Abstract
The dopamine D3 receptor is a target of pharmacotherapeutic interest in a variety of neurological disorders including schizophrenia, restless leg syndrome, and drug addiction. The high protein sequence homology between the D3 and D2 receptors has posed a challenge to developing D3 receptor-selective ligands whose behavioral actions can be attributed to D3 receptor engagement, in vivo. However, through primarily small molecule structure-activity relationship (SAR) studies, a variety of chemical scaffolds have been discovered over the past two decades that have resulted in several D3 receptor-selective ligands with high affinity and in vivo activity. Nevertheless, viable clinical candidates remain limited. The recent determination of the high-resolution crystal structure of the D3 receptor has invigorated structure-based drug design, providing refinements to the molecular dynamic models and testable predictions about receptor-ligand interactions. This review will highlight recent preclinical and clinical studies demonstrating potential utility of D3 receptor-selective ligands in the treatment of addiction. In addition, new structure-based rational drug design strategies for D3 receptor-selective ligands that complement traditional small molecule SAR to improve the selectivity and directed efficacy profiles are examined.
Keywords: Addiction, crystal structure, structure-activity relationships, pharmacology, receptor homology modeling
1. Introduction –– The dopamine D3 receptor as a target for medication discovery
The neurotransmitter dopamine (DA) exerts it effects via DA receptors with varied signaling transduction mechanisms and expression patterns in the brain. DA receptors belong to the G protein-coupled receptor (GPCR) superfamily and are divided into two subfamilies. The D1-like DA receptors (D1 and D5) couple to stimulatory Gs proteins and enhance adenylyl cyclase (AC) activity and increase cytosolic cyclic adenosine monophosphate (cAMP) levels. D2-like DA receptors (D2, D3 and D4) couple to inhibitory Gi/o proteins that suppress AC activity and decrease cAMP. Within the D2-like receptor subfamily, the D2 and D3 receptors are the most homologous pair, sharing extensive sequence identity in the transmembrane domain and the putative ligand binding site (Chien et al., 2010).
First cloned and characterized in 1990 (Sokoloff et al., 1990), DA D3 receptors are expressed as post-synaptic receptors as well as autoreceptors (Diaz et al., 2000), providing inhibitory control on neuronal firing rates. The D3 receptor has a higher affinity for DA than the other receptor subtypes and may therefore be sensitive to tonic stimulation (Levesque et al., 1992). However, since D3 receptor antagonists fail to increase locomotor activity or elevate extracellular levels of DA in the nucleus accumbens or striatum, it appears that D3 autoreceptors exert primarily phasic rather than tonic control of DA neurons (Millan et al., 2000; Sokoloff et al., 2006).
The expression of D3 receptors in the human brain is primarily limited to mesolimbic regions, including particularly the ventral striatum, pallidum, nucleus accumbens, islands of Calleja, olfactory tubercle and lateral septum (Cho et al., 2010; Gurevich & Joyce, 1999; Searle et al., 2010). This relatively focal expression of D3 receptors in brain regions that govern motivational behaviors and the reward properties of addictive drugs make the D3 receptor an enticing target for addiction pharmacotherapies. D3 receptors localized in the basolateral nucleus of the amygdala appear to regulate stimulus-reward associations that mediate reinstatement of drug-seeking behavior (Di Ciano, 2008). In the hippocampus, a modest density of D3 receptors have been found that regulate CREB signaling and could produce long-lasting effects on cognition and relapse behavior (Basile et al., 2006). In contrast, D2 receptors feature a broader distribution at higher concentrations, particularly in the dorsal striatum (Gurevich & Joyce, 1999); alteration of D2 receptor signaling is more commonly associated with side-effects that influence locomotor activity, motor coordination, prolactin secretion, and catalepsy (Cho et al., 2010; Millan et al., 1995). Hence, selective targeting of D3 receptor signaling has the potential to provide a more focused therapeutic effect while limiting potential side effects believed to be mediated primarily through D2 receptors.
D3 receptors have additionally been shown to form heteromers with D1 receptors in the striatum. In these synergistic D1-D3 interactions, D3 receptor stimulation potentiates the effects of D1 signaling on neuronal and behavioral processes, including AC activation and locomotor activity (Fiorentini et al., 2008; Marcellino et al., 2008). Additional evidence exists for the functional coupling of D2 and D3 receptors, which may alter the apparent potency of some D2-like agonists (reviewed in Maggio et al., 2009). It is tempting to consider future directions for the development of pharmacotherapeutics targeting these heteromeric complexes. However, it is too soon to know whether these complexes can be accessed by heteromer-selective drugs, in vivo.
The DA D3 receptor has been investigated as a potential target for medication development to treat substance use disorders (SUDs) with a particular focus on cocaine and methamphetamine. In addition to the expression and signaling patterns described above, alterations in D3 receptor expression patterns following drug exposure suggest an important role for D3 signaling in the development of addiction. Enhanced expression of D3 receptors has been shown following acute or chronic exposure to drugs of abuse in human postmortem studies (Mash & Staley, 1999; Segal et al., 1997; Staley & Mash, 1996). Increased expression of D3 receptors in polydrug users is correlated with self-reported drug craving (Boileau et al., 2012). This upregulation of D3 receptors may therefore contribute to the reinforcing effects of drugs of abuse and drug dependence (Le Foll et al., 2003; Segal et al., 1997).
Currently, there are no approved medications to treat cocaine and methamphetamine addiction and thus developing pharmacotherapeutics to complement existing behavioral strategies is a fundamental goal. A commentary highlighting the D3 receptor as a viable target for the development of SUDs, with an emphasis on psychostimulants, has recently appeared (Newman et al., 2012b).
2. D3-selective drug design using small molecule SAR
2.1. Brief review of the D3 receptor pharmacophore template and examples of promising D3-selective agents for preclinical evaluation
Several comprehensive reviews describing dozens of D3-selective agents and derived structure-activity relationships (SAR) have been published recently (Heidbreder & Newman, 2010; Micheli, 2011; Ye et al., 2013). Despite fertile ground for modification of the classic D3 pharmacophore template, several challenges remain in identifying D3-selective ligands with efficacies that can be translated into in vivo models and ultimately therapeutic agents to treat human addiction.
In general, chemical modification, initially using readily available starting materials and simple, high-yielding synthetic strategies, results in libraries of compounds that are tested in in vitro binding and functional assays to develop SAR. Increasing the affinity and selectivity of these molecules toward the target of interest is typically the first goal. Classical medicinal chemistry as well as quantitative SAR (QSAR) and other computational methods, based on small molecule structures have been used to guide subsequent rational drug design to achieve this goal as efficiently as possible. Once molecules have been identified that show high affinity and selectivity for the target, lead optimization proceeds. Used by most labs to modify lead compounds into tools that can be used for in vivo studies, the now-classic Lipinski rule of 5 (Lipinski, 2000; Lipinski et al., 1997) defined four parameters to aim for when designing molecules with drug-like physicochemical properties: a molecular weight (MW) <500, calculated logarithmic partition coefficient (LogP) in the range of 2-5, <5 H-bond donors (OH, NH), and <10 H-bond acceptors (N, O). These criteria have been used to maximize blood brain barrier (BBB) penetration and optimize other pharmacokinetic parameters. However, these specifications are often too strict to comply with the highly “decorated” and potent compounds that are discovered through target-based SAR. This has resulted in an abundance of compounds with high affinity and/or selectivity for their biological target but that are hopelessly large and lipophilic, resulting in a poor absorption, distribution, metabolism, excretion, and toxicity (ADMET) profiles and ultimate failure in the clinic (Hann & Keseru, 2012).
“Fat and flat – the enemies of drug discovery” was recently coined (Robert J. Young, 2013 31st Camerino-Cyprus-Noordwijkerhout Symposium) and clues as to how to achieve molecules that are more likely to be drug-like - but still have the pharmacological specificity required - remains one of the biggest challenges to medicinal chemists, especially for central nervous system (CNS)-active drugs (Hill & Young, 2010; Wager et al., 2010). Hann and Keseru further elaborated on the risks of “molecular obesity” and advised that a “sweet spot” between molecular mass and LogP values exists that narrows the Lipinski parameters even further to compounds with MWs in the range of 250-500 and LogP values in the 2-4 range (Hann & Keseru, 2012). They refer to a set of ADMET “rules of thumb” published in 2008 (Gleeson, 2008) that provides further data to support aiming for molecules with LogP values <4 and MW<400, keeping in mind ionization at physiological pH, especially for CNS-penetrant drugs, where this is particularly important. High MWs not only preclude BBB penetration but increase plasma protein binding and are associated with inhibition of voltage-gated potassium ion channels that control electrical activity within the heart, commonly known as hERG (named from the human Ether-à-go-go-Related Gene that encodes the Kv11.1 protein). hERG channel inhibition is associated with cardiac arrhythmia and QT interval prolongation, therefore hERG binding is an early eliminator of an otherwise potential drug candidate (Gleeson, 2008). Unfortunately, high affinity for the hERG channel is associated with basic molecules, especially lipophilic amines, and for most GPCRs this provides an early confound between the biological target and the off target drug design (Wager et al., 2010).
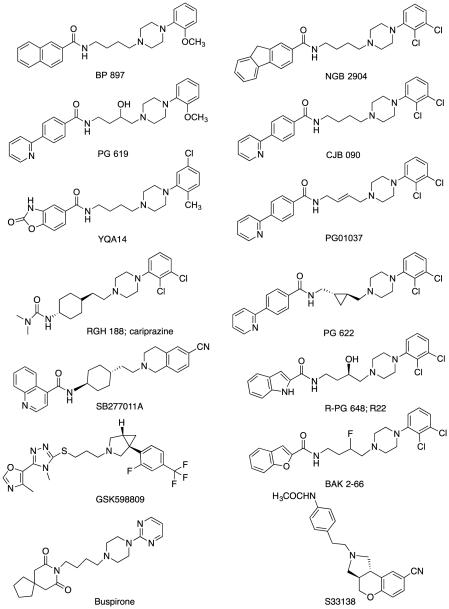
The classic template of the 4-phenylpiperazines, exemplified by the early D3-selective ligands BP 897 (Fig. 1; Pilla et al., 1999) and NGB 2904 (Fig. 1; Yuan et al., 1998), has undergone significant modification to yield some very interesting D3-selective compounds, some of which have reached the clinical trial stage and are discussed in Section 3. The primary goal has been to achieve high-affinity binding to the D3 receptor and to limit or design out “off target” actions, especially at the other D2-family of receptors, and also at 5-HT receptors that share common pharmacophoric elements (e.g. 5-HT1A, 5-HT2A or β-adrenergic). Nevertheless, in some cases, multifunctional compounds that target serotonin 5-HT1A and 5-HT2A receptors have been designed to capitalize on the overlapping SAR and to identify ligands that have potential as treatments for other CNS disorders such as schizophrenia (Butini et al., 2010). In addition, as the template is long to begin with, and addition of molecular “decoration” has added MW and lipophilicity, confounding activity at hERG channels, for example, has precluded further development of otherwise selective and promising agents due to predicted cardiotoxicity. Significant effort has been made in recent years (Bonanomi et al., 2010; Butini et al., 2010; Micheli et al., 2007; Micheli & Heidbreder, 2013) to address this persistent challenge with this class of drugs.
Figure 1.
Chemical structures of D3 receptor antagonists/partial agonists used in preclinical and clinical studies
The early prototypic D3-selective ligands were characterized as antagonists or partial agonists, primarily in cell-based functional assays. It has been noted that the efficacies derived from these D3 functional assays have not always been translated into distinct behavioral actions (Newman et al., 2005). Indeed, compounds that have been described as partial agonists or antagonists often show similar behavioral effects in the preclinical models of addiction (see Heidbreder & Newman, 2010, for further discussion). In addition, the functional potencies in the in vitro assays are often not well correlated to binding affinities at D3 vs. D2. To further complicate interpretation of behavioral results, many D3-selective compounds have less-than-optimal physicochemical properties, solubility, BBB permeability and pharmacokinetics likely due to their high MWs and lipophilicities. These properties may also contribute to the need to use higher doses of drug to observe activity in vivo than might have been predicted from their low nanomolar D3 receptor binding affinities. Several examples of these early preclinical candidates have been compared (Heidbreder & Newman, 2010; Micheli, 2011). The early 2-OMe or 2,3-diCl-4-phenylpiperazine fragment has been substituted with many different substituents and even the phenyl ring has been elaborated on with various heteroaryl ring systems. In the GSK analogues, for example, the 4-phenylpiperazine was first replaced with a tetrahydroisoquinoline in the prototypic SB277011A and later with several atypical bicyclic moieties, including the azabicyclo[3.1.0] hexane moiety found in their clinical candidate GSK598809 (Fig. 1), to be discussed in Section 3.
In most of the D3-selective compounds, the requisite linker that is equivalent to a 4-methylene group chain seen in BP 897 and NGB 2904 remains, but has appeared in many functionalized forms including trans cyclohexyl (e.g., SB277011A), trans olefin (e.g., PG01037) and transcyclopropyl (e.g., PG 622) (Fig. 1; for review see Micheli, 2011). In all of these conformational isomers, the trans isomer is typically more D3-selective than the cis, due to higher affinity at D3 (Grundt et al., 2005; Grundt et al., 2007; Newman et al., 2003; Ye et al., 2013, for review). In addition, modification to this linking chain has uncovered enantioselectivity and a point of separation between D3 and D2 (Newman et al., 2009). For example, with PG 648 (Fig. 1), significant enantioselectivity at D3 (R>S ~15-fold) was not observed for D2 binding (<2-fold difference between enantiomers).
The amide linker has also been replaced with either oxazoles or thiotriazoles that retain the desired pharmacological profile and may provide additional benefits over the amide group in vivo (Micheli et al., 2007). Although an extended aryl amide was thought to be required for high-affinity binding at D3, and also for selectivity over D2 and 5-HT1A, there are several recent examples - especially with the triazole analogs - in which much smaller aryl ring systems provided comparable D3 selectivity and affinity profiles with reduced lipophilicity and molecular weights, which is more favorable for successful drug development (Bonanomi et al., 2010; Micheli et al., 2010a; Micheli et al., 2010b; and others highlighted in Micheli & Heidbreder, 2013). The sulfoxide moiety was also used in other analogues reported in the patent literature to be D3-selective (Micheli & Heidbreder, 2013). Others have also reported successful replacement of the amide function of BP 897 (Jean et al., 2010). It should be noted however, that reducing the amide linker to a secondary or tertiary amine severely decreases D3 receptor affinity and renders the resulting molecules nonselective over D2 receptors (Banala et al., 2011).
A recent review of the patent literature has appeared that describes the formidable contribution, primarily from pharmaceutical companies since 2007 (Micheli & Heidbreder, 2013). In this review, the authors also caution the field to recognize that potency and selectivity ratios can vary across labs and across in vitro assays, making direct comparisons of compounds impossible unless they are evaluated side-by-side. What is not clear from the patent literature is how these novel molecules were designed, especially those that are at substantial variance from the classical pharmacophoric template.
Small molecule comparative molecular field analysis (CoMFA) or comparative molecular similarities index analysis (CoMSIA) and 3D-QSAR studies have been employed in the past (Boeckler et al., 2005; Liu et al., 2011; Lopez et al., 2010; Salama et al., 2007; Wang et al., 2010), however the advent of GPCR homology modeling has opened additional platforms from which to design molecules with theoretically optimized interactions in the targeted receptor binding pocket(s) at the molecular level. As the high-resolution crystal structure of the D3 receptor was recently solved with the D2/D3 antagonist/inverse agonist eticlopride (Chien et al., 2010), a new opportunity for structure-based drug design is available and is being used for the design of novel compounds. Indeed, before the crystal structure of the D3 receptor was published, homology models developed using the structure of bacteriorhodopsin, and later using bovine rhodopsin and the β2-adrenergic receptor structures, provided an excellent basis for D3 receptor homology models (e.g., Hobrath & Wang, 2006; Wang et al., 2010). One example of this strategy is work published by the Gmeiner group in which the crystal structure of the β2-adrenergic receptor was used to derive models for the D2, D3 and D4 receptor subtypes (Ehrlich et al., 2009). Docking analyses led the authors to perform site-directed mutagenesis studies that examined amino acid residues in transmembrane segments (TMs) 2 and 3. By incorporating SAR from their library of small molecules, the individual contributions to D2-subtype selectivity were identified. Importantly, these studies recognized the 4-phenylpiperazine as being the primary recognition moiety for the orthosteric binding site (OBS) for all three members of the D2 family of receptors (Ehrlich et al., 2009).
Other groups have compared homology models for the D3 receptor with homology models for the hERG channel in an attempt to optimize their molecules for high-affinity binding to the D3 receptor while reducing affinity for the hERG channel (Bonanomi et al., 2010; Micheli et al., 2010b; Micheli et al., 2007). This effort has resulted in the discovery of D3-selective antagonists without predicted QT interval prolongation, a very important finding for drug development.
2.2. The design of D3-selective agonists, partial agonists and antagonists
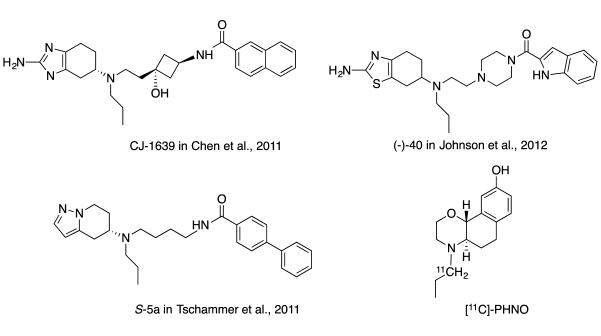
Highly D3 receptor selective agonists have been elusive until recently and are only now beginning to be tested preclinically (Chen et al., 2011; Johnson et al., 2012). These compounds have been designed with a hybrid approach, using a classic D2/D3 agonist (e.g. pramipexole) as the primary pharmacophore (PP) and then elaborating with extended linkers attached via either piperazinylaryl amides or aryl amides to give fully efficacious, D3-selective agonists in vitro (). An additional report (Tschammer et al., 2011) used a similar template to discover compounds with enantioselectivity as well as functional selectivities, another very hot topic for drug discovery, with great interest to relate functional selectivity to behavioral activity in the future. Further characterization of these molecules in vivo will provide critical data to assess the translation of cell-based functional assay results to D3 agonist mediated behaviors.
D3-selective antagonists and partial agonists have been more easily designed as they typically share the classic pharmacophore. Nevertheless, modification of these molecules has thus far not led to tractable SAR regarding efficacies. This pursuit has been partially confounded by the paucity of functional assays that gave consistent efficacy predictions in the first few years of D3 receptor research (Levant, 1997) resulting in a diversity of assays used across labs, producing different measured potencies and efficacies for the same compounds (e.g., BP 897; for review see Newman et al., 2005). To further confound the field, behavioral actions produced by partial agonists vs. antagonists have not been well-defined. Numerous studies suggest that these D3-selective compounds prevent extracellular DA from binding to the receptor, and that this action leads to decreased self-administration and reinstatement to drug-seeking behaviors. However, it is still unclear whether or not an antagonist or partial agonist would be the preferred pharmacotherapy to treat substance use disorders. This has been debated in the literature (e.g., Newman et al., 2012b) and goes beyond the scope of the present review. However, we describe recent examples of preclinical evaluation of D3 receptor antagonists and/or partial agonists in models of addiction to provide evidence that the D3 receptor is a promising target for drug development.
2.3. Recent examples of D3 receptor-selective compounds in preclinical models of addiction
Several classes of chemical structures have been explored in the search for D3-selective ligands. In this section, we discuss some of the major chemical classes that have been explored in SAR studies and tested in animal models of addiction. Because this review is not meant to be a comprehensive evaluation of the preclinical literature, we will focus on reports published since 2008 and recommend to the reader these reviews for earlier work (Heidbreder et al., 2005; Heidbreder & Newman, 2010; Joyce & Millan, 2005; Micheli, 2011; Micheli & Heidbreder, 2013; Newman et al., 2012b; Newman et al., 2005; Xi & Gardner, 2007).
Effective preclinical models are essential for identifying the in vivo profile of novel D3 receptor partial agonists and antagonists as well as advancing the field’s understanding of the role of D3 receptor signaling in drug addiction. Since the cloning of the D3 receptor gene in 1990, animal studies have been crucial to elucidate the in situ functions of D3 receptors. Over the past 15 years, a variety of factors influencing the in vivo selectivity and efficacy of D3 receptor-selective compounds have been identified through behavioral evaluation in a variety of preclinical models, in rodents and nonhuman primates.
A common finding with D3-selective antagonists in behavioral models of addiction is that these compounds are typically ineffective in reducing drug self-administration under low fixed-ratio (FR) schedules, in which typically one (FR1) or two (FR2) lever presses in an operant chamber result in the delivery of the reinforcer (e.g., intravenous cocaine or methamphetamine). These low FR schedules of reinforcement are useful for exploring drug intake patterns, but are not well-suited to measure the reinforcing effects of drugs of abuse (Heidbreder & Newman, 2010; O'Brien & Gardner, 2005). Similarly, D3-selective antagonists are often ineffective in second-order reinforcement schedules, in which a subject responds according to a unit schedule (such as FR10) for a brief stimulus presentation (such as a light) and the unit schedule is then reinforced according to a separate schedule of reinforcement (such as a fixed time interval) via drug delivery. In contrast, progressive ratio (PR) schedules of drug reinforcement - believed to be more sensitive to a drug’s reinforcing effectiveness and motivational effects (Arnold & Roberts, 1997; O'Brien & Gardner, 2005; Richardson & Roberts, 1996) - are sensitive to D3 antagonism.
Other models of addiction are more sensitive to modulation of D3 receptor signaling. Drug-seeking behavior can be attenuated in several models of relapse-like behavior, such as drug-, cue- or stress-induced reinstatement. In these models, animals are trained to self-administer methamphetamine (or another reinforcer) until stable behavior is established. Following this training, the reinforcer is removed from future training sessions, extinguishing the drug-seeking behavior. Robust drug-seeking behavior (i.e., active lever pressing) can be induced by non-contingent exposure to the drug, drug-associated cues, stress or a stress-like trigger such as yohimbine (Shaham et al., 2003). Several studies have reported that D3 antagonism attenuates reinstated drug-seeking behavior.
The subjective, rewarding properties of addictive drugs are also commonly evaluated using conditioned place preference (CPP) tests in which the positive incentive salience of a drug can be measured using a fairly simple Pavlovian learning paradigm (O'Brien & Gardner, 2005; Tzschentke, 2007). D3 receptor antagonists have been reported to block the expression of CPP, but less consistently to attenuate the acquisition of CPP (Beninger & Banasikowski, 2008)
2.3.1. 4-Phenylpiperazines
A large library now exists of D3-selective or D3-preferential antagonists and partial agonists in which the 4-phenylpiperazine is the core structure, as illustrated in Fig. 1. This general molecular template has been explored in multiple SAR studies and several “hits” from this class have shown efficacy in preclinical models of addiction.
BP 897
First reported in 1999 to attenuate cocaine-taking and -seeking behavior in rats (Pilla et al., 1999), BP 897 was one of the first D3-selective phenylpiperazines widely researched in addiction models. Since then, BP 897 has been reported to inhibit cocaine-seeking behavior, morphine CPP, as well as conditioned activity associated with amphetamine, nicotine and cocaine (Garcia-Ladona & Cox, 2003; Le Foll et al., 2005). Recent work has shown that BP 897 did not reduce nicotine self-administration or cue-induced reinstatement to nicotine-seeking behavior (Khaled et al., 2010). BP 897 can attenuate methamphetamine-enhanced brain stimulation, but produces an aversive effect at high doses (Spiller et al., 2008).
BP 897 entered early phase clinical trials for the treatment of addictions to cocaine, nicotine and alcohol as well as for treatments for schizophrenia and Parkinson’s disease (Garcia-Ladona & Cox, 2003). Approximately 70-fold selective for human D3 receptors versus D2 receptors, with apparent partial agonist signaling properties, BP 897 was not successful as a clinical treatment. This may have been due to side effects arising from BP 897’s insufficient selectivity for D3 over D2, 5-HT1A, and α1- and α2-adrenoceptors (Garcia-Ladona & Cox, 2003; Xi & Gardner, 2007).
NGB 2904
Compared to BP 897, NGB 2904 has substantially improved D3 selectivity over D2, HT1A, and α1- and α2-adrenoceptors (Xi & Gardner, 2007). NGB 2904 has been reported to reduce cocaine self-administration under a PR schedule and block reinstatement to cocaine-seeking behavior (Xi & Gardner, 2007). Pretreatment with NGB 2904 attenuated methamphetamine-enhanced brain stimulation reward in rats (Spiller et al., 2008). However, NGB 2904 is poorly water soluble and is highly lipophilic, which likely precluded it from investigation as a clinical candidate (Mason et al., 2010).
PG01037
More than 100-fold selective for D3 over D2, PG01037 was initially reported in 2005 (Grundt et al, 2005). Since then, PG01037 has been investigated in a wide variety of addiction models. PG01037 did not alter methamphetamine or sucrose self-administration under low FR schedules of reinforcement in mice or rats (Caine et al., 2012; Higley et al., 2011b; Orio et al., 2010). However, PG01037 significantly lowered the breakpoint for methamphetamine and sucrose self-administration under PR reinforcement, attenuated methamphetamine-enhanced brain stimulation, and blocked cue-induced reinstatement of methamphetamine seeking (Higley et al., 2011b; Orio et al., 2010). Similarly, low FR schedule cocaine and food self-administration were not altered by PG01037 administration in squirrel monkeys, but it significantly attenuated cocaine’s discrimination stimulus effects and cocaine-induced reinstatement (Achat-Mendes et al., 2010). In an effect similar to genetic knockout of the D3 receptor, treatment with PG01037 in wild-type mice disrupted reconsolidation of cocaine-induced CPP memory (Yan et al., 2013).
PG01037 has improved water solubility over NGB 2904 (Mason et al., 2010). However, it is also a substrate for P-glycoprotein, an ATP-dependent drug efflux pump found at the BBB, which likely limits its potential for translation to the clinic (Mason et al., 2010).
CJB 090
CJB 090 (Fig. 1) is the saturated analogue of PG01037 and somewhat less D3-preferential (50- vs. 133-fold) in binding over D2 receptors (Newman et al., 2005). This compound attenuated methamphetamine self-administration under FR1 reinforcement in rats with long-access (6-hour sessions, 6 days per week) but not short-access (1-hour sessions, 3 days per week). Additionally, under a PR schedule of reinforcement, CJB 090 attenuated self-administration in both the short- and long-access groups (Orio et al., 2010). Pretreatment with CJB 090 significantly decreased cocaine’s discriminative stimulus effects in squirrel monkeys. However, CJB 090 failed to inhibit either self-administration of cocaine or cocaine-induced reinstatement of drug-seeking (Achat-Mendes et al., 2009).
PG 619
PG 619 (Fig. 1) was synthesized and characterized while looking for improvements to the PG01037 structure (Grundt et al., 2005; Grundt et al., 2007). Cocaine self-administration by male rhesus monkeys under a FR30 schedule of reinforcement was not altered by PG 619. However, PG 619 significantly attenuated cocaine-induced reinstatement (Blaylock et al., 2011). Nevertheless, subsequent testing in a rhesus monkey food/drug choice paradigm, PG 619 failed to demonstrate any efficacy in attenuating self-administration of either cocaine or methamphetamine (personal communication from Dr. Michael Nader, Wake Forest University Medical School). In addition, a poor ADME profile in rats (unpublished data) dampened enthusiasm for further development of this agent.
YQA14
Administration of YQA14 (Fig. 1) attenuated cocaine self-administration under PR reinforcement in rats without altering oral sucrose self-administration or locomotor activity at the same tested doses (Song et al., 2012). YQA14 decreased cocaine self-administration under FR1 and PR reinforcement schedules in wild-type mice but had no effect on D3 receptor knockout mice (Song et al., 2012), supporting a D3 receptor mediated effect. Similarly, YQA14 attenuated acquisition and expression of cocaine-induced CPP in WT mice but not D3 receptor knockout mice (Song et al., 2013).
Cariprazine (RGH 188)
Cariprazine (RGH 188; Fig. 1) is a D3-preferring D3/D2 partial agonist in clinical development as an atypical antipsychotic for the treatment of schizophrenia and bipolar mania/mixed episodes (Agai-Csongor et al., 2012; Citrome, 2013). To date, only one study has been published evaluating cariprazine in addiction models. Román et al. (2013) found that oral administration of cariprazine significantly increased cocaine self-administration in rats but inhibited cue-induced reinstatement of cocaine-seeking behavior. The enhanced self-administration effect may be due to a cariprazine-mediated reduction in the rewarding effects of cocaine, necessitating more cocaine infusions to achieve the same reward (Román et al., 2013).
2.3.2. Additional structural templates
SB277011A
One of the D3-selective ligands most extensively studied in addiction models over the past five years, SB277011A was initially described in 2000 (Reavill et al., 2000; Stemp et al., 2000). Previous reviews have covered the effects of SB277011A in preclinical addiction models (Heidbreder et al., 2005; Xi & Gardner, 2007). Since 2008, SB277011A has been tested in multiple models against a variety of different addictive drugs, detailed below.
SB277011A blocked cue-induced reinstatement of nicotine-seeking in rats (Khaled et al., 2010). Pretreatment with SB277011A did not alter methamphetamine self-administration under FR2 schedule of reinforcement but did significantly lower PR breakpoints and inhibit methamphetamine-induced reinstatement (Higley et al., 2011a). SB277011A significantly attenuated methamphetamine-enhanced brain stimulation reward in rats (Spiller et al., 2008). Cue-induced cocaine seeking and incubation of cocaine craving was attenuated by SB277011A administered either systemically or locally into nucleus accumbens or central amygdala in rats (Xi et al., 2013). Administration of SB277011A attenuated morphine-triggered reactivation of cocaine-induced conditioned place preference in adult male rats (Rice et al., 2013) and decreased the conditioned place aversion following naloxone-induced withdrawal from acute morphine administration (Rice et al., 2012a). Infusion of SB277011A into the nucleus accumbens significantly inhibited the expression of morphine-induced context-specific locomotor sensitization (Liang et al., 2011).
GlaxoSmithKline halted clinical development of SB277011A (Xi & Gardner, 2007) after it was determined that the drug has a short half-life and poor oral bioavailability in primates (Austin et al., 2001) despite having favorable pharmacokinetics in the rat (Stemp et al., 2000).
S33138
The hexahydrochromeno[3,4-c]pyrrole, S33138 (Fig. 1) was reported in 2008 in an in-depth series of in vitro and in vivo pharmacological characterizations aimed at assessing its effects as an antipsychotic (Millan et al., 2008a; Millan et al., 2008b; Millan et al., 2008c). Since then, it has been explored to a limited extent in models of addiction. In rats, pretreatment with low doses of S33138 attenuated cocaine-enhanced brain-stimulation reward and cocaine-induced reinstatement of drug-seeking behavior. However, at higher doses, FR2 cocaine and sucrose self-administration behavior was altered, locomotion in a rotarod task was impaired and an aversive-like rightward shift in brain-stimulation reward rate-frequency reward functions was reported (Peng et al., 2009). Ethanol consumption in mice was significantly inhibited by S33138 administration, but only at doses that also decreased water consumption (Rice et al., 2012b).
Buspirone
Buspirone (Fig. 1) has received extensive recent interest as a clinically available drug that may be repurposed for addiction treatment (Le Foll & Boileau, 2013). An atypical anxiolytic, buspirone is a partial agonist at the 5-HT1A receptor, but is also a high-affinity antagonist of DA D3 and D4 receptors (Bergman et al., 2013). In adult male rhesus monkeys, buspirone pretreatment attenuated cocaine self-administration at doses that did not reliably alter food-maintained responding (Bergman et al., 2013; Mello et al., 2013b). Chronic buspirone treatment was also effective in reducing cocaine, nicotine, or combined cocaine+nicotine self-administration in rhesus monkeys (Mello et al., 2013a; Mello et al., 2013b). In rats, buspirone was reduced the anxiolytic effects of withdrawal from cocaine (de Oliveira Citó et al., 2012).
2.3.3. Preclinical support and caveats for translation of the D3 hypothesis to the clinic
D3-selective ligands have been widely studied in addiction models. Among the patterns that emerge, D3 antagonism is clearly most effective in self administration under a PR schedule of reinforcement and in reinstatement paradigms while generally ineffective in FR studies. This suggests that D3 antagonism reduces the rewarding efficacy and motivation to self-administer the drug of abuse, without substantially disrupting general behavior. However, studies that compare D3 antagonist effects on drug-taking to effects on food or sucrose rewards have not been fully explored. In developing potential medications it is important to ascertain whether or not these behavioral effects are specific to drug-induced reward-seeking behaviors or generalize to natural rewards as well.
Developing behavioral models in animals that translate to human behavior is a continual challenge in psychiatric drug development and may be especially challenging in the psychostimulant drug abusing population. Moreover, many of the compounds tested in preclinical models have specific drawbacks that have halted or precluded translation to the clinic. The chemical structures of these compounds are commonly large and lipophilic, which can be problematic in terms of solubility and drug ADMET properties (see Section 2.1 for further discussion). Also, very few studies have detailed the metabolic profiles of these compounds.
Finally, it is not currently clear from preclinical studies whether antagonist or partial agonist effects on D3 receptors is preferable for treatment. More detailed study is needed of in vivo D3 signaling to determine whether there is a functional difference between D3 antagonists or partial agonists in drug-taking or drug-seeking animals; given the D3 receptor’s high affinity for DA, partial agonism may be effectively indistinguishable from antagonism when DA levels are very high, as they are well-known to be during drug-taking and -seeking behaviors. It is anticipated that many of these questions and caveats will be addressable with better drug molecules.
3. Clinical studies targeting the D3 receptor in the treatment of addiction
A critical consideration for clinical trial success is the demonstration that the new drug engages its biological target (e.g. central D3 receptors) at doses that are related to its pharmacological action (e.g., drug craving cessation). Hence, the discovery of a D3-preferential positron emission tomography (PET) ligand to monitor drug occupancy and selectivity at D3 receptors was essential. As described above, selective D3 receptor ligands that are active in vivo have remained a challenge. The further requirements for a PET ligand: 1) high affinity (Ki<1 nM) 2) >30-fold selectivity for the target 3) rapid BBB penetration and 4) limited metabolism has provided significant challenge to identifying a D3-preferntial PET ligand.
[11C]PHNO
Currently, [11C]PHNO (Fig. 2), a D3-prefential agonist has been used in this capacity, although it is perhaps not ideal. First introduced as a nonselective D2/D3 receptor agonist (Horn et al., 1984; Martin et al., 1985), PHNO was developed as a potential agonist treatment for Parkinson’s disease (Koller et al., 1987; Rose & Nashef, 1987). Subsequently [11C]PHNO was first characterized as binding to high affinity states of the D2/D3 receptor in human subjects (Willeit et al., 2006) and later as a D3 receptor agonist (Narendran et al., 2006). It has subsequently been further characterized (Ginovart et al., 2007) and more recently used to demonstrate D3 receptor involvement (Graff-Guerrero et al., 2009a; Graff-Guerrero et al., 2009b; Graff-Guerrero et al., 2008; Willeit et al., 2008) and upregulation in methamphetamine abusers (Boileau et al., 2012). In addition, [11C]PHNO has been used to validate D3 receptor occupancy for the D3 antagonist ABT 925 (Graff-Guerrero et al., 2010). A [11C]PHNO PET study is planned to evaluate whether D3 receptor expression is elevated in smokers versus nonsmokers and whether correlations exist between D3 receptor binding and the reactivity to smoking cues as measured by functional MRI (ClinicalTrials.gov Identifier: NCT01784016). Another planned [11C]PHNO PET study seeks to determine whether the smoking cessation drug varenicline alters D2/D3 receptor binding in tobacco smokers (ClinicalTrials.gov Identifier: NCT01632189).
Figure 2.
Chemical structures of recently described D3 receptor agonists
GSK598809
To date, only a few D3-selective antagonists have progressed to human clinical testing. The first compound from GlaxoSmithKline, GSK598809 (Fig. 1), entered phase 1 clinical trials in 2007 and phase 2 trials for smoking cessation and compulsive overeating (e.g., ClinicalTrials.gov Identifiers: NCT00437632, NCT01188967, NCT00793468, NCT00605241, NCT01039454 ). Cognate analogue GSK618334 also entered phase 1 clinical trials in 2007 (ClinicalTrials.gov Identifiers: NCT00513279, NCT00814957, NCT01036061). To date, 9 trials have been completed with GSK598809 and this compound was deemed to be safe and well tolerated in healthy volunteers (Mugnaini et al., 2013; Searle et al., 2010). Importantly, studies using the D3-preferential PET ligand [11C]PHNO in human volunteers demonstrated that GSK598809 engages with D3-receptor rich regions of the human brain, upon oral administration, in the substantia nigra but not in the dorsal regions of the striatum (Searle et al., 2010). Boileau et al. (2012) determined that methamphetamine polydrug users had higher [11C]PHNO binding in the D3 receptor-rich substantia nigra, globus pallidus, and ventral pallidum, and lower binding in the D2 receptor-rich dorsal striatum compared to healthy controls. [11C]PHNO binding within the substantia nigra correlated with self-reported drug craving (Boileau et al., 2012).
A recent study using GSK598809 and [11C]PHNO sought to predict the ability of GSK598809 to reduce nicotine-seeking/craving behavior in human cigarette smokers and the relationship to D3 occupancy by this D3 antagonist. These results were compared with an ex vivo autoradiography study in rats and the demonstration that GSK598809 dose dependently reduced the expression of nicotine-induced conditioned place preference. Although an absolute translation of these findings was not achieved in this preliminary study, the results suggest that GSK598809 binds to D3 receptors in a dose-dependent manner and support further investigation of GSK598809 for smoking cessation in human subjects. Further translation of preclinical findings that GSK598809 and other D3 antagonists and partial agonists may be effective in reducing methamphetamine and/or cocaine craving in humans has yet to be evaluated. In order to advance to phase 2 clinical trials for cocaine or methamphetamine abuse, further drug-interaction studies must be conducted. In addition, as the D3 antagonists appear to be more effective in reducing drug and/or cue-induced reinstatement to drug seeking in laboratory animals than blocking self-administration of cocaine or methamphetamine, the design of clinical trials must take this into account.
BP1.4979
Bioprojet recently disclosed their novel D3 antagonist BP1.4979 and recruitment for a clinical trial on smoking cessation has begun (ClinicalTrials.gov Identifier: NCT01785147).
Buspirone
Finally, as noted above, the clinically available atypical anxiolytic drug buspirone (Fig. 1) has recently shown promise in preclinical studies (Bergman et al., 2013; Mello et al., 2013a; Mello et al., 2013b; Newman et al., 2012b; Shelton et al., 2013) for the treatment of psychostimulant abuse. Although buspirone is not a D3-selective antagonist, its metabolic profile suggests primary metabolites bind to D3 (and D4) receptors (Bergman et al., 2013). Target engagement using PET imaging and clinical studies are in progress (ClinicalTrials.gov Identifier: NCT01699828) that will inform future exploration of this repurposed drug for treatment of SUDs.
4. The structural basis of D3 over D2 receptor selectivity and the future of rational drug design for D3 receptor-selective ligands
Despite a number of preclinical candidates and a handful of D3-selective antagonists or partial agonists in clinical trials for smoking cessation, the need to identify novel templates and better drug molecules to target the D3 receptor still exists. It is important to note, for example, that in treating SUDs, toxicology and safety studies must be done with the new medication candidate and in the presence of the abused drug e.g. cocaine. Drug combinations can produce untoward side effects that could not be predicted by the drug interacting with its primary target (e.g. D3 receptor) and may be related to off target actions or metabolic vulnerability of a particular structural template. Hence, using all the available structural information to identify new leads remains important in the pursuit of medication candidates. Small molecule drug discovery usually begins with identifying lead compounds that recognize the biological target of interest. The advancement of high-throughput screens (Macarron et al., 2011) and rational lead design and optimization with in silico models (Jorgensen, 2009) have been a more recent source of novel leads.
Common structural features of GPCRs include the 7 TMs, extracellular and intracellular loops (ECLs and ICLs) (Rosenbaum et al., 2009). Specifically for class A rhodopsin-like GPCRs, to which DA receptors belong, a conserved “toggle switch” in TM6 is involved in receptor activation – the impact of the reconfiguration of this switch upon agonist binding is propagated to the intracellular side and triggers the release of a conserved ionic lock between TM3 and TM6, which has been found to stabilize the receptor in an inactive state (Ballesteros et al., 2001a; Shi et al., 2002). Such transitions between active and inactive states have significant impact on the shape and size of the orthosteric ligand binding site; it is computationally complex to design and optimize antagonists or agonists for a particular receptor with desired affinity and efficacy in a structure-based manner.
Due to the high-homology among GPCRs especially within the same family (e.g., between rhodopsin and aminergic receptors), computational models of the D3 receptor were previously built based on the crystal structures of homologous proteins for which crystal structures had been solved (Hobrath & Wang, 2006; Varady et al., 2003). These resulting homology models were then used to characterize points of interaction of small molecules at the binding site, through medicinal chemistry, chimera studies and single point mutations on the receptor (e.g., Ehrlich et al., 2009). Before the crystallographic coordinates of D3 crystal structure were published, a competition was held to determine how well various GPCR modeling and docking procedures performed in identifying key ligand-receptor interactions, comparing these results to the crystal structure of the D3 receptor. The best-performing complex models approached the accuracy of the experimentally determined details, especially in revealing those in the conserved OBS (Kufareva et al., 2011).
Several groups have used homology models of the D3 receptor to design libraries of D3-selective ligands. For example, Levoin and colleagues recently demonstrated that the binding site in their “historical” rhodopsin-based D3 receptor homology model was as well-suited for lead optimization as the crystal structure of the D3 receptor, which explained the successful utilization of the model in their drug discovery processes (Levoin et al., 2011). Similarly, the β-adrenergic receptor-based D3 homology model appeared to perform as well as the D3 receptor crystal structure in retrieving known hit compounds in in silico high-throughput screening trials with a library of 3 million commercially available molecules. Interestingly, the homology model and the crystal structure have different enrichments of the chemical scaffolds, as the OBS in the homology model is slightly more open (Carlsson et al., 2011). In that study, because the compounds were screened against the OBS, none of the hit compounds showed appreciable selectivity for D3 over D2 receptor; nevertheless, novel scaffolds that can be modified to enhance selectivity were uncovered (Carlsson et al., 2011).
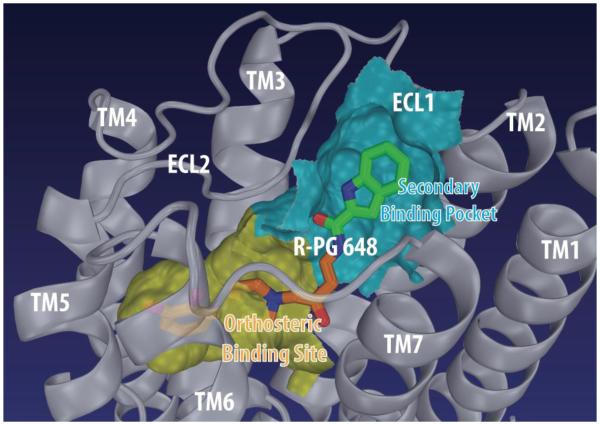
Interestingly, all of the novel hits identified by Carlsson et al. (2011) were found to be antagonists, which raises the question of how we may be able to rationally introduce efficacy into our D3 receptor-selective agents using computational modeling techniques. A recent review evaluating the progress in the structure-based drug design for GPCRs highlighted the advances that are being made with the availability of many high-resolution GPCR structures in both active and inactive states; thus far, most successful investigations have improved ligand binding affinities towards individual receptors but, in most cases, without addressing selectivity or efficacy issues (Congreve et al., 2011). Thus, in comparison, the novelty of our approach is to reveal molecular determinants of D3 over D2 receptor selectivity as well as D3 receptor efficacy. To this end, we have built and refined D3 and D2 receptor models in both inactive and active states. In addition to the D3 receptor structure, stabilized in an inactive conformation by eticlopride, a D3/D2 receptor-selective inverse agonist (Chien et al., 2010), we used the differences in the inactive and active structures of β1 and β2 adrenergic receptors (Cherezov et al., 2007; Rasmussen et al., 2011; Warne et al., 2011) to guide the construction of a D3 receptor model with the OBS in the active conformation. For comparative investigations of D3 vs. D2 receptor, we also correspondingly built and refined D3 receptor-based homology models of the D2 receptor (Chien et al., 2010; Newman et al., 2012a). Using these models, the results of our molecular docking and dynamics studies suggested that the primary pharmacophore (PP) of D3 receptor-selective compounds is bound in the OBS, while the arylamide secondary pharmacophore (SP) is accommodated in a secondary binding pocket (SBP) formed by residues from TMs1, 2, 3, 7 and divergent ECL1 and ECL2 (Chien et al., 2010; Newman et al., 2012a).
To further dissect the contributions of individual pharmacophore components of D3 receptor-selective compounds to the selectivity and efficacy, we incrementally deconstructed our D3 receptor-selective antagonist R22 (R-PG 648, Fig. 1, 3) into “synthons”. Starting with the PP, 2,3-diCl-phenylpiperazine, we added N-alkyl substituents one methylene group at a time toward the amide functional group. We also synthesized the SP, N-n-butyl-indole-2-amide. These synthons were tested for receptor binding affinities and functional efficacies at the D3 and D2 receptors. This study confirmed that the 2,3-diCl-4-phenylpiperazine or its cognate 2-OMe-4-phenylpiperazine served as the PP and that addition of the N-n-butyl linking chain resulted in increased binding affinities at both the D2 and D3 receptors, with essentially no change in selectivity, until the indole amide was added to make the full D3 receptor-selective ligands. Given the near identity of the D3 and D2 receptor residues in the OBS that bind the PP and the linker region, it is expected that the synthons lacking the SP would have little or no selectivity for these two receptors (Newman et al., 2012a).
Figure 3.
Predicted binding mode of R-PG 648 in the dopamine D3 receptor. The primary and secondary pharmacophores of R-PG 648 occupy the orthosteric binding site (OBS) and the secondary binding pocket (SBP), respectively. The OBS and SBP are in surface representation.
Moreover, we also found the N-n-butyl-indole-2-amide had very poor affinity for both the D2 and D3 receptors and was functionally inactive, suggesting that the SP alone does not confer D3 receptor-selectivity. The question thus became: how does D3 selectivity arise in these molecules? Our computational modeling and analysis indicated that substitutions on the terminal 4-phenylpiperazine ring, in combination with the linker, affect the orientation of the PP in the OBS, which consequently influences the exact orientation of the SP. Thus, we proposed that D3 receptor selectivity arises from divergent interactions of the SP within a second binding pocket, separate but affected by the PP in the OBS (Newman et al., 2012a).
Our study also demonstrated that depending on the 4-phenylpiperazine substitution pattern, efficacies could range from a nearly full agonist (2,3-diCl-4-phenylpiperazine) to a very low efficacy partial agonist (e.g., N-n-butyl-2,3-diCl-4-phenylpiperazine or the 2-OMe-4-phenylpiperazine). In the context of accumulated understanding of monoamine receptor activation (Ballesteros et al., 2001b; Shi & Javitch, 2002), our modeling results indicated that efficacy depends on the binding mode in the OBS and suggested that the efficacy of D3 receptor-selective ligands could be manipulated by modifying the PP that binds to the OBS (Newman et al., 2012a).
As noted above, the SBP diverges significantly between the D3 and D2 receptors. Initial chimera studies using R-PG 648 (R22) pointed to a role of the extracellular loops in receptor subtype selectivity (Newman et al., 2009). A more complete study, using eight D2/D3 chimeras and our most D3-selective compound to-date (BAK 2-66, Fig. 1), suggested a appreciable role for the ECL2 in ligand binding: replacing this loop in the D2 receptor with the D3 receptor ECL2 sequence improved binding affinity by 37-fold. However, a more dramatic improvement was seen in chimeras of the D2 receptor that included TMs 1 and 2 and ECL1 of the D3 receptor, resulting in a dramatic 441-fold improvement in binding over the D2 wild-type receptor (Banala et al., 2011). Indeed, the binding affinity for BAK 2-66 at this chimera was nearly four-fold higher than at the wild-type D3 receptor (Banala et al., 2011). Follow-up studies have specifically characterized the divergence in the shape, size, and dynamics of the SBP of D3 and D2 receptors (Michino et al., submitted). The combined computational and experimental results indicated that ECL1 plays a key role in determining the D3 receptor over D2 receptor selectivity of R22. In particular, a single Gly residue in the EL1 was found to be able to interconvert the binding selectivity of R22 in D3 and D2, and suggest the non-conserved ECL1 can influence the orientation of conserved TM2, and thus render different shapes and sizes of the SBP in both D3 and D2 receptors (Michino et al., submitted).
5. Summary
The DA D3 receptor remains an enticing target for addiction pharmacotherapy. A panoply of D3-selective compounds have been tested in vivo, producing promising results in addiction models and supporting the hypothesis that D3 receptor signaling is an important component of the reinforcing aspects of addictive drugs. Promising recent clinical trial results suggest that the D3 receptor remains a viable clinical target, but data are still limited in this regard. Continued development of novel D3-selective compounds is necessary as there are currently no FDA-approved treatments for psychostimulant addiction and no current candidates in phase 3 clinical trials, to our knowledge. The new molecular tools discussed in this review hold great promise in identifying new D3-preferential ligands for clinical development.
This is a very exciting time in GPCR research and structure-based drug design. Many crystal structures of both active and inactive receptor states are now available, providing important clues to address the drug discovery issues that were difficult to tackle without high-resolution structural information, such as selectivity and efficacy. The efforts of medicinal chemists have already optimized many lead molecules, but there is still a need for “molecular tweaking” to convert research tools into viable medications. Combining crystal structure-informed computational models with, for example, the hERG channel model (Micheli et al., 2010a; Micheli et al., 2010b) will further refine small molecule medicinal chemistry and enhance the likelihood of designing viable clinical candidates. Models of increasing complexity can be combined with molecular pharmacology techniques and may be able to identify unexplored (allosteric?) binding sites that will ultimately provide improved drug-like molecule design for the future.
Acknowledgements
This work was supported in part by the NIDA Intramural Research Program (AHN) and DA023694 (LS). TMK is supported by an NIH IRTA post-doctoral fellowship and CB is supported by an NIH post-baccalaureate fellowship. AHN and LS would like to acknowledge the members of our labs, past and present, and our wonderful collaborators who have helped move our D3 receptor program forward.
Abbreviations
- DA
Dopamine
- GPCR
G protein-coupled receptor
- AC
adenylyl cyclase
- cAMP
cyclic adenosine monophosphate
- SUDs
substance use disorders
- SAR
structure-activity relationships
- QSAR
quantitative structure-activity relationships
- MW
molecular weight
- LogP
logarithmic partition coefficient
- BBB
blood brain barrier
- ADMET
absorption, distribution, metabolism, excretion, and toxicity
- CNS
central nervous system
- hERG
human Ether-à-go-go-Related Gene
- CoMFA
comparative molecular field analysis
- CoMSIA
comparative molecular similarities index analysis
- TM
transmembrane segment
- OBS
orthosteric binding site
- FR
fixed-ratio
- PR
progressive ratio
- CPP
conditioned place preference
- PET
positron emission tomography
- ECL
extracellular loop
- ICL
intracellular loop
- PP
primary pharmacophore
- SP
secondary pharmacophore
- SBP
secondary binding pocket
Footnotes
Conflicts of interest. The authors have no conflicts of interest to declare.
VI. References
- Achat-Mendes C, Grundt P, Cao J, Platt DM, Newman AH, Spealman RD. Dopamine D3 and D2 receptor mechanisms in the abuse-related behavioral effects of cocaine: studies with preferential antagonists in squirrel monkeys. Journal of Pharmacology and Experimental Therapeutics. 2010;334:556–565. doi: 10.1124/jpet.110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achat-Mendes C, Platt DM, Newman AH, Spealman RD. The dopamine D3 receptor partial agonist CJB 090 inhibits the discriminative stimulus but not the reinforcing or priming effects of cocaine in squirrel monkeys. Psychopharmacology. 2009;206:73–84. doi: 10.1007/s00213-009-1581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agai-Csongor E, Domany G, Nogradi K, Galambos J, Vago I, Keseru GM, et al. Discovery of cariprazine (RGH-188): a novel antipsychotic acting on dopamine D3/D2 receptors. Bioorganic and Medicinal Chemistry Letters. 2012;22:3437–3440. doi: 10.1016/j.bmcl.2012.03.104. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacology Biochemistry and Behavior. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Austin NE, Baldwin SJ, Cutler L, Deeks N, Kelly PJ, Nash M, et al. Pharmacokinetics of the novel, high-affinity and selective dopamine D3 receptor antagonist SB-277011 in rat, dog and monkey: in vitro/in vivo correlation and the role of aldehyde oxidase. Xenobiotica. 2001;31:677–686. doi: 10.1080/00498250110056531. [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Jensen AD, Liapakis G, Rasmussen SG, Shi L, Gether U, et al. Activation of the beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. Journal of Biological Chemistry. 2001a;276:29171–29177. doi: 10.1074/jbc.M103747200. [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Shi L, Javitch JA. Structural mimicry in G protein-coupled receptors: implications of the high-resolution structure of rhodopsin for structure-function analysis of rhodopsin-like receptors. Molecular Pharmacology. 2001b;60:1–19. [PubMed] [Google Scholar]
- Banala AK, Levy BA, Khatri SS, Furman CA, Roof RA, Mishra Y, et al. N-(3-fluoro-4-(4-(2-methoxy or 2,3-dichlorophenyl)piperazine-1-yl)butyl)arylcarboxamides as selective dopamine D3 receptor ligands: critical role of the carboxamide linker for D3 receptor selectivity. Journal of Medicinal Chemistry. 2011;54:3581–3594. doi: 10.1021/jm200288r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile M, Lin R, Kabbani N, Karpa K, Kilimann M, Simpson I, et al. Paralemmin interacts with D3 dopamine receptors: implications for membrane localization and cAMP signaling. Archives of Biochemistry and Biophysics. 2006;446:60–68. doi: 10.1016/j.abb.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Banasikowski TJ. Dopaminergic mechanism of reward-related incentive learning: focus on the dopamine D(3) receptor. Neurotoxicity Research. 2008;14:57–70. doi: 10.1007/BF03033575. [DOI] [PubMed] [Google Scholar]
- Bergman J, Roof RA, Furman CA, Conroy JL, Mello NK, Sibley DR, et al. Modification of cocaine self-administration by buspirone (buspar®): potential involvement of D3 and D4 dopamine receptors. International Journal of Neuropsychopharmacology. 2013;16:445–458. doi: 10.1017/S1461145712000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaylock BL, Gould RW, Banala A, Grundt P, Luedtke RR, Newman AH, et al. Influence of cocaine history on the behavioral effects of Dopamine D(3) receptor-selective compounds in monkeys. Neuropsychopharmacology. 2011;36:1104–1113. doi: 10.1038/npp.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckler F, Ohnmacht U, Lehmann T, Utz W, Hubner H, Gmeiner P. CoMFA and CoMSIA investigations revealing novel insights into the binding modes of dopamine D3 receptor agonists. Journal of Medicinal Chemistry. 2005;48:2493–2508. doi: 10.1021/jm049269+. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, et al. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. Journal of Neuroscience. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanomi G, Braggio S, Capelli AM, Checchia A, Di Fabio R, Marchioro C, et al. Triazolyl azabicyclo[3.1.0]hexanes: A class of potent and selective dopamine D(3) receptor antagonists. ChemMedChem. 2010;5:705–715. doi: 10.1002/cmdc.201000026. [DOI] [PubMed] [Google Scholar]
- Butini S, Campiani G, Franceschini S, Trotta F, Kumar V, Guarino E, et al. Discovery of bishomo(hetero)arylpiperazines as novel multifunctional ligands targeting dopamine D(3) and serotonin 5-HT(1A) and 5-HT(2A) receptors. Journal of Medicinal Chemistry. 2010;53:4803–4807. doi: 10.1021/jm100294b. [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Barrett AC, Collins GT, Grundt P, Newman AH, et al. Cocaine self-administration in dopamine D(3) receptor knockout mice. Experimental and Clinical Psychopharmacology. 2012;20:352–363. doi: 10.1037/a0029135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J, Coleman RG, Setola V, Irwin JJ, Fan H, Schlessinger A, et al. Ligand discovery from a dopamine D3 receptor homology model and crystal structure. Nature Chemical Biology. 2011;7:769–778. doi: 10.1038/nchembio.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Collins GT, Levant B, Woods J, Deschamps JR, Wang S. CJ-1639: A Potent and Highly Selective Dopamine D3 Receptor Full Agonist. ACS Medicinal Chemistry Letters. 2011;2:620–625. doi: 10.1021/ml200100t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien EY, Liu W, Zhao Q, Katritch V, Han GW, Hanson MA, et al. Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science. 2010;330:1091–1095. doi: 10.1126/science.1197410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DI, Zheng M, Kim KM. Current perspectives on the selective regulation of dopamine D(2) and D(3) receptors. Archives of Pharmacal Research. 2010;33:1521–1538. doi: 10.1007/s12272-010-1005-8. [DOI] [PubMed] [Google Scholar]
- Citrome L. Cariprazine : chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability. Expert Opinion on Drug Metabolism & Toxicology. 2013;9:193–206. doi: 10.1517/17425255.2013.759211. [DOI] [PubMed] [Google Scholar]
- Congreve M, Langmead CJ, Mason JS, Marshall FH. Progress in structure based drug design for G protein-coupled receptors. Journal of Medicinal Chemistry. 2011;54:4283–4311. doi: 10.1021/jm200371q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Citó M. d. C., da Silva FC, Silva MI, Moura BA, Macedo DS, Woods DJ, et al. Reversal of cocaine withdrawal-induced anxiety by ondansetron, buspirone and propranolol. Behavioural Brain Research. 2012;231:116–123. doi: 10.1016/j.bbr.2012.01.056. [DOI] [PubMed] [Google Scholar]
- Di Ciano P. Drug seeking under a second-order schedule of reinforcement depends on dopamine D3 receptors in the basolateral amygdala. Behavioral Neuroscience. 2008;122:129–139. doi: 10.1037/0735-7044.122.1.129. [DOI] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, et al. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. Journal of Neuroscience. 2000;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich K, Gotz A, Bollinger S, Tschammer N, Bettinetti L, Harterich S, et al. Dopamine D2, D3, and D4 selective phenylpiperazines as molecular probes to explore the origins of subtype specific receptor binding. Journal of Medicinal Chemistry. 2009;52:4923–4935. doi: 10.1021/jm900690y. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Molecular Pharmacology. 2008;74:59–69. doi: 10.1124/mol.107.043885. [DOI] [PubMed] [Google Scholar]
- Garcia-Ladona FJ, Cox BF. BP 897, a selective dopamine D3 receptor ligand with therapeutic potential for the treatment of cocaine-addiction. CNS Drug Reviews. 2003;9:141–158. doi: 10.1111/j.1527-3458.2003.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S, et al. Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. Journal of Cerebral Blood Flow and Metabolism. 2007;27:857–871. doi: 10.1038/sj.jcbfm.9600411. [DOI] [PubMed] [Google Scholar]
- Gleeson MP. Generation of a set of simple, interpretable ADMET rules of thumb. Journal of Medicinal Chemistry. 2008;51:817–834. doi: 10.1021/jm701122q. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Mamo D, Shammi CM, Mizrahi R, Marcon H, Barsoum P, et al. The effect of antipsychotics on the high-affinity state of D2 and D3 receptors: a positron emission tomography study With [11C]-(+)-PHNO. Archives of General Psychiatry. 2009a;66:606–615. doi: 10.1001/archgenpsychiatry.2009.43. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Mizrahi R, Agid O, Marcon H, Barsoum P, Rusjan P, et al. The dopamine D2 receptors in high-affinity state and D3 receptors in schizophrenia: a clinical [11C]-(+)-PHNO PET study. Neuropsychopharmacology. 2009b;34:1078–1086. doi: 10.1038/npp.2008.199. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Redden L, Abi-Saab W, Katz DA, Houle S, Barsoum P, et al. Blockade of [11C](+)-PHNO binding in human subjects by the dopamine D3 receptor antagonist ABT-925. International Journal of Neuropsychopharmacology. 2010;13:273–287. doi: 10.1017/S1461145709990642. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, et al. Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Human Brain Mapping. 2008;29:400–410. doi: 10.1002/hbm.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, et al. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. Journal of Medicinal Chemistry. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, et al. Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. Journal of Medicinal Chemistry. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20:60–80. doi: 10.1016/S0893-133X(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Hann MM, Keseru GM. Finding the sweet spot: the role of nature and nurture in medicinal chemistry. Nature Reviews: Drug Discovery. 2012;11:355–365. doi: 10.1038/nrd3701. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, et al. The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Research Brain Research Reviews. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Annals of the New York Academy of Sciences. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Kiefer SW, Li X, Gaal J, Xi ZX, Gardner EL. Dopamine D(3) receptor antagonist SB277011A inhibits methamphetamine self-administration and methamphetamine-induced reinstatement of drug-seeking in rats. European Journal of Pharmacology. 2011a;659:187–192. doi: 10.1016/j.ejphar.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi ZX, et al. PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. Journal of Psychopharmacology. 2011b;25:263–273. doi: 10.1177/0269881109358201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AP, Young RJ. Getting physical in drug discovery: a contemporary perspective on solubility and hydrophobicity. Drug Discovery Today. 2010;15:648–655. doi: 10.1016/j.drudis.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Hobrath JV, Wang S. Computational elucidation of the structural basis of ligand binding to the dopamine 3 receptor through docking and homology modeling. Journal of Medicinal Chemistry. 2006;49:4470–4476. doi: 10.1021/jm0501634. [DOI] [PubMed] [Google Scholar]
- Horn AS, Hazelhoff B, Dijkstra D, de Vries JB, Mulder TB, Timmermans P, et al. The hydroxy-hexanydronaphthoxazines: a new group of very potent and selective dopamine agonists. Journal of Pharmacy and Pharmacology. 1984;36:639–640. doi: 10.1111/j.2042-7158.1984.tb04918.x. [DOI] [PubMed] [Google Scholar]
- Jean M, Renault J, Levoin N, Danvy D, Calmels T, Berrebi-Bertrand I, et al. Synthesis and evaluation of amides surrogates of dopamine D3 receptor ligands. Bioorganic and Medicinal Chemistry Letters. 2010;20:5376–5379. doi: 10.1016/j.bmcl.2010.07.096. [DOI] [PubMed] [Google Scholar]
- Johnson M, Antonio T, Reith ME, Dutta AK. Structure-activity relationship study of N(6)-(2-(4-(1H-Indol-5-yl)piperazin-1-yl)ethyl)-N(6)-propyl-4,5,6,7-tetrahydroben zo[d]thiazole-2,6-diamine analogues: development of highly selective D3 dopamine receptor agonists along with a highly potent D2/D3 agonist and their pharmacological characterization. Journal of Medicinal Chemistry. 2012;55:5826–5840. doi: 10.1021/jm300268s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen WL. Efficient drug lead discovery and optimization. Accounts of Chemical Research. 2009;42:724–733. doi: 10.1021/ar800236t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JN, Millan MJ. Dopamine D3 receptor antagonists as therapeutic agents. Drug Discovery Today. 2005;10:917–925. doi: 10.1016/S1359-6446(05)03491-4. [DOI] [PubMed] [Google Scholar]
- Khaled MA, Farid Araki K, Li B, Coen KM, Marinelli PW, Varga J, et al. The selective dopamine D3 receptor antagonist SB 277011-A, but not the partial agonist BP 897, blocks cue-induced reinstatement of nicotine-seeking. International Journal of Neuropsychopharmacology. 2010;13:181–190. doi: 10.1017/S1461145709991064. [DOI] [PubMed] [Google Scholar]
- Koller W, Herbster G, Gordon J. PHNO, a novel dopamine agonist, in animal models of parkinsonism. Movement Disorders. 1987;2:193–199. doi: 10.1002/mds.870020306. [DOI] [PubMed] [Google Scholar]
- Kufareva I, Rueda M, Katritch V, Stevens RC, Abagyan R, participants GD. Status of GPCR modeling and docking as reflected by community-wide GPCR Dock 2010 assessment. Structure. 2011;19:1108–1126. doi: 10.1016/j.str.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Boileau I. Repurposing buspirone for drug addiction treatment. International Journal of Neuropsychopharmacology. 2013;16:251–253. doi: 10.1017/S1461145712000995. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. Increased dopamine D3 receptor expression accompanying behavioral sensitization to nicotine in rats. Synapse. 2003;47:176–183. doi: 10.1002/syn.10170. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacological Reviews. 1997;49:231–252. [PubMed] [Google Scholar]
- Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, et al. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levoin N, Calmels T, Krief S, Danvy D, Berrebi-Bertrand I, Lecornte JM, et al. Homology Model Versus X-ray Structure in Receptor-based Drug Design: A Retrospective Analysis with the Dopamine D3 Receptor. ACS Medicinal Chemistry Letters. 2011;2:293–297. doi: 10.1021/ml100288q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Zheng X, Chen J, Li Y, Xing X, Bai Y, et al. Roles of BDNF, dopamine D(3) receptors, and their interactions in the expression of morphine-induced context-specific locomotor sensitization. European Neuropsychopharmacology. 2011;21:825–834. doi: 10.1016/j.euroneuro.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. Journal of Pharmacological and Toxicological Methods. 2000;44:235–249. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Liu J, Li Y, Zhang S, Xiao Z, Ai C. Studies of New Fused Benzazepine as Selective Dopamine D3 Receptor Antagonists Using 3D-QSAR, Molecular Docking and Molecular Dynamics. International Journal of Molecular Sciences. 2011;12:1196–1221. doi: 10.3390/ijms12021196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez L, Selent J, Ortega R, Masaguer CF, Dominguez E, Areias F, et al. Synthesis, 3D-QSAR, and structural modeling of benzolactam derivatives with binding affinity for the D(2) and D(3) receptors. ChemMedChem. 2010;5:1300–1317. doi: 10.1002/cmdc.201000101. [DOI] [PubMed] [Google Scholar]
- Macarron R, Banks MN, Bojanic D, Burns DJ, Cirovic DA, Garyantes T, et al. Impact of high-throughput screening in biomedical research. Nature Reviews: Drug Discovery. 2011;10:188–195. doi: 10.1038/nrd3368. [DOI] [PubMed] [Google Scholar]
- Maggio R, Aloisi G, Silvano E, Rossi M, Millan MJ. Heterodimerization of dopamine receptors: new insights into functional and therapeutic significance. Parkinsonism & Related Disorders. 2009;15(Suppl 4):S2–7. doi: 10.1016/S1353-8020(09)70826-0. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Ferre S, Casado V, Cortes A, Le Foll B, Mazzola C, et al. Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. Journal of Biological Chemistry. 2008;283:26016–26025. doi: 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GE, Williams M, Pettibone DJ, Zrada MM, Lotti VJ, Taylor DA, et al. Selectivity of (+)-4-propyl-9-hydroxynaphthoxazine [(+)-PHNO] for dopamine receptors in vitro and in vivo. Journal of Pharmacology and Experimental Therapeutics. 1985;233:395–401. [PubMed] [Google Scholar]
- Mash DC, Staley JK. D3 dopamine and kappa opioid receptor alterations in human brain of cocaine-overdose victims. Annals of the New York Academy of Sciences. 1999;877:507–522. doi: 10.1111/j.1749-6632.1999.tb09286.x. [DOI] [PubMed] [Google Scholar]
- Mason CW, Hassan HE, Kim KP, Cao J, Eddington ND, Newman AH, et al. Characterization of the transport, metabolism, and pharmacokinetics of the dopamine D3 receptor-selective fluorenyl- and 2-pyridylphenyl amides developed for treatment of psychostimulant abuse. Journal of Pharmacology and Experimental Therapeutics. 2010;333:854–864. doi: 10.1124/jpet.109.165084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Fivel PA, Kohut SJ. Effects of chronic buspirone treatment on nicotine and concurrent nicotine+cocaine self-administration. Neuropsychopharmacology. 2013a;38:1264–1275. doi: 10.1038/npp.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Fivel PA, Kohut SJ, Bergman J. Effects of chronic buspirone treatment on cocaine self-administration. Neuropsychopharmacology. 2013b;38:455–467. doi: 10.1038/npp.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli F. Recent advances in the development of dopamine D3 receptor antagonists: a medicinal chemistry perspective. ChemMedChem. 2011;6:1152–1162. doi: 10.1002/cmdc.201000538. [DOI] [PubMed] [Google Scholar]
- Micheli F, Arista L, Bertani B, Braggio S, Capelli AM, Cremonesi S, et al. Exploration of the amine terminus in a novel series of 1,2,4-triazolo-3-yl-azabicyclo[3.1.0]hexanes as selective dopamine D3 receptor antagonists. Journal of Medicinal Chemistry. 2010a;53:7129–7139. doi: 10.1021/jm100832d. [DOI] [PubMed] [Google Scholar]
- Micheli F, Arista L, Bonanomi G, Blaney FE, Braggio S, Capelli AM, et al. 1,2,4-Triazolyl azabicyclo[3.1.0]hexanes: a new series of potent and selective dopamine D(3) receptor antagonists. Journal of Medicinal Chemistry. 2010b;53:374–391. doi: 10.1021/jm901319p. [DOI] [PubMed] [Google Scholar]
- Micheli F, Bonanomi G, Blaney FE, Braggio S, Capelli AM, Checchia A, et al. 1,2,4-triazol-3-yl-thiopropyl-tetrahydrobenzazepines: a series of potent and selective dopamine D(3) receptor antagonists. Journal of Medicinal Chemistry. 2007;50:5076–5089. doi: 10.1021/jm0705612. [DOI] [PubMed] [Google Scholar]
- Micheli F, Heidbreder C. Dopamine D3 receptor antagonists: a patent review (2007 - 2012) Expert Opinion on Therapeutic Patents. 2013;23:363–381. doi: 10.1517/13543776.2013.757593. [DOI] [PubMed] [Google Scholar]
- Michino M, Donthamsetti P, Beuming T, Banala AK, Duan L, Roux T, et al. A single glycine in extracellular loop 1 interconverts the pharmacological specificity of dopamine D2 and D3 receptors. Molecular Pharmacology, submitted. 2013 doi: 10.1124/mol.113.087833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Gobert A, Newman-Tancredi A, Lejeune F, Cussac D, Rivet JM, et al. S33084, a novel, potent, selective, and competitive antagonist at dopamine D(3)-receptors: I. Receptorial, electrophysiological and neurochemical profile compared with GR218,231 and L741,626. Journal of Pharmacology and Experimental Therapeutics. 2000;293:1048–1062. [PubMed] [Google Scholar]
- Millan MJ, Loiseau F, Dekeyne A, Gobert A, Flik G, Cremers TI, et al. S33138 (N-[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1] benzopyrano[3,4-c]pyrrol-2(3H)-yl)-ethyl]phenyl-acetamide), a preferential dopamine D3 versus D2 receptor antagonist and potential antipsychotic agent: III. Actions in models of therapeutic activity and induction of side effects. Journal of Pharmacology and Experimental Therapeutics. 2008a;324:1212–1226. doi: 10.1124/jpet.107.134536. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Mannoury la Cour C. Novi F, Maggio R, Audinot V, Newman-Tancredi A, et al. S33138 [N-[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1]-benzopyrano[3,4-c]pyrrol-2(3H )-yl)-ethyl]phenylacetamide], a preferential dopamine D3 versus D2 receptor antagonist and potential antipsychotic agent: I. Receptor-binding profile and functional actions at G-protein-coupled receptors. Journal of Pharmacology and Experimental Therapeutics. 2008b;324:587–599. doi: 10.1124/jpet.107.126706. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Peglion JL, Vian J, Rivet JM, Brocco M, Gobert A, et al. Functional correlates of dopamine D3 receptor activation in the rat in vivo and their modulation by the selective antagonist, (+)-S 14297: 1. Activation of postsynaptic D3 receptors mediates hypothermia, whereas blockade of D2 receptors elicits prolactin secretion and catalepsy. Journal of Pharmacology and Experimental Therapeutics. 1995;275:885–898. [PubMed] [Google Scholar]
- Millan MJ, Svenningsson P, Ashby CR, Jr., Hill M, Egeland M, Dekeyne A, et al. S33138 [N-[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1]-benzopyrano[3,4-c]pyrrol-2(3H )-yl)-ethyl]phenylacetamide], a preferential dopamine D3 versus D2 receptor antagonist and potential antipsychotic agent. II. A neurochemical, electrophysiological and behavioral characterization in vivo. Journal of Pharmacology and Experimental Therapeutics. 2008c;324:600–611. doi: 10.1124/jpet.107.132563. [DOI] [PubMed] [Google Scholar]
- Mugnaini M, Iavarone L, Cavallini P, Griffante C, Oliosi B, Savoia C, et al. Occupancy of brain dopamine D3 receptors and drug craving: a translational approach. Neuropsychopharmacology. 2013;38:302–312. doi: 10.1038/npp.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, et al. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006;60:485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- Newman AH, Beuming T, Banala AK, Donthamsetti P, Pongetti K, LaBounty A, et al. Molecular determinants of selectivity and efficacy at the dopamine D3 receptor. Journal of Medicinal Chemistry. 2012a;55:6689–6699. doi: 10.1021/jm300482h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Blaylock BL, Nader MA, Bergman J, Sibley DR, Skolnick P. Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochemical Pharmacology. 2012b;84:882–890. doi: 10.1016/j.bcp.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Cao J, Bennett CJ, Robarge MJ, Freeman RA, Luedtke RR. N-(4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl, butenyl and butynyl)arylcarboxamides as novel dopamine D(3) receptor antagonists. Bioorganic and Medicinal Chemistry Letters. 2003;13:2179–2183. doi: 10.1016/s0960-894x(03)00389-5. [DOI] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Cyriac G, Deschamps JR, Taylor M, Kumar R, et al. N-(4-(4-(2,3-dichloro- or 2-methoxyphenyl)piperazin-1-yl)butyl)heterobiarylcarboxamides with functionalized linking chains as high affinity and enantioselective D3 receptor antagonists. Journal of Medicinal Chemistry. 2009;52:2559–2570. doi: 10.1021/jm900095y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. Journal of Medicinal Chemistry. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacology & Therapeutics. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Orio L, Wee S, Newman AH, Pulvirenti L, Koob GF. The dopamine D3 receptor partial agonist CJB090 and antagonist PG01037 decrease progressive ratio responding for methamphetamine in rats with extended-access. Addiction Biology. 2010;15:312–323. doi: 10.1111/j.1369-1600.2010.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng XQ, Ashby CR, Jr., Spiller K, Li X, Li J, Thomasson N, et al. The preferential dopamine D3 receptor antagonist S33138 inhibits cocaine reward and cocaine-triggered relapse to drug-seeking behavior in rats. Neuropharmacology. 2009;56:752–760. doi: 10.1016/j.neuropharm.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, et al. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, et al. Pharmacological actions of a novel, high-affinity, and selective human dopamine D(3) receptor antagonist, SB-277011-A. Journal of Pharmacology and Experimental Therapeutics. 2000;294:1154–1165. [PubMed] [Google Scholar]
- Rice OV, Gardner EL, Heidbreder CA, Ashby CR., Jr. The acute administration of the selective dopamine D(3) receptor antagonist SB277011A reverses conditioned place aversion produced by naloxone precipitated withdrawal from acute morphine administration in rats. Synapse. 2012a;66:85–87. doi: 10.1002/syn.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice OV, Heidbreder CA, Gardner EL, Schonhar CD, Ashby CR., Jr. The selective D3 receptor antagonist SB277011A attenuates morphine-triggered reactivation of expression of cocaine-induced conditioned place preference. Synapse. 2013;67:469–475. doi: 10.1002/syn.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice OV, Patrick J, Schonhar CD, Ning H, Ashby CR., Jr. The effects of the preferential dopamine D(3) receptor antagonist S33138 on ethanol binge drinking in C57BL/6J mice. Synapse. 2012b;66:975–978. doi: 10.1002/syn.21575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of Neuroscience Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Román V, Gyertyan I, Saghy K, Kiss B, Szombathelyi Z. Cariprazine (RGH-188), a D(3)-preferring dopamine D(3)/D(2) receptor partial agonist antipsychotic candidate demonstrates anti-abuse potential in rats. Psychopharmacology. 2013;226:285–293. doi: 10.1007/s00213-012-2906-7. [DOI] [PubMed] [Google Scholar]
- Rose FC, Nashef L. Parkinson's disease: further steps forward. Gerontology. 1987;33:369–373. doi: 10.1159/000212905. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama I, Hocke C, Utz W, Prante O, Boeckler F, Hubner H, et al. Structure-selectivity investigations of D2-like receptor ligands by CoMFA and CoMSIA guiding the discovery of D3 selective PET radioligands. Journal of Medicinal Chemistry. 2007;50:489–500. doi: 10.1021/jm0611152. [DOI] [PubMed] [Google Scholar]
- Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, et al. Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biological Psychiatry. 2010;68:392–399. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Segal DM, Moraes CT, Mash DC. Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Research Molecular Brain Research. 1997;45:335–339. doi: 10.1016/s0169-328x(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Hendrick ES, Beardsley PM. Efficacy of buspirone for attenuating cocaine and methamphetamine reinstatement in rats. Drug and Alcohol Dependence. 2013;129:210–216. doi: 10.1016/j.drugalcdep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Javitch JA. The binding site of aminergic G protein-coupled receptors: the transmembrane segments and second extracellular loop. Annual Review of Pharmacology and Toxicology. 2002;42:437–467. doi: 10.1146/annurev.pharmtox.42.091101.144224. [DOI] [PubMed] [Google Scholar]
- Shi L, Liapakis G, Xu R, Guarnieri F, Ballesteros JA, Javitch JA. Beta2 adrenergic receptor activation. Modulation of the proline kink in transmembrane 6 by a rotamer toggle switch. Journal of Biological Chemistry. 2002;277:40989–40996. doi: 10.1074/jbc.M206801200. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS & Neurological Disorders Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, et al. YQA14: a novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice. Addiction Biology. 2012;17:259–273. doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Zhang HY, Peng XQ, Su RB, Yang RF, Li J, et al. Dopamine D3 receptor deletion or blockade attenuates cocaine-induced conditioned place preference in mice. Neuropharmacology. 2013;72:82–87. doi: 10.1016/j.neuropharm.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Peng XQ, Newman AH, Ashby CR, Jr., Heidbreder C, et al. The selective dopamine D3 receptor antagonists SB277011A and NGB 2904 and the putative partial D3 receptor agonist BP-897 attenuate methamphetamine-enhanced brain stimulation reward in rats. Psychopharmacology. 2008;196:533–542. doi: 10.1007/s00213-007-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. Journal of Neuroscience. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemp G, Ashmeade T, Branch CL, Hadley MS, Hunter AJ, Johnson CN, et al. Design and synthesis of trans-N-[4-[2-(6-cyano-1,2,3, 4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolinecarboxamide (SB-277011): A potent and selective dopamine D(3) receptor antagonist with high oral bioavailability and CNS penetration in the rat. Journal of Medicinal Chemistry. 2000;43:1878–1885. doi: 10.1021/jm000090i. [DOI] [PubMed] [Google Scholar]
- Tschammer N, Elsner J, Goetz A, Ehrlich K, Schuster S, Ruberg M, et al. Highly potent 5-aminotetrahydropyrazolopyridines: enantioselective dopamine D3 receptor binding, functional selectivity, and analysis of receptor-ligand interactions. Journal of Medicinal Chemistry. 2011;54:2477–2491. doi: 10.1021/jm101639t. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction Biology. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Varady J, Wu X, Fang X, Min J, Hu Z, Levant B, et al. Molecular modeling of the three-dimensional structure of dopamine 3 (D3) subtype receptor: discovery of novel and potent D3 ligands through a hybrid pharmacophore- and structure-based database searching approach. Journal of Medicinal Chemistry. 2003;46:4377–4392. doi: 10.1021/jm030085p. [DOI] [PubMed] [Google Scholar]
- Wager TT, Hou X, Verhoest PR, Villalobos A. Moving beyond rules: the development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. ACS Chemical Neuroscience. 2010;1:435–449. doi: 10.1021/cn100008c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Mach RH, Luedtke RR, Reichert DE. Subtype selectivity of dopamine receptor ligands: insights from structure and ligand-based methods. Journal of Chemical Information and Modeling. 2010;50:1970–1985. doi: 10.1021/ci1002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne T, Moukhametzianov R, Baker JG, Nehme R, Edwards PC, Leslie AG, et al. The structural basis for agonist and partial agonist action on a beta(1)-adrenergic receptor. Nature. 2011;469:241–244. doi: 10.1038/nature09746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S, et al. First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: A [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology. 2008;33:279–289. doi: 10.1038/sj.npp.1301400. [DOI] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, et al. High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biological Psychiatry. 2006;59:389–394. doi: 10.1016/j.biopsych.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Reviews. 2007;13:240–259. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Li X, Li J, Peng XQ, Song R, Gaal J, et al. Blockade of dopamine D3 receptors in the nucleus accumbens and central amygdala inhibits incubation of cocaine craving in rats. Addiction Biology. 2013;18:665–677. doi: 10.1111/j.1369-1600.2012.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Kong H, Wu EJ, Newman AH, Xu M. Dopamine D3 receptors regulate reconsolidation of cocaine memory. Neuroscience. 2013;241:32–40. doi: 10.1016/j.neuroscience.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye N, Neumeyer JL, Baldessarini RJ, Zhen X, Zhang A. Update 1 of: Recent progress in development of dopamine receptor subtype-selective agents: potential therapeutics for neurological and psychiatric disorders. Chemical Reviews. 2013;113:PR123–178. doi: 10.1021/cr300113a. [DOI] [PubMed] [Google Scholar]
- Yuan J, Chen X, Brodbeck R, Primus R, Braun J, Wasley JW, et al. NGB 2904 and NGB 2849: two highly selective dopamine D3 receptor antagonists. Bioorganic and Medicinal Chemistry Letters. 1998;8:2715–2718. doi: 10.1016/s0960-894x(98)00469-7. [DOI] [PubMed] [Google Scholar]