Abstract
With the unprecedented progresses of biomedical nanotechnology during the past few decades, conventional drug delivery systems (DDSs) have been involved into smart DDSs with stimuli-responsive characteristics. Benefiting from the response to specific internal or external triggers, those well-defined nanoplatforms can increase the drug targeting efficacy, in the meantime, reduce side effects/toxicities of payloads, which are key factors for improving patient compliance. In academic field, variety of smart DDSs have been abundantly demonstrated for various intriguing systems, such as stimuli-responsive polymeric nanoparticles, liposomes, metals/metal oxides, and exosomes. However, these nanoplatforms are lack of standardized manufacturing method, toxicity assessment experience, and clear relevance between the pre-clinical and clinical studies, resulting in the huge difficulties to obtain regulatory and ethics approval. Therefore, such relatively complex stimulus-sensitive nano-DDSs are not currently approved for clinical use. In this review, we highlight the recent advances of smart nanoplatforms for targeting drug delivery. Furthermore, the clinical translation obstacles faced by these smart nanoplatforms have been reviewed and discussed. We also present the future directions and perspectives of stimuli-sensitive DDS in clinical applications.
Keywords: Controlled release, Smart nanoplatform, Drug delivery system (DDS), Biomaterials, Nanomedicine.
1. Introduction
To enhance their therapeutic effects and reduce the related side effects, active drug molecules should selectively accumulate in the disease area for a prolonged period with high controllability. Drug delivery refers to the approaches, formulations, technologies, and systems for transporting therapeutics in the body as needed to safely and efficiently achieve their desired therapeutic effects 1. Conventional drug delivery systems (DDSs) are often accompanied by systemic side effects that mainly attributable to their nonspecific bio-distribution and uncontrollable drug release characteristics. To overcome these limitations, advanced controlled DDSs have been developed to achieve the release of payloads at the target sites in a spatial controlled manner. In comparison to the conventional DDSs, the smart controlled DDSs can effectively reduce the dosage frequency, while maintaining the drug concentration in targeted organs/tissues for a longer period of time. In this sense, the controlled DDSs provide broad insights and fascinating properties for decreasing drug concentration fluctuation, reducing drug toxicities and improving therapeutic efficacy.
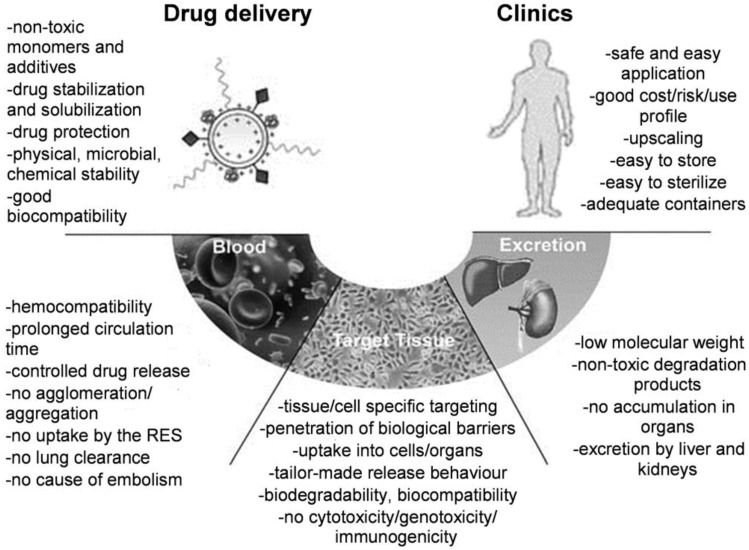
Due to their unique nanoscale properties and specific bio-functions, various nanomaterials provide intriguing benefits and new opportunities for the smart DDSs. For example, nanoparticle-based DDSs can selectively accumulate and specifically bind to the disease target with controlled release behavior. Although recent research and reviews have developed and summarized several novel aspects for nanomaterials that being used as smart drug carriers 1-6, few of them have been finally translated into clinics for real-world applications 7-9. To our point of view, there are several essential components should be taken into account to ensure clinical potential for future marketing (Figure 1) 10. On one hand, the design of nanomaterials as drug carriers should address the following key issues: (i) sufficient biocompatibility and biodegradability; (ii) good stability in physiological conditions; and (iii) high drug loading capacity and low toxicity. On the other hand, besides the primary requirement for safety and therapeutic efficacy, industrial scale-up for DDSs is also a prerequisite for this type of novel nanomaterials in clinical applications as shown in Figure 1.
Figure 1.
Essential components for design of polymeric drug delivery systems (DDSs). “RES” is the abbreviation of “Reticuloendothelial System”. Reproduced with permission from reference 10.
To date, a myriad of materials, such as polymers, lipids and inorganic materials, have been developed and served as drug carriers to control the release behavior of payloads 11-16, making the drugs “smart”. In this review, we summarize the well-defined smart carriers, including the smart polymer carriers, liposome, organic-inorganic hybrid smart nanoparticles, exosomes, and other nanomaterials, for controlled drug release. Moreover, the clinical application potentialities of controlled drug delivery nanoplatforms, as well as the obstacles faced by those nanoplatforms in clinical translation have been reviewed and discussed.
2. Design rationale of smart drug delivery nanoplatforms
It is well known that drugs should ideally be released at the target sites in a controlled manner to enhance their therapeutic efficiency, meanwhile, to reduce the side effects. Inheriting from the controlled release nanoplatforms, the loaded drugs can act “smart”. Since the phase transition of polyacrylamide gels was observed by Tanaka in 1978 17, the research on phase transition of polymeric gels was greatly boosted. Almost in the same period, thermal-sensitive liposomes was first reported for drug delivery 18. Gradually, the stimuli-responsive biomaterials have been developed and widely used for controlled drug delivery. With the development of nanotechnology and nanomaterials, drugs can also be conjugated with different nanoparticles (see section 3.3). By utilizing the excellent size and surface properties, the nanomaterials are acting as one of the most promising smart DDSs.
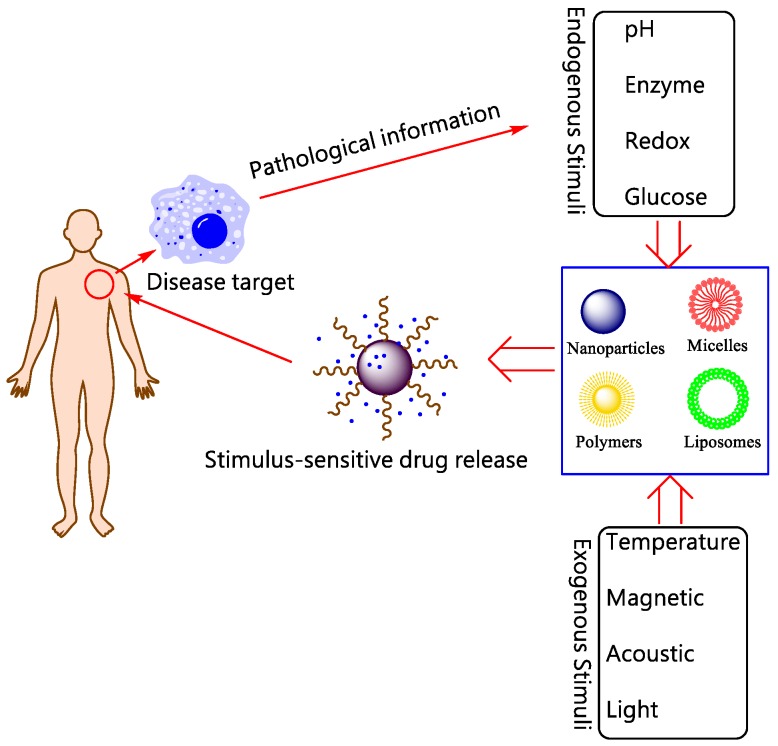
In this review, “Smart DDSs” is determined as the process that the drugs are not released before reaching target tissues/ organs (or with extremely slow rate), and only released with proper rates at the sites of action. Although the drug molecules themselves sometimes can be smart components, here we primarily discuss about smart DDSs by means of nanotechnology to carry the drug molecules. The delicate design of smart nanoplatform enables the release of payloads at specific tissues in systemic administration. Currently, the drug-loaded nanoplatform ensures that the drug will not freely extravasate during the blood circulation, but only release at the targets where the nanocarriers accumulate by active or passive targeting strategy. These elaborately designed smart or stimuli-responsive nanoplatforms can respond to endogenous and/ or exogenous stimulus as shown in Figure 2. The endogenous triggers such as pH variations, hormone level, enzyme concentration, small bio-molecules, glucose or redox gradient 19, 20, which are related to the disease pathological characteristics. While the exogenous triggers, including temperature, magnetic field, ultrasound (US), light, electric pulse/ high energy radiation, can also be used to trigger or enhance the drug release at diseased areas. The rationales of different stimuli-responsive nanoplatforms will be briefly discussed as below.
Figure 2.
Schematic illustration for stimuli-responsive DDSs.
2.1 pH-responsive
Among different types of stimuli, pH is one of the most frequently used triggers for drug release 21-24. The conventional pH-responsive carriers are based on significant variation of pH values in different organs, such as stomach (pH≈ 2) and intestinal tract (pH≈ 7). For example, Eudragit S100 coated citrus pectin nanoparticles (E-CPNs) were prepared for the colon specific targeting of 5-Fluorouracil (5-FU) 25. The designed carriers can sensitively differentiate delicate pH changes in specific disease sites, such as inflammatory, ischemic, and tumor tissues, even in different organelles, like endosomes and lysosomes. One typical example is the pH-responsive nanocarriers for solid tumor targeting 26. The extracellular pH in normal tissue and blood is usually maintained at around 7.4. However, because of high rate of glycolysis, the mean extracellular pH values in various solid tumors are usually below 7.0 27, 28. The low pH in the tumor extracellular matrix can be used as a specific stimulus in controlled DDSs. Furthermore, pH difference can also be found in organelles such as in endosomes and lysosomes, in which the pH value is lower than other intracellular organelles with the pH range from 4.5 to 5.5 21, 28. Thus, the pH variety is the key design rationale for the development of advanced DDSs.
Although the stimulus of pH is widely used in smart DDS, it still needs to be combined with other stimuli, such as temperature, redox, to achieve extremely precise and specific release at the target sites 29-31.
2.2 Redox-responsive
Redox responsive stimuli have gained great attention for disease therapy and widely used in intracellular DDSs 19, 32. The redox potential in microenvironments is multivariate in different tissues, which can be exploited to design redox-sensitive delivery systems. The design and fabrication of nanoparticles responsive to Glutathione (GSH) can be a promising approach for targeting drug delivery 33. The GSH reduction is a well-known redox system within cancer cells. On one hand, concentrations of GSH in blood and normal extracellular matrices are reported to be 2-20 μM, at the same time GSH levels within cancer cell ranges from 2 to 10 mM, which is 100- to 500- fold higher than the normal ranges 34. Such significant difference in GSH level between cancer and normal cell has made redox responsive delivery systems to be an attractive strategy to design the DDSs for targeting specific tumor intracellular sites. On the other hand, by utilizing the high accumulation of reactive oxygen species (ROS) in some disease tissues, ROS-response DDS is also an effective mechanism to finely control the targeted drug release. It has been reported the mucosal ROS concentrations in inflammatory tissues and colon cancer were 10- to 100- fold higher than that of normal tissues 19, 35. The exciting specificity and accuracy have been shown by the developed redox stimuli-responsive DDSs, however, it is difficult to achieve the specific redox molecular mechanism based controllability due to the complex biological environment and hetereogeniety of tumor cells.
2.3 Enzyme-responsive
Enzymes used as triggers in the design of smart DDSs have been an emerging field in recent years owing to its unique superiorities, such as substrate specificity and high selectivity under mild conditions 36-39. Since enzymes (such as glycosidases, lipase, phospoholipases or proteases) are related to almost all the biological and metabolic process, they can be exploited to achieve enzyme-mediated drug release by the bio-catalytic action at the cancer or inflammation tissues 38. A major challenge in enzyme-responsive DDSs is to precisely control the initial response time of the systems.
2.4 Temperature-responsive
Temperature is one of the most convenient and effective factors to control the drug release compared with other stimuli 40-42. Normally, pathophysiological conditions, such as inflammation and tumors, have higher temperatures than normal tissues 43. Considering the temperature difference between cancer tissues and normal tissues, functionalized nanoparticles can be triggered to enhance their drug release in tumors 44, 45. Another temperature- responsive strategy is that the tumor site could be heated by external triggers (US, magnetic field, etc.) to improve the drug release within the tumor vasculature microenvironment 34. In general, thermo-sensitive nanocarriers are designed to retain their payloads around the physiological temperature of 37°C, and release the payloads rapidly when temperature is increased higher than 40-45°C. Towards the thermo-responsive nanoplatforms, the current challenge is to maintain the safety of the platforms without sacrificing their sensitivity to slight temperature changes 19.
2.5 Light, magnetic, and US responsive
Light-responsive systems represent a way to trigger drug release at the desired target by external light illumination. Photo sensitive carriers can achieve the on-off drug release event because the nanostructure may open or close when stimulated by either a one-time or repeatable light irradiation 19. However, considering the limitation of light wavelength for practical therapy, light penetration depth currently restrains the non-invasive applications for deep tissues 46.
Magnetic stimuli may provide a non-invasive approach to temporally and spatially control of the carriers to the targets and release drugs under programmable exposure of external magnetic field 47-49. For example, the most commonly-used core/shell magnetic nanoparticles (MNPs) exhibit a variety of unique magnetic properties. The large surface-to-volume ratio of MNPs provides abundant active sites for biomolecule conjugation, thus allowing precise design and engineering in order to gain their intended smart functions by applying a localized external magnetic field, such as long-lasting circulation in the blood stream, target specificity to lesion tissues, and therapeutic delivery. Furthermore, when these nano-scaled MNPs were encapsulated in colloidal carriers, such as micelles, liposomes, or solid nanoparticles 34, 50-53, the composite structures might become sensitive to an external magnetic field to realize multifunctional formulations for both diagnostic and therapeutic purposes 54-60.
Recently, US has been extensively used in clinics for diagnosis and therapy due to its intrinsic tissue penetration and high safety. The development of ultrasonic sensitive nanocarriers for ultrasonography expands US techniques to be a unique and effective method to capture drug carriers and trigger drug release at the desired sites by tuning the US frequency, duty cycles and time of exposure 61.
2.6 Other responsive systems
Apart from the above-mentioned stimuli for smart DDSs, glucose 62-64 and electro-responsive systems 65-68 have also been employed to control the release of payloads within nanocarriers. Moreover, sensitivity to hybrid stimulus can further improve drug delivery accuracy. Dual stimuli-responsive DDSs are most common systems and have been investigated, such as thermo- and pH- responsive systems 69, 70, thermo- and light- responsive systems 71, 72, redox- and pH- -responsive systems 30, 31, ultrasonic and magnetic responsive systems 73-77.
In order to realize the smart characteristic of DDSs, a wide range of stimuli that are able to trigger the drug release at target place and expected time has been assembled in various nano-architectures. To ascertain the viability of these strategies, evidence for regulation of the response to each stimulus would be needed both in vitro and in vivo. In this review, we mainly discuss smart nanoplatforms with the most promising clinical potential in the application of stimuli-responsive DDSs. Smart DDSs for controlled drug release (e.g., polymers, liposome, organic-inorganic hybrid biomaterials and exosomes) are discussed. Although smart nanoplatforms can be widely used in several diseased, such as neoplastic diseases, diabetes, infections, cardiovascular diseases and inflammatory diseases, this review is mainly focused on carcinoma diseases for the future clinical translation of smart DDSs.
3. Smart nanoscale DDS
3.1 Polymeric nanoparticles
Polymers with the ability to respond to external physical or chemical stimulus are defined as smart or stimuli-responsive polymers. Half century ago, smart polymers have been utilized to control the release of biologically active cargos, which played a very important role in the research and development of nanomedicines. As introduced in previous section, various external stimuli have been reported in smart polymeric systems, including US, pH, and magnetic fields 1, 7, 16. The physical/ chemical properties of polymers can smartly transform in responding to those stimuli. The drug release rate can be controlled by the intensity of applied stimuli to the fabricated carriers.
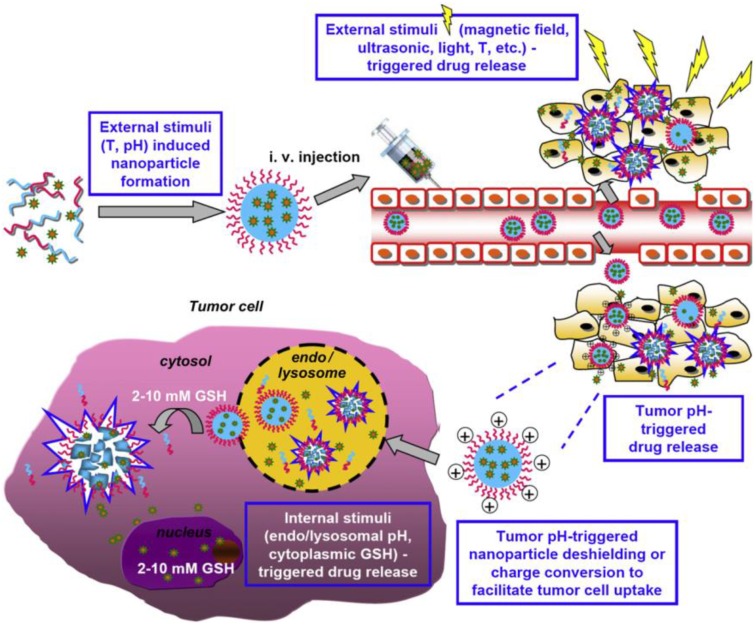
These smart polymer materials can be divided into two categories: single stimulus and dual-/ multi-stimuli responsive polymers 29. Single stimulus that induces protonation, hydrolytic cleavage or (supra) molecular conformational change in the polymer nanomaterials, which can be further categorized as exogenous and/or endogenous stimulus 19. The exogenous stimuli such as temperature, magnetic field, ionic strength, US intensity or electric pulses can trigger conformation changes due to on-off polymer chains 78. Factors like pH, enzyme concentration, hormone level, redox gradient, and small bio-molecules are summarized as endogenous stimuli 79. Figure 3 shows the process of using dual and multi-stimuli smart polymeric nanomaterials for controlling the drug release in solid tumors 29. Among a variety of stimuli that have been utilized, pH, redox, enzymes, light, and temperature have recently emerged as promising triggering motif for the design of smart polymeric DDSs 20, 29, 79.
Figure 3.
Dual and multi-stimuli (e.g., T (temperature), pH, magnetic field, US, and light) smart polymeric materials used for smart drugs in solid tumors. Reproduced with permission from reference 29.
Normally, pH-responsive polymers are a class of polyelectrolytes with ionizable groups. With the change of environmental pH or the ionic composition, smart polyelectrolytes are ionized and can dramatically change their conformation for drug release. Many researches have focused on the design rationale of pH environment activated nanoparticles for smart DDSs 21, 26, 27, 80-82. Generally, there are two strategies to control drug release from polymers in the tumor vicinity. One is that the nanocarriers can be activated to release cargos by pH variety when arriving at slightly acidic tumor sites. The other strategy is that surface charge of polymer could be positively switchable to be internalized by targeted cells, as shown in Figure 4.
Figure 4.
Schematic representation of the two strategies to control drug release from polymers in the tumor extracellular pH (pHe) environment. (A) The polymer carrier maintains its stealth function while circulating in the blood circulation. (B) Upon arrival at slightly acidic tumor sites through the EPR effect, the stealth polymer carrier is activated by pH to release its cargos. (C) Surface charge reversal to a positive charge when the stealth polymer carrier arrived at tumor vicinity, and drugs were released.
The pH-induced smart polymeric DDSs have been greatly developed in recent years, leading to controllable release at the tumor sites 83-85. One of the typical drug controlled release system is smart pH-sensitive polymeric micelles 85. The hydrazone bond was used to conjugate doxorubicin (DOX) with poly (styrene-co-maleic anhydride) (SMA) derivative, which was modified by adipic dihydrazide (ADH). Then disulfiram (DSF) was encapsulated into the micelles to be formed by the self-assembly of SMA-ADH-DOX (SAD) conjugate. The system was designed to treat multidrug resistance (MDR) cancer, and the result showed that the pH-sensitive DDS enabled a fast release of DSF. It could inhibit the activity of P-glycoprotein (P-gp) and rehabilitate the signaling pathways of cell apoptotic, while DOX conjugated by hydrazone bond was released with pH-dependent process. In another study, Hennink et al. reported the thermosensitive polymeric micelles as promising nanoplatform for pH-triggered drug release. The smart micelles were prepared and interfacially crosslinked via hydrazone bonds, which were rapidly de-crosslinked at pH 5.0 at 37 oC 86. However, delivery systems triggered by pH variation solely for a faster drug release might not be sufficient, therefore surface charge switchable polymeric nanocarriers were designed to enhance tumor drug delivery, especially for drug-resistant cells or nucleic acid drugs. The charged surface transformation from negative to positive can be utilized to enhance cellular uptake in virtue of the strengthened interaction between polymer nanocarriers and cellular membrane. Wang and co-workers 87-91 have designed several surface charge reversal polymer nanocarriers for drug, protein and gene delivery. As shown in Figure 5, a kind of core-shell-corona polymer carriers called PIC⊕NP/Pt@PPC-DA nanoparticles have been designed. Strikingly, it was able to enhance the platinum drug accumulation in response to tumor pH variation with prolonged circulation time in the blood 91.
Figure 5.
(A) Schematic illustration of the fabrication of PIC ⊕NP/Pt@PPC-DA pH-responsive nanoparticles; (B) Schematic illustration of the procedure of drug release from polymer nanoparticles. (i) non-specific interactions are scarcely happened between ⊕NP/Pt@PPC-DA and serum components; (ii) nanoparticles accumulate at the tumor site owing to the enhanced permeability and retention effect; (iii) the positively charged ⊕NP/Pt nanoparticles are exposed again to enhance the Pt(IV) pro-drug internalization; (iv) the Pt(IV) pro-drug is rapidly released from ⊕NP/Pt nanoparticles. Reproduced with permission from reference 91.
In addition to the polyelectrolytes, a variety of self-assembled or novel polymeric structures have been developed for controlled drug delivery, such as micelles, polymer-gels, polymer-somes, and dendritic polymers 92, 93. Specifically, pH-sensitive polymer system was also used for tumor imaging. The novel ultra pH-sensitive (UPS) fluorescent nanoprobes were prepared by Gao's group 94, 95. These nanoprobes were silent during the blood circulation, while strongly activated (>300-fold) in response to the acidic extracellular pH in tumors or in the neovasculature. Potentially, the UPS platform and fluorescence imaging can provide high-resolution delineation of primary and metastatic tumors to achieve complete tumor resections during surgery.
It is well noted that intracellular GSH can trigger the exchange of thiol-disulfide bond. By utilizing this property, polymers with disulfide bond can be disintegrated and the loaded drugs can be liberated rapidly from the carriers when stimulated by GSH 96. In general, there are two methods for disulfide bonds to be used in polymer systems. One is the modification of disulfide bond on the backbone chains of polymer. The other is to use disulfide bonds as cross-linkers within the polymeric network 97. As an example that shows in Figure 6, disulfide bond was used as effective cross-linker during the preparation of novel redox-degradable hyperbranched polyglycerols 98. A glycerol monomer 2-((2-(oxiran-2-ylmethoxy) ethyl) disulfanyl) ethan-1-ol (SSG) was synthesized and polymerized via ring-opening polymerization to prepare redox-responsive polyglycerols (PSSGs). There are some free low molecular weight thiols presented in serum albumin and plasma, therefore, the disulfide bonds might not be stable in blood plasma. Thus it might affect the specific response efficiency of the redox-responsive polymers.
Figure 6.
Schematic illustration for the redox-responsive degradation process of PSSG, Reproduced with permission from reference 98.
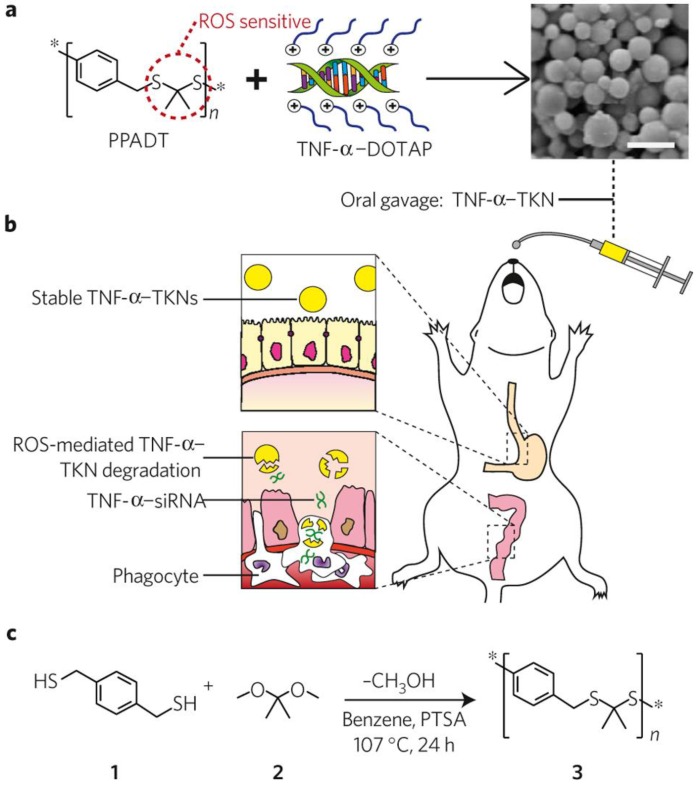
In addition to the application in cancer treatment, redox responsive polymer systems have also been applied to diseases that associated with intestinal inflammation. Wilson and coworkers first prepared a delivery vehicle termed thioketal nanoparticles (TKNs) for small interfering RNA (siRNA) (Figure 7) 35. TKNs were formulated by poly- (1,4-phenyleneacetone dimethylene thioketal) (PPADT), which was able to degrade selectively in response to ROS. This may lead to the siRNA release under the high levels ROS of intestinal inflammation after oral delivery 99. It has been demonstrated that orally administered TKNs loaded with siRNA could diminish TNF-messenger RNA levels in the colon and protected mice from ulcerative colitis. Therefore, TKNs might provide a new approach for the treatment of numerous gastrointestinal diseases related to intestinal inflammation through oral administration.
Figure 7.
Schematic illustration of thioketal nanocarriers formulated by ROS-sensitive polymer to release siRNA at sites of intestinal inflammation through oral administration. (a) Firstly, TNF-α-siRNA were prepared with the cationic lipid DOTAP (1, 2-dioleoyl-3-trimethylammonium-propane), and then TNF-α-TKNs were obtained through adding TNF-α-DOTAP complexes to an organic solution containing PPADT. Right image shows the scanning electron micrograph of TNF-α-TKNs with the scale bar of 1.5 μm. (b) TNF-α-TKNs remained to be stable in the gastrointestinal tract when delivered orally, and TNF-α-siRNA were protected and prevented its release unless at sites of intestinal inflammation, where infiltrating phagocytes produce unusually high levels of ROS. (c) PPADT 3 was synthesized by the acetal exchange reaction. PTSA represented p-toluenesulfonic acid. Reproduced with permission from reference 35.
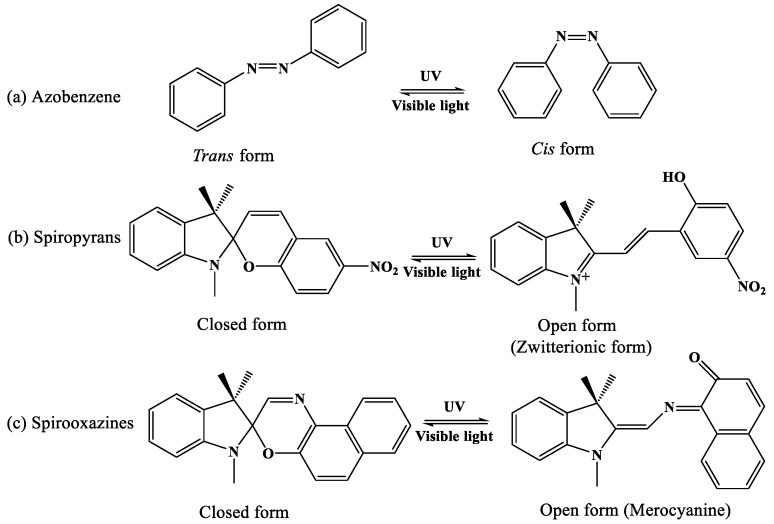
Light-responsive polymeric systems can be achieved by inducing photochromic moieties or photochemical reaction 100. As the photochromism moieties have different absorption spectra, the reversible transition between two isomeric species will emerge. These reversible transition processes are commonly activated by the irradiation of visible or ultraviolet light, and the typical photochromic compounds used in polymeric systems include azobenzene, spiropyran, and spirooxazine 100. As shown in Figure 8, photodynamic therapy (PDT) is one kind of minimally invasive treatment that combines light at appropriate wavelengths with a photosensitizer (photoactive drug) to destroy target cells by producing highly toxic ROS 101. Zheng's group fabricated novel photosensitizers through coupling the plasmonic nanoparticles with palladium-photosensitizers. This design enables the use of the same laser wavelength to stimulate both of the PDT and imaging features originated from surface-enhanced Raman scattering (SERS), which indicates potentiality for dosimetry of photosensitizer concentration and light dose through SERS monitoring 102. Long wavelength responsive polymer system can also be used for photothermal therapy (PTT). One kind of porphysome nanovesicles used for biophotonic imaging and photothermal therapy were generated by porphyrin bilayers, and the redshifted porphysomes could further to be responsive at 760 nm 103. Besides, organic photosensitizers of upconversion nanoparticle for biological applications were reported extensively 104. Li and coworkers prepared original high-effective upconversion nanocapsules based on triplet- triplet annihilation (TTA) 105, which was capable of loading both sensitizer and annihilator into BSA-dextran stabilized oil droplets. These results showed that the nanocapsules could be successfully applied to lymph node imaging in vivo of living mice.
Figure 8.
Typical photochromic compounds used in photo-responsive polymer systems.
Temperature-responsive polymer also attracts extensive attention due to the phase transition induced by alternation of external environment. Various polymers are proven to exhibit critical solubility temperature (CST) 106, among which poly-N-isopropylacrylamide (PNIPAAm) is frequently used due to its lower critical solution temperature (LCST) at 32°C. The polymer side-chain isopropyl groups can be easily hydrated or dehydrated to induce a reversible variety of the hydrophilicity or hydrophobicity 7.
Glucose-responsive polymers are widely explored due to their potential applications in diabetes 107. The current treatment of diabetics to control blood glucose levels is normally realized by long-term perioral administration or daily insulin injections, which results in a poor compliance to the patients. The research of smart polymers response to glucose might hold promise for diabetics with new therapeutic regimen. Since polymers like chitosan and dextrin can be degraded by means of special enzymes secreted by bacteria, it prompts the enzyme responsive-polymer system to be hotspot 22.
Some natural polymers can also be used as controlled biomaterial for smart drug release, such as chitosan with their blends, and cyclodextrin with their derivatives 108, 109. All in all, polymers are the most studied and promising controlled release candidates for the clinical application. Some of them have been investigated at the preclinical status 7. They are not only used as smart nanocarriers for DDSs, but also with great potential applications in bio-imaging and diagnosis 22.
3.2 Liposomes
The description of swollen phospholipid systems were first reported by Alec Bangham and colleagues in 1965 110. Since then, a variety of enclosed phospholipid bilayer structures consisting of single bilayers were described as “liposomes” 111. In 1971, Gregoriadis et al. firstly used liposomes as drug delivery systems 112. Then, with the development of new preparation technology, the large unilamellar liposomes (LUV) can be obtained by extrusion of multilamellar vesicles through polycarbonate filters. Especially, when the diameter of liposome was decreased within the scope of 100 nm or less, liposomes have been widely used as advanced DDSs in numerous clinical trials such as anti-cancer, anti-inflammatory, anti-fungal drugs, and gene medicines 113, 114. Some liposome formulations have been approved for commercial use as shown in Table 1. Doxil®, the first Food and Drug Administration (FDA) approved nanomedicine delivery system is based on PEGylated liposomes 115. Except for liposomes on the market, a number of lipidic nanoparticles are in the pipeline from concept to clinical application, which indicates that the liposomes used as drug carrier may be well-developed for clinical acceptance. Inspired by the promising clinical applications, the development of smart liposomes is now hot topic in nanomedicine, which can be easily stimulated by several triggers such as temperature, pH gradients, enzymes changes, US and light, etc. 116, 117. These novel smarter liposome delivery systems may exhibit better potentiality in future clinical application.
Table 1.
Liposomes approved in clinics.
| Applications | Indication | Drug formulation | Company | Status | Ref |
|---|---|---|---|---|---|
| Doxil | Ovarian cancer, AIDS-related Kaposi's sarcoma, breast cancer, myeloma |
PEGylated liposomal DOX | Ortho Biotech | Approved 1995 by FDA | [115, 118] |
| Amphotec | Deep fungal infection | Amphotericin B liposome | Ben Venue Lab | Approved 1996 by FDA | [119] |
| DaunoXome | HIV-associated Kaposi's sarcoma |
Liposomal daunorubicin | Galen Ltd. | Approved 1996 by FDA | [120] |
| AmBisome | Fungal infection, Cryptococcal Meningitis, visceral leishmaniasis | Liposomal amphotericin B | Gilead Sciences, Inc. | Approved 1997 by FDA | [119] |
| Depocyt | Lymphocytic meningitis | Liposomal cytarabine | Pacira Pharmaceuticals, Inc. | Approved 1999 by FDA | [121] |
| Myocet | Metastatic breast cancer | Non-PEGylated liposomal DOX | Sopherion therapeutics, Inc. | Approved 2000 by EMEA | [122] |
| Visudyne | Choroidal neovascularization symptoms | Non-PEGylated liposomal verteporfin | Novarits Pharma | Approved 2000 by FDA | [123] |
| Lipusu | Ovarian cancer, non-small cell lung cancer | Liposomal PTX | Luye Pharma | Approved 2003 by CFDA | [124] |
| MARQIBO KIT | Acute lymphoblastic leukaemia | Liposomal vincristine | Talon Therapeutics, Inc. | Approved 2012 by FDA | [125] |
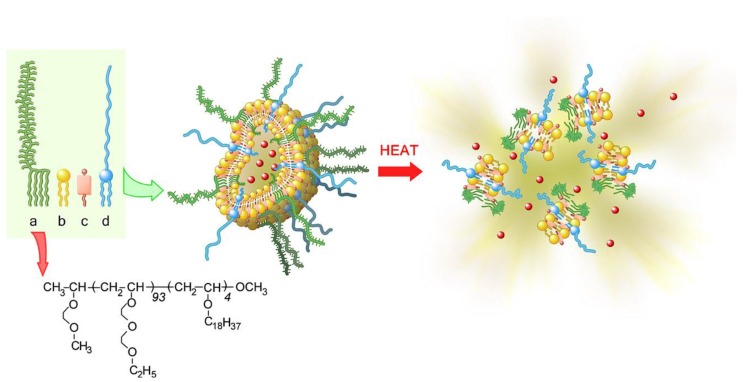
Though various stimuli can be used as a trigger to control drug release of liposomes, temperature stimuli might be an important trigger from the point of safety and facility 126, 127. As a smart drug carrier system, ThermoDox, temperature-sensitive DOX liposomes developed by the company Celsion may be the closest formulation to the clinic so far. Taking advantage of the dipalmitoylphosphatidylcholine (DPPC) lipid crystallization melting temperature at 41.5°C, the doxorubicin can be released from ThermoDox at this temperature 126. Radiofrequency ablation (RFA) was also used to activate DOX release from ThermoDox. The liver cancer-targeted ThermoDox DDS showed an improved safety profile compared to free doxorubicin in a Phase I clinical trial. Although the results of Phase III clinical trials with ThermoDox were not completely satisfied because the life span after treatment by ThermoDox failed to reach the threshold of 33% 19, the strategy of temperature-sensitive liposomes provide a promising clinical future for the smart DDS. To improve the control of drug release in response to mild heating, thermosensitive polymers were used to modify liposomes to produce temperature-sensitive polymeric liposomes. A typical example of ultra-temperature-sensitive liposomes based on thermosensitive block copolymer has been developed by Kono's group. The synthesized poly [2-(2-ethoxy) ethoxyethyl vinyl ether (EOEOVE)] as shown in Figure 9 is a promising biomaterial for the construction of temperature-sensitive liposomes. The poly (EOEOVE) modified liposomes showed an even higher sensitivity to temperature than poly (N-isopropylacrylamide), which can further enhance the tumor-selectivity and therapy effectiveness of payloads.
Figure 9.
Schematic diagram depicting the construction of temperature-sensitive liposomes with thermo-sensitive poly(EOEOVE)-OD4 (Octadecyl vinyl ether), and heat-triggered release of DOX from liposomes. (a) poly(EOEOVE)-OD4, (b) EYPC (Egg yolk phosphatidylcholine), (c) cholesterol, (d) PEG-DSPE ((polyethylene glycol)- distearoyl phosphatidylethanolamine). Reproduced with permission from reference 127.
The fabrication of liposome complex further prompts the development of smart liposomes. For example, after being loaded with MNPs and exposed under a magnetic field, the magnetic liposomes were endowed with multifunctional properties such as vessel effect, surface effect, biocompatibility, targeting effect and easy recovery property. Plank and coworkers designed a kind of folate receptor targeted magnetic liposomes. Under the external magnetic field exposure, magnetic hyperthermia-triggered the drug release and localized high concentration in tumor tissues, resulting in great improvement of the anticancer efficacy 128.
Other recent advances in smart liposomes include the use of low pH environment for pH-triggered approaches 129-132, the use of enzyme as a trigger in enzyme-sensitive liposomes 133, 134 and US responsive liposomes 135-137. Besides, light as a stimulus has been widely investigated in photosensitive liposomes 138-140. Moreover, liposomes can be a platform for co-delivery of magnetic resonance imaging (MRI) agents and therapeutic drugs 141-143. As an important smart drug carrier, stimuli-sensitive liposomes represent a pathway towards the design of nanocarriers with significantly improved efficacy. Although successful in vivo applications of these systems still remain a challenge, it is believed that more clinical products based on smart liposomal platform will come to the fore in the near future.
3.3 Organic-inorganic hybrid smart nanoparticles
Organic-inorganic hybrid smart biomaterials refer to the materials combined with the characteristics of organic and inorganic materials, which can respond to stimuli after hybridization. The hybridization materials can be constructed by connecting organic or polymer molecules with nano-metal particles or nano-oxides like silica and titanium dioxide 144, 145. Mesoporous silica materials as smart DDSs have attracted extensive attention in the past decade 145-148. In addition, gold nanoparticles (AuNPs) have widely been explored for photothermal therapy (PTT) in the biomedical field 149-154. Their specific surface chemistry with facile functional modification provides the hybridization with more possibilities 145. Besides, upconversion nanoparticles 155, 156, magnetic-sensitive nanocrystals in liposomes 157, 158, US-responsive liposome with perfluorocarbon bubbles 159 and photoacoustic nanoparticles 160 can also be used as hybrid smart nano-DDSs for controlled drug release.
Although mesoporous silica nanoparticles (MSNPs) possess a large loading capacity, the loaded drugs are always easily released immediately after administration. Similar with conventional therapy methods, this may definitely lead to lower therapeutic efficacy and severe side-effects due to the off-target effect. In order to minimize the premature release of the payloads before reaching the target site, different organic molecules or polymers have been used as smart gatekeepers on the pore outlets to prevent the drugs from leaking out the carriers until the carrier is exposed to internal or external stimuli 161. The outer layer can be operated by the stimuli, such as pH, temperature, photo irradiation, redox potential, electromagnetic fields, and biomolecules (Figure 10) 162, which may adjust the drug release speed from the pores of MSNPs. The strategy of modifying MSNPs with organic molecules to be smart is analogous to smart polymers. The first enzyme-sensitive cap on MSNPs was described in 2008 163. The MSNPs was functionalized by cyclodextrin (CD) torus with a PEG thread and connected by an enzyme cleavable site. The drug was released when the enzyme-responsive bond was cleaved in the presence of esterase. Mondragón et al. 164 prepared two novel MSNPs hybrid with poly-L-lysine outer surface systems in two different anchoring strategies. One strategy was to utilize the formation of urea bonds, while another one was focus on attachment by amide bonds. Almost no cargo has been released in water for both nanoparticles. After introducing proteases into the release medium, a notable payload released because poly-L-lysine cap on the surface smartly enzyme responded to the enzyme in a controlled manner. Other organic constituents have also been used to modify inorganic compounds to fabricate smart hybrid materials, such as polypeptides, polyesters and polysaccharides 165, 166. They can act as capping agents after grafting them to the entrance of the pores of MSNPs, and cargos will be released till the existence of protease, esterase, galactosidase and so on 161. Compared with other inorganic nanoparticles, although MSNPs served as organic-inorganic hybrid smart DDS and shows superior biocompatibility, the pharmacokinetics and pharmacodynamics of MSNPs should be further evaluated.
Figure 10.
The various stimuli that induce the MSNP-hybridized DDSs to release the cargos. Reproduced with permission from reference 162.
Gold nanoparticles (AuNPs) such as nanoshells, nanocages, nanorods, or composite nanostructures have been widely developed as PTT agents 154, 167-169. However, as inorganic nanomaterials, AuNPs are not biodegradable and gradually accumulate in the body after systemic administration. Such problem may be solved by surface modification of the organic functional groups. Kojima and coworkers have prepared various liposomes complexes with AuNPs. Results reveal that the liposomes promote the formation of stable dispersions of AuNPs under isotonic conditions 170. Poly(ethylene glycol) (PEG)-attached poly(amidoamine) (PAMAM) dendrimers were also used to encapsulated AuNPs for PTT, and the result demonstrated that these dendrimers with AuNPs exhibited strong cytotoxicity against human cervical cancer (HeLa) cells under visible light irradiation 171. Wang's group developed a novel drug delivery system that tethers DOX modified onto the surface of AuNPs (DOX-Hyd@AuNPs) with a PEG spacer via an acid-labile linkage, which was proved to overcome multidrug resistance (MDR) in cancer cells. This strategy presents an effective way to overcome MDR in cancer cells, meanwhile probing the intracellular microenvironment to realize the controlled drug release characterisitcs 153.
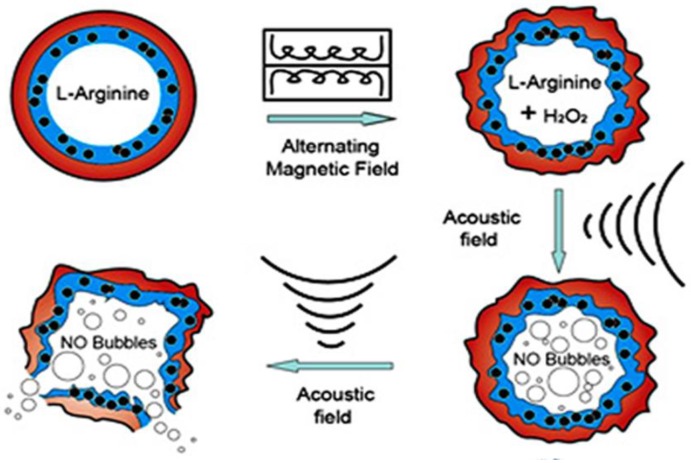
Since magnetic nanoparticles (MNPs) can be manipulated under the influence of an external alternating magnetic field (AMF), they have been utilized in numerous applications related to drug and gene delivery, diagnostics and therapeutics. When MNPs are elaborately assembled with other multiple triggers, the magnetic composites can be developed as synergistic or sequential drug delivery systems to significantly increase delivery efficacy and reduce side effects. For example, when superparamagnetic iron oxide (SPIO) Fe3O4 nanoparticles were embedded into the shell of microbubbles, the SPIO-inclusion microbubbles can be used not only as ultrasound and magnetic resonance dual imaging contrast agents, but also can be manipulated instantaneously by the moderate US irradiation. By external ultrasonic controlling, nanoparticles can be delivered into targeted cells noninvasively and effectively 75, 172. Furthermore, polymeric double-layer shelled microspheres doped with both SPIO Fe3O4 nanoparticles and L-arginine can be smartly controlled by external magnetic force. Under magnetic field exposure, the encapsulated Fe3O4 nanoparticles in the shell induce a change between “open” and “closed” states to allow the exchange of the arginine solution inside and H2O2 solution outside the microcontainers. As shown in Figure 11, once the arginine contacts with H2O2, NO gas can be continuously generated in the microcontainers. The microcontainers can thus be viewed as smart “microreactors” for synthesis of the interior NO gas 73, 173 for US imaging and NO agents related disease therapy.
Figure 11.
Schematic illustration of microvesicles encapsulated magnetic nanoparticles and glucose oxidase for dual-stimuli responsive programmable delivery system. The encapsulated glucose-specific enzyme firstly catalyzes glucose into gluconic acid and H2O2. The subsequent alternating magnetic field increases the porosity of the polymer shell, leading to the reaction between H2O2 and L-arginine to produce nitric oxides. Reproduced with permission from reference 73.
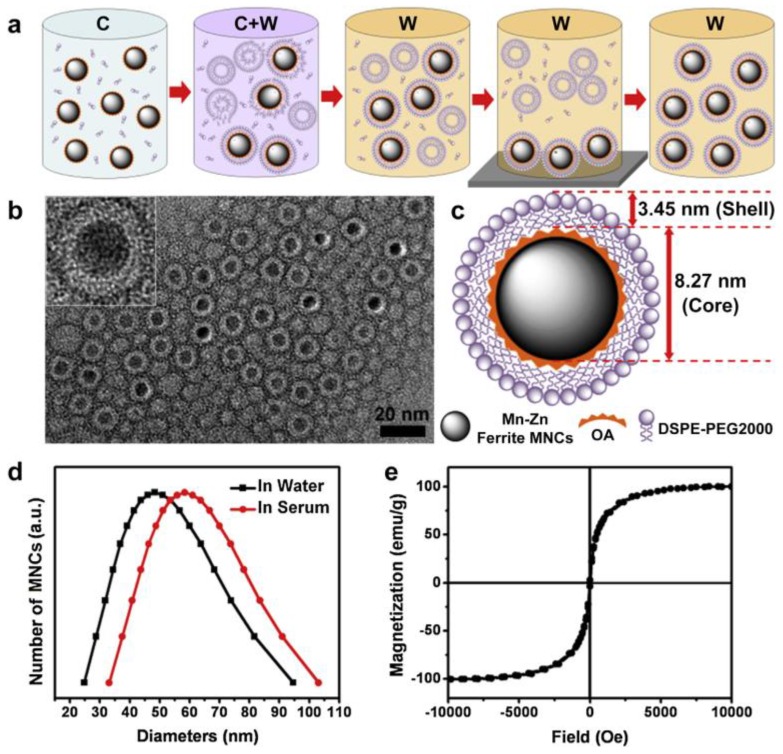
Another important biomedical application of hybrid MNPs is in the field of theranostics. It is well known that the SPIO can be used as T1 or T2 contrast agent for magnetic resonance imaging (MRI). At the same time, SPIO can be fabricated as carrier to load anticancer drugs to the target sites by external magnetic field control. Both the release of chemical drug and the hyperthermia of SPIO triggered by the external magnetic field are beneficial for tumor therapy 50. As mentioned above, one strategy of smart SPIO-based nanomaterials for theranostics is a combination of MRI and hyperthermic therapy. However, challenges are still ahead to increase both the imaging and therapy efficiency in one single SPIO platform. Xie et al. 55 successfully developed an effective magnetic nanocrystals (MNCs)-mediated theranostics strategy by using MRI and AMF in hyperthermic therapy. The PEGylated Mn-Zn ferrite nanocrystals showed excellent performance in the magnetism, relaxivity coefficient, and specific absorptionrate (SAR) value (Figure 12). Real time MRI monitoring by AMF indicated the effective suppress of the tumor growth. Moreover, the MRI monitoring guided smart magnetic DDSs can realize the accurate long-lasting tumor hyperthermia.
Figure 12.
(a) Schematic diagram to describe the synthesis of PEGylated Mn-Zn ferrite MNCs (C: chloroform; W: water). (b) TEM image of PEGylated MNCs with core-shell structure, and the white layer surrounding the magnetic cores indicating the DSPE-PEG2000 coating layer. (c) Schematic illustration of PEGylated MNCs with 8.27 nm magnetic core and 3.45 nm lipid shell. (d) DLS curve of PEGylated MNCs in water and serum. (e) Hysteresis loops at room temperature for PEGylated MNCs. Reproduced with permission from reference 55.
Despite of its the fast progress, the hybrid smart nano-DDS in all-in-one strategy still has some aspects to be improved, such as the intrinsic low sensitivity, high cost of manufacture, toxicity and non-biodegradable nature. The current developmental stage of theranostic hybrid smart DDSs are still in their early stage.
3.4 Exosomes
Exosomes are nano-sized membrane vesicles secreted by special cells related tissues or cells when responding to the endogenous or exogenous stimuli. The application of exosome is rapidly developed in recent years as one kind of biomaterials for drug delivery. For example, exosomes can be directed to specific tissues, while maintaining their biological functions 174-178. As important mediators of intercellular communication and regulators of the cellular niche, exosomes appear to play an important role in many disease processes, most notably cancers and inflammation. Many features suggest that the exosomes are ideal drug delivery vehicles. Firstly, exosomes can load with specific drugs to serve as carriers for personalized medicine. Since exosomes released from various types of host cells, they show different biological effects and targeting specificities. Secondly, exosomes are well biocompatible with the lowest cytotoxicity. For instance, Batrakova and coworkers have developed a novel exosome-based delivery system as catalase-loaded exosomes (exoCAT) to treat Parkinson's disease (PD) 179. Exosomes reformed upon sonication and extrusion, or permeabilization with saponin resulted in high loading efficiency, sustained release, and catalase preservation against proteases degradation. Thus, it can serve as a versatile strategy to treat inflammatory and neurodegenerative disorders. Exosomes can also be used to deliver chemotherapeutics such as DOX to tumor tissues. Nie's group has utilized the mouse immature dendritic cells (imDCs) to produce exosome, then the exosomal membrane protein (Lamp2b) fused to αv integrin-specific iRGD peptide (CRGDKGPDC) and DOX were loaded into purified exosomes from imDCs via electroporation 180. iRGD exosomes have demonstrated highly efficient targeting. The growth of tumor was well inhibited without overt toxicity, which could be a promising strategy for clinical applications.
In a nutshell, exosomes may serve as a promising candidate for smart drug delivery nanocarrier due to non-cytotoxic effect, nature targeting characteristics, and a high drug loading capacity. However, as an emerging area, the application of exosomes as DDS has not been largely explored and there are still many challenges ahead. One of the major challenges is to maintain their biological properties during the therapeutics loading process. Although the large-scale production of exosomes can be gained by the oncogenic immortalization of human stem cells 181, it is still need to achieve large-scale production with ideal reproducibility from the appropriate type of exosomes. Moreover, main challenges for current research on exosomes are data collection and classification, as well as the establishment of related evaluation and test methods, which are key issues need to be addressed for clinical application.
4. What are the obstacles for smart nanoplatform in potential clinical applications?
A great number of smart DDSs have been developed over the last decades 8, 182, 183. Until now, some nanocarriers have been approved in clinic, including liposomes, nano-suspension, polymer nanoparticles, nanocapsule, micelles, etc. For instance, the typical nanomedicines that approved for clinical applications are, Doxil (Liposomal DOX), Lipusu (Liposomal PTX), and Abraxane (Nanoparticulate albumin/PTX), to name a few 184. Visudyne, as a nanomedicine for photodynamics therapy, was the only nanomedicine with stimuli-responsive nanoplatform concept approved by Food and Drug Administration (FDA). However, other stimuli-responsive DDSs are still at clinical stage, as shown in Table 2. These approved formulations have been adequately evaluated and deeply optimized over the years. One significant characteristic of these promising clinical nanocarriers relies on the simple formulations. Contradictory, in order to obtain smart properties, most of developed smart DDSs were designed with sophisticated structures and formulations, which is difficult to scale up for industrial production. Therefore, the design simplicity is still one of the key points for successful drug carrier translation in these smart carriers. Besides, an extremely large numbers of optimizations and improvements experiments are needed for the translation of each stimulus from preclinical experimental models to routine clinical practice. Especially, endogenous triggers (such as pH gradient, enzyme concentration) are indeed difficult to control because the tremendous variation from one patient to another. Although exogenous stimuli responsive systems are much easier to be controlled and is much more promising for clinic, major improvements would be required to solve problems related to normal tissue damage and tissue-penetration depth 19. A clinically useful formulation should possess the properties of reproducibility, scale up possibility, and verifiability. Accordingly, more attentions should be paid to the development of advanced approaches that can precisely control over the preparation process, for the purpose of generating nanocarriers with required features, high batch-to-batch reproducibility, and industrial scale-up feasibility. The standard production methods and stimuli dosage control may finally accelerate the translation of smart drugs from the bench to the bedside.
Table 2.
Stimuli-responsive drug-delivery systems in clinical trials.
| Applications | Indication | Stimulus/Drug formulation | Company | Status | Ref |
|---|---|---|---|---|---|
| ThermoDox | Breast cancer, primary liver cancer | Thermosensitive liposomal doxorubicin | Celsion Corporation | Phase II/III | [185] |
| Opaxio | Ovarian cancer | Enzyme-activated polymeric NP | Cell Therapeutics, Inc. | Phase III | [186] |
| NanoTherm | Glioblastoma, prostata cancer, eosphageal cancer, pancreatic cancer | Magnetic sensitive iron oxide NPs | MagForce Nanotechnologies AG | Phase I/II | [187] www.magforce.de |
| AuroShell | Intracranial tumors | Thermosensitive gold nanoshell | Nanospectra Biosciences | Phase I | [188, 189] |
4.1 Difficulties in the translation of sugar-coating nanoplatform into clinical products
Although several excellent review articles are available 3, 7-9, 162, to our point of view, there are still lots of obstacles ahead for smart drug delivery systems from bench to bed. Fundamental research has been extensively explored on “clinical prospects”, but some of them might never enter into clinic. The possible obstacles have been described as below:
Firstly, “quality by design” (QBD) means a kind of scientific, risk based, and active drug development method, and the design run throughout from the concept of product to industrialization. QBD has been a consensus in drug manufacturing, which is also useful for the development of smart DDSs. Many of the nanotherapeutics are administered by intravenous injection. Despite of their potential for increasing the half-life of drugs, the nanocarriers face a series of complex biological barriers that greatly limit the site-specific targeting. Most of nanoplatforms will not succeed in clinics if the biological barriers faced by the nanoparticles are not conquered 184. Biological barriers such as opsonization, the mononuclear phagocyte system (MPS), the high intratumoral pressure, cellular internalization, escaping from endosomal and lysosomal compartments as well as drug efflux pumps will not only hinder the accumulation of nanocarriers at target sites, but also limit the therapeutic outcomes 190. In addition to the substantial challenges demonstrated by each individual biological barrier, other factors such as administration routes, disease types, and disease progression should also be considered in the design of smart DDS because these barriers vary in complexity in vivo.
Secondly, the principles of pharmaceutical development are safety, effective, quality controllability, and patient compliance. The safety issues as well as toxicity are still priority needs to be fulfilled by the smart DDSs. There are many issues need to be addressed, including the safety of the nanomaterials, their biodegradability and their metabolites. For potential new drug candidates, the safety tests including biocompatibility, acute toxicity, subacute toxicity, carcinogenicity, developmental toxicity, immunotoxicology, genotoxicity, and irritation studies to blood vessel are required. The pharmacodynamics and pharmacokinetics involved in the absorption, distribution, metabolism, excretion (ADME) process should also be well studied. Unfortunately, there are limited publications of such studies on the orally administrated nanoscale DDSs, and current research is much more focused on “smart” instead of “basics”. Moreover, compared with conventional DDS, stimuli-responsive DDSs maybe more prone to inter-subject variability in their performance, thus raising issues with reproducibility in safety/ efficacy. Researchers also need to pay more attention and energy to this topic from the market point of view.
Thirdly, major issues for clinical translation of nanomedicine include industrial scale-up validation, batch-to-batch reproducibility, as well as controllability of nanomaterials' physicochemical properties. Verification of the methodology should be confirmed for the novel smart nano-DDS, such as linearity, stability, specificity, precision, repeatability. Thus, reproducibility and product analysis are key obstacles for a Good Manufacturing Practice (GMP)-compliant, industrial scale-up production of smart DDSs. In summary, all these issues challenge the clinical applications of stimulus-sensitive DDSs. These issues for smart DDSs must be solved to ensure the successful clinical translation.
Finally, in order to make a real impact for nanotechnology to be integrated with pharmaceutical industry, it is necessary to formulate a clear regulatory framework to approve new nanomedicine products. Though a number of nanomedicines have been approved by the FDA and European Medicines Agency (EMA), frameworks for the regulation of nano-enabled medical products acceptable for pharmaceutical and medical-device companies and public are not available yet 191. The guidance be named “Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology” was released by FDA in 2014, which is the only official regulation that can be referenced for nanomedicine industry. Despite the scope of nanomaterials are defined in the guidance, which is far from sufficient for nano-pharmaceutical industry 192. Regulations are not only essential for defining concept of nanomedicine products, but also significant for the characterization of nanomedicine and quality control, as well as clinical trials and the approval process. In the same way, standardized methods will accelerate the research and translation of smart DDSs. Although the National Cancer Institute (NCI) and FDA have done a lot of work on standardization of nanomedicine characterizations and analyses, there are still a long way to create standardized definitions and protocols to characterize nanomedicines for clinics.
4.2 Current limitations between small animal evaluation and clinical effect of nanoplatforms
Animal research is an indispensable part in preclinical studies. The appropriate animal models are of great importance for in vivo therapeutic evaluation of drugs. The complexity of nanomaterials places higher demands on animal models, especially for smart DDSs. Though there are tremendous amount of publications about advanced DDSs, little has been published on the relevance of safety and efficacy evaluation of smart DDSs in animals to be predictive clinical effect in humans 8, 193. Small animal models, like mouse, rats and rabbits, are indispensable tools for understanding the molecular basis and pathology of diseases. Nevertheless, current animal models fail to accurately reproduce human diseases because of inbreeding. Each human being is unique, therefore, the diversity and complexity of the disease have been created. Accordingly, mouse models of various diseases need fully consider their complexity by bringing in population diversity into disease modelling rather than relying on inbred strains of laboratory animals 194, 195. Furthermore, to make the results of animal evaluation of nanoplatforms be valuable for the clinical research, the following points must be considered. At first, a specific disease should be evaluated on different animal species. A single model usually cannot reflect the complexity species of patient population. The animal models evaluation standards of different diseases must be established. Second, a full assessment should be done with drug dosage and administration to ensure a good relativity among the in vitro experiments, animal evaluation and human trials. Last but not the least, for the advanced DDSs, it is reasonable to develop the effective evaluation strategies for a good appreciation of toxicology, pharmacokinetics and pharmacodynamics based on animal models.
5. Conclusion and perspective
With the development of material science, pharmaceutical sciences and biomedical science, various controlled releasing nanomaterials will be used for smart DDSs in the future. Although smart nano-DDSs have shown to be much more efficient in both diagnosis and therapy, potential druggability still needs to be evaluated before the smart DDSs reach to clinics. It will be an enormous challenge for researchers to improve preclinical research of advanced DDSs to reproducible and translatable production to clinical-trial success. Nevertheless, it is must be kept in mind that patients treatments are the ultimate purpose of all our efforts. Future work about smart DDSs for controlled drug delivery should be focused on the study of clinical translation to ensure more stimulus- sensitive nanomedicine to be clinically utilized.
Acknowledgments
This work was supported by the National Important Science Research Program of China (Nos. 2013CB733804), National Natural Science Foundation of China for Key Project of International Cooperation (61420106012), National High Technology Research and Development Program (“863” Program) of China (2013AA032205), National Natural Science Foundation of China (31370019, 81473160), and Collaborative Innovation Center of Suzhou Nano Science and Technology as well as Collaborative Innovation Center of Dendrobium Industrialization of Anhui Province. Dr. Dongfei Liu from University of Helsinki, Dr. Yang Shi from South China University of Technology, as well as Dr. Yi Chen from Southeast University are acknowledged for helping us with revision of the manuscript and valuable discussions.
References
- 1.Hrubý M, Filippov SK, Štěpánek P. Smart polymers in drug delivery systems on crossroads: Which way deserves following? European Polymer Journal. 2015;65:82–97. [Google Scholar]
- 2.Kopeček J, Yang J. Hydrogels as smart biomaterials. Polymer International. 2007;56:1078–98. [Google Scholar]
- 3.Lee BK, Yun YH, Park K. Smart nanoparticles for drug delivery: Boundaries and opportunities. Chemical Engineering Science. 2015;125:158–64. doi: 10.1016/j.ces.2014.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamrungsap S, Zhao Z, Chen T, Wang L, Li C, Fu T. et al. Nanotechnology in therapeutics: a focus on nanoparticles as a drug delivery system. Nanomedicine. 2012;7:1253–71. doi: 10.2217/nnm.12.87. [DOI] [PubMed] [Google Scholar]
- 5.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Advanced Drug Delivery Reviews. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 6.Couvreur P. Nanoparticles in drug delivery: past, present and future. Advanced Drug Delivery Reviews. 2013;65:21–3. doi: 10.1016/j.addr.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Lorenzo C, Concheiro A. Smart drug delivery systems: from fundamentals to the clinic. Chemical Communications. 2014;50:7743–65. doi: 10.1039/c4cc01429d. [DOI] [PubMed] [Google Scholar]
- 8.Crommelin DJ, Florence AT. Towards more effective advanced drug delivery systems. International Journal of Pharmaceutics. 2013;454:496–511. doi: 10.1016/j.ijpharm.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Holzapfel BM, Reichert JC, Schantz J-T, Gbureck U, Rackwitz L, Nöth U. et al. How smart do biomaterials need to be? A translational science and clinical point of view. Advanced Drug Delivery Reviews. 2013;65:581–603. doi: 10.1016/j.addr.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Grund S, Bauer M, Fischer D. Polymers in drug delivery—state of the art and future trends. Advanced Engineering Materials. 2011;13:B61–B87. [Google Scholar]
- 11.Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C. et al. 25th anniversary article: rational design and applications of hydrogels in regenerative medicine. Advanced Materials. 2014;26:85–124. doi: 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang H-I, Yeh M-K. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. International Journal of Nanomedicine. 2012;7:49–60. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids and Surfaces B: Biointerfaces. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Rossi F, Ferrari R, Castiglione F, Mele A, Perale G, Moscatelli D. Polymer hydrogel functionalized with biodegradable nanoparticles as composite system for controlled drug delivery. Nanotechnology. 2014;26:015602. doi: 10.1088/0957-4484/26/1/015602. [DOI] [PubMed] [Google Scholar]
- 15.Shimoni O, Postma A, Yan Y, Scott AM, Heath JK, Nice EC. et al. Macromolecule functionalization of disulfide-bonded polymer hydrogel capsules and cancer cell targeting. ACS Nano. 2012;6:1463–72. doi: 10.1021/nn204319b. [DOI] [PubMed] [Google Scholar]
- 16.Stumpel JE, Gil ER, Spoelstra AB, Bastiaansen CW, Broer DJ, Schenning AP. Stimuli-Responsive Materials Based on Interpenetrating Polymer Liquid Crystal Hydrogels. Advanced Functional Materials. 2015;25:3314–20. [Google Scholar]
- 17.Tanaka T. Collapse of gels and the critical endpoint. Physical Review Letters. 1978;40:820. [Google Scholar]
- 18.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–3. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 19.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nature Materials. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 20.Kelley EG, Albert JN, Sullivan MO, Epps III TH. Stimuli-responsive copolymer solution and surface assemblies for biomedical applications. Chemical Society Reviews. 2013;42:7057–71. doi: 10.1039/c3cs35512h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Huang Y, Kumar A, Tan A, Jin S, Mozhi A. et al. pH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechnology Advances. 2014;32:693–710. doi: 10.1016/j.biotechadv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Ganesh VA, Baji A, Ramakrishna S. Smart functional polymers-a new route towards creating a sustainable environment. RSC Advances. 2014;4:53352–64. [Google Scholar]
- 23.Gao W, Chan JM, Farokhzad OC. pH-responsive nanoparticles for drug delivery. Molecular Pharmaceutics. 2010;7:1913–20. doi: 10.1021/mp100253e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu P, Yu H, Guo C, Cui Z, Chen X, Yin Q. et al. Reversal of doxorubicin resistance in breast cancer by mitochondria-targeted pH-responsive micelles. Acta Biomaterialia. 2015;14:115–24. doi: 10.1016/j.actbio.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Subudhi MB, Jain A, Jain A, Hurkat P, Shilpi S, Gulbake A. et al. Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5-Fluorouracil. Materials. 2015;8:832–49. doi: 10.3390/ma8030832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stubbs M, McSheehy PM, Griffiths JR, Bashford CL. Causes and consequences of tumour acidity and implications for treatment. Molecular Medicine Today. 2000;6:15–9. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- 27.Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nature Reviews Drug Discovery. 2011;10:767–77. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 28.Lee ES, Oh KT, Kim D, Youn YS, Bae YH. Tumor pH-responsive flower-like micelles of poly (L-lactic acid)-b-poly (ethylene glycol)-b-poly (L-histidine) Journal of Controlled Release. 2007;123:19–26. doi: 10.1016/j.jconrel.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng R, Meng F, Deng C, Klok H-A, Zhong Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 2013;34:3647–57. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 30.Pan Y-J, Chen Y-Y, Wang D-R, Wei C, Guo J, Lu D-R. et al. Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials. 2012;33:6570–9. doi: 10.1016/j.biomaterials.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Zhong P, Meng F, Cheng R, Deng C, Feijen J. et al. Redox and pH-responsive degradable micelles for dually activated intracellular anticancer drug release. Journal of Controlled Release. 2013;169:171–9. doi: 10.1016/j.jconrel.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Huo M, Yuan J, Tao L, Wei Y. Redox-responsive polymers for drug delivery: from molecular design to applications. Polymer Chemistry. 2014;5:1519–28. [Google Scholar]
- 33.Wang J, Sun X, Mao W, Sun W, Tang J, Sui M. et al. Tumor Redox Heterogeneity-Responsive Prodrug Nanocapsules for Cancer Chemotherapy. Advanced Materials. 2013;25:3670–6. doi: 10.1002/adma.201300929. [DOI] [PubMed] [Google Scholar]
- 34.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nature Reviews Drug Discovery. 2014;13:813–27. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N. Orally delivered thioketal nanoparticles loaded with TNF-α-siRNA target inflammation and inhibit gene expression in the intestines. Nature Materials. 2010;9:923–8. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen MM, Carlini AS, Chien MP, Sonnenberg S, Luo C, Braden RL. et al. Enzyme-Responsive Nanoparticles for Targeted Accumulation and Prolonged Retention in Heart Tissue after Myocardial Infarction. Advanced Materials. 2015;27:5547–52. doi: 10.1002/adma.201502003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Callmann CE, Barback CV, Thompson MP, Hall DJ, Mattrey RF, Gianneschi NC. Therapeutic Enzyme-Responsive Nanoparticles for Targeted Delivery and Accumulation in Tumors. Advanced Materials. 2015;27:4611–5. doi: 10.1002/adma.201501803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De La Rica R, Aili D, Stevens MM. Enzyme-responsive nanoparticles for drug release and diagnostics. Advanced Drug Delivery Reviews. 2012;64:967–78. doi: 10.1016/j.addr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 39.[39] Lock LL, Tang Z, Keith D, Reyes C, Cui H. Enzyme-Specific Doxorubicin Drug Beacon as Drug-Resistant Theranostic Molecular Probes. ACS Macro Letters. 2015;4:552–5. doi: 10.1021/acsmacrolett.5b00170. [DOI] [PubMed] [Google Scholar]
- 40.Shi Y, van den Dungen ET, Klumperman B, van Nostrum CF, Hennink WE. Reversible Addition-Fragmentation Chain Transfer Synthesis of a Micelle-Forming, Structure Reversible Thermosensitive Diblock Copolymer Based on the N-(2-Hydroxy propyl) Methacrylamide Backbone. ACS Macro Letters. 2013;2:403–8. doi: 10.1021/mz300662b. [DOI] [PubMed] [Google Scholar]
- 41.Shi Y, van Steenbergen MJ, Teunissen EA, Novo Ls, Gradmann S, Baldus M. et al. Π-Π stacking increases the stability and loading capacity of thermosensitive polymeric micelles for chemotherapeutic drugs. Biomacromolecules. 2013;14:1826–37. doi: 10.1021/bm400234c. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Cardoso RM, Van Nostrum CF, Hennink WE. Anthracene functionalized thermosensitive and UV-crosslinkable polymeric micelles. Polymer Chemistry. 2015;6:2048–53. [Google Scholar]
- 43.Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. Journal of Controlled Release. 2010;148:135–46. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 44.Adelsberger J, Kulkarni A, Jain A, Wang W, Bivigou-Koumba AM, Busch P. et al. Thermoresponsive PS-b-PNIPAM-b-PS micelles: aggregation behavior, segmental dynamics, and thermal response. Macromolecules. 2010;43:2490–501. [Google Scholar]
- 45.Zhao Y, Fan X, Liu D, Wang Z. PEGylated thermo-sensitive poly (amidoamine) dendritic drug delivery systems. International Journal of Pharmaceutics. 2011;409:229–36. doi: 10.1016/j.ijpharm.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Lal S, Clare SE, Halas NJ. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Accounts of Chemical Research. 2008;41:1842–51. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 47.Sun J, Zhang Y, Chen Z, Zhou J, Gu N. Fibrous Aggregation of Magnetite Nanoparticles Induced by a Time-Varied Magnetic Field. Angewandte Chemie International Edition. 2007;46:4767–70. doi: 10.1002/anie.200604474. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Zhang Y, Wang C, Xu R, Chen Z, Gu N. Magnetically sensitive alginate-templated polyelectrolyte multilayer microcapsules for controlled release of doxorubicin. The Journal of Physical Chemistry C. 2010;114:7673–9. [Google Scholar]
- 49.Chen Z, Yin J-J, Zhou Y-T, Zhang Y, Song L, Song M. et al. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. Acs Nano. 2012;6:4001–12. doi: 10.1021/nn300291r. [DOI] [PubMed] [Google Scholar]
- 50.Fang K, Song L, Gu Z, Yang F, Zhang Y, Gu N. Magnetic field activated drug release system based on magnetic PLGA microspheres for chemo-thermal therapy. Colloids and Surfaces B: Biointerfaces. 2015;136:712–20. doi: 10.1016/j.colsurfb.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 51.Yang F, Zhang X, Song L, Cui H, Myers JN, Bai T. et al. Controlled Drug Release and Hydrolysis Mechanism of Polymer-Magnetic Nanoparticle Composite. ACS Applied Materials & Interfaces. 2015;7:9410–9. doi: 10.1021/acsami.5b02210. [DOI] [PubMed] [Google Scholar]
- 52.Hu K, Sun J, Guo Z, Wang P, Chen Q, Ma M. et al. A novel magnetic hydrogel with aligned magnetic colloidal assemblies showing controllable enhancement of magnetothermal effect in the presence of alternating magnetic field. Advanced Materials. 2015;27:2507–14. doi: 10.1002/adma.201405757. [DOI] [PubMed] [Google Scholar]
- 53.Wang F, Kim D-K, Yoshitake T, Johansson S, Bjelke B, Muhammed M. et al. Diffusion and clearance of superparamagnetic iron oxide nanoparticles infused into the rat striatum studied by MRI and histochemical techniques. Nanotechnology. 2010;22:015103. doi: 10.1088/0957-4484/22/1/015103. [DOI] [PubMed] [Google Scholar]
- 54.Yue-Jian C, Juan T, Fei X, Jia-Bi Z, Ning G, Yi-Hua Z. et al. Synthesis, self-assembly, and characterization of PEG-coated iron oxide nanoparticles as potential MRI contrast agent. Drug Development and Industrial Pharmacy. 2010;36:1235–44. doi: 10.3109/03639041003710151. [DOI] [PubMed] [Google Scholar]
- 55.Xie J, Zhang Y, Yan C, Song L, Wen S, Zang F. et al. High-performance PEGylated Mn-Zn ferrite nanocrystals as a passive-targeted agent for magnetically induced cancer theranostics. Biomaterials. 2014;35:9126–36. doi: 10.1016/j.biomaterials.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Xiong F, Chen Y, Chen J, Yang B, Zhang Y, Gao H. et al. Rubik-like magnetic nanoassemblies as an efficient drug multifunctional carrier for cancer theranostics. Journal of Controlled Release. 2013;172:993–1001. doi: 10.1016/j.jconrel.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 57.Song L, Zang F, Song M, Chen G, Zhang Y. Effective PEGylation of Fe3O4 nanomicelles for in vivo MR imaging. Journal of Nanoscience and Nanotechnology. 2015;15:4111–8. doi: 10.1166/jnn.2015.9803. [DOI] [PubMed] [Google Scholar]
- 58.Liu D, Wu W, Chen X, Wen S, Zhang X, Ding Q. et al. Conjugation of paclitaxel to iron oxide nanoparticles for tumor imaging and therapy. Nanoscale. 2012;4:2306–10. doi: 10.1039/c2nr11918h. [DOI] [PubMed] [Google Scholar]
- 59.Yang H-W, Hua M-Y, Liu H-L, Huang C-Y, Tsai R-Y, Lu Y-J. et al. Self-protecting core-shell magnetic nanoparticles for targeted, traceable, long half-life delivery of BCNU to gliomas. Biomaterials. 2011;32:6523–32. doi: 10.1016/j.biomaterials.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi K, Nakamura M, Sakamoto W, Yogo T, Miki H, Ozaki S. et al. Superparamagnetic nanoparticle clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment. Theranostics. 2013;3:366–76. doi: 10.7150/thno.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paris JL, Cabañas MV, Manzano M, Vallet-Regí M. Polymer-Grafted Mesoporous Silica Nanoparticles as Ultrasound-Responsive Drug Carriers. ACS Nano. 2015;9:11023–33. doi: 10.1021/acsnano.5b04378. [DOI] [PubMed] [Google Scholar]
- 62.Guo Q, Zhang T, An J, Wu Z, Zhao Y, Dai X. et al. Block versus Random Amphiphilic Glycopolymer Nanopaticles as Glucose-Responsive Vehicles. Biomacromolecules. 2015;16:3345–56. doi: 10.1021/acs.biomac.5b01020. [DOI] [PubMed] [Google Scholar]
- 63.Wu Q, Wang L, Yu H, Wang J, Chen Z. Organization of glucose-responsive systems and their properties. Chemical Reviews. 2011;111:7855–75. doi: 10.1021/cr200027j. [DOI] [PubMed] [Google Scholar]
- 64.Gu Z, Aimetti AA, Wang Q, Dang TT, Zhang Y, Veiseh O. et al. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano. 2013;7:4194–201. doi: 10.1021/nn400630x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murdan S. Electro-responsive drug delivery from hydrogels. Journal of Controlled Release. 2003;92:1–17. doi: 10.1016/s0168-3659(03)00303-1. [DOI] [PubMed] [Google Scholar]
- 66.Yun J, Im JS, Lee Y-S, Kim H-I. Electro-responsive transdermal drug delivery behavior of PVA/PAA/MWCNT nanofibers. European Polymer Journal. 2011;47:1893–902. [Google Scholar]
- 67.Ying X, Wang Y, Liang J, Yue J, Xu C, Lu L. et al. Angiopep-Conjugated Electro-Responsive Hydrogel Nanoparticles: Therapeutic Potential for Epilepsy. Angewandte Chemie International Edition. 2014;53:12436–40. doi: 10.1002/anie.201403846. [DOI] [PubMed] [Google Scholar]
- 68.Curcio M, Spizzirri UG, Cirillo G, Vittorio O, Picci N, Nicoletta FP. et al. On demand delivery of ionic drugs from electro-responsive CNT hybrid films. RSC Advances. 2015;5:44902–11. [Google Scholar]
- 69.Schmaljohann D. Thermo-and pH-responsive polymers in drug delivery. Advanced Drug Delivery Reviews. 2006;58:1655–70. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, Guo R, Yang M, Jiang X, Liu B. Thermo and pH Dual-Responsive Nanoparticles for Anti-Cancer Drug Delivery. Advanced Materials. 2007;19:2988–92. [Google Scholar]
- 71.Zhang Z, Wang J, Chen C. Near-Infrared Light-Mediated Nanoplatforms for Cancer Thermo-Chemotherapy and Optical Imaging. Advanced Materials. 2013;25:3869–80. doi: 10.1002/adma.201301890. [DOI] [PubMed] [Google Scholar]
- 72.Jochum FD, Theato P. Thermo-and light responsive micellation of azobenzene containing block copolymers. Chemical Communications. 2010;46:6717–9. doi: 10.1039/c0cc01288b. [DOI] [PubMed] [Google Scholar]
- 73.Yang F, Chen P, He W, Gu N, Zhang X, Fang K. et al. Bubble microreactors triggered by an alternating magnetic field as diagnostic and therapeutic delivery devices. Small. 2010;6:1300–5. doi: 10.1002/smll.201000173. [DOI] [PubMed] [Google Scholar]
- 74.Yang F, Hu S, Zhang Y, Cai X, Huang Y, Wang F. et al. A Hydrogen Peroxide-Responsive O2 Nanogenerator for Ultrasound and Magnetic-Resonance Dual Modality Imaging. Advanced Materials. 2012;24:5205–11. doi: 10.1002/adma.201202367. [DOI] [PubMed] [Google Scholar]
- 75.Yang F, Zhang M, He W, Chen P, Cai X, Yang L. et al. Controlled release of Fe3O4 nanoparticles in encapsulated microbubbles to tumor cells via sonoporation and associated cellular bioeffects. Small. 2011;7:902–10. doi: 10.1002/smll.201002185. [DOI] [PubMed] [Google Scholar]
- 76.Yang F, Li M, Cui H, Wang T, Chen Z, Song L. et al. Altering the response of intracellular reactive oxygen to magnetic nanoparticles using ultrasound and microbubbles. Science China Materials. 2015;58:467–80. [Google Scholar]
- 77.Cai X, Yang F, Gu N. Applications of magnetic microbubbles for theranostics. Theranostics. 2012;2:103–12. doi: 10.7150/thno.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Delcea M, Möhwald H, Skirtach AG. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Advanced Drug Delivery Reviews. 2011;63:730–47. doi: 10.1016/j.addr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 79.Stuart MAC, Huck WT, Genzer J, Müller M, Ober C, Stamm M. et al. Emerging applications of stimuli-responsive polymer materials. Nature Materials. 2010;9:101–13. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 80.Gao GH, Li Y, Lee DS. Environmental pH-sensitive polymeric micelles for cancer diagnosis and targeted therapy. Journal of Controlled Release. 2013;169:180–4. doi: 10.1016/j.jconrel.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 81.Du J-Z, Mao C-Q, Yuan Y-Y, Yang X-Z, Wang J. Tumor extracellular acidity-activated nanoparticles as drug delivery systems for enhanced cancer therapy. Biotechnology Advances. 2014;32:789–803. doi: 10.1016/j.biotechadv.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 82.Meng F, Zhong Y, Cheng R, Deng C, Zhong Z. pH-sensitive polymeric nanoparticles for tumor-targeting doxorubicin delivery: concept and recent advances. Nanomedicine. 2014;9:487–99. doi: 10.2217/nnm.13.212. [DOI] [PubMed] [Google Scholar]
- 83.Liu R, Li D, He B, Xu X, Sheng M, Lai Y. et al. Anti-tumor drug delivery of pH-sensitive poly (ethylene glycol)-poly (L-histidine-)-poly (L-lactide) nanoparticles. Journal of Controlled Release. 2011;152:49–56. doi: 10.1016/j.jconrel.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 84.Li H, Li M, Chen C, Fan A, Kong D, Wang Z. et al. On-demand combinational delivery of curcumin and doxorubicin via a pH-labile micellar nanocarrier. International Journal of Pharmaceutics. 2015;495:572–8. doi: 10.1016/j.ijpharm.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 85.Duan X, Xiao J, Yin Q, Zhang Z, Yu H, Mao S. et al. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS Nano. 2013;7:5858–69. doi: 10.1021/nn4010796. [DOI] [PubMed] [Google Scholar]
- 86.Shi Y, van Nostrum CF, Hennink WE. Interfacially Hydrazone Cross-linked Thermosensitive Polymeric Micelles for Acid-Triggered Release of Paclitaxel. ACS Biomaterials Science & Engineering. 2015;1:393–404. doi: 10.1021/acsbiomaterials.5b00006. [DOI] [PubMed] [Google Scholar]
- 87.Du JZ, Sun TM, Song WJ, Wu J, Wang J. A Tumor-Acidity-Activated Charge-Conversional Nanogel as an Intelligent Vehicle for Promoted Tumoral-Cell Uptake and Drug Delivery. Angewandte Chemie International Edition. 2010;122:3703–8. doi: 10.1002/anie.200907210. [DOI] [PubMed] [Google Scholar]
- 88.Du J-Z, Du X-J, Mao C-Q, Wang J. Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. Journal of the American Chemical Society. 2011;133:17560–3. doi: 10.1021/ja207150n. [DOI] [PubMed] [Google Scholar]
- 89.Yuan YY, Mao CQ, Du XJ, Du JZ, Wang F, Wang J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Advanced Materials. 2012;24:5476–80. doi: 10.1002/adma.201202296. [DOI] [PubMed] [Google Scholar]
- 90.Yang X-Z, Du J-Z, Dou S, Mao C-Q, Long H-Y, Wang J. Sheddable ternary nanoparticles for tumor acidity-targeted siRNA delivery. ACS Nano. 2011;6:771–81. doi: 10.1021/nn204240b. [DOI] [PubMed] [Google Scholar]
- 91.Yang XZ, Du XJ, Liu Y, Zhu YH, Liu YZ, Li YP. et al. Rational design of polyion complex nanoparticles to overcome cisplatin resistance in cancer therapy. Advanced Materials. 2014;26:931–6. doi: 10.1002/adma.201303360. [DOI] [PubMed] [Google Scholar]
- 92.Mo R, Sun Q, Xue J, Li N, Li W, Zhang C. et al. Multistage pH-Responsive Liposomes for Mitochondrial-Targeted Anticancer Drug Delivery. Advanced Materials. 2012;24:3659–65. doi: 10.1002/adma.201201498. [DOI] [PubMed] [Google Scholar]
- 93.Ju C, Mo R, Xue J, Zhang L, Zhao Z, Xue L. et al. Sequential intra-intercellular nanoparticle delivery system for deep tumor penetration. Angewandte Chemie International Edition. 2014;53:6253–8. doi: 10.1002/anie.201311227. [DOI] [PubMed] [Google Scholar]
- 94.Zhou K, Wang Y, Huang X, Luby-Phelps K, Sumer BD, Gao J. Tunable, Ultrasensitive pH-Responsive Nanoparticles Targeting Specific Endocytic Organelles in Living Cells. Angewandte Chemie International Edition. 2011;50:6109–14. doi: 10.1002/anie.201100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y, Zhou K, Huang G, Hensley C, Huang X, Ma X. et al. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nature Materials. 2014;13:204–12. doi: 10.1038/nmat3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun H, Guo B, Cheng R, Meng F, Liu H, Zhong Z. Biodegradable micelles with sheddable poly (ethylene glycol) shells for triggered intracellular release of doxorubicin. Biomaterials. 2009;30:6358–66. doi: 10.1016/j.biomaterials.2009.07.051. [DOI] [PubMed] [Google Scholar]
- 97.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. Journal of Controlled Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 98.Son S, Shin E, Kim B-S. Redox-Degradable Biocompatible Hyperbranched Polyglycerols: Synthesis, Copolymerization Kinetics, Degradation, and Biocompatibility. Macromolecules. 2015;48:600–9. [Google Scholar]
- 99.Kountouras J, Chatzopoulos D, Zavos C. Reactive oxygen metabolites and upper gastrointestinal diseases. Hepato-gastroenterology. 2000;48:743–51. [PubMed] [Google Scholar]
- 100.Wang W, Lin J, Cai C, Lin S. Optical properties of amphiphilic copolymer-based self-assemblies. European Polymer Journal. 2015;65:112–31. [Google Scholar]
- 101.Lovell JF, Liu TW, Chen J, Zheng G. Activatable photosensitizers for imaging and therapy. Chemical Reviews. 2010;110:2839–57. doi: 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- 102.Farhadi A, Roxin Á, Wilson BC, Zheng G. Nano-enabled SERS reporting photosensitizers. Theranostics. 2015;5:469. doi: 10.7150/thno.10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL. et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nature Materials. 2011;10:324–32. doi: 10.1038/nmat2986. [DOI] [PubMed] [Google Scholar]
- 104.Wang F, Banerjee D, Liu Y, Chen X, Liu X. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst. 2010;135:1839–54. doi: 10.1039/c0an00144a. [DOI] [PubMed] [Google Scholar]
- 105.Liu Q, Yin B, Yang T, Yang Y, Shen Z, Yao P. et al. A general strategy for biocompatible, high-effective upconversion nanocapsules based on triplet-triplet annihilation. Journal of the American Chemical Society. 2013;135:5029–37. doi: 10.1021/ja3104268. [DOI] [PubMed] [Google Scholar]
- 106.Liu T-Y, Hu S-H, Liu D-M, Chen S-Y, Chen I-W. Biomedical nanoparticle carriers with combined thermal and magnetic responses. Nano Today. 2009;4:52–65. [Google Scholar]
- 107.Kim H, Kang YJ, Kang S, Kim KT. Monosaccharide-responsive release of insulin from polymersomes of polyboroxole block copolymers at neutral pH. Journal of the American Chemical Society. 2012;134:4030–3. doi: 10.1021/ja211728x. [DOI] [PubMed] [Google Scholar]
- 108.Bonnet V, Gervaise C, Djedaïni-Pilard F, Furlan A, Sarazin C. Cyclodextrin nanoassemblies: a promising tool for drug delivery. Drug Discovery Today. 2015;20:1120–6. doi: 10.1016/j.drudis.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 109.Ryu JH, Hong S, Lee H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomaterialia. 2015;27:101–15. doi: 10.1016/j.actbio.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 110.Bangham A, Standish MM, Watkins J. Diffusion of univalent ions across the lamellae of swollen phospholipids. Journal of Molecular Biology. 1965;13:238–IN27. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 111.Deamer DW. From “Banghasomes” to liposomes: A memoir of Alec Bangham, 1921-2010. The FASEB Journal. 2010;24:1308–10. doi: 10.1096/fj.10-0503. [DOI] [PubMed] [Google Scholar]
- 112.Gregoriadis G, Ryman B. Liposomes as carriers of enzymes or drugs: a new approach to the treatment of storage diseases. Biochemical Journal. 1971;124:58P. doi: 10.1042/bj1240058p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y. et al. Liposome: classification, preparation, and applications. Nanoscale Research Letters. 2013;8:102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Torchilin V. Liposomes in drug delivery. Fundamentals and Applications of Controlled Release Drug Delivery: Springer; 2012. p289-328. [Google Scholar]
- 115.Barenholz YC. Doxil®—the first FDA-approved nano-drug: lessons learned. Journal of Controlled Release. 2012;160:117–34. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 116.Bibi S, Lattmann E, Mohammed AR, Perrie Y. Trigger release liposome systems: local and remote controlled delivery? Journal of Microencapsulation. 2012;29:262–76. doi: 10.3109/02652048.2011.646330. [DOI] [PubMed] [Google Scholar]
- 117.Sawant RR, Torchilin VP. Liposomes as 'smart'pharmaceutical nanocarriers. Soft Matter. 2010;6:4026–44. [Google Scholar]
- 118.Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Research. 2002;4:95. doi: 10.1186/bcr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clemons KV, Stevens DA. Comparative efficacies of four amphotericin B formulations—Fungizone, Amphotec (Amphocil), AmBisome, and Abelcet against systemic murine aspergillosis. Antimicrobial Agents and Chemotherapy. 2004;48:1047–50. doi: 10.1128/AAC.48.3.1047-1050.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Petre CE, Dittmer DP. Liposomal daunorubicin as treatment for Kaposi's sarcoma. International Journal of Nanomedicine. 2007;2:277. [PMC free article] [PubMed] [Google Scholar]
- 121.Chamberlain MC. Neurotoxicity of intra-CSF liposomal cytarabine (DepoCyt) administered for the treatment of leptomeningeal metastases: a retrospective case series. Journal of Neuro-oncology. 2012;109:143–8. doi: 10.1007/s11060-012-0880-x. [DOI] [PubMed] [Google Scholar]
- 122.Nicolini A, Giardino R, Carpi A, Ferrari P, Anselmi L, Colosimo S. et al. Metastatic breast cancer: an updating. Biomedicine & Pharmacotherapy. 2006;60:548–56. doi: 10.1016/j.biopha.2006.07.086. [DOI] [PubMed] [Google Scholar]
- 123.Barnes LD, Giuliano EA, Ota J. Cellular localization of Visudyne® as a function of time after local injection in an in vivo model of squamous cell carcinoma: an investigation into tumor cell death. Veterinary Ophthalmology. 2010;13:158–65. doi: 10.1111/j.1463-5224.2010.00775.x. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Q, Huang X-E, Gao L-L. A clinical study on the premedication of paclitaxel liposome in the treatment of solid tumors. Biomedicine & Pharmacotherapy. 2009;63:603–7. doi: 10.1016/j.biopha.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 125.Silverman JA, Deitcher SR. Marqibo®(vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemotherapy and Pharmacology. 2013;71:555–64. doi: 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen K-J, Chaung E-Y, Wey S-P, Lin K-J, Cheng F, Lin C-C. et al. Hyperthermia-mediated local drug delivery by a bubble-generating liposomal system for tumor-specific chemotherapy. ACS Nano. 2014;8:5105–15. doi: 10.1021/nn501162x. [DOI] [PubMed] [Google Scholar]
- 127.Kono K, Ozawa T, Yoshida T, Ozaki F, Ishizaka Y, Maruyama K. et al. Highly temperature-sensitive liposomes based on a thermosensitive block copolymer for tumor-specific chemotherapy. Biomaterials. 2010;31:7096–105. doi: 10.1016/j.biomaterials.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 128.Pradhan P, Giri J, Rieken F, Koch C, Mykhaylyk O, Döblinger M. et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. Journal of Controlled Release. 2010;142:108–21. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 129.Simões S, Moreira JN, Fonseca C, Düzgüneş N, Pedroso de Lima MC. On the formulation of pH-sensitive liposomes with long circulation times. Advanced Drug Delivery Reviews. 2004;56:947–65. doi: 10.1016/j.addr.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 130.Obata Y, Tajima S, Takeoka S. Evaluation of pH-responsive liposomes containing amino acid-based zwitterionic lipids for improving intracellular drug delivery in vitro and in vivo. Journal of Controlled Release. 2010;142:267–76. doi: 10.1016/j.jconrel.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 131.Chiang Y-T, Lo C-L. pH-responsive polymer-liposomes for intracellular drug delivery and tumor extracellular matrix switched-on targeted cancer therapy. Biomaterials. 2014;35:5414–24. doi: 10.1016/j.biomaterials.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 132.Cuomo F, Lopez F, Ceglie A, Maiuro L, Miguel MG, Lindman B. pH-responsive liposome-templated polyelectrolyte nanocapsules. Soft Matter. 2012;8:4415–20. [Google Scholar]
- 133.[133] Wan Y, Han J, Fan G, Zhang Z, Gong T, Sun X. Enzyme-responsive liposomes modified adenoviral vectors for enhanced tumor cell transduction and reduced immunogenicity. Biomaterials. 2013;34:3020–30. doi: 10.1016/j.biomaterials.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 134.Zhu G, Mock JN, Aljuffali I, Cummings BS, Arnold RD. Secretory phospholipase A2 responsive liposomes. Journal of Pharmaceutical Sciences. 2011;100:3146–59. doi: 10.1002/jps.22530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang S-L. Ultrasound-responsive liposomes. Liposomes: Methods and Protocols, Volume 1: Pharmaceutical Nanocarriers; 2010. pp. 113–28. [DOI] [PubMed] [Google Scholar]
- 136.Yudina A, De Smet M, Lepetit-Coiffe M, Langereis S, Van Ruijssevelt L, Smirnov P. et al. Ultrasound-mediated intracellular drug delivery using microbubbles and temperature-sensitive liposomes. Journal of Controlled Release. 2011;155:442–8. doi: 10.1016/j.jconrel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 137.Klibanov AL, Shevchenko TI, Raju BI, Seip R, Chin CT. Ultrasound-triggered release of materials entrapped in microbubble-liposome constructs: a tool for targeted drug delivery. Journal of Controlled Release. 2010;148:13–7. doi: 10.1016/j.jconrel.2010.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Paasonen L, Sipilä T, Subrizi A, Laurinmäki P, Butcher SJ, Rappolt M. et al. Gold-embedded photosensitive liposomes for drug delivery: triggering mechanism and intracellular release. Journal of Controlled Release. 2010;147:136–43. doi: 10.1016/j.jconrel.2010.07.095. [DOI] [PubMed] [Google Scholar]
- 139.Leung SJ, Romanowski M. Light-activated content release from liposomes. Theranostics. 2012;2:1020–36. doi: 10.7150/thno.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Skupin-Mrugalska P, Piskorz J, Goslinski T, Mielcarek J, Konopka K, Düzgüneş N. Current status of liposomal porphyrinoid photosensitizers. Drug Discovery Today. 2013;18:776–84. doi: 10.1016/j.drudis.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 141.Mikhaylov G, Mikac U, Magaeva AA, Itin VI, Naiden EP, Psakhye I. et al. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nature Nanotechnology. 2011;6:594–602. doi: 10.1038/nnano.2011.112. [DOI] [PubMed] [Google Scholar]
- 142.de Smet M, Heijman E, Langereis S, Hijnen NM, Grüll H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: an in vivo proof-of-concept study. Journal of Controlled Release. 2011;150:102–10. doi: 10.1016/j.jconrel.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 143.de Smet M, Langereis S, van den Bosch S, Grüll H. Temperature-sensitive liposomes for doxorubicin delivery under MRI guidance. Journal of Controlled Release. 2010;143:120–7. doi: 10.1016/j.jconrel.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 144.Sanchez C, Julián B, Belleville P, Popall M. Applications of hybrid organic-inorganic nanocomposites. Journal of Materials Chemistry. 2005;15:3559–92. [Google Scholar]
- 145.Yang P, Gai S, Lin J. Functionalized mesoporous silica materials for controlled drug delivery. Chemical Society Reviews. 2012;41:3679–98. doi: 10.1039/c2cs15308d. [DOI] [PubMed] [Google Scholar]
- 146.Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chemical Society Reviews. 2012;41:2590–605. doi: 10.1039/c1cs15246g. [DOI] [PubMed] [Google Scholar]
- 147.Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Advanced Materials. 2012;24:1504–34. doi: 10.1002/adma.201104763. [DOI] [PubMed] [Google Scholar]
- 148.Vivero-Escoto JL, Slowing II, Trewyn BG, Lin VSY. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small. 2010;6:1952–67. doi: 10.1002/smll.200901789. [DOI] [PubMed] [Google Scholar]
- 149.Kim D, Park S, Lee JH, Jeong YY, Jon S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. Journal of the American Chemical Society. 2007;129:7661–5. doi: 10.1021/ja071471p. [DOI] [PubMed] [Google Scholar]
- 150.Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chemical Society Reviews. 2012;41:2740–79. doi: 10.1039/c1cs15237h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mieszawska AJ, Mulder WJ, Fayad ZA, Cormode DP. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Molecular Pharmaceutics. 2013;10:831–47. doi: 10.1021/mp3005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sun T-M, Wang Y-C, Wang F, Du J-Z, Mao C-Q, Sun C-Y. et al. Cancer stem cell therapy using doxorubicin conjugated to gold nanoparticles via hydrazone bonds. Biomaterials. 2014;35:836–45. doi: 10.1016/j.biomaterials.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 153.Wang F, Wang Y-C, Dou S, Xiong M-H, Sun T-M, Wang J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. Acs Nano. 2011;5:3679–92. doi: 10.1021/nn200007z. [DOI] [PubMed] [Google Scholar]
- 154.Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chemical Society Reviews. 2012;41:2256–82. doi: 10.1039/c1cs15166e. [DOI] [PubMed] [Google Scholar]
- 155.Wang C, Cheng L, Liu Z. Drug delivery with upconversion nanoparticles for multi-functional targeted cancer cell imaging and therapy. Biomaterials. 2011;32:1110–20. doi: 10.1016/j.biomaterials.2010.09.069. [DOI] [PubMed] [Google Scholar]
- 156.Tsang M-K, Bai G, Hao J. Stimuli responsive upconversion luminescence nanomaterials and films for various applications. Chemical Society Reviews. 2015;44:1585–607. doi: 10.1039/c4cs00171k. [DOI] [PubMed] [Google Scholar]
- 157.Amstad E, Kohlbrecher J, Müller E, Schweizer T, Textor M, Reimhult E. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. Nano Letters. 2011;11:1664–70. doi: 10.1021/nl2001499. [DOI] [PubMed] [Google Scholar]
- 158.Sailor MJ, Park JH. Hybrid nanoparticles for detection and treatment of cancer. Advanced Materials. 2012;24:3779–802. doi: 10.1002/adma.201200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hagisawa K, Nishioka T, Suzuki R, Maruyama K, Takase B, Ishihara M. et al. Thrombus-targeted perfluorocarbon-containing liposomal bubbles for enhancement of ultrasonic thrombolysis: in vitro and in vivo study. Journal of Thrombosis and Haemostasis. 2013;11:1565–73. doi: 10.1111/jth.12321. [DOI] [PubMed] [Google Scholar]
- 160.Lu W, Huang Q, Ku G, Wen X, Zhou M, Guzatov D. et al. Photoacoustic imaging of living mouse brain vasculature using hollow gold nanospheres. Biomaterials. 2010;31:2617–26. doi: 10.1016/j.biomaterials.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Giret S, Wong Chi Man M, Carcel C. Mesoporous-Silica-Functionalized Nanoparticles for Drug Delivery. Chemistry-A European Journal. 2015;21:13850–65. doi: 10.1002/chem.201500578. [DOI] [PubMed] [Google Scholar]
- 162.Baek S, Singh RK, Khanal D, Patel KD, Lee E-J, Leong KW. et al. Smart multifunctional drug delivery towards anticancer therapy harmonized in mesoporous nanoparticles. Nanoscale. 2015;7:14191–216. doi: 10.1039/c5nr02730f. [DOI] [PubMed] [Google Scholar]
- 163.Patel K, Angelos S, Dichtel WR, Coskun A, Yang Y-W, Zink JI. et al. Enzyme-responsive snap-top covered silica nanocontainers. Journal of the American Chemical Society. 2008;130:2382–3. doi: 10.1021/ja0772086. [DOI] [PubMed] [Google Scholar]
- 164.Mondragón L, Mas N, Ferragud V, de la Torre C, Agostini A, Martínez-Máñez R. et al. Enzyme-responsive intracellular-controlled release using silica mesoporous nanoparticles capped with ε-Poly-L-lysine. Chemistry-A European Journal. 2014;20:5271–81. doi: 10.1002/chem.201400148. [DOI] [PubMed] [Google Scholar]
- 165.Coll C, Mondragón L, Martínez-Máñez R, Sancenón F, Marcos MD, Soto J. et al. Enzyme-Mediated Controlled Release Systems by Anchoring Peptide Sequences on Mesoporous Silica Supports. Angewandte Chemie International Edition. 2011;50:2138–40. doi: 10.1002/anie.201004133. [DOI] [PubMed] [Google Scholar]
- 166.Bernardos A, Mondragón L, Javakhishvili I, Mas N, de la Torre C, Martínez-Máñez R. et al. Azobenzene Polyesters Used as Gate-Like Scaffolds in Nanoscopic Hybrid Systems. Chemistry-A European Journal. 2012;18:13068–78. doi: 10.1002/chem.201200787. [DOI] [PubMed] [Google Scholar]
- 167.Liu H, Liu T, Wu X, Li L, Tan L, Chen D. et al. Targeting gold nanoshells on silica nanorattles: a drug cocktail to fight breast tumors via a single irradiation with near-infrared laser light. Advanced Materials. 2012;24:755–61. doi: 10.1002/adma.201103343. [DOI] [PubMed] [Google Scholar]
- 168.Dong W, Li Y, Niu D, Ma Z, Gu J, Chen Y. et al. Facile synthesis of monodisperse superparamagnetic Fe3O4 core@ hybrid@ Au shell nanocomposite for bimodal imaging and photothermal therapy. Advanced Materials. 2011;23:5392–7. doi: 10.1002/adma.201103521. [DOI] [PubMed] [Google Scholar]
- 169.Yeh Y-C, Creran B, Rotello VM. Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale. 2012;4:1871–80. doi: 10.1039/c1nr11188d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kojima C, Hirano Y, Yuba E, Harada A, Kono K. Preparation and characterization of complexes of liposomes with gold nanoparticles. Colloids and Surfaces B: Biointerfaces. 2008;66:246–52. doi: 10.1016/j.colsurfb.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 171.Umeda Y, Kojima C, Harada A, Horinaka H, Kono K. PEG-attached PAMAM dendrimers encapsulating gold nanoparticles: growing gold nanoparticles in the dendrimers for improvement of their photothermal properties. Bioconjugate Chemistry. 2010;21:1559–64. doi: 10.1021/bc1001399. [DOI] [PubMed] [Google Scholar]
- 172.Yang F, Li Y, Chen Z, Zhang Y, Wu J, Gu N. Superparamagnetic iron oxide nanoparticle-embedded encapsulated microbubbles as dual contrast agents of magnetic resonance and ultrasound imaging. Biomaterials. 2009;30:3882–90. doi: 10.1016/j.biomaterials.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 173.Yang F, Li M, Liu Y, Wang T, Feng Z, Cui H. et al. Glucose and magnetic-responsive approach toward in situ nitric oxide bubbles controlled generation for hyperglycemia theranostics. Journal of Controlled Release. 2016;228:87–95. doi: 10.1016/j.jconrel.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 174.Sun D, Zhuang X, Zhang S, Deng Z-B, Grizzle W, Miller D. et al. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Advanced Drug Delivery Reviews. 2013;65:342–7. doi: 10.1016/j.addr.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 175.Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. Journal of Controlled Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.van Dommelen SM, Vader P, Lakhal S, Kooijmans S, van Solinge WW, Wood MJ. et al. Microvesicles and exosomes: opportunities for cell-derived membrane vesicles in drug delivery. Journal of Controlled Release. 2012;161:635–44. doi: 10.1016/j.jconrel.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 177.Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M. A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2014;1846:75–87. doi: 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 178.Tan A, Rajadas J, Seifalian AM. Exosomes as nano-theranostic delivery platforms for gene therapy. Advanced Drug Delivery Reviews. 2013;65:357–67. doi: 10.1016/j.addr.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 179.Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z. et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. Journal of Controlled Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ. et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–90. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 181.Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo A. et al. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. Journal of Translational Medicine. 2011;9:1471–82. doi: 10.1186/1479-5876-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kopeček J. Smart and genetically engineered biomaterials and drug delivery systems. European Journal of Pharmaceutical Sciences. 2003;20:1–16. doi: 10.1016/s0928-0987(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 183.Lavan DA, McGuire T, Langer R. Small-scale systems for in vivo drug delivery. Nature Biotechnology. 2003;21:1184–91. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 184.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature Biotechnology. 2015;33:941–51. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lindner LH, Hossann M, Vogeser M, Teichert N, Wachholz K, Eibl H. et al. Dual role of hexadecylphosphocholine (miltefosine) in thermosensitive liposomes: active ingredient and mediator of drug release. Journal of Controlled Release. 2008;125:112–20. doi: 10.1016/j.jconrel.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 186.Shaffer SA, Baker-Lee C, Kennedy J, Lai MS, de Vries P, Buhler K. et al. In vitro and in vivo metabolism of paclitaxel poliglumex: identification of metabolites and active proteases. Cancer Chemotherapy and Pharmacology. 2007;59:537–48. doi: 10.1007/s00280-006-0296-4. [DOI] [PubMed] [Google Scholar]
- 187.Gil PR, Hühn D, Loretta L, Sasse D, Parak WJ. Nanopharmacy: Inorganic nanoscale devices as vectors and active compounds. Pharmacological Research. 2010;62:115–25. doi: 10.1016/j.phrs.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 188.Schwartz JA, Shetty AM, Price RE, Stafford RJ, Wang JC, Uthamanthil RK. et al. Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model. Cancer Research. 2009;69:1659–67. doi: 10.1158/0008-5472.CAN-08-2535. [DOI] [PubMed] [Google Scholar]
- 189.[189] Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine: Nanotechnology, Biology and Medicine. 2013;9:1–14. doi: 10.1016/j.nano.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ferrari M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends in Biotechnology. 2010;28:181–8. doi: 10.1016/j.tibtech.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Helmus MN. The need for rules and regulations. Nature Nanotechnology. 2007;2:333–4. doi: 10.1038/nnano.2007.165. [DOI] [PubMed] [Google Scholar]
- 192.Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. Journal of Controlled Release. 2015;200:138–57. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 193.Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483:531–3. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 194.Hunter KW. Mouse models of cancer: does the strain matter? Nature Reviews Cancer. 2012;12:144–9. doi: 10.1038/nrc3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Teicher BA. Tumor models for efficacy determination. Molecular Cancer Therapeutics. 2006;5:2435–43. doi: 10.1158/1535-7163.MCT-06-0391. [DOI] [PubMed] [Google Scholar]