Abstract
The cysteine dioxygenase (Cdo1)-null and the cysteine sulfinic acid decarboxylase (Csad)-null mouse are not able to synthesize hypotaurine/taurine by the cysteine/cysteine sulfinate pathway and have very low tissue taurine levels. These mice provide excellent models for studying the effects of taurine on biological processes. Using these mouse models, we identified betaine:homocysteine methyltransferase (BHMT) as a protein whose in vivo expression is robustly regulated by taurine. BHMT levels are low in liver of both Cdo1-null and Csad-null mice, but are restored to wild-type levels by dietary taurine supplementation. A lack of BHMT activity was indicated by an increase in the hepatic betaine level. In contrast to observations in liver of Cdo1-null and Csad-null mice, BHMT was not affected by taurine supplementation of primary hepatocytes from these mice. Likewise, CSAD abundance was not affected by taurine supplementation of primary hepatocytes, although it was robustly upregulated in liver of Cdo1-null and Csad-null mice and lowered to wild-type levels by dietary taurine supplementation. The mechanism by which taurine status affects hepatic CSAD and BHMT expression appears to be complex and to require factors outside of hepatocytes. Within the liver, mRNA abundance for both CSAD and BHMT was upregulated in parallel with protein levels, indicating regulation of BHMT and CSAD mRNA synthesis or degradation.
Keywords: Taurine, Betaine, Betaine:homocysteine methyltransferase, Cysteine dioxygenase, Cysteine sulfinic acid decarboxylase, Liver, Hepatocytes
Introduction
Taurine is present in rodent and human tissues at concentrations in the range of 5–30 μmol/g (Roman et al. 2013; Wójcik et al. 2010). Taurine plays important roles in diverse biological processes, such as cell volume regulation, conjugation of bile acids, novel modifications of mitochondrial tRNAs, antioxidant defenses, membrane stabilization, modulation of ion movement, modulation of neurotransmitter activity, and development of the central nervous and visual systems (Huxtable 1992; Ripps and Shen 2012). Taurine appears to protect cells against various types of injury as a result of its effects on cellular homeostasis and has been reported to have preventive properties for coronary heart disease and diabetes (Agca et al. 2014; Ito et al. 2012; Wójcik et al. 2010). Despite the many functional properties of taurine, the physiological and biochemical mechanisms mediating the actions of taurine are poorly understood.
The CDO/CSAD-dependent pathway of taurine biosynthesis is the major route for taurine synthesis in mammals. Cysteine dioxygenase (CDO), encoded by the Cdo1 gene, catalyzes the addition of molecular oxygen to cysteine to form cysteinesulfinate, which is either metabolized via transamination to yield pyruvate and sulfite or decarboxylated by cysteine sulfinic acid decarboxylase (CSAD) to hypotaurine, with hypotaurine being further oxidized to taurine. Hence, Cdo1 knockout mice or Csad knockout mice have a very limited capacity to synthesize taurine and very low tissue taurine levels when fed a taurine-free diet. Compared to the taurine transporter (Taut, or SLC6A6) knockout mouse that has been used as a model of taurine deficiency (Warskulat et al. 2007), the Cdo1 and Csad knockout mice are more taurine-deficient, particularly in liver which is active in taurine biosynthesis (Park et al. 2014; Roman et al. 2013; Ueki et al. 2011). For example, the hepatic taurine concentration in Taut-null mice was about 30 % of wild-type, whereas that in Cdo1-null mice is about 5 % of wild-type levels (Roman et al. 2013; Warskulat et al. 2007).
Our laboratory has been using the Cdo1-null mouse model to further understand the physiological functions of taurine and the consequences of its depletion, including the role of taurine in the regulation of gene expression. In this paper, we show that taurine deficiency in the Cdo1-null mouse is associated with an increase in betaine and other molecules that serve as organic osmolytes, and we identify the downregulation of betaine:homocysteine methyltransferase (BHMT) expression as the probable mechanism for promoting elevated betaine concentrations in taurine-deficient tissues. Furthermore, we show that taurine supplementation of Cdo1-null mice or of Csad-null mice restores hepatic levels of BHMT to wild-type levels, whereas addition of taurine to isolated primary hepatocytes from Cdo1-null mice does not, suggesting the possibility that regulation of BHMT and CSAD expression by taurine requires input from extrahepatic tissues.
Materials and methods
Animals and diets
Cdo1−/− and Cdo1+/+ mice for this study were generated by crossing C57BL/6 Cdo1+/− male and female mice as described previously (Roman et al. 2013; Ueki et al. 2011). All experimental procedures involving Cdo1+/− mice and their offspring were conducted with the approval of the Cornell University Institutional Animal Care and Use Committee (#2009-0138). Mice were housed in a room maintained at 23 °C and 45–50 % humidity, with a 10-h dark period (from 20:00 to 6:00 h). Pups and dams had unrestricted access to a basal taurine-free diet from birth to postnatal day 21 when pups were removed from their dams. Mice used for isolation of hepatocytes continued to receive the taurine-free basal diet. For studies that included high-fat/taurine-free and high-fat/taurine-supplemented diets in addition to the basal/taurine-free diet (Table 1), mice assigned to the same diet were housed together (2–4 mice per cage) from the time of weaning, fed the basal diet from postnatal day 21 through 37, and given the assigned treatment diets from postnatal day 38 until postnatal day 63 when the liver was collected.
Table 1.
Composition of basal (taurine-free), high-fat (taurine-free) and high-fat + taurine diets
| Basal diet (AIN93G with reduced cystine) | High-fat diet (modified AIN93Ga) | High fat + taurine diet (modified AIN93Ga) | |
|---|---|---|---|
| Ingredient | g/kg diet | g/kg diet | g/kg diet |
| Cornstarch | 398.986 | 142.753 | 137.753 |
| Casein (>85 % protein) | 200 | 246 | 246 |
| Dextrinized cornstarch | 132 | 162.36 | 162.36 |
| Sucrose | 100 | 123 | 123 |
| Soybean oil (no additives) | 70 | 86.1 | 86.1 |
| Fiber (cellulose) | 50 | 61.5 | 61.5 |
| Mineral mix (AIN-93M-MX) | 35 | 43.05 | 43.05 |
| Vitamin mix (AIN-93-VX) | 10 | 12.3 | 12.3 |
| L-Cystine | 1.5 | 1.845 | 1.845 |
| Choline bitartrate (41.1 % choline) | 2.5 | 3.075 | 3.075 |
| Tert-butylhydroquinone | 0.014 | 0.017 | 0.017 |
| Lard | 0 | 118 | 118 |
| Taurine | 0 | 0 | 5 |
High-fat diets were generated by replacing cornstarch with lard on an equi-caloric basis; other ingredients were adjusted to maintain amount constant as a percentage of total calories; caloric density of high fat diets was 4432 kcal/kg diet compared to 3758 kcal/kg diet for the basal diet
It should be noted that the high-fat diets with and without taurine supplementation were designed for a study of energy metabolism in these mice (J. Niewiadomski, H. Roman, L. Hirschberger and M. Stipanuk, unreported results). However, the macronutrient composition of the diet did not appear to affect any of the parameters measured related to taurine metabolism because measurements were consistently similar for liver of mice fed the taurine-free basal and taurine-free high-fat diets (see Results).
Liver samples from Csad-null mice were obtained from third generation homozygous Csad knockout mice born to homozygous Csad knockout dams as described by Park et al. (2014). Chimeric mice were generated on a C57BL/6 background and back-crossed twice to C57BL/6. All experimental procedures involving Csad-null mice were conducted with the approval of the Institutional Animal Care and Use Committee of the New York State Institute for Basic Research in Developmental Disabilities (#392). Mice were fed a taurine-free nonpurified rodent diet (Lab-Diet, PMI Nutrition International, St. Louis, MO). Taurine-supplemented Csad-null offspring and their dams received taurine in their drinking water (1 g taurine per 100 ml), whereas taurine-deficient Csad-null offspring and their dams were not supplemented with taurine. Thus, taurine-deficient Csad-null mice were exposed to taurine deficiency both during gestation and after birth, whereas taurine-supplemented Csad-null mice were exposed to taurine both during gestation and continuously after birth. Liver was collected from Csad-null offspring, both taurine-supplemented and taurine-deficient, when mice had reached 8 weeks of age.
Liver collection
Liver from Cdo-null and wild-type mice (62–68 days of age, both male and female) fed either the basal, high-fat, or high-fat + taurine diet (n = 6 for each sex/genotype/diet grouping; 72 mice total) for the 3.5 weeks prior to sample collection was harvested for measurement of taurine levels; the abundances of BHMT and CSAD protein and mRNA; and analysis of polar metabolites. Mice were euthanized with an overdose of isoflurane between 10:00 and 14:00 h. Liver was removed and immediately frozen in liquid nitrogen and then stored at −80 °C until samples were analyzed.
Liver from taurine-supplemented Csad-null and unsupplemented Csad-null mice was obtained when mice were 56 days of age (n = 3 for each sex/genotype/diet grouping. 12 mice total). Mice were euthanized by an overdose of avertin (375 mg/kg, i.p.) between 10:00 and 14:00 h. Liver was removed and immediately frozen in liquid nitrogen and then stored at −80 °C until samples were analyzed.
Western blotting
Frozen liver samples were homogenized in four volumes of lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA and 0.5 % Nonidet P-40) supplemented with 1 × Complete Protease Inhibitor Cocktail (Roche) and 1 × PhosSTOP phosphatase inhibitor (Roche). Homogenates were centrifuged at 18,000×g for 20 min at 4 °C, and the supernatants were used for determination of the abundances of BHMT and CSAD or CDO.
Aliquots of cell lysates or liver supernatant containing 30 μg of total protein were separated by SDS-PAGE (12 %, w/v, polyacrylamide) and then the protein bands were transferred onto a 0.45-μm Immobilon-FL PVDF membrane (Millipore Corp.). Membranes were blocked using blocking buffer for near infrared fluorescent westerns (LI-COR Biosciences) and blotted for immunoreactive proteins. Sources and dilutions of primary antibodies were as follows: anti-CSAD (1:8000; gift from Dr. Marcel Tappaz, INSERM, France), anti-BHMT (1:1000; Thermo Fisher Scientific), anti-CDO generated in the Stipanuk laboratory (Stipanuk et al. 2008), anti-FMO3 (1:500; Thermo Fisher Scientific), anti-GDE2 (1:500; Santa Cruz Biotechnology), anti-AKR1B3 (1:500; Santa Cruz Biotechnology), anti-β-tubulin (1:500; Santa Cruz Biotechnology); and anti-GAPDH (1:1000; Proteintech Group). An infrared fluorescent dye-labeled secondary antibody (IRDye, LI-COR Biosciences) and the Odyssey direct infrared imaging system and software (LI-COR Biosciences) were used to visualize and quantify the relative abundance of each protein. Protein abundances were normalized by β-tubulin or GAPDH abundance and then expressed as fold of the wild-type control value.
Measurement of taurine and hypotaurine
For the Cdo1-null and wild-type mice, taurine and hypotaurine in liver samples were measured by HPLC as described previously by Ueki et al. (2011). For the Csad-null and wild-type mice, liver samples were analyzed for taurine by HPLC as described previously by Park et al. (2014).
Measurement of mRNA relative abundance
RNA was isolated using the RNeasy mini kit according to the manufacturer’s directions (Qiagen). Complementary DNA was reverse transcribed using Applied Biosystems High Capacity cDNA kit (Applied Biosystems) and quantified using Power Sybr Green (Applied Biosystems) in conjunction with a Roche 480 Lightcycler (Roche Diagnostics). Forward and reverse primer sequences are as follows: BHMT forward 5′-CGGCTTCAGAAAAACATGG-3′ reverse 5′-TCTGCCAGATTCCTTTCTGG-3′; CSAD forward 5′-CCAGTGCCTCTGAGAAGGTC-3′ reverse 5′-TGACACTGTAGTGAATCACAGTCC-3′; CDO forward 5′-GATTCTGTGCTGGGGTGAA-3′ reverse 5′-CAGTGGGAGTCCGTGTGAT-3′; β-actin forward 5′-CTAAGGCCAACCGTGAAAAG-3′ reverse 5′-ACCAGAGGCATACAGGGACA-3′. Values for CDO, BHMT, and CSAD mRNA were normalized to values for β-actin mRNA.
LC/MS analysis of liver metabolites
To extract metabolites, a weighed amount of frozen liver was homogenized in ice-cold 80 % methanol/water (200 μl per 5 mg liver), followed by dilution with one additional volume of ice-cold 80 % methanol/water (200 μl per 5 mg liver), vortexing, sitting on ice for 10 min, and centrifugation at 20,000×g at 4 °C for 10 min. A 200 μl aliquot of the supernatant was transferred to a microcentrifuge tube and dried in a SpeedVac (Thermo Scientific). Dried extracts were stored at −80 °C until the LC–MS analysis was done. For LC–MS, samples were reconstituted into water at (30 μl water per 5 mg liver), diluted with an additional 30 μl acetonitrile/methanol (1:1, v/v), and centrifuged at 20,000×g at 4 °C for 3 min; 4 μl of the final supernatant was injected into the LC–MS system.
The LC–MS system consisted of an Ultimate 3000 UHPLC (Dionex) coupled to a Q Exactive-Mass spectrometer (QE-MS, Thermo Scientific). For analysis of polar metabolites, hydrophilic interaction LC (HILIC) was run with an Xbridge amide column (100 × 2.1 mm i.d., 3.5 μm; Waters) as described previously (Liu et al. 2014). The QE-MS was equipped with a HESI probe. Relevant parameters were: heater temperature, 120 °C; sheath gas, 30; auxiliary gas, 10; sweep gas, 3; spray voltage, 3.6 kV for positive mode and 2.5 kV for negative mode. Capillary temperature was set at 320 °C, and S-lens was 55. The QE-MS scan range was 60–900 (m/z). The resolution was set at 70,000. The maximum injection time (max IT) was 200 ms. Automated gain control (AGC) was targeted at 3 × 106 ions. Peak extraction and peak area integration were performed as described previously (Liu et al. 2014).
Studies with primary hepatocytes
Cdo1−/− and Cdo1+/+ male mice (42–100 days of age) that had been fed the basal diet since weaning were euthanized with an overdose of isoflurane, and hepatocytes were quickly isolated by collagenase perfusion, density gradient centrifugation through Percoll and repeated low speed centrifugation in ice-cold medium to remove contaminating red blood cells. Viability of isolated hepatocytes was determined by trypan blue exclusion and was routinely greater than 90 %.
Isolated hepatocytes were plated on collagen-coated 6-well plates (4 × 104 cells per cm2) in Dulbecco’s Modified Eagle’s medium (DMEM, Gibco #12800-017, containing 1 mM pyruvate, 25 mM glucose and 4 mM glutamine) supplemented with 10 % (vol/vol) fetal bovine serum (FBS), 20,000 units penicillin and 20 mg streptomycin per liter, and an additional 1 mM sodium pyruvate. Hepatocytes were then incubated at 37 °C in an atmosphere of 5 % CO2. At 2 h after plating, the plating medium was replaced with fresh culture medium consisting of DMEM supplemented with 1 mM sodium pyruvate, 20,000 units penicillin and 20 mg streptomycin per liter, 0.2 % (wt/vol) of bovine serum albumin fraction V (instead of FBS), 100 nM dexamethasone and 1 nM insulin (Sigma-Aldrich #I9278). After 24 h, culture medium was replaced with treatment medium (total volume of 2.5 ml per well). Treatment medium was identical to the culture medium except that it also included 0.05 mM bathocuproine disulfonic acid (BCS, Sigma-Aldrich) and 0.3 mM L-cysteine (Cys, MP Biomedicals, Inc.), and in the case of taurine-supplemented cells, either 0.05 or 0.5 mM taurine (Acros Organics). Hepatocytes were cultured in the treatment medium for 24 or 48 h prior to harvest.
For measurement of taurine and hypotaurine levels in cells, hepatocytes were harvested by washing with ice-cold PBS, treating with 0.25 % trypsin (Gibco #25200) to release cells, and harvesting hepatocytes into ice-cold PBS. The cell suspension was centrifuged at 1600 × g for 10 min at 4 °C to obtain the pelleted cells, which were immediately frozen and stored at −80 °C until they were analyzed. At the time of analysis, frozen hepatocytes were thawed on ice and resuspended in 0.1 M phosphate buffer, pH 7.5. The cell suspension was then sonicated for three 5-s intervals at 4 °C to prepare cell homogenates that were used to measure total protein and taurine and hypotaurine levels. For measurement of protein abundance by western blotting, hepatocytes were washed twice with ice-cold PBS and harvested into lysis buffer (50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.5 % Nonidet P-40) supplemented with 1 × Complete Protease Inhibitor Cocktail (Roche) and PhosSTOP phosphatase inhibitor (Roche). The lysate was frozen and stored at −80 °C until analysis. Analyses were done as described above for the Cdo1-null mouse study.
Protein assays
Total cellular protein in hepatocyte lysates and soluble protein in the supernatant fractions from liver homogenates were determined using the BCA Protein Assay Kit (Thermo Scientific/Pierce) using bovine serum albumin (BSA) as the standard.
Statistical analysis
Results of measurements are expressed as mean ± SEM for 3–6 individual mice or separate hepatocyte preparations. Data for male and female mice were analyzed separately. Results for Cdo1-null mice and hepatocytes were analyzed as a full factorial least squares model using JMP version 10 (SAS, Cary, NC). Differences were considered significant at p ≤ 0.05 for main effects (genotype, treatment). Post hoc individual pairwise comparisons of least squares means in the model were made using Tukey’s comparisons; comparisons were considered significant at p < 0.05. Data for taurine and hypotaurine levels, data for the hepatic abundances of BHMT protein and mRNA abundances, and all data for metabolites were log transformed prior to statistical analyses. Hepatic abundances of CSAD protein and mRNA were transformed to square roots prior to statistical analysis. For analysis of taurine treatment effects in the Csad-null mice, Student’s t test was used; comparisons were considered significant at p < 0.05.
Results
BHMT is downregulated in liver of taurine-deficient Cdo1-null mice and restored to wild-type levels by taurine supplementation
The initial stimulus for this work was our desire to identify a protein that we have consistently observed to be markedly and consistently downregulated in liver of Cdo1-null mice compared to wild-type littermates. This unidentified protein was detected with β-actin antibody in immunoblots of liver extracts but not in extracts of other tissues of Cdo1-null or wild-type mice fed taurine-free semi-purified diets, suggesting the protein might be one that is expressed predominantly in liver. The unknown protein ran with an apparent molecular mass of 45 kDa compared to the 42 kDa β-actin band but was clearly separated from it. We used a proteomic screen we had previously run for primary hepatocytes from Cdo1-null mice and their wild-type littermates (iTRAQ, Cornell University Proteomics and Mass Spectrometry Core Facility) to see if this screen identified any protein with a molecular mass near 45 kDa that was lower in Cdo1-null mice than in wild-type mice. The only protein meeting both criteria was BHMT. As shown in Fig. 1a, we confirmed by western blotting with a specific BHMT antibody that BHMT in liver of Cdo1-null mice was lower (an average of 0.5-fold) than in liver of wild-type mice. Furthermore, we observed that BHMT in liver of Cdo1-null mice returned to wild-type levels when the mice were fed a taurine-supplemented diet, suggesting that hepatic BHMT expression is downregulated in response to taurine deficiency. BHMT mRNA abundance underwent a similar fold change as shown in Fig. 1b.
Fig. 1.
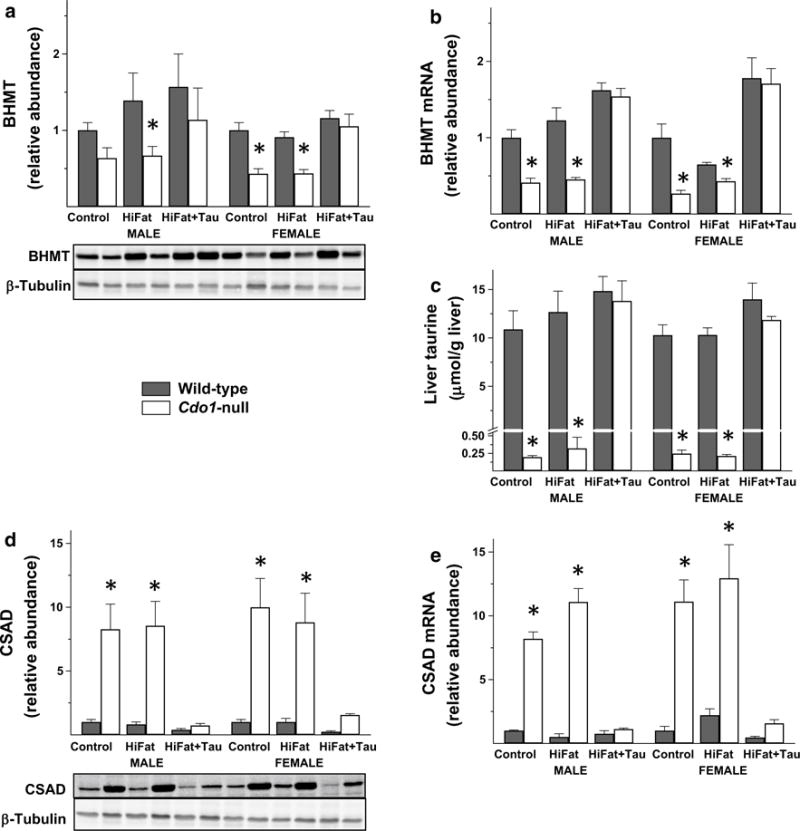
BHMT protein abundance (a), BHMT mRNA abundance (b), taurine level (c), CSAD protein abundance (d) and CSAD mRNA abundance (e) in liver from Cdo1-null and wild-type mice fed either a basal, high-fat, or high-fat + taurine diet. Values shown in bar graphs are mean ± SEM for six mice and are expressed as fold the value for wild-type mice of the same sex fed the basal diet; asterisk indicates the value for the Cdo1-null mice is significantly different (p < 0.05) than that for wild-type mice of the same sex and dietary treatment group based on post hoc Tukey’s comparison tests. Representative western blots are shown below the bar graphs for protein abundances
As shown in Fig. 1c, taurine was very low in liver of Cdo1-null mice fed taurine-free diets, being only 1.9–2.5 % of levels in wild-type mice fed a taurine-free diet. Taurine supplementation resulted in normal hepatic taurine levels in Cdo1-null mice. In contrast, taurine supplementation had no significant effect on hepatic taurine levels of wild-type mice that had an active pathway for taurine synthesis. Furthermore, hepatic taurine levels were not different between wild-type mice fed the (taurine-free) basal or taurine-free high-fat diet or between Cdo1-null mice fed the (taurine-free) basal or taurine-free high-fat diet. Consistent with the previously reported effect of taurine status on hepatic CSAD expression (Roman et al. 2013; Ueki et al. 2011), the CSAD protein and mRNA relative abundances were markedly elevated (an average of ninefold for CSAD protein and 12-fold for CSAD mRNA) in liver from Cdo1-null mice fed the taurine-free diet, and this was returned to wild-type levels when Cdo1-null mice were fed a taurine-supplemented diet (Fig. 1d, e).
Notably, the macronutrient composition of the diet (i.e., taurine-free basal versus taurine-free high-fat) had no effect on hepatic BHMT protein or mRNA abundance, taurine level, or CSAD protein or mRNA abundance, further suggesting that taurine status was the major determinant of the observed differences in BHMT expression levels.
To further explore the relationship between taurine levels and the levels of other organic osmolytes in murine liver, we ran a metabolomics profile on liver samples from six male rats per genotype-diet group. As shown in Table 2, Cdo1-null mice, whether fed the basal or the high-fat diet, had very low hepatic taurine levels, consistent with the dramatic reductions reported in Fig. 1c for the HPLC analysis of taurine. Levels of betaine, choline, glycerophosphocholine and carnosine, molecules that function as organic osmolytes, were significantly higher in the Cdo1-null mice fed either the basal or high-fat taurine-free diet; of these, the greatest fold differences were observed for betaine. Interestingly, all of these differences disappeared when taurine was added to the diet (i.e., the high-fat + taurine groups), indicating that the lack of taurine biosynthesis in the Cdo1-null mice triggered the changes in the levels of betaine and other organic osmolytes. The higher concentration of betaine along with the lower abundance of BHMT in liver of taurine-deficient mice compared to taurine-supplemented mice suggests that the downregulation of BHMT expression in response to taurine depletion has functional consequences on the metabolism of betaine that allows the intracellular betaine concentration to increase.
Table 2.
Fold differences in taurine, organic osmolytes and bile acids in liver of male wild-type and Cdo1-null mice fed basal, high-fat, or taurine-supplemented high-fat diets
| Metabolites | Genotype-diet groups relative abundance of metabolites | Significance level for main effects and 2-way interaction as analyzed by a general linear model F(5,24)† | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Wild-type Basal | Cdo1-null Basal | Wild-type HiFat | Cdo1-null HiFat | Wild-type HiFat + Tau | Cdo1-null HiFat + Tau | Genotype | Diet | GxD | |
| Taurine | 1 ± 0.06a*,† | 0.059 ± 0.005b | 0.97 ± 0.10a | 0.047 ± 0.002b | 0.94 ± 0.17a | 1.16 ± 0.06a | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| Betaine | 1 ± 0.05b | 2.33 ± 0.13a | 1.16 ± 0.10b | 2.66 ± 0.40a | 1.06 ± 0.10b | 1.38 ± 0.26b | p < 0.0001 | p = 0.0099 | p = 0.0209 |
| Choline | 1 ± 0.08b | 1.88 ± 0.11a | 1.04 ± 0.05b | 1.63 ± 0.21a | 1.00 ± 0.01b | 0.83 ± 0.08b | p < 0.0001 | p = 0.0099 | p = 0.0210 |
| sn-Glycerol-3-phosphocholine | 1 ± 0.12b | 2.05 ± 0.14a | 1.01 ± 0.08b | 1.83 ± 0.27a | 0.93 ± 0.10b | 0.82 ± 0.12b | p = 0.0010 | p = 0.0006 | p = 0.0038 |
| Carnosine | 1 ± 0.05b | 2.26 ± 0.27a | 1.24 ± 0.14b | 2.63 ± 0.17a | 1.08 ± 0.14b | 0.94 ± 0.08b | p < 0.0001 | p < 0.0001 | p = 0.0001 |
| myo-Inositol | 1 ± 0.05ab | 1.19 ± 0.06ab | 0.94 ± 0.04b | 1.34 ± 0.15a | 1.05 ± 0.06ab | 1.13 ± 0.08ab | p = 0.0014 | ||
| Glucitol/Iditol/Galactitol | 1 ± 0.09a | 0.65 ± 0.08ab | 0.47 ± 0.04b | 0.45 ± 0.02b | 0.46 ± 0.05b | 0.41 ± 0.09b | p = 0.0621 | p = 0.0006 | |
All values for metabolite concentrations are expressed relative to the average value for the wild-type/basal diet group, which was set as 1.0. Data are expressed as mean ± SEM
All values were transformed to log10 values prior to statistical analysis. Columns on the right give p values for the main effects and their interaction as analyzed by a general linear model for the two categorical variables and their interaction using JMP, version 11 (SAS, Cary, NC). Values not followed by the same superscript letter are significantly different at p < 0.05 as determined by the Tukey’s post hoc comparison test. Italic values indicates metabolites with concentration changes in response to taurine deficiency
These observations for CSAD and BHMT led us to investigate the abundance of other proteins that might be involved in maintenance of cellular osmolarity. Thus, we looked at the abundances of several other proteins that could regulate organic osmolyte levels: flavin containing monooxygenase 3 (FMO3), glycerophosphocholine phosphodiesterase (GDPD5), and aldose reductase (AKR1B3). FMO3 has been implicated in the oxidation of trimethylamine to trimethylamine oxide, which is an important osmolyte in some species (Bennett et al. 2013; Yancey et al. 2002). Glycerophosphocholine is an abundant osmolyte in the kidney, and its degradation by GDPD5 is inhibited by NaCl-induced cell shrinkage, allowing glycerophosphocholine to accumulate (Gallazzini et al. 2008). Aldose reductase (aldo–keto reductase family 1, member B3; Akr1b3) metabolizes various endogenous and exogenous aldehydes including a key reaction in the conversion of glucose to sorbitol, which also serves as an organic osmolyte in some cells (Yancey 2005; Burger-Kentischer et al. 1999). However, neither FMO3, GDPD5 nor AKR1B3 was differentially abundant in liver of Cdo1-null versus wild-type mice or affected by high-fat or taurine-supplemented diets (data not shown). Thus, BHMT appears to be somewhat unique in being regulated in response to taurine status. It should be noted that regulation of BHMT abundance is not related to tissue hypotaurine pools, as dietary taurine restores hepatic levels of taurine but not of hypotaurine in Cdo1-null mice (Roman et al. 2013).
In vitro supplementation of taurine does not increase BHMT or CSAD levels in primary hepatocytes from taurine-deficient Cdo1-null mice
Hepatocytes isolated from Cdo1-null mice and wild-type mice were incubated with basal DMEM or DMEM supplemented with either 0.05 mM or 0.5 mM taurine for 24 h. As shown in Fig. 2a, hepatocytes from Cdo1-null mice had little taurine when cultured in taurine-free medium but actually had higher levels than wild-type cells when they were cultured in taurine-supplemented medium. In contrast, hypotaurine levels (Fig. 2b) remained below 1 % of wild-type levels in hepatocytes from Cdo1-null mice, regardless of taurine supplementation, consistent with the inability of Cdo1-null mice to convert cysteine sulfinate to hypotaurine.
Fig. 2.
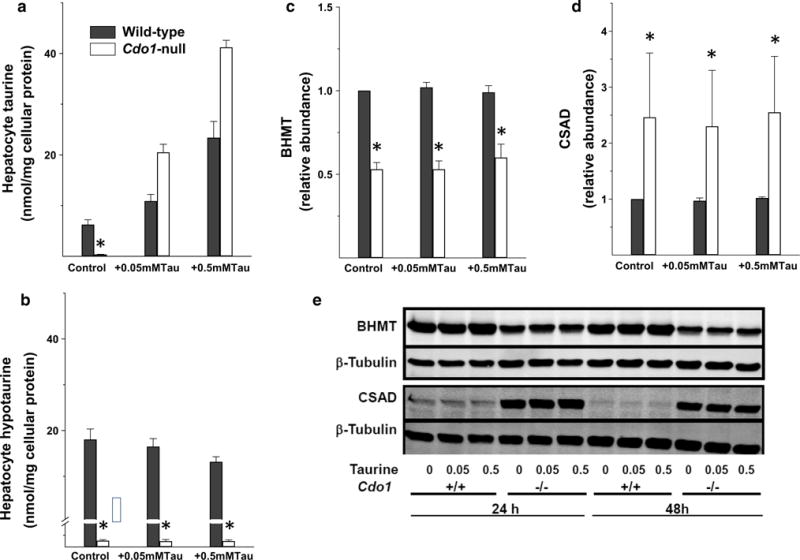
Taurine (a) and hypotaurine (b) levels, BHMT protein abundance (c), CSAD protein abundance (d), and representative western blots for BHMT and CSAD (e) in primary hepatocytes from Cdo-null (−/−) and wild-type (+/+) mice after culture in basal taurine-free medium or in medium supplemented with 0.05 or 0.5 mM taurine. Values shown in bar graphs are mean ± SEM for three separate hepatocyte preparations for cells cultured for 24 h with treatment medium; values are expressed as fold the value for hepatocytes from wild-type mice cultured in basal medium. Asterisk indicates the value for hepatocytes from Cdo1-null mice is significantly different (p < 0.05) than that for hepatocytes from wild-type mice based on a post hoc Tukey’s comparison tests. Bars not denoted by the same letter are significantly different (p < 0.05) by post hoc Tukey’s comparison. The representative western blots are shown for hepatocytes cultured for 24 h and for 48 h in treatment medium
As observed for liver from Cdo1-null and wild-type mice, the abundance of BHMT in hepatocytes from Cdo1-null mice was only 55 % of the wild-type level (Fig. 2c, e). Likewise, the levels of CSAD were higher in hepatocytes from Cdo1-null mice than in hepatocytes from wild-type mice (Fig. 2d, e). However, in contrast to observations for the effects of dietary taurine supplementation in vivo on the abundances of BHMT and CSAD in mouse liver, the abundances of BHMT and of CSAD in cultured hepatocytes were not affected by supplementation of the medium with either 0.05 or 0.5 mM taurine for 24 or 48 h (Fig. 2c–e). The failure of taurine to restore either BHMT or CSAD abundance in hepatocytes from Cdo1-null mice to their wild-type levels suggests that expression of BHMT and CSAD in response to taurine status requires more than a change in taurine concentration for appropriate signaling and protein expression.
BHMT in liver of taurine-deficient Csad-null mice is increased by taurine supplementation
To further investigate the effect of taurine status on hepatic BHMT expression, we examined the effect of taurine supplementation on taurine and BHMT levels in liver of Csad-null mice. As shown in Fig. 3a–c, taurine levels were very low in Csad-null mice fed the taurine-free diet, and both taurine level and BHMT protein and mRNA abundances were significantly increased in these mice when taurine was provided in the drinking water. Notably, hepatic taurine levels in the Csad-null mice ranged from 6 to 13 % of the levels present in taurine-supplemented mice and, thus, were not quite as depleted as those in Cdo1-null mice. BHMT abundance in Csad-null mice fed the taurine-free diet were 67 and 46 % of the BHMT abundance in Csad-null mice that were supplemented with taurine, similar to the fold difference observed in Cdo1-null mice with and without taurine supplementation. Hepatic BHMT mRNA levels in Csad-null mice fed the taurine-free diet were even lower, only 25 % of those in the taurine-supplemented group. Because these mice are maintained as the homozygous knockouts, there are no wild-type littermates that can be used for direct comparison. However, wild-type mice fed the taurine-free diet had hepatic taurine levels (not shown) that were identical to those of the taurine-supplemented Csad-null mice, showing that taurine supplementation was effective in repleting hepatic taurine levels. In addition, the BHMT protein abundances in male and female Csad-null mice supplemented with taurine were greater than those of wild-type mice by 28 % for male mice and 68 % for female mice (data not shown), showing that taurine supplementation was effective in restoring BHMT values to wild-type levels or higher.
Fig. 3.
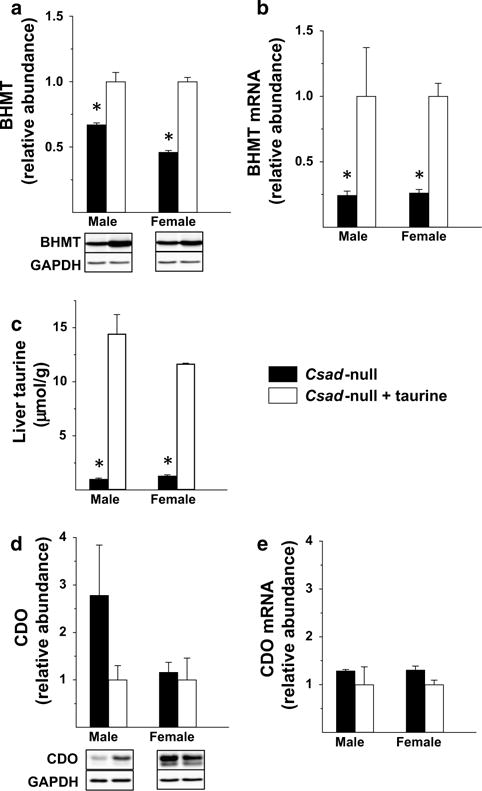
BHMT protein abundance (a), BHMT mRNA abundance (b), taurine level (c), CDO protein abundance (d) and CDO mRNA abundance (e) in liver from Csad-null mice fed either a taurine-free basal diet and supplemented without taurine or with taurine (1 g taurine per 100 mL drinking water). Values shown in bar graphs are mean ± SEM for three mice and are expressed as fold the value for taurine-supplemented mice of the same sex; asterisk indicates the value for the taurine-deficient Csad-null mice is significantly different (p < 0.05) than that for taurine-supplemented Csad-null mice of the same sex based on Student’s t test. Representative western blots are shown below the bar graphs for protein abundances
We also looked at CDO protein and mRNA levels to determine if taurine status had an effect on CDO expression as it does on CSAD expression. As shown in Fig. 2d, e, hepatic CDO protein and mRNA abundances were not significantly different between taurine-supplemented and taurine-deficient Csad-null mice. Despite a large apparent difference in the mean CDO protein abundance in taurine-supplemented and unsupplemented Csad-null male mice, there was a high variance in observed levels and some levels were actually higher in taurine-free mice than in taurine-supplemented mice as shown by the representative blot. A lack of effect of taurine on CDO expression is also supported by the similar CDO abundances in the Csad-null mice and wild-type mice of the same strain (data not shown).
Discussion
Using two different mouse models of deficient taurine synthesis along with taurine-free and taurine-supplemented diets, we have identified BHMT as a protein whose expression is strongly regulated in the mouse in vivo in response to the mouse’s taurine status. Reduction of BHMT abundance in liver was associated with an increase in betaine concentration, suggesting that this regulation of BHMT may be important for generating alternative organic nonperturbing osmolytes to compensate for the loss of taurine. On the other hand, reduction of BHMT abundance in liver could also result in decreased remethylation of homocysteine resulting in increased transsulfuration of serine to form α-ketobutyrate + cysteine (Fig. 4). In wild-type mice, the cysteine formed by transsulfuration would be further metabolized to cysteine sulfinate, hypotaurine and finally taurine. Both mechanisms could potentially be important in wild-type mice.
Fig. 4.
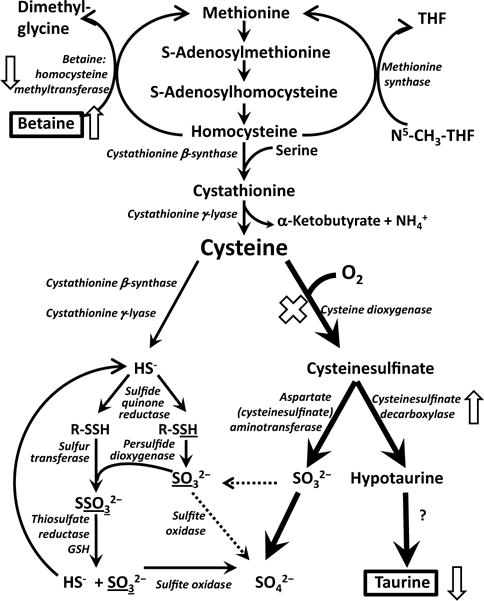
Overview of methionine and cysteine metabolism with the effects of taurine deficiency secondary to deletion of the Cdo1 gene on CSDC and BHMT abundances and betaine levels shown by arrows
BHMT mRNA and protein levels have been shown to be responsive to osmotic conditions in studies with H4IIE rat hepatoma cells. In these hepatoma cells, Bhmt expression was suppressed by hyperosmotic conditions that caused cell shrinkage; and the reverse occurred under hypoosmotic conditions, accompanied by a decrease in intracellular betaine (Schäfer et al. 2007). Of the organic osmolytes that were elevated in our taurine-deficient Cdo1-null mice, the largest molar increase was of betaine, demonstrating a likely important role for betaine in maintaining osmotic balance in taurine-deficient liver. Based on the fold changes observed in osmolyte concentrations in this study and the normal concentrations of these metabolites in liver of C57BL/6 mice (Hoffmann et al. 2013; Mong et al. 2011), we calculate that taurine level was decreased by 12,300 nmol/g in Cdo1-null mouse liver, whereas betaine, glycerophosphocholine, choline and carnosine were increased by about 3000, 600, 280, and 200 nmol/g, respectively. Clearly, no single metabolite could make up for the magnitude of the loss of taurine, but the elevation in betaine by itself made up for 24 % of the taurine loss, whereas the combination of glycerophosphocholine, choline and carnosine compensated for only 9 % of the taurine loss.
Although it is possible that a lower level of hepatic betaine could affect the remethylation of homocysteine to methionine, no differences in liver homocysteine and methionine levels are observed in Cdo1-null mice compared to wild-type mice or in either Cdo1-null mice or wild-type mice supplemented with taurine (Roman et al. 2013). Furthermore, in the metabolomics data collected in this study, there were no differences in hepatic methionine levels among any of the treatment groups (data not shown), whereas levels of choline and glycerol-3-phosphocholine were significantly higher in the taurine-deficient Cdo1-null mice (Table 2). Thus, data from the Cdo1-null mouse indicate that a 50 % decrease in BHMT does not have a major effect on transmethylation/transsulfuration pathways, at least when the diet contains adequate methionine and B-vitamins. This is notably in contrast to the consequences of a total loss of BTMT, as the Bhmt-null mouse has been reported to have a much higher fold elevation of hepatic betaine, elevated liver total homocysteine levels, low levels of choline and glycerophosphocholine in the liver, and fatty liver (Teng et al. 2011).
Because CSAD has been previously shown to be strongly upregulated in taurine-deficient cats and rodents (Rentschler et al. 1986; De La Rosa and Stipanuk 1985), sulfur-amino acid-deficient rodents (Bagley and Stipanuk 1994; Bella et al. 1999a, b) and in Cdo1-null mice (Roman et al. 2013; Ueki et al. 2011), it seems possible that the regulation of Csad and Bhmt expression in response to taurine status may share a common mechanism. Interestingly, in contrast to marked upregulation of Taut expression, Csad expression was not upregulated in rat kidney or whole rat brain in response to salt loading (Bitoun et al. 2001; Bitoun and Tappaz 2000), suggesting that a common mechanism would be unlikely to depend on changes in cell volume or osmolarity.
A common mechanism of regulation of Csad and Bhmt expression is suggested by recent reports of the effects of bile acids and of small heterodimer partner (Shp; also known as nuclear receptor subfamily 0, group b, member 2, or Nr0b2) on each of these proteins. Both Bhmt expression and Csad expression were lower in mice supplemented with dietary cholate but higher in mice that received cholestyramine, suggesting a link with bile acid metabolism (Kerr et al. 2013; Tsuchiya et al. 2015). Similarly, both Bhmt and Csad expression were shown to be suppressed by SHP (NR0B2). Hepatic Csad mRNA expression was increased and the hepatic concentration of hypotaurine, the product of the reaction catalyzed by CSAD, was elevated in Shp (Nr0b2)-null mice (Kerr et al. 2013). Similarly, hepatic Bhmt expression (mRNA) was 2.8-fold the wild-type level, BHMT protein abundance and activity were higher, and the level of betaine, the substrate of BHMT, was lower in liver of Shp-null mice (Tsuchiya et al. 2015). Shp re-expression in the Shp-null mice lowered the BHMT mRNA abundance and increased the betaine level in liver of the Shp-null mice (Tsuchiya et al. 2015). Although these reports suggest a possible common mechanism for regulation of Bhmt and Csad expression by bile acids that acts through SHP and although the Cdo1- and Csad-null mice used here would have alterations in their bile acid pools due to a lack of taurine for bile acid conjugation, this mechanism cannot account for our observations of regulation of Csad and Bhmt expression in opposite directions in response to taurine deficiency and taurine supplementation (i.e., an upregulation of Csad expression and downregulation of Bhmt expression in the face of taurine depletion).
Further insight into the effects of taurine depletion on regulation of CSAD and BHMT levels comes from the observations that the addition of taurine to primary hepatocytes from Cdo1-null mice did not return levels of these proteins to wild-type levels as occurred when taurine was included in the diet of whole mice. This was true despite taurine supplementation increasing cellular taurine levels in Cdo1-null hepatocytes to levels higher than those in unsupplemented wild-type cells and to levels similar to those in supplemented wild-type cells. These observations suggest that the upregulation of CSAD and the downregulation of BHMT in liver of Cdo1-null mice is likely not a direct response to hepatic taurine concentration but instead may depend on hormones or mediators released by other cell types in the liver or by nonhepatic tissues. It is possible, of course, that the composition of the culture medium could also obscure effects, particularly if they are mediated by changes in osmolyte levels.
To answer the question of whether CDO abundance is influenced by taurine status, separate from the well-established effects on cysteine on CDO abundance via regulation of its ubiquitination and degradation, we measured the abundance of CDO in liver of the Csad-null mice. No effect of taurine supplementation was observed in this model of taurine depletion, despite the fact that taurine supplementation restored hepatic taurine to wild-type levels. The lack of effect of taurine supplementation in this model suggests that taurine does not affect CDO levels in the mouse. Our results are consistent with previous observations for CDO activity in taurine-sufficient versus taurine-depleted cats (Rentschler et al. 1986) and with the similar levels of Cdo1 mRNA reported for the Csad-null mice used here (Park et al. 2014) but are in marked contrast to the eightfold increase in CDO abundance in response to taurine supplementation reported by Jiang et al. (2014) in a mouse model of homocystinuria (i.e., Cbs-null mice that carry two copies of the human CBS gene that are expressed at a low level). It should be noted that the homocystinuric mice had hepatic taurine levels that were more than 75 % those of wild-type or cysteine-supplemented mice and, thus, were not nearly as taurine deficient as the Cdo1- and Csad-null mice. Differences between the models might account for the different observations because our work suggests that the effect of taurine status on the regulation of CSAD and BHMT levels is complex and involves signaling events that occur in vivo but not in isolated hepatocytes. Nevertheless, our results in Cdo1- and Csad-null mice supports previously reported observations that taurine supplementation has no effect on CDO expression in wild-type mice and isolated hepatocytes (Kwon and Stipanuk 2001; Ueki et al. 2011).
In conclusion, we report that the mRNA and protein expression levels of hepatic BHMT, like hepatic CSAD, are markedly regulated in response to taurine intake in mice with genetic disruption of the taurine biosynthetic pathway. BHMT protein and mRNA abundances were downregulated to about 50 and 25 %, respectively, of wild-type levels in our severely taurine-deficient mice. Although CSAD expression has been shown to be regulated in a dose–response manner to dietary protein, which would be consistent with a dose–response to tissue taurine levels, (Stipanuk et al. 2002), a dose–response association of degree of taurine depletion and extent of BHMT reduction remains to be established.
In contrast to CSAD, CDO which catalyzes the first step of the taurine biosynthetic pathway was not upregulated in response to the severe taurine deficiency present in unsupplemented Cdo1- and Csad-null mice. This supports our earlier suggestion that CDO is regulated predominantly in response to cysteine concentration by a mechanism that serves to control body cysteine levels (Dominy et al. 2006a, b, 2008; Cresenzi et al. 2003; Stipanuk et al. 2004; Stipanuk et al. 2009).
In taurine-deficient mice, the downregulation of BHMT allows an increase in betaine concentration, which may be important for maintenance of a sufficient level of nonperturbing osmolytes in cells. In wild-type mice, such a regulatory mechanism could also contribute to the supply of cysteine for taurine biosynthesis by suppressing remethylation and enhancing transsulfuration of homocysteine. The failure of taurine addition to isolated hepatocytes to affect the altered expression of either BHMT or CSAD, despite restoration of normal taurine levels, however, suggests that the expression of these proteins is regulated by a mechanism that requires more than a change in hepatocyte taurine concentration. Further research is necessary to elucidate the mechanism by which BHMT and CSAD expression is regulated in response to taurine status.
Acknowledgments
Funding This project was supported by Grants DK056649, CA168997 and AI110613 from the National Institutes of Health and by the New York State Office for People with Developmental Disabilities. HJ was supported by a “Mobility Plus” fellowship from the Ministry of Science and Higher Education (MNISW), Republic of Poland. The content is solely the responsibility of the authors.
Abbreviations
- BHMT
Betaine:homocysteine methyltransferase
- CDO
Cysteine dioxygenase
- CSAD
Cysteine sulfinic acid decarboxylase
- SHP
Small heterodimer partner
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All experimental procedures involving Cdo1+/− mice and their offspring were conducted with the approval of the Cornell University Institutional Animal Care and Use Committee (#2009-0138). All experimental procedures involving Csad-null mice were conducted with the approval of the Institutional Animal Care and Use Committee of the New York State Institute for Basic Research in Developmental Disabilities (#392).
References
- Agca CA, Tuzcu M, Hayirli A, Sahin K. Taurine ameliorates neuropathy via regulating NF-κB and Nrf2/HO-1 signaling cascades in diabetic rats. Food Chem Toxicol. 2014;71:116–121. doi: 10.1016/j.fct.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Bagley PJ, Stipanuk MH. The activities of rat hepatic cysteine dioxygenase and cysteinesulfinate decarboxylase are regulated in a reciprocal manner in response to dietary casein level. J Nutr. 1994;124:2410–2421. doi: 10.1093/jn/124.12.410. [DOI] [PubMed] [Google Scholar]
- Bella DL, Hahn C, Stipanuk MH. Effects of nonsulfur and sulfur amino acids on the regulation of hepatic enzymes of cysteine metabolism. Am J Physiol. 1999a;277(1 Pt 1):E144–E153. doi: 10.1152/ajpendo.1999.277.1.E144. [DOI] [PubMed] [Google Scholar]
- Bella DL, Hirschberger LL, Hosokawa Y, Stipanuk MH. Mechanisms involved in the regulation of key enzymes of cysteine metabolism in rat liver in vivo. Am J Physiol. 1999b;276(2 Pt 1):E326–E335. doi: 10.1152/ajpendo.1999.276.2.E326. [DOI] [PubMed] [Google Scholar]
- Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitoun M, Tappaz M. Gene expression of taurine transporter and taurine biosynthetic enzymes in brain of rats with acute or chronic hyperosmotic plasma. A comparative study with gene expression of myo-inositol transporter, betaine transporter and sorbitol biosynthetic enzyme. Brain Res Mol Brain Res. 2000;77:10–18. doi: 10.1016/s0169-328x(00)00034-6. [DOI] [PubMed] [Google Scholar]
- Bitoun M, Levillain O, Tappaz M. Gene expression of the taurine transporter and taurine biosynthetic enzymes in rat kidney after antidiuresis and salt loading. Pflugers Arch. 2001;442:87–95. doi: 10.1007/s004240000506. [DOI] [PubMed] [Google Scholar]
- Burger-Kentischer A, Müller E, März J, Fraek ML, Thurau K, Beck FX. Hypertonicity-induced accumulation of organic osmolytes in papillary interstitial cells. Kidney Int. 1999;55:1417–1425. doi: 10.1046/j.1523-1755.1999.00382.x. [DOI] [PubMed] [Google Scholar]
- Cresenzi CL, Lee JI, Stipanuk MH. Cysteine is the metabolic signal responsible for dietary regulation of hepatic cysteine dioxygenase and glutamate cysteine ligase in intact rats. J Nutr. 2003;133:2697–2702. doi: 10.1093/jn/133.9.2697. [DOI] [PubMed] [Google Scholar]
- De La Rosa J, Stipanuk MH. The effect of taurine depletion with guanidinoethanesulfonate on bile acid metabolism in the rat. Life Sci. 1985;36:1347–1351. doi: 10.1016/0024-3205(85)90039-6. [DOI] [PubMed] [Google Scholar]
- Dominy JE, Jr, Hirschberger LL, Coloso RM, Stipanuk MH. In vivo regulation of cysteine dioxygenase via the ubiquitin-26S proteasome system. Adv Exp Med Biol. 2006a;583:37–47. doi: 10.1007/978-0-387-33504-9_4. [DOI] [PubMed] [Google Scholar]
- Dominy JE, Jr, Hirschberger LL, Coloso RM, Stipanuk MH. Regulation of cysteine dioxygenase degradation is mediated by intracellular cysteine levels and the ubiquitin-26 S proteasome system in the living rat. Biochem J. 2006b;394(Pt 1):267–273. doi: 10.1042/BJ20051510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominy JE, Jr, Hwang J, Guo S, Hirschberger LL, Zhang S, Stipanuk MH. Synthesis of amino acid cofactor in cysteine dioxygenase is regulated by substrate and represents a novel post-translational regulation of activity. J Biol Chem. 2008;283:12188–12201. doi: 10.1074/jbc.M800044200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallazzini M, Ferraris JD, Burg MB. GDPD5 is a glycerophosphocholine phosphodiesterase that osmotically regulates the osmoprotective organic osmolyte GPC. Proc Natl Acad Sci USA. 2008;105:11026–11031. doi: 10.1073/pnas.0805496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann L, Brauers G, Gehrmann T, Häussinger D, Mayatepek E, Schliess F, Schwahn BC. Osmotic regulation of hepatic betaine metabolism. Am J Physiol Gastrointest Liver Physiol. 2013;304:G835–G846. doi: 10.1152/ajpgi.00332.2012. [DOI] [PubMed] [Google Scholar]
- Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Ito T, Schaffer SW, Azuma J. The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids. 2012;42:1529–1539. doi: 10.1007/s00726-011-0883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Stabler SP, Allen RH, Abman SH, Maclean KN. Altered hepatic sulfur metabolism in cystathionine β-synthase-deficient homocystinuria: regulatory role of taurine on competing cysteine oxidation pathways. FASEB J. 2014;28:4044–4054. doi: 10.1096/fj.14-253633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr TA, Matsumoto Y, Matsumoto H, Xie Y, Hirschberger LL, Stipanuk MH, Anakk S, Moore DD, Watanabe M, Kennedy S, Davidson NO. Cysteine sulfinic acid decarboxylase regulation: a role for farnesoid X receptor and small heterodimer partner in murine hepatic taurine metabolism. Hepatol Res. 2013 doi: 10.1111/hepr.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YH, Stipanuk MH. Cysteine regulates expression of cysteine dioxygenase and gamma-glutamylcysteine synthetase in cultured rat hepatocytes. Am J Physiol Endocrinol Metab. 2001;280:E804–E815. doi: 10.1152/ajpendo.2001.280.5.E804. [DOI] [PubMed] [Google Scholar]
- Liu X, Ser Z, Locasale JW. Development and quantitative evaluation of a high-resolution metabolomics technology. Anal Chem. 2014;86:2175–2184. doi: 10.1021/ac403845u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong MC, Chao CY, Yin MC. Histidine and carnosine alleviated hepatic steatosis in mice consumed high saturated fat diet. Eur J Pharmacol. 2011;653:82–88. doi: 10.1016/j.ejphar.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Park E, Park SY, Dobkin C, Schuller-Levis G. Development of a novel cysteine sulfinic acid decarboxylase knockout mouse: dietary taurine reduces neonatal mortality. J Amino Acids. 2014 doi: 10.1155/2014/346809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentschler LA, Hirschberger LL, Stipanuk MH. Response of the kitten to dietary taurine depletion: effects on renal reabsorption, bile acid conjugation and activities of enzymes involved in taurine synthesis. Comp Biochem Physiol B. 1986;84:319–325. doi: 10.1016/0305-0491(86)90084-2. [DOI] [PubMed] [Google Scholar]
- Ripps H, Shen W. Review: taurine: a “very essential” amino acid. Mol Vis. 2012;18:2673–2686. [PMC free article] [PubMed] [Google Scholar]
- Roman HB, Hirschberger LL, Krijt J, Valli A, Kožich V, Stipanuk MH. The cysteine dioxgenase knockout mouse: altered cysteine metabolism in nonhepatic tissues leads to excess H2S/HS(−) production and evidence of pancreatic and lung toxicity. Antioxid Redox Signal. 2013;19:1321–1336. doi: 10.1089/ars.2012.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer C, Hoffmann L, Heldt K, Lornejad-Schäfer MR, Brauers G, Gehrmann T, Garrow TA, Häussinger D, Mayatepek E, Schwahn BC, Schliess F. Osmotic regulation of betaine homocysteine-S-methyltransferase expression in H4IIE rat hepatoma cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1089–G1098. doi: 10.1152/ajpgi.00088.2006. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Londono M, Lee JI, Hu M, Yu AF. Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J Nutr. 2002;132:3369–3378. doi: 10.1093/jn/132.11.3369. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Hirschberger LL, Londono MP, Cresenzi CL, Yu AF. The ubiquitin-proteasome system is responsible for cysteine-responsive regulation of cysteine dioxygenase concentration in liver. Am J Physiol Endocrinol Metab. 2004;286:E439–E448. doi: 10.1152/ajpendo.00336.2003. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Dominy JE, Jr, Ueki I, Hirschberger LL. Measurement of cysteine dioxygenase activity and protein abundance. Curr Protoc Toxicol. 2008;38:6.15.1–6.15.25. doi: 10.1002/0471140856.tx0615s38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanuk MH, Ueki I, Dominy JE, Jr, Simmons CR, Hirschberger LL. Cysteine dioxygenase: a robust system for regulation of cellular cysteine levels. Amino Acids. 2009;37:55–63. doi: 10.1007/s00726-008-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YW, Mehedint MG, Garrow TA, Zeisel SH. Deletion of betaine-homocysteine S-methyltransferase in mice perturbs choline and 1-carbon metabolism, resulting in fatty liver and hepatocellular carcinomas. J Biol Chem. 2011;286:36258–36267. doi: 10.1074/jbc.M111.265348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki I, Roman HB, Valli A, Fieselmann K, Lam J, Peters R, Hirschberger LL, Stipanuk MH. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am J Physiol Endocrinol Metab. 2011;301:E668–E684. doi: 10.1152/ajpendo.00151.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warskulat U, Heller-Stilb B, Oermann E, Zilles K, Haas H, Lang F, Häussinger D. Phenotype of the taurine transporter knockout mouse. Methods Enzymol. 2007;428:439–458. doi: 10.1016/S0076-6879(07)28025-5. [DOI] [PubMed] [Google Scholar]
- Wójcik OP, Koenig KL, Zeleniuch-Jacquotte A, Costa M, Chen Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis. 2010;208:19–25. doi: 10.1016/j.atherosclerosis.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol. 2005;208(Pt 15):2819–2830. doi: 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]
- Yancey PH, Blake WR, Conley J. Unusual organic osmolytes in deep-sea animals: adaptations to hydrostatic pressure and other perturbants. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:667–676. doi: 10.1016/s1095-6433(02)00182-4. [DOI] [PubMed] [Google Scholar]
- Tsuchiya H, Costa KA, Lee S, Renga B, Jaeschke H, Yang Z, Orena SJ, Goedken MJ, Zhang Y, Kong B, Lebofsky M, Rudraiah S, Smalling R, Guo G, Fiorucci S, Zeisel SH, Wang L. Interactions between nuclear receptor SHP and FOXA1 maintain oscillatory homocysteine homeostasis in mice. Gastroenterol. 2015;148:1012–1023. doi: 10.1053/j.gastro.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]


