Abstract
Epithelial stem cells are regulated through a complex interplay of signals from diffusible ligands, cellular interactions, and attachment to the extracellular matrix. The development of Drosophila models of epithelial stem cells and their associated niche has made it possible to dissect the contribution of each of these factors in vivo, during both basal homeostasis and in response to acute damage such as infection. Studies of Drosophila epithelial stem cells have also provided insight into the mechanisms by which a healthy population of stem cells are maintained throughout adulthood by demonstrating, for example, that stem cells have a finite lifespan and may be displaced by replacement cells competing for niche occupancy. Here, we summarize the literature on each of the known Drosophila epithelial stem cells, with a focus on the two most well characterized types, the follicle stem cells (FSCs) in the ovary and the intestinal stem cells (ISCs) in the posterior midgut. Several themes have emerged from these studies, which suggest that there may be a common set of features among niches in a variety of epithelia. For example, unlike the simpler Drosophila germline stem cell niches, both the FSC and ISC niches produce multiple, partially redundant, niche signals, some of which activate pathways such as Wnt/Wingless, Hedgehog, and EGF that also regulate mammalian epithelial tissue renewal. Further study into these relatively new stem cell models will be of use in understanding both the specifics of epithelial regeneration and the diversity of mechanisms that regulate adult stem cells in general.
Introduction
Epithelial stem cells are central to the physiology of regenerative epithelia such as the intestinal lining, epidermis, cornea, and breast. Stem cells in these tissues divide regularly to self-renew and replenish lost cells following normal cell turn over or tissue damage, and are thus crucial for homeostasis and repair. In addition, as the most upstream progenitors of cells in the tissue, epithelial stem cells are a natural point of regulation and an ideal target for regenerative medicine-based therapies. Moreover, defects in stem cell function and regulation underlie many aspects of aging and disease. Therefore, understanding how epithelial stem cells behave in their natural environment will provide insight into the fundamental mechanisms that guide epithelial tissue biology and medicine.
The development of invertebrate models has provided an opportunity to study the behavior and genetic regulation of epithelial stem cells in great detail. Studies of these systems have provided a range of insights, including the identification of signaling networks that promote self-renewal and coordinate stem cell output with tissue demand, the discovery of cellular interactions that ensure that a healthy population of stem cells are maintained throughout life, and descriptions of the structure and function of the epithelial stem cell niche. Here, we review these findings with a focus on the two most well-studied models, the Drosophila follicle stem cells (FSCs) and intestinal stem cells (ISCs).
Studying stem cells in vivo in Drosophila
Adult stem cells reside in distinct microenvironments, or niches, that promote their self-renewal and regulate their activity.1,2 The Drosophila male and female germline stem cell niches have been characterized in detail, and these studies found that the niche is both necessary for maintaining the stem cell fate of resident stem cells, and sufficient for inducing the stem cell fate in non-stem cells that enter the niche.3,4 This highlights the exquisite sensitivity of stem cells to changes in their local environment and thus the importance of studying stem cells in their native, in vivo, context. In Drosophila, the relatively simple tissue architecture and the high ratio of stem cells to surrounding non-stem cells, combined with the wide variety of genetic tools available, greatly facilitate the process of identifying and genetically manipulating stem cells in vivo.
The gold standard for identifying stem cells in vivo is lineage analysis,1 in which a stem cell lineage is labeled with a genetically heritable marker and the patterns of labeled cells are followed over time. In Drosophila, lineage analysis techniques typically use flp/FRT mediated mitotic recombination to induce expression of a marker such as GFP or LacZ in one half of a dividing cell’s lineage (reviewed in5). In addition, recently developed variations allow both halves of a dividing cell’s lineage to be traced with different markers or differential labeling of subpopulations within the lineage.6–9 A variant of lineage analysis, called mosaic analysis, is used to genetically modify the cells within a marked clone. Several systems for mosaic analysis are available for different purposes, typically either causing cells within the clone to become homozygous for an allele of interest or to overexpress a transgene. For both lineage analysis and mosaic analysis, spatial and temporal control over clone induction is achieved using tissue-specific or inducible promoters combined, in some cases, with the UAS/Gal4/Gal80 expression system common to Drosophila studies.5
Follicle Stem Cells
Follicle stem cells (previously called somatic stem cells) are the progenitors of the epithelia that surround each developing follicle in the Drosophila ovary (see10 for review of oogenesis). Drosophila ovaries are composed of discrete substructures called ovarioles, and follicles are continually produced during adulthood from a specialized structure at the tip of each ovariole called the germarium. The process begins with the production of a new germ cell by a germline stem cell at the anterior tip of the germarium. Germ cells undergo four incomplete mitoses to become interconnected 16-cell cysts as they migrate posteriorly through a meshwork of stromal “escort cells.”11,12 When the cysts reach the follicle stem cells at the mid-point of the germarium, they go through several transitions within a short period of time that must be tightly coordinated for proper follicle production. First, the germ cells in a 16-cell cyst exit mitosis and the oocyte enters meiosis. The remaining 15 germ cells will become polyploid nurse cells that support oocyte development and provide organelles and other cytoplasmic products to the egg. Following the initiation of meiosis, the cysts widen and move into a single file, shed their escort cell covering, and become encapsulated by undifferentiated “pre-follicle” cells. As the cysts continue to move toward the posterior, the pre-follicle cells divide and differentiate gradually into a single-layered, polarized epithelium. Little is known about the mechanisms that orchestrate this series of events, but it is very likely that regulation of the FSCs and their niches is an important part of the process.
There are exactly two actively dividing FSCs per germarium in wildtype ovaries6,13 and they can be unambiguously identified using a combination of criteria. First, FSCs are always one of the anterior-most labeled cells in a mature FSC clone.13 Second, FSCs are consistently found in the same position: one FSC is on each side of the germarium, in contact with the basement membrane, and just posterior to the region 2a cyst at the boundary between region 2a and region 2b.6 Third, FSCs have a unique triangular shape with a broad basal surface, and a lateral surface that tapers toward the apical side of the cell. In ideal cases, shape and position can reliably distinguish FSCs from nearby escort cells, which have chevron-shaped nuclei, and pre-follicle cells in region 2b, which have broader apical domains (Fig. 1).6 However, differences in shape can be subtle and are often not sufficient to distinguish FSCs from pre-follicle cells immediately adjacent to the FSC niche. Lastly, FSCs usually have lower levels of Fasciclin III than pre-follicle cells in region 2b;14 however this criterion is also unreliable on its own because expression levels in FSCs and pre-follicle cells can vary. Moreover, the levels of Fasciclin III expression in pre-follicle cells at the region 2a/region 2b boundary and FSCs are indistinguishable.15 Thus, combining three criteria—anterior-most position within an FSC clone, location at the region 2a/region 2b boundary in contact with the basement membrane, and triangular cell shape—provides the most accurate method for identification of FSCs.
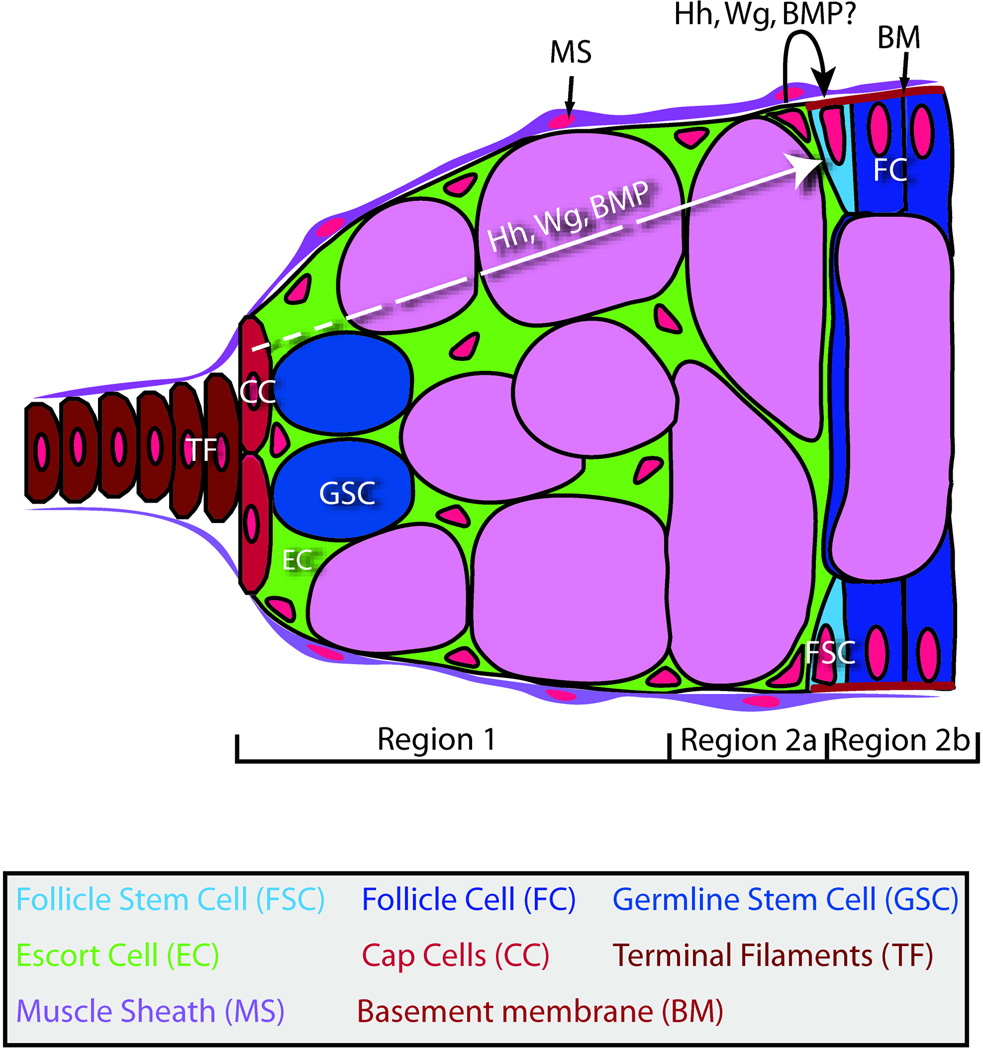
Figure 1. The FSC niche.
FSCs (light blue) reside at the region 2a/region 2b border against the basement membrane (red) and in direct contact with escort cells (green) and their immediate daughters (dark blue). It is unclear whether FSCs ever contact germ cells (pink). The germarium is surrounded by a muscle sheath (purple) that has no known role in FSC niche function. Hedgehog (Hh), Wingless (Wg), and BMP signalling are all required for FSC maintenance and the signaling ligands are produced by either the cap cells (dark pink), the escort cells, or both. It is not known whether signals from the germline or follicle cells promote FSC maintenance or regulate its activity.
The FSC lineage consists of eight transient divisions downstream of the FSC division, and encompasses all follicle cells, including subtypes such as the stalk and polar cells, but not escort cells or any other cell type in the ovary.13 Each FSC produces approximately 50% of the follicle cells in the ovariole, indicating that both FSCs are equally active and, for the majority of follicles in a wildtype ovariole, the follicle cells are derived almost exclusively from a single daughter cell from each FSC.15 FSC daughter cells move away from the niche either towards the posterior into region 2b, or laterally along the region 2a/2b border toward the opposite FSC niche. An individual FSC produces both posterior-migrating cells (pmcs) and lateral cross-migrating cells (cmcs) in roughly alternating succession6 and the two FSCs are usually out of phase with each other so that one FSC daughter cell of each type colonizes each incoming cyst. This causes cells within the two lineages to acquire different fates and presumably helps to distribute the cells across the entire surface of the cyst.15 These observations suggest that FSC output is generally coordinated both between the two FSC niches and with demand from the germline. However, deviations from the standard patterns of pre-follicle cell migration are somewhat common, indicating that the cyst encapsulation process is tolerant of perturbations.15
FSC maintenance, self-renewal, and competition
Although FSCs are usually long-lived, they are occasionally lost from the niche and replaced by a daughter of the remaining stem cell.13 The rate of interniche replacement can be precisely measured by generating mitotic clones in adult ovaries at low frequency, so that most germaria have no more than one FSC labeled, and measuring the changes in the frequency of labeled FSCs. Interniche replacement causes heterogeneous germaria, which start out with one labeled FSC and one unlabeled FSC, to become homogeneous, so the decrease in the frequency of heterogeneous germaria over time can be related to stem cell half-life.5 Using this method, FSCs in wildtype tissue have an observable half-life of approximately 12 days.6 Notably, FSCs may also sometimes be replaced by their own genetically identical daughter cell, but these events are not detectable with current lineage tracing methods.
Elucidating the mechanism of FSC maintenance is essential for understanding the workings of the FSC niche. A study of stem cell replacement in wildtype tissue found that cmcs regularly come into contact with the FSC on the opposite side of the germarium and are thus the most likely source of new stem cells following FSC loss. In addition, cmcs appear to be capable of displacing a stem cell from its niche suggesting that cmcs compete with resident stem cells for niche occupancy.6 Thus, while most cmcs complete their lateral migration and incorporate into the growing epithelium, cmcs may also participate in stem cell replacement by occupying vacated niches, and by competing with FSCs in occupied niches, displacing those that are less fit. Several genetic mosaic studies, summarized below, have provided substantial insight into the mechanism of FSC maintenance by identifying mutations that reduce the half-life of the mutant stem cell.
Hedgehog signaling
The Hedgehog (Hh) ligand is produced by the germline stem cell niche cells and escort cells16,17 and diffuses to the FSC niche. Hh signaling has several functions in FSCs and pre-follicle cells. First, Hh signaling is required for FSC maintenance, as indicated by the observation that FSCs lacking smoothened, a positive regulator of Hh signaling, have a reduced half-life.18–20 Second, Hh regulates proliferation of cells in the early part of the FSC lineage. This was first revealed by the observation that global overexpression of Hh from a heat shock promoter results in excess pre-follicle cells in the niche region whereas global reduction of Hh in hhts flies shifted to the non-permissive temperature causes an underproduction of follicle cells.16 Subsequent studies found that these follicle cell over- and under-production phenotypes could be recapitulated in FSC clones lacking patched (a negative regulator of Hh signaling) or smoothened, respectively.18 However, it remains unclear whether Hh regulates proliferation of FSCs, early pre-follicle cells, or both. Third, Hh signaling may promote competence to acquire the stalk cell fate in early pre-follicle cells because Hh overexpression causes expanded stalks between follicles outside the germarium.16
Wingless signaling
Like Hh, Wingless (Wg) signaling regulates both stem cell maintenance and proliferation in the FSC lineage.21 FSCs that lack a positive regulator of Wg signaling, either disheveled or armadillo, produce fewer follicle cells, indicating a proliferation defect in FSCs, their daughters, or both, and mutant FSCs are very rapidly lost from the niche. In contrast, removal of a negative regulator of Wg signaling, either axin or shaggy, from FSCs causes overproliferation of cells in the FSC lineage but, unexpectedly, mutant FSCs are also prematurely lost. This may indicate that FSC self-renewal requires a precise intermediate level of Wg signaling. Alternately, reduced Wg signaling may cause premature FSC loss by one mechanism, such as reduced affinity for the niche or impaired self-renewal, while overactive Wg signaling causes premature loss by another mechanism, such as reduced competition for niche occupancy.
Bone morphogenic pathway signaling
The BMP pathway is essential for FSC maintenance, but does not seem to regulate proliferation or differentiation in the FSC lineage.19 FSCs mutant for one of the BMP pathway receptors punt, thickveins, or saxophone, or for a positive pathway regulator, either mothers against dpp, or Medea, cannot transduce a BMP signal and are prematurely lost from the niche. In contrast, FSCs overexpressing a constitutively activated form of thickveins have a prolonged lifespan. Overexpression of activated thickveins also rescues the shortened lifespan of disheveled−/− FSCs, restoring it to wildtype levels, but does not rescue the shortened lifespan of smoothened−/− FSCs, suggesting that BMP signaling may act in conjunction with Wg signaling but in parallel to Hh signaling.
Interestingly, the loss-of-function phenotype in FSCs for Medea differs from those of other BMP pathway components in several ways. First, FSCs mutant for Medea have a significantly shorter lifespan than FSCs mutant for other BMP pathway components. Second, loss of Medea, but not other BMP pathway components, reduces the rate of follicle cell proliferation. Lastly, overexpression of the anti-apoptotic gene, p35, partially rescues the decreased lifespan of Medea−/− FSCs but has no effect on FSCs lacking other BMP pathway components. This suggests that, in addition to acting in the BMP pathway, Medea transduces a second unknown signal that promotes FSC survival and proliferation in the FSC lineage.
Adherens junctions and integrins
Two cell adhesion complexes, adherens junctions and integrins have been shown to be essential for FSC maintenance within the niche. Shotgun (DE-cadherin), and armadillo (β-catenin), which form the core of the adherens junction complex are both present in FSCs and are localized to the plasma membrane.22 Discrete foci of shotgun protein accumulates between FSCs and escort cells, suggesting that FSCs are attached to escort cells through adherens junctions. Like armadillo−/− FSC clones, shotgun−/− FSC clones are lost very rapidly after clone induction, indicating a crucial, potentially non-redundant role for adherens junctions in FSC maintenance. Moreover, in a study of cyclinE (cycE) regulation, FSC clones mutant for cycE and overexpressing shotgun were more frequent than those mutant for cycE only, suggesting that high levels of cell adhesion may be able to compensate for other defects.23
FSCs are also anchored to the basement membrane through integrins, and FSCs mutant for either both α-integrins in the Drosophila genome (multiple edematous wings and inflated), or the β-integrin, myospheroid, have a reduced half-life.24 Likewise, FSCs lacking laminin A, the basement membrane ligand for integrins, also have a reduced half-life, although less so than FSCs lacking integrins, presumably because laminins are deposited extracellularly, so nearby wildtype cells can partially compensate for underproduction by mutant cells in a clone. Adherens junctions and integrins are likely important for FSC maintenance because they anchor FSCs to the niche. However, these cell adhesion complexes may also promote self-renewal through their influence on the shape of the FSC or by helping to deliver niche signals to FSCs.
Epigenetic and microRNA regulation
Differentiation in the FSC lineage is a step-wise process25 in which cells gradually lose their potential to acquire different cell fates. Follicle cells also become more epigenetically rigid with each cell generation,26 suggesting that chromatin modifications help facilitate these developmental transitions. Though little is known about the epigenetic differences between FSCs and their daughters, several chromatin remodeling factors have been found to be essential for normal FSC maintenance in the niche. FSC clones mutant for domino,27 the Drosophila SWR1 homolog, scrawny,28 a ubiquitin-specific protease, or the Polycomb repressors Posterior sex combs and Suppressor of zeste 2,29 all had significantly reduced half-lives, indicating that these genes are required for normal FSC maintenance. FSCs mutant for both Posterior sex combs and Suppressor of zeste 2 also formed ectopic undifferentiated tumors that extruded basally from the germarium, which were partially suppressed by knocking down Wg signaling. Lastly, FSC clones mutant for dicer-1, which is required for processing microRNA, had reduced half-lives and were smaller than their wild type twin spots.30 These results suggest that both chromatin modification and microRNA function regulate FSC maintenance and development in the FSC lineage.
Future directions for the study of FSCs
Taken together, genetic mosaic studies of FSCs and their lineage have demonstrated that a wide range of cellular processes are important for the maintenance of an epithelial stem cell in vivo. It will be interesting to further investigate how each of these cellular processes contribute to FSC maintenance, whether through promoting retention of the FSC in the niche, self-renewal, or competition for niche occupancy. Studies of the germline stem cell niches were greatly facilitated by descriptions of the cellular and sub-cellular differences between the stem cells and their immediate daughters.31 Equivalent studies in the FSC lineage are just beginning, and further characterization will be necessary before some of these distinctions between niche retention, self-renewal, and niche competition can be made.
Much also remains to be learned about the composition and structure of the FSC niche. The genetic mosaic studies discussed above suggest that escort cells anchor the FSCs in the niche and possibly also provide niche signals, but it has been difficult to confirm these hypotheses by genetically manipulating the escort cells alone. Escort cells were once believed to turn over regularly at the region 2a/region 2b border,11 suggesting that the contribution of escort cells to the FSC niche must be provided by multiple transient escort cells acting in aggregate. However, a recent live-imaging study found that, while at least some escort cells are dynamic, capable of migrating and changing shape, cell division and cell death are rare.12 It remains unclear, however, whether all escort cells are equivalent or a specialized subset provides the FSC niche function. Also unclear is whether the escort cells contacting FSCs are static and post-mitotic, like germline stem cell niche cells, or capable of migration and occasional cell division. Further characterization of escort cells using the newly-developed long-term live imaging techniques and mosaic analysis will address these questions. Lastly, very little is known about the contribution, if any, of FSC daughter cells to niche structure and function. Some FSC maintenance genes, such as integrins and E-cadherin, are also required for the integrity of the follicle epithelium and it will be interesting to investigate whether part of the instability of FSC clones lacking these genes is caused by a breakdown of the tissue architecture downstream from the FSC niche.
Intestinal stem cells
Approximately 800–1000 intestinal stem cells (ISCs) are evenly distributed among the 10,000 cells in the posterior midgut epithelium, which extends from the stomach region to the junction of the midgut and hindgut.32 The midgut epithelium is a polarized, pseudostratified monolayer attached to a basement membrane and surrounded on the outside by visceral muscle (Fig. 2). ISCs were originally identified by lineage analysis32,33 and, like FSCs, were found to have a triangular shape with a broad basal surface attached to the basement membrane. Delta expression is a widely-used, reliable marker of the ISC fate, although a small fraction of ISCs in wildtype tissue are Delta(−).34 In addition, as with any marker, Delta may be unreliable in mutant tissues because some mutations could affect Delta expression but not ISC fate, or vice versa.
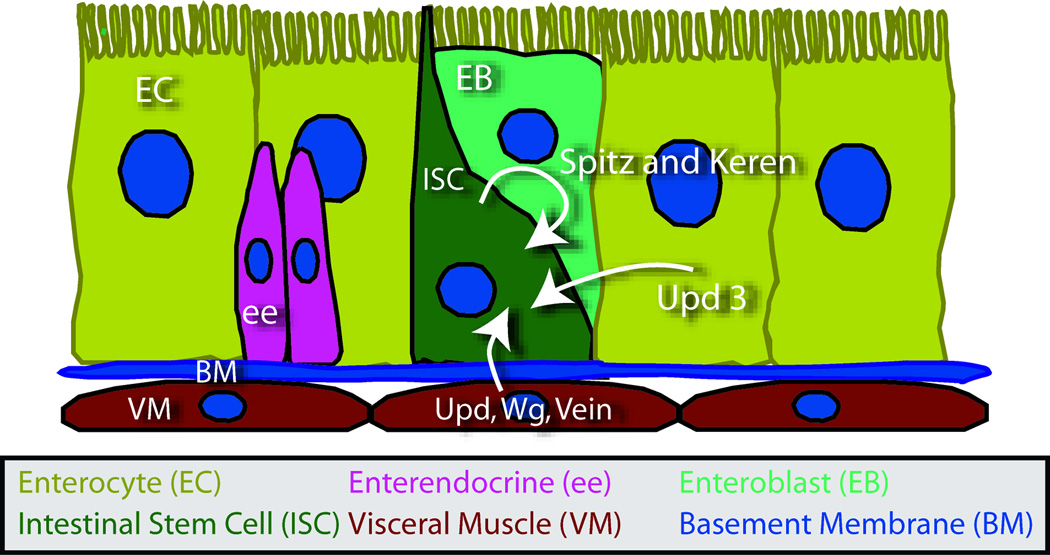
Figure 2. The ISC niche.
The ISCs reside throughout the posterior midgut against the basement membrane (blue) and in direct contact with enteroblasts (light green), enterocytes (yellow) and, occasionally, enteroendocrine cells (pink). The midgut is surrounded by a visceral muscle that is a key part of the ISC niche. Wingless, EGF and JAK/STAT signaling are all required for ISC maintenance and the signaling ligands are produced by several sources. During basal homeostasis, the visceral muscle produces Unpaired, Wingless and Vein, whereas diploid epithelial cells, which are probably ISCs, produce Spitz and Keren, and a few scattered enterocytes produce Unpaired 3. During infection, enterocytes increase production of JAK/STAT ligands, which act on both ISCs and the visceral muscle to increase ISC proliferation.
ISCs divide regularly to produce immature diploid daughter cells, called enteroblasts, that differentiate without further division into either the hormone producing enteroendocrine cells, identified by Prospero expression, or the nutrient-absorbing enterocyte cells, identified by their large, polyploid nuclei and Pdm1 expression.32,33,35 A single ISC is capable of producing both enterocyte precursors and enteroendocrine precursors, although enterocyte precursors are produced much more frequently. Interestingly, enteroendocrine precursors are typically generated in pairs from two consecutive ISC divisions, suggesting that a feedback mechanism exists to regulate enteroendocrine precursor production.34
Genetic regulation of ISCs during homeostasis
Interest in ISCs has grown enormously since they were identified in 2006 and several studies, summarized below, have substantially improved our understanding of ISC maintenance, proliferation and differentiation.
Notch signaling
Notch signaling promotes asymmetric division by inducing differentiation in one of the two daughters of an ISC division. The Notch ligand Delta is segregated to both daughter cells during mitosis but is significantly down-regulated in the non-stem cell daughter by the time cytokinesis completes.34 Both daughter cells express Notch but, because Delta is enriched in the ISC, only the non-stem cell daughter receives enough Notch signal to acquire the enteroblast fate. This asymmetry is reinforced by the Notch repressor, hairless, which attenuates Notch signaling in the ISC.36 Interestingly, ISCs that have recently produced an enterocyte invariably express higher levels of Delta than ISCs that have recently produced an enteroendocrine cell, suggesting that the level of Notch signaling received by the enteroblast at the ISC division determines its ultimate fate.34 This model is supported by clonal analysis studies which found that ISCs overexpressing Delta almost exclusively produce enterocytes, whereas those lacking either Delta or Notch produce tumors that are mosaic for enteroendocrine and ISC-like cells.33,34
Wingless signaling
Wingless, EGF, and JAK/STAT are ISC niche signals that function with partial redundancy to promote ISC proliferation and maintenance.37,38 The Wingless ligand is produced by the surrounding visceral muscle and acts directly on ISCs to promote proliferation and self-renewal.39 ISC clones mutant for positive regulators of Wingless signaling, armadillo or dishevelled, have a reduced half-life, indicating that Wingless signaling is required in ISCs for maintenance in the niche. In addition, ISCs mutant for armadillo or disheveled have a reduced a rate of cell division whereas those mutant for a negative regulator, either shaggy39 or APC35, form tumors, indicating that Wingless regulates ISC proliferation. However, armadillo−/− and disheveled−/− ISCs still produce both enterocytes and enteroendocrine cells, indicating that Wingless signaling is dispensable for differentiation in the ISC lineage.38
JAK/STAT signaling
During basal homeostasis, JAK/STAT ligands are produced by at least two sources. Unpaired is expressed by the visceral muscle37 and Unpaired 3 is expressed by a few scattered enterocytes.40 The JAK/STAT receptor, Domeless, is expressed in the visceral muscle, ISCs, and enteroblasts but not enteroendocrine cells or enterocytes.37 Like Wingless signaling, JAK/STAT signaling is required in ISCs for maintenance, as indicated by the observation that domeless−/− ISCs are lost from the tissue more rapidly than wildtype ISCs37. JAK/STAT also acts in conjunction with wingless to promote basal ISC proliferation. However, unlike Wngless, JAK/STAT is also required for proper enteroblast differentiation.37,40,41 Specifically, high levels of JAK/STAT promote the enteroendocrine fate whereas low levels promote the enterocyte fate.
EGF signaling
EGF ligands come from different sources and have overlapping function. The EGF ligand Vein, is produced by the visceral muscle and diffuses across the basement membrane to the ISCs whereas the EGF ligands Spitz and Keren are produced by basal diploid epithelial cells that, based on their position, are likely to be ISCs.38,42 Like JAK/STAT and Wingless signaling, reducing EGF signaling in ISCs, either by downregulating the production of one or more ligands or by eliminating EGFR or other pathway components from ISCs, causes both reduced proliferation and ISC loss.38,42 EGFR is expressed only in the ISCs and EBs, and ISC clones mutant for Egfr or ras, a positive regulator of EGF signaling, can contain both enteroendocrine cells and enterocytes, indicating that EGF signaling is not essential for differentiation.38
Taken together, these studies indicate that Wingless, JAK/STAT and EGF signaling have partially overlapping roles in promoting ISC maintenance and proliferation during basal homeostasis. The ligands for these signaling pathways come from both the post-mitotic visceral muscle cells as well as the much more dynamic cells of the intestinal epithelium. This suggests that the ISC niche is not a single immutable structure in the tissue but is instead a composite of several different cell types and extracellular structures. Moreover, as described below, both the composition of the niche and the relative importance of each niche component may change during basal homeostasis or in response to damage.
ISC regulation in response to infection and repair
One of the primary functions of the intestinal epithelium is to provide protection against pathogens and other damaging agents ingested by the fly. In Drosophila, commensal yeast and bacteria are managed in healthy intestinal mucosa by a sensing mechanism that modulates reactive oxygen species (ROS) production in enterocytes through regulation of the NADPH oxidase enzyme, Duox.43,44 Enteric infection by gram-negative pathogenic bacteria such as Erwinia and Pseudomonas dramatically upregulate this system, causing a burst of ROS that, in addition to fighting the infection directly, initiates the innate immune response and increases the rate of cell turn over in the ISC lineage.45 The primary effector of the innate immune response in the intestine is the Imd pathway, which activates production of specific antimicrobial peptides,46 whereas the signals that increase cell turn over during infection are the same ISC niche signals that regulate basal homeostasis. Infected enterocytes produce all three unpaired ligands, which act both directly on ISCs to stimulate proliferation40 and indirectly, by stimulating the production of EGF ligands by the muscle,47 which then stimulate ISC proliferation. In addition to upregulation of these niche signals, activation of the JNK pathway, which has little function in basal homeostasis, helps to further amplify the stress signal produced by infection.40,48
While we have focused on the response to infection here, other studies have found that ROS, JNK, and other signals such as PVR also mediate the stress-response to physical damage and aging.49–53 In addition, several recent studies have investigated the mechanism by which the stress response system is coordinated with basal homeostasis so that it can be rapidly activated in response to infection or injury and then downregulated when the stress is removed. The AP-1 transcription factor FOS, which integrates homeostatic EGF signaling with stress-responsive JNK signaling is one point of co-regulation.54 Another layer of regulation is provided by Yorkie, a transcription factor in the Hippo pathway, that is repressed in ISCs during basal homeostasis. Upon infection or injury, damage-response signals such as JNK derepress Yorkie, allowing it to upregulate niche signals such as Unpaired and activate additional genes that promote proliferation and survival.55–58 These studies demonstrate how the complex interplay of damage response and homeostatic signaling provides flexibility for the ISC niche.
Other Drosophila models of epithelial regeneration
Several other Drosophila models have emerged recently that provide an opportunity to further understand the range of possible strategies for epithelial tissue renewal. For example, the hindgut is an attractive model for the study of facultative epithelial stem cells.59 Unlike the ovarian and posterior midgut epithelia, the hindgut epithelium does not turn over regularly during basal homeostasis. However, a small population of cells in a ring at the anterior edge of the tissue are capable of dividing in response to severe tissue damage. Further study of this phenomenon will provide insight into how epithelial progenitors transition between quiescent and proliferative states and how acute damage responses compare to constitutive homeostatic renewal. In addition, recent studies have suggested that both the gastric region of the intestine60 and the Malpighian tubules,61 which provide a kidney-like function, are maintained by stem cells and that daughters are capable of long and complex migrations. Further study of these systems may elucidate additional mechanisms for epithelial regeneration. Lastly, developmental studies have identified mechanisms for the establishment and maintenance of the stem/progenitor cell fate. In the developing tracheal epithelium, differentiated epithelial cells undergo a programmed dedifferentiation to facilitate branching and further growth62 whereas in the developing intestine, a novel type of transient niche forms to preserve the undifferentiated state of the future adult stem cells.63
The mechanism of stem cell replacement
Several different Drosophila stem cell types, including germline stem cells and FSCs, turn over regularly during adulthood.1 Studies of stem cell replacement found that partially labeled stem cell populations tend toward homogeneity over time, even as the overall numbers of stem cells per unit tissue remain unchanged, indicating that stem cells can be lost and replaced by the daughter of a remaining stem cell. In theory, stem cell replacement could be either a stochastic process, in which stem cells are occasionally lost at random from the niche, or it could be genetically dependent, in which stem cell loss is caused by a mutation, most likely arising in either the stem cell or the replacement cell.
While the early studies could not distinguish between these possibilities, subsequent studies identified mutations that caused homozygous mutant stem cells to be lost more rapidly.64 This indicated that stem cell loss could be genetically dependent and suggested that at least some of the stem cell turn over in wildtype tissue was due to spontaneous mutations that altered stem cell half-life. Moreover, several of the mutations that were identified are in genes that control stem cell retention in the niche or self-renewal, suggesting that stem cells bearing these mutations were lost more rapidly because of a cell-autonomous defect. In these cases, it is likely the mutant stem cell would vacate the niche regardless of whether a replacement cell was available to take its place.
However, other replacement events may be non-autonomous, occurring only because a fitter replacement cell is present and outcompetes the resident stem cell for niche occupancy. This form of replacement was first suggested by observations of FSC clonal patterns,6 and was subsequently observed and proven to be genetically dependent in the female65 and male66,67 germline stem cell niches. Thus, it is likely that, in Drosophila niches, stochastic loss as well as genetically dependent autonomous and non-autonomous loss all occur during basal homeostasis and are part of a strategy to preserve the health of the stem cell population. Further studies that compare the half-life of mutant stem cells in wildtype and various mutant contexts will elucidate the relative importance of each of these forms of replacement. Notably, a recent study found that mammalian intestinal stem cells also regularly undergo replacement, and the authors demonstrated that the pattern of replacement in wildtype tissue is consistent with stochastic loss.68 It will be interesting to see whether genetic mosaic studies reveal that replacement in this system can also be genetically dependent.
Conclusion
The tremendous advances provided by the characterization of the male and female germline stem cell niches established the value of Drosophila as a model for studying adult stem cells. The more recent development of epithelial stem cell models demonstrates not only that there is more diversity of niche architecture than was previously appreciated, but also the versatility of Drosophila as a model system for investigating this diversity. Germline stem cells are found in prominent, immobile niches, whereas epithelial stem cells reside among fluid and strikingly uniform cell populations. In addition, differentiation in epithelial lineages often occurs slowly, over the course of several divisions, and thus the differences between stem cell and daughter are very subtle. Yet, despite this uniformity, ISC and FSC divisions still regularly produce asymmetric outcomes. It is currently unclear how stem cell fate is preserved while daughter cell fates diverge in these contexts and this central question of niche biology will have to be resolved for the epithelial niche.
As Drosophila and mammalian epithelial stem cells are becoming more well understood, a set of features is emerging that is common among epithelial stem cells and their niches and may be important for the process of self-renewal. First, intrinsic differences, such as cell shape, may differentiate FSCs from their daughters, allowing them to respond differently to essentially the same microenvironment. Specifically, both FSCs and ISCs, but not their daughters, have a triangular shape with a broad basal surface and narrow apical surface. Interestingly, mammalian intestinal stem cells also seem to have a very similar basal-to-apical triangular morphology, suggesting that this feature may be conserved.69 Second, studies of both Drosophila epithelia, as discussed above, and mammalian epithelia such as the interfollicular epithelium70 and the hair follicle71 indicate that broad contact with the basement membrane correlates with the stem cell state, though this feature alone does not seem to be sufficient for asymmetric segregation of cell fates. Third, unlike the germline stem cell niches, which are simpler, epithelial niches use multiple signals that have partially overlapping function and are derived from multiple sources. This redundancy may add a robustness and flexibility that allows epithelial tissues to rapidly adapt to physiological and environmental changes. Further studies into the fascinating class of epithelial-type niches will expand our understanding of adult stem cell biology and provide a foundation for the rational design of therapeutics and diagnostics.
Acknowledgments
P.S.H. is supported by the California Institute for Regenerative Medicine (Grant Number TG2-01153), A. C. is supported by the Eugene Cota-Robles Fellowship, and T.G.N is supported by Award Number R01GM097158 from the National Institute Of General Medical Sciences.
Footnotes
Further Reading/Resources
Stembook: http://www.stembook.org
The Society for Developmental Biology Education Section: http://www.sdbonline.org/index.php?option=com_content&task=section&id=6&Itemid=62%3E
Interactive fly: http://www.sdbonline.org/fly/aimain/1aahome.htm
Nature Education: Genetics: http://www.nature.com/scitable/topic/genetics-5
| Subtopic | Article title |
|---|---|
| Tissue Stem Cells and Niches | Invertebrate Midgut |
| Tissue Stem Cells and Niches | Vertebrate Epithelium |
| Gametogenesis | Oogenesis |
Contributor Information
Pankaj Sahai-Hernandez, University of California, San Francisco.
Angela Castanieto, University of California, San Francisco.
Todd Gregory Nystul, University of California, San Francisco, todd.nystul@ucsf.edu.
References
- 1.Morrison S, Spradling AC. Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 4.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 5.Fox D, Morris L, Nystul T, Spradling A. Lineage analysis of stem cells. StemBook. 2009 [PubMed] [Google Scholar]
- 6.Nystul T, Spradling A. An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell. 2007;1:277–285. doi: 10.1016/j.stem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Griffin R, Sustar A, Bonvin M, Binari R, del Valle Rodriguez A, Hohl AM, Bateman JR, Villalta C, Heffern E, Grunwald D, et al. The twin spot generator for differential Drosophila lineage analysis. Nat Methods. 2009;6:600–602. doi: 10.1038/nmeth.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potter CJ, Tasic B, Russler EV, Liang L, Luo L. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell. 2010;141:536–548. doi: 10.1016/j.cell.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu HH, Chen CH, Shi L, Huang Y, Lee T. Twin-spot MARCM to reveal the developmental origin and identity of neurons. Nat Neurosci. 2009;12:947–953. doi: 10.1038/nn.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spradling AC. Ch. 1. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. Vol. 1. Cold Spring Harbor Press; 1993. pp. 1–70. [Google Scholar]
- 11.Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell. 2005;9:501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Morris LX, Spradling AC. Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development. 2011;138:2207–2215. doi: 10.1242/dev.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margolis J, Spradling AC. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- 14.Spradling AC, de Cuevas M, Drummond-Barbosa D, Keyes L, Lilly M, Pepling M, Xie T. The Drosophila germarium: stem cells, germ line cysts, and oocytes. Cold Spring Harb Symp Quant Biol. 1997;62:25–34. [PubMed] [Google Scholar]
- 15.Nystul T, Spradling AC. Regulation of epithelial stem cell replacement and follicle formation in the Drosophila ovary. Genetics. 2010;184:503–515. doi: 10.1534/genetics.109.109538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forbes AJ, Lin H, Ingham PW, Spradling AC. hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development. 1996;122:1125–1135. doi: 10.1242/dev.122.4.1125. [DOI] [PubMed] [Google Scholar]
- 17.Hartman TR, Zinshteyn D, Schofield HK, Nicolas E, Okada A, O'Reilly AM. Drosophila Boi limits Hedgehog levels to suppress follicle stem cell proliferation. J Cell Biol. 2010;191:943–952. doi: 10.1083/jcb.201007142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Kalderon D. Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature. 2001;410:599–604. doi: 10.1038/35069099. [DOI] [PubMed] [Google Scholar]
- 19.Kirilly D, Spana EP, Perrimon N, Padgett RW, Xie T. BMP signaling is required for controlling somatic stem cell self-renewal in the Drosophila ovary. Developmental Cell. 2005;9:651–662. doi: 10.1016/j.devcel.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Vied C, Kalderon D. Hedgehog-stimulated stem cells depend on non-canonical activity of the Notch co-activator Mastermind. Development. 2009;136:2177–2186. doi: 10.1242/dev.035329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song X, Xie T. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development. 2003;130:3259–3268. doi: 10.1242/dev.00524. [DOI] [PubMed] [Google Scholar]
- 22.Song X, Xie T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc Natl Acad Sci USA. 2002;99:14813–14818. doi: 10.1073/pnas.232389399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Kalderon D. Cyclin E-dependent protein kinase activity regulates niche retention of Drosophila ovarian follicle stem cells. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0909272106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Reilly AM, Lee H-H, Simon MA. Integrins control the positioning and proliferation of follicle stem cells in the Drosophila ovary. J Cell Biol. 2008;182:801–815. doi: 10.1083/jcb.200710141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franz A, Riechmann V. Stepwise polarisation of the Drosophila follicular epithelium. Developmental Biology. 2010;338:136–147. doi: 10.1016/j.ydbio.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Skora AD, Spradling AC. Epigenetic stability increases extensively during Drosophila follicle stem cell differentiation. Proc Natl Acad Sci USA. 2010;107:7389–7394. doi: 10.1073/pnas.1003180107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xi R, Xie T. Stem cell self-renewal controlled by chromatin remodeling factors. Science. 2005;310:1487–1489. doi: 10.1126/science.1120140. [DOI] [PubMed] [Google Scholar]
- 28.Buszczak M, Paterno S, Spradling AC. Drosophila Stem Cells Share a Common Requirement for the Histone H2B Ubiquitin Protease Scrawny. Science. 2008 doi: 10.1126/science.1165678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Han Y, Xi R. Polycomb group genes Psc and Su(z)2 restrict follicle stem cell self-renewal and extrusion by controlling canonical and noncanonical Wnt signaling. Genes Dev. 2010;24:933–946. doi: 10.1101/gad.1901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin Z, Xie T. Dcr-1 Maintains Drosophila Ovarian Stem Cells. Curr Biol. 2007;17:539–544. doi: 10.1016/j.cub.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 31.Fuller MT, Spradling AC. Male and Female Drosophila Germline Stem Cells: Two Versions of Immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- 32.Ohlstein B, Spradling AC. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 33.Micchelli C, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 34.Ohlstein B, Spradling AC. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 35.Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009;136:2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- 36.Bardin AJ, Perdigoto CN, Southall TD, Brand AH, Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development. 2010;137:705–714. doi: 10.1242/dev.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin G, Xu N, Xi R. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells. J Mol Cell Biol. 2010;2:37–49. doi: 10.1093/jmcb/mjp028. [DOI] [PubMed] [Google Scholar]
- 38.Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol. 2011;354:31–43. doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 39.Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455:1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- 40.Jiang H, Patel PH, Kohlmaier A, Grenley MO, Mcewen DG, Edgar BA. Cytokine/Jak/Stat Signaling Mediates Regeneration and Homeostasis in the Drosophila Midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 42.Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8:84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ha EM. A Direct Role for Dual Oxidase in Drosophila Gut Immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 44.Ryu J-H, Ha E-M, Oh C-T, Seol J-H, Brey PT, Jin I, Lee DG, Kim J, Lee D, Lee W-J. An essential complementary role of NF-κB pathway to microbicidal oxidants in Drosophila gut immunity. Embo J. 2006;25:3693–3701. doi: 10.1038/sj.emboj.7601233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 2010;8:152. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biteau B, Hochmuth C, Jasper H. JNK Activity in Somatic Stem Cells Causes Loss of Tissue Homeostasis in the Aging Drosophila Gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biteau B, Karpac J, Supoyo S, DeGennaro M, Lehmann R, Jasper H. Lifespan Extension by Preserving Proliferative Homeostasis in Drosophila. PLoS Genetics. 2010;6:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi N-H, Kim J-G, Yang D-J, Kim Y-S, Yoo M-A. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi Y-J, Hwang M-S, Park J-S, Bae S-K, Kim Y-S, Yoo M-A. Age-related upregulation of Drosophila caudal gene via NF-kappaB in the adult posterior midgut. Biochimica et biophysica acta. 2008;1780:1093–1100. doi: 10.1016/j.bbagen.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Park J-S, Kim Y-S, Yoo M-A. The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila. Aging. 2009;1:637–651. doi: 10.18632/aging.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biteau B, Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development. 2011;138:1045–1055. doi: 10.1242/dev.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci U S A. 2010;107:21064–21069. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fox DT, Spradling AC. The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell. 2009;5:290–297. doi: 10.1016/j.stem.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh SR, Zeng X, Zheng Z, Hou SX. The adult Drosophila gastric and stomach organs are maintained by a multipotent stem cell pool at the foregut/midgut junction in the cardia (proventriculus) Cell Cycle. 2011;10:1109–1120. doi: 10.4161/cc.10.7.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh SR, Liu W, Hou SX. The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell. 2007;1:191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weaver M, Krasnow MA. Dual origin of tissue-specific progenitor cells in Drosophila tracheal remodeling. Science. 2008;321:1496–1499. doi: 10.1126/science.1158712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kirilly D, Xie T. The Drosophila ovary: an active stem cell community. Cell Res. 2007;17:15–25. doi: 10.1038/sj.cr.7310123. [DOI] [PubMed] [Google Scholar]
- 65.Jin Z, Kirilly D, Weng C, Kawase E, Song X, Smith S, Schwartz J, Xie T. Differentiation-defective stem cells outcompete normal stem cells for niche occupancy in the Drosophila ovary. Cell Stem Cell. 2008;2:39–49. doi: 10.1016/j.stem.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Issigonis M, Tulina N, de Cuevas M, Brawley C, Sandler L, Matunis E. JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science. 2009;326:153–156. doi: 10.1126/science.1176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheng XR, Brawley CM, Matunis E. Dedifferentiating spermatogonia outcompete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell. 2009;5:191–203. doi: 10.1016/j.stem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 69.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 70.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blanpain C, Lowry W, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]