), 5-alpha reductase inhibitor (5-alphaRIs, e.g., dutasteride), and anti-muscarinic agents (e.g., tolterodine), either alone or in combination.[35] However, the use of α1 blockers and/or 5-alphaRIs has been associated with sexual dysfunctions and ejaculatory disorders,[36] which may worsen any sexual dysfunction that might be caused by BPH and LUTS.
Several clinical studies have assessed PDE-5 inhibitors including vardenafil, sildenafil, and tadalafil in reducing LUTS; however, tadalafil is the only PDE-5 inhibitor approved by the FDA in 2011 for the treatment of signs and symptoms of BPH, as well as in men with both ED and signs and symptoms of BPH.[37] In this context, 12-week treatment with once daily 5 mg tadalafil had demonstrated significant improvements in the total International Prostate Symptom Score (IPSS), BPH impact index, and IIEF. These improvements took place regardless of the age, previous treatment with α1 adrenergic blocker, BPH-LUTS severity at baseline, or ED status.[37] Using integrated data analyses from four placebo-controlled clinical studies, Brock et al.[38] have shown that tadalafil-induced improvement for LUTS is independent of improvements in ED; where the unidirectional path analysis model suggested that 70% of the total IPSS score improvement was derived from a direct treatment effect, while 30% of the total IPSS score was an indirect treatment effect via IIEF improvement. This supports the suggestion of dual mechanisms of actions of tadalafil on LUTSs/BPH, and EDs.
Combination therapy has also been tried using PDE-5 inhibitors and α1 blockers or 5-alphaRIs. A systematic review and meta-analysis of seven publications involving 515 patients showed that the combined use of PDE-5 inhibitors and α1 blockers resulted in additive favorable effects in men with ED and LUTS compared with PDE-5 inhibitor monotherapy, suggesting that alpha-blockers may enhance the efficacy of PDE-5 inhibitors, which is beneficial for the treatment of ED and LUTS.[39] Similar results were obtained when tadalafil was co-administered with the 5-alphaRI (finasteride), where the results obtained from an international, randomized, double-blind parallel study showed that the co-administration of tadalafil/finasteride provided early improvement in LUTSs in men with BPH and prostatic enlargement. Tadalafil/finasteride co-administration also improved erectile function in men who have comorbid ED.[40]
In summary, the combination of PDE-5 inhibitors and α1 blockers or 5-alphaRI is becoming a standard treatment for the management of LUTS associated with BPH and ED.
Pulmonary hypertension
Pulmonary hypertension (PH) is a general term indicating an abnormal increase of BP on the right side of the heart. The high pressure could be caused by a medical condition such as chronic lung disease, blood clots in the blood vessels of the lungs (thromboembolism), or LV muscle or valve diseases. This type of PH was called “secondary PH,” but is now referred to as PH. Pulmonary arterial hypertension (PAH), “used to be called primary PH,” occurs due to pathological changes in the resistance of pulmonary circulation where the blood vessels in the lung become thick and narrow, resisting blood flow.[41] The exposure of the right ventricle to pressure overload by chronic PH from any cause will result in an initial adaptive response of myocardial hypertrophy, which is followed by progressive contractile dysfunction and consequently heart failure.[41]
Three classes of drugs have been developed and approved for the treatment of PAH: Prostanoids (prostacyclin or prostacyclin analogues), endothelin-1 receptor antagonists (ERAs, e.g., bosentan, sitaxsentan, and ambrisentan), and PDE-5 inhibitors (sildenafil and tadalafil). All the three classes of medications have been shown to favorably affect hemodynamic parameters as well as to improve functional capacity and exercise tolerance.[42]
In 16-weeks, double-blind, placebo-controlled study of 405 patients, 40 mg tadalafil once daily was well tolerated and provided the best results in terms of improved exercise capacity and quality of life measures and reduced clinical worsening.[43] Sildenafil produced similar results. Moreover, the addition of sildenafil to the therapy of neonates with advanced PAH progression during bosentan monotherapy was associated with a significant improvement of WHO functional class and improved exercise capacity.[44]
When the long-term outcomes were assessed in a meta-analysis of 24 articles with 3758 patients, it was found that only intravenous prostacyclin has a proven survival benefit, particularly in patients with severe disease. PDE-5 inhibitors did not improve mortality. However, there are several limitations to this study including a relatively small sample size and short-time follow-up.[45]
Thus, irrespective of the proved efficacy of PDE-5 inhibitors in improving hemodynamic parameters as well as functional capacity and exercise tolerance in PAH, the long-term mortality and duration of survival improvement are uncertain and require further research.
Conclusion
The discovery of the mechanisms responsible for NO actions provided the humanity with many clues for understanding normal physiological functions and the treatment of many diseases. There is no doubt that the discovery of PDE-5 inhibitors is a milestone in innovation and drug discovery. The list of the medical indications of this group is expanding and we would expect greater advances in this respect. The discovery of long-acting PDE-5 inhibitors such as tadalafil and the daily dose regimen has undoubtedly proven to be highly effective in the management of ED from a variety of causes. The use of PDE-5 inhibitors seems to provide better choice for the treatment of BPH as other drugs used for this condition such as α1 blockers and/or 5-alphaRIs have been associated with sexual dysfunctions and ejaculatory disorders. However, the combined use of PDE-5 inhibitors and α1 blockers resulted in additive favorable effects compared with PDE-5 inhibitors monotherapy. Irrespective of the proved efficacy of PDE-5 inhibitors in improving hemodynamic parameters as well as functional capacity and exercise tolerance in PAH, the long-term mortality and duration of survival improvement are uncertain and require further research. Theoretically, PDE-5 inhibitors would provide many beneficial effects through their anti-hypertrophic effect on cardiac muscles and anti-thrombotic effect, thus protecting cardiac functions. However, many clinical trials are underway evaluating the effectiveness of PDE inhibitors in different sets of cardiac diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Furchgott RF. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983;53:557–73. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- 2.Steinhorn BS, Loscalzo J, Michel T. Nitroglycerin and Nitric Oxide – A rondo of themes in cardiovascular therapeutics. N Engl J Med. 2015;373:277–80. doi: 10.1056/NEJMsr1503311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keravis T, Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br J Pharmacol. 2012;165:1288–305. doi: 10.1111/j.1476-5381.2011.01729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Förstermann U, Sessa WC. Nitric oxide synthases: Regulation and function. Eur Heart J. 2012;33:829–37. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62:525–63. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keravis T, Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br J Pharmacol. 2012;165:1288–305. doi: 10.1111/j.1476-5381.2011.01729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgado M, Cairrão E, Santos-Silva AJ, Verde I. Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cell Mol Life Sci. 2012;69:247–66. doi: 10.1007/s00018-011-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamboa A, Shibao C, Diedrich A, Choi L, Pohar B, Jordan J, et al. Contribution of endothelial nitric oxide to blood pressure in humans. Hypertension. 2007;49:170–7. doi: 10.1161/01.HYP.0000252425.06216.26. [DOI] [PubMed] [Google Scholar]
- 9.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–42. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Wang Q, Wu Y, Moriasi C, Liu Z, Dai X, et al. Endothelial cell-specific liver kinase B1 deletion causes endothelial dysfunction and hypertension in mice in vivo. Circulation. 2014;129:1428–39. doi: 10.1161/CIRCULATIONAHA.113.004146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lasker GF, Pankey EA, Kadowitz PJ. Modulation of soluble guanylate cyclase for the treatment of erectile dysfunction. Physiology (Bethesda) 2013;28:262–9. doi: 10.1152/physiol.00001.2013. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YH, Casadei B. Sub-cellular targeting of constitutive NOS in health and disease. J Mol Cell Cardiol. 2012;52:341–50. doi: 10.1016/j.yjmcc.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Simon JN, Duglan D, Casadei B, Carnicer R. Nitric oxide synthase regulation of cardiac excitation-contraction coupling in health and disease. J Mol Cell Cardiol. 2014;73:80–91. doi: 10.1016/j.yjmcc.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: Ten years after, and continuing. Circ Res. 2003;93:388–98. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 15.Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu G, et al. Oxidative stress regulates left ventricular PDE5 expression in the failing heart. Circulation. 2010;121:1474–83. doi: 10.1161/CIRCULATIONAHA.109.906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M, Koitabashi N, Nagayama T, Rambaran R, Feng N, Takimoto E, et al. Expression, activity, and pro-hypertrophic effects of PDE5A in cardiac myocytes. Cell Signal. 2008;20:2231–6. doi: 10.1016/j.cellsig.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pokreisz P, Vandenwijngaert S, Bito V, Van den Bergh A, Lenaerts I, Busch C, et al. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation. 2009;119:408–16. doi: 10.1161/CIRCULATIONAHA.108.822072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radomski MW, Palmer RM, Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A. 1990;87:5193–7. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smolenski A. Novel roles of cAMP/cGMP-dependent signaling in platelets. J Thromb Haemost. 2012;10:167–76. doi: 10.1111/j.1538-7836.2011.04576.x. [DOI] [PubMed] [Google Scholar]
- 20.Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9:154–69. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- 21.Rudd RM, Gellert AR, Studdy PR, Geddes DM. Inhibition of exercise-induced asthma by an orally absorbed mast cell stabilizer (M and B 22,948) Br J Dis Chest. 1983;77:78–86. [PubMed] [Google Scholar]
- 22.Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006;147(Suppl 1):S252–7. doi: 10.1038/sj.bjp.0706495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990;170:843–50. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- 24.Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992;326:90–4. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 25.Meldrum DR, Burnett AL, Dorey G, Esposito K, Ignarro LJ. Erectile hydraulics: Maximizing inflow while minimizing outflow. J Sex Med. 2014;11:1208–20. doi: 10.1111/jsm.12457. [DOI] [PubMed] [Google Scholar]
- 26.Corona G, Mondaini N, Ungar A, Razzoli E, Rossi A, Fusco F. Phosphodiesterase type 5 (PDE5) inhibitors in erectile dysfunction: The proper drug for the proper patient. J Sex Med. 2011;8:3418–32. doi: 10.1111/j.1743-6109.2011.02473.x. [DOI] [PubMed] [Google Scholar]
- 27.Yuan J, Zhang R, Yang Z, Lee J, Liu Y, Tian J, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: A systematic review and network meta-analysis. Eur Urol. 2013;63:902–12. doi: 10.1016/j.eururo.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Lombardi G, Nelli F, Celso M, Mencarini M, Del Popolo G. Treating erectile dysfunction and central neurological diseases with oral phosphodiesterase type 5 inhibitors. Review of the literature. J Sex Med. 2012;9:970–85. doi: 10.1111/j.1743-6109.2011.02615.x. [DOI] [PubMed] [Google Scholar]
- 29.Burnett AL. The role of nitric oxide in erectile dysfunction: Implications for medical therapy. J Clin Hypertens (Greenwich) 2006;8(12 Suppl 4):53–62. doi: 10.1111/j.1524-6175.2006.06026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruzziches R, Francomano D, Gareri P, Lenzi A, Aversa A. An update on pharmacological treatment of erectile dysfunction with phosphodiesterase type 5 inhibitors. Expert Opin Pharmacother. 2013;14:1333–44. doi: 10.1517/14656566.2013.799665. [DOI] [PubMed] [Google Scholar]
- 31.Nehra A, Jackson G, Miner M, Billups KL, Burnett AL, Buvat J, et al. The Princeton III Consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc. 2012;87:766–78. doi: 10.1016/j.mayocp.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shon JH, Ku HY, Bae SY, Oh MK, Yeo CW, Bae SK, et al. The disposition of three phosphodiesterase type 5 inhibitors, vardenafil, sildenafil, and udenafil, is differently influenced by the CYP3A5 genotype. Pharmacogenet Genomics. 2011;21:820–8. doi: 10.1097/FPC.0b013e32834b79e6. [DOI] [PubMed] [Google Scholar]
- 33.Borer J, Armstrong P. Food and drug administration cardiovascular and renal drugs advisory committee. Proceedings of the 99th meeting of the food and drug administration cardiovascular and renal drugs advisory committee; May 29th and 30th, 2003. Circulation. 2003;107:e9052. doi: 10.1161/01.CIR.0000082691.46188.C8. [DOI] [PubMed] [Google Scholar]
- 34.Miner M, Rosenberg MT, Perelman MA. Treatment of lower urinary tract symptoms in benign prostatic hyperplasia and its impact on sexual function. Clin Ther. 2006;28:13–25. doi: 10.1016/j.clinthera.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Bullock TL, Andriole GL GL., Jr Emerging drug therapies for benign prostatic hyperplasia. Expert Opin Emerg Drugs. 2006;11:111–23. doi: 10.1517/14728214.11.1.111. [DOI] [PubMed] [Google Scholar]
- 36.Gacci M, Eardley I, Giuliano F, Hatzichristou D, Kaplan SA, Maggi M, et al. Critical analysis of the relationship between sexual dysfunctions and lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol. 2011;60:809–25. doi: 10.1016/j.eururo.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 37.Carson CC, Rosenberg M, Kissel J, Wong DG. Tadalafil – A therapeutic option in the management of BPH-LUTS. Int J Clin Pract. 2014;68:94–103. doi: 10.1111/ijcp.12305. [DOI] [PubMed] [Google Scholar]
- 38.Brock GB, McVary KT, Roehrborn CG, Watts S, Ni X, Viktrup L, et al. Direct effects of tadalafil on lower urinary tract symptoms versus indirect effects mediated through erectile dysfunction symptom improvement: Integrated data analyses from 4 placebo controlled clinical studies. J Urol. 2014;191:405–11. doi: 10.1016/j.juro.2013.09.057. [DOI] [PubMed] [Google Scholar]
- 39.Yan H, Zong H, Cui Y, Li N, Zhang Y. The efficacy of PDE5 inhibitors alone or in combination with alpha-blockers for the treatment of erectile dysfunction and lower urinary tract symptoms due to benign prostatic hyperplasia: A systematic review and meta-analysis. J Sex Med. 2014;11:1539–45. doi: 10.1111/jsm.12499. [DOI] [PubMed] [Google Scholar]
- 40.Casabé A, Roehrborn CG, Da Pozzo LF, Zepeda S, Henderson RJ, Sorsaburu S, et al. Efficacy and safety of the coadministration of tadalafil once daily with finasteride for 6 months in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia. J Urol. 2014;191:727–33. doi: 10.1016/j.juro.2013.09.059. [DOI] [PubMed] [Google Scholar]
- 41.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, et al. Right ventricular function and failure: Report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–91. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 42.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: A report of the American College of Cardiology Foundation task force on expert consensus documents and the American heart association: Developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the pulmonary hypertension association. Circulation. 2009;119:2250–94. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 43.Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, et al. Pulmonary arterial hypertension and response to tadalafil (PHIRST) study group. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 44.Douwes JM, Roofthooft MT, Van Loon RL, Ploegstra MJ, Bartelds B, Hillege HL, et al. Sildenafil add-on therapy in paediatric pulmonary arterial hypertension, experiences of a national referral centre. Heart. 2014;100:224–30. doi: 10.1136/heartjnl-2013-304895. [DOI] [PubMed] [Google Scholar]
- 45.Ryerson CJ, Nayar S, Swiston JR, Sin DD. Pharmacotherapy in pulmonary arterial hypertension: A systematic review and meta-analysis. Respir Res. 2010;11:12. doi: 10.1186/1465-9921-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]