Abstract
Purpose
To examine the relationships among tear osmolarity, tear film stability, and several measures of dry eye (DE) symptoms in a multivariable analysis.
Methods
A cross-sectional study was conducted with 137 subjects (68 non–contact lens [CL] wearers and 69 soft CL wearers) recruited from a university campus. Tear breakup time (TBUT) was measured noninvasively (NITBUT) and with fluorescein (FTBUT). Tear osmolarity was measured by an osmometer. Dry eye symptoms were assessed using the Dry Eye Flow Chart and several different questionnaires.
Results
Subjects ranged in age from 18 to 67 years, with a mean of 28 years. Subjects had a mean (SD) osmolarity of 293 (10) mOsm/L, NITBUT of 14.1 (10.9) seconds, and FTBUT of 14.8 (12.6) seconds. Shorter NITBUT and FTBUT were significantly associated with female sex (p = 0.001 and p = 0.027, respectively) and Asian ethnicity (p = 0.030 and p = 0.004, respectively). There were no clinically significant relationships between tear osmolarity and FTBUT, NITBUT, or DE symptoms. Higher Dry Eye Flow Chart score (i.e., worse symptoms) was associated with older age (p < 0.001), female sex (p = 0.014), CL wear (p < 0.001), shorter NITBUT (p < 0.001), and shorter FTBUT (p = 0.028). The sensitivities and specificities for using clinical measurements to diagnose moderate to severe DE were as follows: osmolarity, 0.67 and 0.46, respectively; NITBUT, 0.72 and 0.52, respectively; and FTBUT, 0.68 and 0.57, respectively.
Conclusions
In a population of asymptomatic, mild and moderate DE patients, increased tear osmolarity was not significantly associated with reported symptom severity and frequency. Tear osmolarity, NITBUT, and FTBUT exhibited similar sensitivities and specificities when used to diagnose moderate to severe DE.
Keywords: tear film stability, tear breakup time, tear osmolarity, corneal staining, dry eye disease, dry eye syndrome, ethnicity, race, sex
Tear hyperosmolarity and tear film instability are key factors in the development of dry eye (DE) disease.1 Low aqueous flow (aqueous-deficient DE) or high aqueous evaporation (evaporative DE) can both lead to tear hyperosmolarity, which is associated with many pathophysiological changes. Experimentally induced “dry eye,” with its attendant tear hyperosmolarity, has been shown to stimulate ocular surface inflammation through increased expression and production of proinflammatory cytokines and chemokines in the tears2–6 and to result in corneal epithelial cell death by apoptosis.7 Ocular surface disease and tear hyperosmolarity have also been associated with goblet cell loss,8,9 which can lead to altered mucin expression10 and tear film instability.8,11 Ocular surface cooling attributed to aqueous evaporation, which has also been associated with tear hyperosmolarity, may stimulate sensory nerve fibers innervating the ocular surface, including polymodal nociceptors, mechanonociceptors, or cold thermoreceptors, and generate sensations of ocular discomfort.12,13
Although tear osmolarity has gained widespread acceptance as a diagnostic for DE disease,14–23 recent findings suggest that tear osmolarity measurements do not possess the very high sensitivity and specificity claimed by some studies15,24 and that it may have limited value in the diagnosis of DE.25 Similarly, although tear film instability has been linked to symptoms of dryness and discomfort in several studies,16,26–30 other studies have found contradictory evidence.14,15,31–33 Even among tear film stability assessments, it is not clear how well correlated are noninvasive and invasive (i.e., using sodium fluorescein dye) tear breakup times (TBUTs), or whether the two methods are even assessing the same type or aspect of tear breakup.27,29,32,34,35 Additionally, information on the relationship between tear osmolarity and tear film stability is emerging, with some studies reaching opposing conclusions.11,15,19,24,36–40
Clearly, there is a lack of consensus on many facets of the relationships among tear osmolarity, tear film stability, and dryness symptoms.41,42 This study proposes to provide additional evidence by (1) examining the relationships among tear osmolarity, TBUT (noninvasive and with fluorescein), and dryness symptoms measured using both subjective symptom rating scale questionnaires and a previously published categorical classification tool43 and (2) determining whether these relationships differ with subject demographic characteristics or contact lens (CL) use. Our goal is to gain further understanding of the complex interrelationships among common clinical and subjective assessments used in the diagnosis and treatment of DE disease.
METHODS
Subjects
This study was conducted at the University of California, Berkeley (UCB) Clinical Research Center (CRC) in the spring of 2010. Informed consent was obtained from all study participants, and the study adhered to the tenets of the Declaration of Helsinki. The study protocol was approved by an institutional review board (UCB Committee for the Protection of Human Subjects).
Potential CL-wearing subjects aged 18 years and older with no history of ocular disease were recruited from the UCB campus and surrounding community by telephone calls to subjects registered in the CRC database from participation in past studies. We enrolled 50 subjects from the CRC database and an additional 19 CL-wearing subjects from fliers posted on the UCB campus. We then recruited an approximately equal number (n = 68) of non-CL wearers who were otherwise from the same study population. All CL wearers discontinued lens wear at least 24 hours before the visit.
A subset (n = 50) of the CL-wearing group who had documented corneal staining history from a minimum of three previous studies in the CRC database were classified for additional subgroup analysis as follows: active stainer for those who presented with corneal staining in either eye at 50% or more of their previous visits to the CRC, nonstainer for those who presented with corneal staining in either eye at 20% or fewer of their visits, and occasional stainer if corneal staining was observed in 21 to 49% of their visits. The subgroup analysis of this group was conducted to determine if corneal staining history may be more informative than a single visit assessment, particularly among those with mild to moderate symptoms whose clinical signs often do not correlate with symptoms.
Measurements and Procedures
Participants completed the UCB CRC Dry Eye Flow Chart (DEFC), which has been described in detail elsewhere43 (Lundgrin EL, et al. 2008; IOVS;484:ARVO E-Abstract 4831; Tran N, et al. 2009; Am Acad Optom: E-Abstract 119; Li W, et al. 2014; Am Acad Optom Hot Topic Session Scientific Paper), upon arrival at the CRC. In short, the DEFC has the participants describe their symptoms using one of the following categories: 1, no dryness symptoms; 2, mild symptoms without significant discomfort; 3, symptoms with significant discomfort, but never/rarely interfering with daily activities (e.g., reading, computer use, CL wear); 4, symptoms with significant discomfort, sometimes interfering with daily activities; 5, symptoms with significant discomfort, usually/always interfering with daily activities. At this same visit, 100-point visual analog rating scale questionnaires for both average and end-of-day dryness severity (0, no dryness; 100, intolerable dryness) and frequency (0, never/rarely; 100, usually/always) were also administered.
After the questionnaires were completed, noninvasive TBUT (NITBUT) was assessed using the Carl Zeiss Humphrey Atlas Corneal Topography System (Carl Zeiss Meditec AG, Germany), followed by measurement of tear osmolarity using the TearLab Osmolarity System (TearLab Corporation, San Diego, CA). Biomicroscopy (SL 120 Slit Lamp, Carl Zeiss Meditec AG) and 2 µL of 0.35% sodium fluorescein delivered using a micropipette were used to measure invasive TBUT (fluorescein TBUT [FTBUT]),44 followed by clinical grading of corneal and conjunctival staining using the Brien Holden Vision Institute grading scales.45
Noninvasive TBUT and FTBUT were measured three times per eye, with a minimum of 30 seconds between measurements, and averaged. Tear breakup was considered to have occurred when the reflected mires just became distorted (for NITBUT) or when a black spot or line on the perched tear film became visible (for FTBUT). If presence of tear debris or reflex tearing was suspected, measurements were repeated. If tear breakup did not occur after 60 seconds, TBUT was truncated at 60 seconds for statistical analysis. Osmolarity was measured once in each eye, from tear samples taken from the lower lateral tear meniscus.
Statistical Methods
After a thorough exploratory analysis, multivariable linear mixed-effects models were used to identify the significant factors associated with each of the outcomes (NITBUT, FTBUT, osmolarity, DEFC score), while accounting for the internal correlations engendered by measuring both eyes of each subject. Because only a single subject reported a grade of 5 on the DEFC (symptoms usually/always interfere with activities), that subject was included in the group reporting grade 4 (symptoms sometimes interfere with activities) for statistical analysis. Models were selected by considering F-test p values, evaluating the clinical relevance of the estimated effect magnitudes, examining residual and other diagnostic plots, and comparing nested models by log-likelihood and nonnested models by the Akaike Information Criterion. In a subgroup analysis, multivariable models were fit using the data from CL-wearing subjects who were categorized based on known corneal staining history from the CRC database. Receiver operating characteristic analysis was performed to determine the optimum thresholds of potential diagnostic variables for distinguishing subjects with no/mild DE symptoms (DEFC scores 1 to 3) from those with moderate-to-severe symptoms (DEFC scores 4 and 5) and to estimate the sensitivity and specificity of those diagnostics. Optimum diagnostic thresholds were taken to be those values corresponding to the points on the receiver operating characteristic curves closest in Euclidean distance to the upper left corner of the plot, that is, the point of theoretical 100% sensitivity and specificity.
RESULTS
Subject Characteristics
One hundred thirty-nine subjects were enrolled in the study, with 137 subjects (274 eyes) completing all measurements and questionnaires. One subject was disqualified because of concurrent enrollment in another ophthalmic clinical study, and one subject failed to achieve osmolarity readings above the minimum detectable value of 275 mOsm/L, as recommended by TearLab. Demographic and subject characteristics, and descriptive statistics for the primary outcomes, are shown in Tables 1 to 3. Subjects were predominantly female (71%), ranged in age from 18 to 67 years and averaged about 28 years, and were about 45% Asian and 55% non-Asian. The distribution of ages was slightly skewed toward the younger end (18 to 25 years) because of the UCB campus–based study population from which we sampled.
TABLE 1.
Subject characteristics and descriptive statistics for the main outcomes
| Age, y | Minutes awake |
NITBUT, s | FTBUT, s | Osmolarity, mOsm/L |
DE severity | DE frequency | |||
|---|---|---|---|---|---|---|---|---|---|
| Avg. | EOD | Avg. | EOD | ||||||
| Mean | 28.10 | 193.80 | 14.11 | 14.77 | 292.80 | 24.48 | 37.25 | 24.04 | 34.49 |
| SD | 13.11 | 110.40 | 10.93 | 12.64 | 10.22 | 22.49 | 29.81 | 23.81 | 29.71 |
| Median | 22 | 165 | 10.9 | 9.7 | 292 | 18 | 33 | 15 | 27 |
| Range | 18–67 | 45–615 | 1.0–60.0 | 1.7–60.0 | 275–329 | 0–78 | 0–96 | 0–87 | 0–96 |
Dry eye variables on a 0-to-100 rating scale (0, no dryness; 100, extreme dryness).
EOD, end of day.
TABLE 3.
Distribution of DEFC scores
| DEFC score | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| n | 44 | 13 | 8 | 71 | 1 |
Subjects included 68 non-CL wearers and 69 CL wearers, all of whom wore only soft (conventional or silicone hydrogel) lenses with no overnight wear and had maintained their current wearing patterns (hours per day, days per week) for more than 6 months. Subjects in the CL-wearing group had a mean (SD) of 5.5 (1.84) days per week of wear, and most CL subjects (87%) had worn CLs for more than 1 year.
The following sections present the results of the multivariable analyses of the main outcome measures of tear film stability (NITBUT and FTBUT), osmolarity, and DEFC score.
Tear Film Stability
Both NITBUT and FTBUT were transformed by natural logarithm to better approximate normality for statistical tests. Eight subjects had NITBUT longer than 60 seconds for one or more of the three measurements taken per eye. For FTBUT, 16 subjects had measurements longer than 60 seconds for one or more of the three measurements. All measurements longer than 60 seconds were truncated at 60 seconds before averaging.
Multivariable models for ln(NITBUT) and ln(FTBUT) revealed that shorter TBUT was significantly associated with female sex (p = 0.001 and p = 0.027, respectively) and Asian ethnicity (p = 0.030 and p = 0.004, respectively; Table 4). The ln(NITBUT) model estimates female subjects to have about 25.0% faster breakup times than male subjects on average and Asians to have about 10.9% faster breakup times than non-Asians. Similarly, the multivariable model of ln(FTBUT) estimates female subjects to have about 20.2% faster breakup times than male subjects on average and Asians to have about 23.8% faster breakup times than non-Asians. Neither age, time awake before measurement, nor CL wear was associated with either NITBUT or FTBUT, and among CL wearers, neither days per week nor total duration of wear (years) was related to NITBUT or FTBUT.
TABLE 4.
Covariate estimates and p values from multivariable models with ln(NITBUT) and ln(FTBUT) as outcome variables
| ln(NITBUT) | ln(FTBUT) | |||
|---|---|---|---|---|
| Effect | Estimate | p | Estimate | p |
| Intercept | 2.241 | <0.001 | 2.166 | <0.001 |
| Sex | 0.287 | 0.001 | 0.225 | 0.027 |
| Ethnicity | 0.171 | 0.030 | 0.272 | 0.004 |
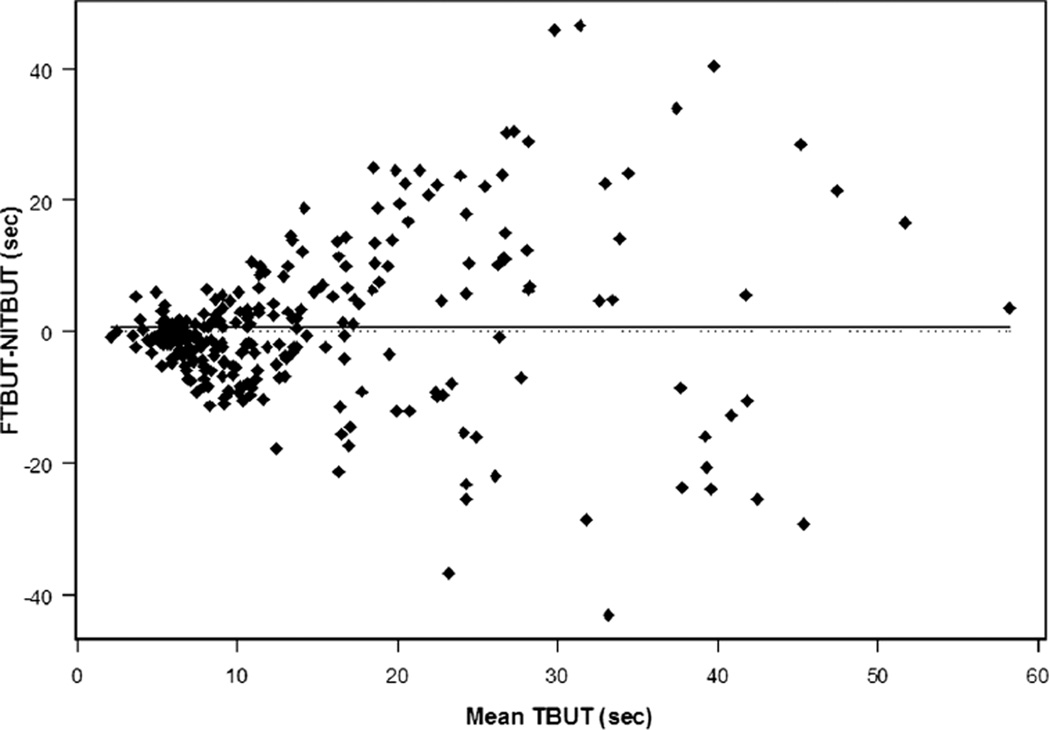
Noninvasive TBUT and FTBUT were moderately correlated (Pearson ρ = 0.503). Fluorescein TBUT tended to be slightly longer than NITBUT by about 0.65 seconds, although individual differences between these two measures of tear film stability exhibited substantial variability, with limits of agreement of (−23.03, 24.34) seconds overall. The variability in the differences between NITBUT and FTBUT was much greater for stable tear films (breakup times >10 seconds), as shown in the difference-versus-mean plot in Fig. 1. Limits of agreement (FTBUT − NITBUT) were wider for breakup times longer than 10 seconds compared with 10 seconds or less, for both CL wearers ([−33.80, 38.11] seconds vs. [–15.42, 8.20] seconds, respectively) and nonwearers ([−15.90, 30.80] seconds vs. [−12.55, 6.48] seconds, respectively). Because the numerical variance is lower than 10 seconds by definition, we also calculated the coefficient of variation (CV), which scales the variance relative to the size of the mean. An examination of the CV within each TBUT method, stratifying on 10 seconds, confirmed greater variability within each method for more stable tear films (CV, 0.30 vs. 0.51 among lens wearers; CV, 0.32 vs. 0.53 among non–lens wearers).
FIGURE 1.
The difference (FTBUT − NITBUT) versus the mean of NITBUT and FTBUT for all subjects.
Tear Osmolarity
Multivariable model estimates indicate statistically significantly higher osmolarity among Asians (p = 0.043) by about 1.6 mOsm/L compared with non-Asians and statistically significantly higher osmolarity by about 1.0 mOsm/L for every 5 seconds shorter FTBUT (p ≤ 0.001) (Table 5). Although statistically significant, these differences are not large enough to be of clinical relevance. Osmolarity was not significantly related to NITBUT, age, sex, or time awake before measurement. No significant relationships were found between osmolarity and dryness symptoms assessed with either the DEFC or the 100-point rating scales. Osmolarity was not significantly related to CL wear, and among CL wearers, osmolarity was not significantly related to days per week or total duration (years) of wear. There were no significant interactions among the explanatory variables for osmolarity.
TABLE 5.
Covariate estimates and p values from the multivariable model with osmolarity as the outcome variable
| Effect | Osmolarity | |
|---|---|---|
| Estimate | p | |
| Intercept | 301.265 | <0.001 |
| Ethnicity | −1.583 | 0.043 |
| ln(FTBUT) | −3.177 | <0.001 |
DEFC Score
Multivariable models of DEFC score revealed significant associations between higher DEFC score (i.e., worse symptoms) and older age (p < 0.001), female sex (p = 0.014), longer time awake (p = 0.034), CL wear (p < 0.001), either shorter NITBUT or shorter FTBUT (not both in the same model as they are moderately collinear; p < 0.001 and p = 0.0035, respectively), and some measure of greater DE symptoms (all highly collinear, so only one per model), including severity or frequency of dryness, on average or at the end of the day, or use of drops as a proxy for having DE symptoms (all p ≤ 0.01; Table 6). Dry Eye Flow Chart score was not significantly related to osmolarity or ethnicity and, among CL wearers, was not significantly related to days per week or total duration (years) of CL wear. There were no significant interactions among the explanatory variables for DEFC score.
TABLE 6.
Covariate estimates and p values from multivariable model with DEFC score as the outcome variable using either ln(NITBUT) (model 1) or ln(FTBUT) (model 2) as covariates
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Effect | Estimate | p | Estimate | p |
| Intercept | 2.101 | <0.001 | 1.807 | <0.001 |
| Age | 0.009 | <0.001 | 0.009 | <0.001 |
| Sex | −0.194 | 0.014 | −0.237 | 0.015 |
| Minutes awake | 0.0004 | 0.034 | 0.0003 | 0.035 |
| CL wear | 0.668 | <0.001 | 0.636 | <0.001 |
| ln(NITBUT) | −0.255 | <0.001 | ||
| ln(FTBUT) | −0.124 | 0.004 | ||
| DE average severity | 0.029 | <0.001 | 0.030 | <0.001 |
Auxiliary Analysis: Subgroup of CL Wearers with Corneal Staining History
Fifty of the CL-wearing subjects had known corneal staining history from participation in three or more previous CRC studies (10 active stainers, 8 occasional stainers, and 32 nonstainers). Multivariable mixed-effects models were fit using this subgroup of study participants for each of the major outcomes. The best multivariable models of ln(NITBUT) and ln(FTBUT) showed that shorter TBUT was significantly associated with active corneal stainers (p = 0.010 and p < 0.001, respectively) and use of eye drops (p < 0.001 and p = 0.011, respectively), and both models estimated nonstainers to have about 2 seconds longer TBUTs on average compared with active stainers. The best multivariable model of osmolarity in this subgroup showed higher tear osmolarity to be significantly associated with active corneal stainers (p = 0.002) and shorter ln(FTBUT) (p = 0.004), but the estimated effect sizes were clinically negligible. Dry Eye Flow Chart score was not significantly related to corneal staining history in this subgroup.
Dry Eye Diagnosis: Sensitivity and Specificity
We can classify subjects with DEFC score greater than or equal to 4 as having moderate to severe symptoms (i.e., symptoms with sufficient discomfort to interfere with activities like CL wear, reading, or computer use) and those with DEFC score less than 4 as having no/mild symptoms and no interference with visual activities. The sensitivities and specificities of NITBUT, FTBUT, osmolarity, corneal staining history, and symptom questionnaire responses were estimated relative to this functional, binary categorization of the DEFC score (Table 7).
TABLE 7.
Sensitivities, specificities, and thresholds for distinguishing binary DEFC score (no/mild symptoms vs. debilitating symptoms)
| Diagnostic | Threshold | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|
| NITBUT | 11.0 s | 0.72 (0.62–0.83) | 0.52 (0.40–0.64) |
| FTBUT | 8.7 s | 0.68 (0.57–0.79) | 0.57 (0.45–0.69) |
| Osmolarity | 294 mOsm/L | 0.67 (0.56–0.76) | 0.46 (0.34–0.58) |
| Corneal staining Hx | Present at >20% of visits | 0.29 (0.19–0.40) | 0.83 (0.74–0.92) |
| DE severity (0–100) | |||
| Average | 16 | 0.81 (0.71–0.90) | 0.71 (0.60–0.82) |
| End of day | 31 | 0.79 (0.70–0.86) | 0.80 (0.70–0.90) |
| DE frequency (0–100) | |||
| Average | 16 | 0.74 (0.63–0.84) | 0.78 (0.68–0.88) |
| End of day | 19 | 0.85 (0.76–0.93) | 0.71 (0.60–0.82) |
CI, confidence interval; Hx, history.
In general, osmolarity, TBUTs, and the other variables measured in this study had moderate to poor sensitivity and specificity for diagnosing DE, with the exception of average and end-of-day dryness severity and frequency ratings. Interestingly, when Asians and non-Asians were considered separately, sensitivities and specificities for NITBUT and FTBUT were virtually the same for the two ethnic groups but at slightly different optimum thresholds: non-Asians were diagnosed as having moderate DE with NITBUT less than 10.0 seconds or FTBUT less than 9.2 seconds; Asians were diagnosed as having debilitating DE with NITBUT less than 9.0 seconds or FTBUT less than 6.7 seconds.
We also examined the sensitivity and specificity of NITBUT and FTBUT for identifying those subjects with no/mild symptoms versus moderate symptoms in the subset of subjects with unstable tear films, which was defined according to the common clinical criterion of TBUT less than or equal to 10 seconds. Table 8 shows that although sensitivities for both NITBUT (44%) and FTBUT (55%) were low, NITBUT had a much higher specificity (78%) than did FTBUT (50%).
TABLE 8.
Sensitivities, specificities, and thresholds for distinguishing binary DEFC score (no/mild symptoms vs. debilitating symptoms) by tear film stability assessments, for the subset of subjects with unstable tear films (TBUT ≤ 10 seconds)
| Diagnostic | nTotal | nDE | nnon-DE | Threshold, s | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|
| NITBUT (≤10 s) | 50 | 32 | 18 | 7.1 | 0.44 (0.27–0.61) | 0.78 (0.59–0.97) |
| FTBUT (≤10 s) | 55 | 33 | 22 | 6.5 | 0.55 (0.38–0.72) | 0.50 (0.29–0.71) |
Summary of Results
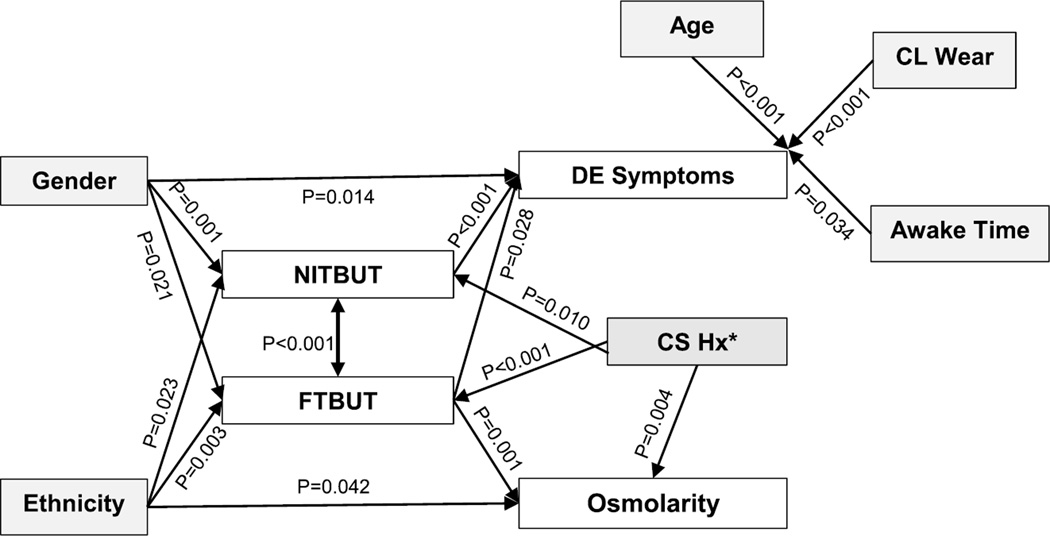
The Directed Acyclic Graph shown in Fig. 2 summarizes the many results of this analysis. Female sex, Asian ethnicity, increased severity or frequency of DE symptoms, and higher DEFC score were all significantly associated with both shorter NITBUT and shorter FTBUT. In addition, higher DEFC score was significantly associated with older age, female sex, longer time awake, and CL wear. Shorter FTBUT was significantly associated with higher osmolarity but at a clinically negligible level; NITBUT was not associated with osmolarity. Osmolarity and DE symptoms were not significantly related. In the subgroup of CL wearers with known corneal staining history, both NITBUT and FTBUT were significantly longer among nonstainers, and osmolarity was significantly lower, although by a clinically negligible amount on average.
FIGURE 2.
Directed Acyclic Graph depicting the main results of the analysis. Note in particular that although FTBUT is significantly related to both osmolarity and dry eye symptoms, osmolarity and symptoms were not significantly related to each other. CS Hx, corneal staining history. Corneal staining history was only available for n = 50 contact lens wearers in the study.
DISCUSSION
In this study of 18- to 67-year-old participants with ocular discomfort ranging from none to moderately significant, tear osmolarity had a weak association with ln(FTBUT) but had no significant relationship with symptoms assessed with the DEFC or the 100-point rating-scale questionnaires. The statistical relationship between higher tear osmolarity and shorter FTBUT is consistent with previous reports,46 but in the current study, the magnitude of the effect was of no clinical significance (1 mOsm/L higher per 5 seconds of shorter FTBUT). The relationship between osmolarity and ocular dryness symptoms has been debated.14,16,22,24,25,33,47 Many studies that linked tear hyperosmolarity to cell damage had exposed epithelial tissues (in animal and human models) to osmolarity environments between 313 and 500 mOsm/L,3,6–8 even near 1000 mOsm/L in one study,11 whereas participants in our study averaged 292 mOsm/L and ranged between 276 and 329 mOsm/L. Although it is possible that the lower average osmolarity in our study group is the reason for the lack of association with symptoms, it is difficult to overlook the fact that 71 of 137 study participants reported significant discomfort that sometimes interfered with their daily activities (DEFC = 4). However, there was only one participant who reported significant discomfort that always interfered with daily activities (DEFC = 5), so perhaps the relationship between osmolarity and symptoms would have been stronger had we had more participants with very severe symptoms. This explanation appears less likely considering that other studies have reported a weak correlation between tear osmolarity and Ocular Surface Disease Index score in subjects with higher mean tear osmolarity than in this study.19,22 It should be noted that unlike the Ocular Surface Disease Index or Standard Patient Evaluation of Eye Dryness questionnaires that require patients to specify types of symptoms, the DEFC asks about symptoms in a very general sense, and some published studies that used the DEFC have reported a significant association between DEFC score and clinical outcomes such as NITBUT and corneal staining43 (Lundgrin EL, et al. 2008; IOVS;484:ARVO E-Abstract 4831; Tran N, et al. 2009; Am Acad Optom: E-Abstract 119; Li W, et al. 2014; Am Acad Optom Hot Topic Session Scientific Paper).
The weak relationship between osmolarity and DE symptoms may also be explained by intraperson variability in tear osmolarity over time, which is thought to be greater in symptomatic subjects15,18; in the present study, measurements were collected at only one time point and may not reflect fluctuations in osmolarity, particularly among those with more severe symptoms.
Finally, it has been suggested that localized areas of tear evaporation over the corneal surface may trigger corneal sensory nerve endings to induce symptoms of ocular discomfort,12,13 but the osmolarity of the tears sampled from the tear meniscus may not be the same as the osmolarity in the localized areas of evaporation on the cornea. Consider, as an example, the “black line” formed near the lid margins after fluorescein instillation is believed to separate the tear meniscus from the perched tear film over the cornea.48 It has also been suggested that the osmolarity in the meniscus varies depending on location.49 Therefore, if the meniscus contains more aqueous than localized areas of evaporation on the cornea, osmolarity from the meniscus would be less than that in exposed regions on the cornea, resulting in a lower measurement than expected in a symptomatic patient.
Although the best multivariable models of ln(NITBUT) and ln(FTBUT) included identical sets of covariates, these two measures of tear film stability were only moderately correlated with each other. For unstable tear films with TBUTs less than 10 seconds, intermethod differences tended to be small, and tear film stability can be quantified by either method. As breakup times became longer (up to 60 seconds) with more stable tear films, the agreement between NITBUT and FTBUT weakened. These results are consistent with a previous study.34 Tear breakup time values greater than 60 seconds were truncated and set to 60 seconds in our study for practical reasons. As a result, the mean TBUT is expected to be shorter than had truncation not been performed. We do not expect the truncation to affect the trend seen in the less than or equal to 10 seconds group but may expect to see even slightly less correlation between NITBUT and FTBUT in the greater than 10 seconds group because more participants had FTBUT greater than 60 seconds than NITBUT greater than 60 seconds (n = 16 and 8, respectively) and because the potential upper range could be much higher than 60 seconds for some participants. We conducted a post hoc analysis to examine the impact of a different TBUT truncation limit, because TBUT recording methods can vary by study or equipment. We conducted the analysis with a 24-second cutoff (i.e., all TBUT measurements >24 seconds were analyzed as 24 seconds) to mimic the limit set by the Oculus Keratograph 5 (Oculus, Inc, Washington). The results demonstrated that although the median TBUTs remained the same as with a 60-second cutoff, the means were reduced by about 2 seconds. Therefore, the median TBUT is a more robust measure of tear film stability, particularly when comparing TBUT measures using different instruments and cutoffs. It is unclear whether the NITBUT results may have been different using other methods, such as interferometry (e.g., Keeler Tearscope), automated systems (e.g., Oculus Keratograph 5 NIKBUT feature), or keratometers with an increased number of mires (e.g., Medmont E300 Topgrapher), but to date, there is no evidence that any of these systems is superior in accuracy.
Female sex was associated with a less stable tear film and increased severity and frequency of dryness symptoms compared with male sex. The role of low androgen and high estrogen levels in reduced lacrimal and meibomian gland function has been published extensively and supports the association between female sex and reduced tear film stability and accompanying DE symptoms.50–58 It has also been suggested that the use of makeup among female subjects could be a risk factor for tear film instability59 and DE,60 but this was not directly investigated in our study. Because we did not instruct participants to discontinue use of eye makeup before the study, it is possible that the presence of eye makeup confounded the relationship between female sex and both tear film stability and symptoms.
The sensitivities and specificities of various clinical procedures for diagnosing DE based on the DEFC score were determined by comparing subjects with no/mild symptoms to those with moderate to severe symptoms. We found that these commonly accepted diagnostic procedures had poor to moderate sensitivity and specificity. For example, the sensitivity and specificity for NITBUT and FTBUT were similar to or worse than those in previous reports.14,29,35,61,62 When TBUTs were less than or equal to 10 seconds, NITBUT and FTBUT had poor sensitivity (44 and 55%, respectively), suggesting that among patients with significant DE who are tested, about half of them will have longer TBUTs than the threshold (7.1 and 6.5 seconds, respectively). Interestingly, NITBUT had a much higher specificity (78%) compared with FTBUT (50%), suggesting that NITBUT was less likely to measure below the threshold for those without moderate symptoms. At a threshold of 294 mOsm/L, the sensitivity of tear osmolarity (67%) was similar to previous reports but the specificity (46%) was lower than previously published.23,63 These results suggest that tear osmolarity is somewhat better at correctly identifying those with severe symptoms than tear film stability, but those without severe symptoms still have a moderate probability of having tear osmolarity greater than 294 mOsm/L. All dryness severity and frequency ratings were relatively sensitive and specific, which is expected because the measure of performance is based on symptoms. In general, these results suggest that there is no gold standard among the clinical tests performed in this study for diagnosing DE in a population with a wide range of dryness symptoms.
We also found that, although sensitivities and specificities for FTBUT were similar between Asians and non-Asians, their optimum thresholds were noticeably different (6.7 seconds for Asians and 9.2 seconds for non-Asians). Fluorescein TBUT is the most common method of assessing tear film stability in clinical practice, and a threshold of 10 seconds is commonly accepted. These results show that the optimum threshold is probably somewhat lower than 10 seconds, and in particular, the threshold for Asian patients should be set much lower as many Asians with FTBUT between about 7 and 10 seconds do not have dryness symptoms.
Among the CL wearers with corneal staining history, the group of “active stainers” who presented with corneal staining at half or more of previous visits was significantly associated with shorter TBUTs by about 2 seconds but not associated with greater tear osmolarity or symptoms assessed with the DEFC to any clinically important degree, compared with “nonstainers.” Whether the active stainers have a compromised tear film that makes the ocular surface vulnerable to epithelial cell damage or an irregular corneal epithelial surface that is unable to maintain an even perched tear film remains to be elucidated.
In summary, this study found that higher tear osmolarity was statistically, but not clinically, significantly associated with longer FTBUT and Asian ethnicity and not significantly related to NITBUT or to symptoms assessed with either the DEFC or the 100-point rating scales. Shorter NITBUT and FTBUT, however, were significantly associated with moderate dryness symptoms. The sensitivities and specificities of NITBUT, FTBUT, and osmolarity to detect moderate DE were similar and indicated only moderate diagnostic value. Asian ethnicity was significantly associated with shorter TBUTs and higher tear osmolarity, and female sex was also associated with shorter TBUTs and with DE symptoms. The use of CLs and CL-wearing patterns did not show relationships with our main outcome measures possibly because of the study’s requirement of a 24-hour washout period with no use of CLs or ophthalmic solutions. Overall, the results of this study provide additional evidence on some of the complex interrelationships among common clinical and subjective assessments used in the diagnosis of DE disease, including TBUT, tear osmolarity, and dryness symptoms.
TABLE 2.
Subject demographics
| Sex | Ethnicity | Eye drops | |||
|---|---|---|---|---|---|
| Female | Male | Asian | Non-Asian | No | Yes |
| 97 | 40 | 61 | 76 | 106 | 31 |
Acknowledgments
This study was supported by a National Eye Institute Mentored Clinical Scientist Development Program Award (K12) (TNY), a Roberta Smith Research Fund (MCL), and a Clinical Research Unrestricted Fund (MCL).
The authors thank TearLab for supplying test cards for osmolarity measurements and Drs. Nina Tran and Harry Green for assisting with data collection.
Footnotes
Commercial relationship interest: None
REFERENCES
- 1.Definition and Classification Subcommittee of the International Dry Eye WorkShop. The epidemiology of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 3.Li DQ, Chen Z, Song XJ, Luo L, Pflugfelder SC. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:4302–4311. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- 4.Corrales RM, Stern ME, De Paiva CS, Welch J, Li DQ, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47:3293–3302. doi: 10.1167/iovs.05-1382. [DOI] [PubMed] [Google Scholar]
- 5.Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- 6.Li DQ, Luo L, Chen Z, Kim HS, Song XJ, Pflugfelder SC. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82:588–596. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo L, Li DQ, Pflugfelder SC. Hyperosmolarity-induced apoptosis in human corneal epithelial cells is mediated by cytochrome c and MAPK pathways. Cornea. 2007;26:452–460. doi: 10.1097/ICO.0b013e318030d259. [DOI] [PubMed] [Google Scholar]
- 8.Gilbard JP, Rossi SR, Gray KL, Hanninen LA, Kenyon KR. Tear film osmolarity and ocular surface disease in two rabbit models for keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 1988;29:374–378. [PubMed] [Google Scholar]
- 9.Pflugfelder SC, Tseng SC, Yoshino K, Monroy D, Felix C, Reis BL. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology. 1997;104:223–235. doi: 10.1016/s0161-6420(97)30330-3. [DOI] [PubMed] [Google Scholar]
- 10.Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–1011. [PubMed] [Google Scholar]
- 11.Liu H, Begley C, Chen M, Bradley A, Bonanno J, McNamara NA, Nelson JD, Simpson T. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009;50:3671–3679. doi: 10.1167/iovs.08-2689. [DOI] [PubMed] [Google Scholar]
- 12.Belmonte C, Gallar J. Cold thermoreceptors, unexpected players in tear production and ocular dryness sensations. Invest Ophthalmol Vis Sci. 2011;52:3888–3892. doi: 10.1167/iovs.09-5119. [DOI] [PubMed] [Google Scholar]
- 13.Parra A, Gonzalez-Gonzalez O, Gallar J, Belmonte C. Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. Pain. 2014;155:1481–1491. doi: 10.1016/j.pain.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Lemp MA, Bron AJ, Baudouin C, Benitez Del Castillo JM, Geffen D, Tauber J, Foulks GN, Pepose JS, Sullivan BD. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151:792–798. doi: 10.1016/j.ajo.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan BD, Crews LA, Sonmez B, de la Paz MF, Comert E, Charoenrook V, de Araujo AL, Pepose JS, Berg MS, Kosheleff VP, Lemp MA. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 2012;31:1000–1008. doi: 10.1097/ICO.0b013e318242fd60. [DOI] [PubMed] [Google Scholar]
- 16.Julio G, Lluch S, Cardona G, Fornieles A, Merindano D. Item by item analysis strategy of the relationship between symptoms and signs in early dry eye. Curr Eye Res. 2012;37:357–364. doi: 10.3109/02713683.2012.654884. [DOI] [PubMed] [Google Scholar]
- 17.Gilbard JP, Farris RL, Santamaria J., 2nd Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch Ophthalmol. 1978;96:677–681. doi: 10.1001/archopht.1978.03910050373015. [DOI] [PubMed] [Google Scholar]
- 18.Keech A, Senchyna M, Jones L. Impact of time between collection and collection method on human tear fluid osmolarity. Curr Eye Res. 2013;38:428–436. doi: 10.3109/02713683.2013.763987. [DOI] [PubMed] [Google Scholar]
- 19.Versura P, Profazio V, Campos EC. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res. 2010;35:553–564. doi: 10.3109/02713683.2010.484557. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki M, Massingale ML, Ye F, Godbold J, Elfassy T, Vallabhajosyula M, Asbell PA. Tear osmolarity as a biomarker for dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51:4557–4561. doi: 10.1167/iovs.09-4596. [DOI] [PubMed] [Google Scholar]
- 21.Jacobi C, Jacobi A, Kruse FE, Cursiefen C. Tear film osmolarity measurements in dry eye disease using electrical impedance technology. Cornea. 2011;30:1289–1292. doi: 10.1097/ICO.0b013e31821de383. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, Geerling G, Pepose JS, Kosheleff V, Porreco A, Lemp MA. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51:6125–6130. doi: 10.1167/iovs.10-5390. [DOI] [PubMed] [Google Scholar]
- 23.Tomlinson A, Khanal S, Ramaesh K, Diaper C, McFadyen A. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006;47:4309–4315. doi: 10.1167/iovs.05-1504. [DOI] [PubMed] [Google Scholar]
- 24.Messmer EM, Bulgen M, Kampik A. Hyperosmolarity of the tear film in dry eye syndrome. Dev Ophthalmol. 2010;45:129–138. doi: 10.1159/000315026. [DOI] [PubMed] [Google Scholar]
- 25.Amparo F, Jin Y, Hamrah P, Schaumberg DA, Dana R. What is the value of incorporating tear osmolarity measurement in assessing patient response to therapy in dry eye disease? Am J Ophthalmol. 2014;157:69.e2–77.e2. doi: 10.1016/j.ajo.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begley CG, Chalmers RL, Abetz L, Venkataraman K, Mertzanis P, Caffery BA, Snyder C, Edrington T, Nelson D, Simpson T. The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Invest Ophthalmol Vis Sci. 2003;44:4753–4761. doi: 10.1167/iovs.03-0270. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Chen Q, Chen W, Cui L, Ma H, Lu F. Tear dynamics and corneal confocal microscopy of subjects with mild self-reported office dry eye. Ophthalmology. 2011;118:902–907. doi: 10.1016/j.ophtha.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 28.Lane SS, Dubiner HB, Epstein RJ, Ernest PH, Greiner JV, Hardten DR, Holland EJ, Lemp MA, McDonald JE, 2nd, Silbert DI, Blackie CA, Stevens CA, Bedi R. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31:396–404. doi: 10.1097/ICO.0b013e318239aaea. [DOI] [PubMed] [Google Scholar]
- 29.Goto T, Zheng X, Klyce SD, Kataoka H, Uno T, Karon M, Tatematsu Y, Bessyo T, Tsubota K, Ohashi Y. A new method for tear film stability analysis using videokeratography. Am J Ophthalmol. 2003;135:607–612. doi: 10.1016/s0002-9394(02)02221-3. [DOI] [PubMed] [Google Scholar]
- 30.Gumus K, Crockett CH, Rao K, Yeu E, Weikert MP, Shirayama M, Hada S, Pflugfelder SC. Noninvasive assessment of tear stability with the tear stability analysis system in tear dysfunction patients. Invest Ophthalmol Vis Sci. 2011;52:456–461. doi: 10.1167/iovs.10-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23:762–770. doi: 10.1097/01.ico.0000133997.07144.9e. [DOI] [PubMed] [Google Scholar]
- 32.Best N, Drury L, Wolffsohn JS. Clinical evaluation of the Oculus Keratograph. Cont Lens Anterior Eye. 2012;35:171–174. doi: 10.1016/j.clae.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan BD, Crews LA, Messmer EM, Foulks GN, Nichols KK, Baenninger P, Geerling G, Figueredo F, Lemp MA. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol. 2012;92:161–166. doi: 10.1111/aos.12012. [DOI] [PubMed] [Google Scholar]
- 34.Cho P, Douthwaite W. The relation between invasive and noninvasive tear break-up time. Optom Vis Sci. 1995;72:17–22. doi: 10.1097/00006324-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Goto T, Zheng X, Okamoto S, Ohashi Y. Tear film stability analysis system: introducing a new application for videokeratography. Cornea. 2004;23:S65–S70. doi: 10.1097/01.ico.0000136685.88489.70. [DOI] [PubMed] [Google Scholar]
- 36.Szalai E, Berta A, Szekanecz Z, Szucs G, Modis L., Jr Evaluation of tear osmolarity in non-Sjogren and Sjogren syndrome dry eye patients with the TearLab system. Cornea. 2012;31:867–871. doi: 10.1097/ICO.0b013e3182532047. [DOI] [PubMed] [Google Scholar]
- 37.Balakrishnan P, Green H, Lin M. Ethnic differences in tear film stability are not related to tear osmolarity. Optom Vis Sci. 2010;87 E-abstract 105838. [Google Scholar]
- 38.Aragona P, Di Stefano G, Ferreri F, Spinella R, Stilo A. Sodium hyaluronate eye drops of different osmolarity for the treatment of dry eye in Sjogren’s syndrome patients. Br J Ophthalmol. 2002;86:879–884. doi: 10.1136/bjo.86.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolkoff P, Nojgaard JK, Troiano P, Piccoli B. Eye complaints in the office environment: precorneal tear film integrity influenced by eye blinking efficiency. Occup Environ Med. 2005;62:4–12. doi: 10.1136/oem.2004.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mudgil P. Hyperosmolarity and tear film stability: are they related? Invest Ophthalmol Vis Sci. 2012;53 E-abstract 555. [Google Scholar]
- 41.Research Subcommittee of the International Dry Eye WorkShop. Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:179–193. doi: 10.1016/s1542-0124(12)70086-1. [DOI] [PubMed] [Google Scholar]
- 42.Nichols JJ, Willcox MD, Bron AJ, Belmonte C, Ciolino JB, Craig JP, Dogru M, Foulks GN, Jones L, Nelson JD, Nichols KK, Purslow C, Schaumberg DA, Stapleton F, Sullivan DA. The TFOS International Workshop on Contact Lens Discomfort: executive summary. Invest Ophthalmol Vis Sci. 2013;54:TFOS7–TFOS13. doi: 10.1167/iovs.13-13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran N, Graham AD, Lin MC. Ethnic differences in dry eye symptoms: effects of corneal staining and length of contact lens wear. Cont Lens Anterior Eye. 2013;36:281–288. doi: 10.1016/j.clae.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Borchman D, Foulks GN, Yappert MC, Mathews J, Leake K, Bell J. Factors affecting evaporation rates of tear film components measured in vitro. Eye Contact Lens. 2009;35:32–37. doi: 10.1097/ICL.0b013e318193f4fc. [DOI] [PubMed] [Google Scholar]
- 45.Terry RL, Schnider CM, Holden BA, Cornish R, Grant T, Sweeney D, La Hood D, Back A. CCLRU standards for success of daily and extended wear contact lenses. Optom Vis Sci. 1993;70:234–243. doi: 10.1097/00006324-199303000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Labbe A, Terry O, Brasnu E, Van Went C, Baudouin C. Tear film osmolarity in patients treated for glaucoma or ocular hypertension. Cornea. 2012;31:994–999. doi: 10.1097/ICO.0b013e31823f8cb6. [DOI] [PubMed] [Google Scholar]
- 47.Caffery B, Chalmers RL, Marsden H, Nixon G, Watanabe R, Harrison W, Mitchell GL. Correlation of tear osmolarity and dry eye symptoms in convention attendees. Optom Vis Sci. 2014;91:142–149. doi: 10.1097/OPX.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 48.Miller KL, Polse KA, Radke CJ. Black-line formation and the “perched” human tear film. Curr Eye Res. 2002;25:155–162. doi: 10.1076/ceyr.25.3.155.13478. [DOI] [PubMed] [Google Scholar]
- 49.Bron AJ, Yokoi N, Gaffney EA, Tiffany JM. A solute gradient in the tear meniscus. I. A hypothesis to explain Marx’s line. Ocul Surf. 2011;9:70–91. doi: 10.1016/s1542-0124(11)70014-3. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan DA. Sex and sex steroid influences on the dry eye syndrome. In: Pflugfelder SC, Beuerman RW, Stern ME, editors. Dry Eye and Ocular Surface Disorders. New York, NY: Marcel Dekker; 2004. pp. 165–190. [Google Scholar]
- 51.Han SB, Hyon JY, Woo SJ, Lee JJ, Kim TH, Kim KW. Prevalence of dry eye disease in an elderly Korean population. Arch Ophthalmol. 2011;129:633–638. doi: 10.1001/archophthalmol.2011.78. [DOI] [PubMed] [Google Scholar]
- 52.Lu P, Chen X, Liu X, Kang Y, Xie Q, Ke L, Wei X. Dry eye syndrome in elderly Tibetans at high altitude: a population-based study in China. Cornea. 2008;27:545–551. doi: 10.1097/ICO.0b013e318165b1b7. [DOI] [PubMed] [Google Scholar]
- 53.Jie Y, Xu L, Wu YY, Jonas JB. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye (Lond) 2009;23:688–693. doi: 10.1038/sj.eye.6703101. [DOI] [PubMed] [Google Scholar]
- 54.Moss SE, Klein R, Klein BE. Incidence of dry eye in an older population. Arch Ophthalmol. 2004;122:369–373. doi: 10.1001/archopht.122.3.369. [DOI] [PubMed] [Google Scholar]
- 55.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118:1264–1268. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 56.Malet F, Le Goff M, Colin J, Schweitzer C, Delyfer MN, Korobelnik JF, Rougier MB, Radeau T, Dartigues JF, Delcourt C. Dry eye disease in French elderly subjects: the Alienor Study. Acta Ophthalmol. 2014;92:e429–e436. doi: 10.1111/aos.12174. [DOI] [PubMed] [Google Scholar]
- 57.Uchino M, Nishiwaki Y, Michikawa T, Shirakawa K, Kuwahara E, Yamada M, Dogru M, Schaumberg DA, Kawakita T, Takebayashi T, Tsubota K. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology. 2011;118:2361–2367. doi: 10.1016/j.ophtha.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 58.McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998;105:1114–1119. doi: 10.1016/S0161-6420(98)96016-X. [DOI] [PubMed] [Google Scholar]
- 59.Malik A, Claoué C. Transport and interaction of cosmetic product material within the ocular surface: beauty and the beastly symptoms of toxic tears. Cont Lens Anterior Eye. 2012;35:247–259. doi: 10.1016/j.clae.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Guillon M, Maissa C. Dry eye symptomatology of soft contact lens wearers and nonwearers. Optom Vis Sci. 2005;82:829–834. doi: 10.1097/01.opx.0000178060.45925.5d. [DOI] [PubMed] [Google Scholar]
- 61.Mengher LS, Bron AJ, Tonge SR, Gilbert DJ. A non-invasive instrument for clinical assessment of the pre-corneal tear film stability. Curr Eye Res. 1985;4:1–7. doi: 10.3109/02713688508999960. [DOI] [PubMed] [Google Scholar]
- 62.Vitali C, Moutsopoulos HM, Bombardieri S. The European Community Study Group on diagnostic criteria for Sjogren’s syndrome. Sensitivity and specificity of tests for ocular and oral involvement in Sjogren’s syndrome. Ann Rheum Dis. 1994;53:637–647. doi: 10.1136/ard.53.10.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farris RL. Tear osmolarity—a new gold standard? Adv Exp Med Biol. 1994;350:495–503. doi: 10.1007/978-1-4615-2417-5_83. [DOI] [PubMed] [Google Scholar]