Abstract
In recent years, the knowledge about the control of tumor microenvironment has increased and emerged as an important player in tumorigenesis. The role of normal stromal cells in the tumor initiation and progression has brought our vision in to the forefront of cell-to-cell communication. In this review, we focus on the mechanism of communication between stromal and tumor cells, which is based on the exchange of extracellular vesicles (EVs). We describe several, evergrowing, pieces of evidence that EVs transfer messages through their miRNA, lipid, protein and nucleic acid contents. A better understanding of this sophisticated method of communication between normal cancer cells may lead to developing novel approaches for personalized diagnostics and therapeutics.
Keywords: extracellular vesicles, exosomes, mesenchymal stem cells, microRNA, MSCs, Lipids, proteins, RNA, tumor microenvironment
One form of intercellular communication is through the exchange of secreted cell membrane fragments known as extracellular vesicles (EVs) into the extracellular space.1,2 The interest in understanding the role of EVs in cancer started in the late 1970s, when studies showed the secretion of EVs in both normal and cancer cells3–6. A correlation between elevated blood EVs levels in cancer patients7–9 and other studies implicated EVs as potential diagnosis markers for cancer.10–12 This has shifted recent research to focus on whether EVs play a supportive role in cancer pathology, including effects associated with cancer initiation, progression, angiogenesis and metastasis.13
An important factor in the support of the tumor microenvironment is the cell–cell communication between stromal cells and transformed cancer cells. The role of gap junctions in transport of cellular communicators14 and juxtacrine regulation based on direct communication is well documented.15,16 Recently, Tsuyada et al.17 demonstrated the cancer-stroma signaling circuit, in which breast cancer cells stimulate the expression of chemokine CCL2 in normal fibroblasts that become cancer activated fibroblasts. In turn, this stimulates the stemness of breast cancer cells constituting the cancer-stroma-cancer signaling circuit.18,19
Recent studies have demonstrated that EVs exchange cargo of small RNA, mRNA, proteins, lipids and other regulatory molecules, between breast cancer cells and stromal cells.20,21 They are recognized as being involved in regulating a variety of extracellular signals and paracrine signaling,22,23 including breast cancer invasiveness.24,25 A dynamic interaction between stromal cells, cancer cells and the tumor microenvironment facilitates tumor progression (Figs 1 and 2)
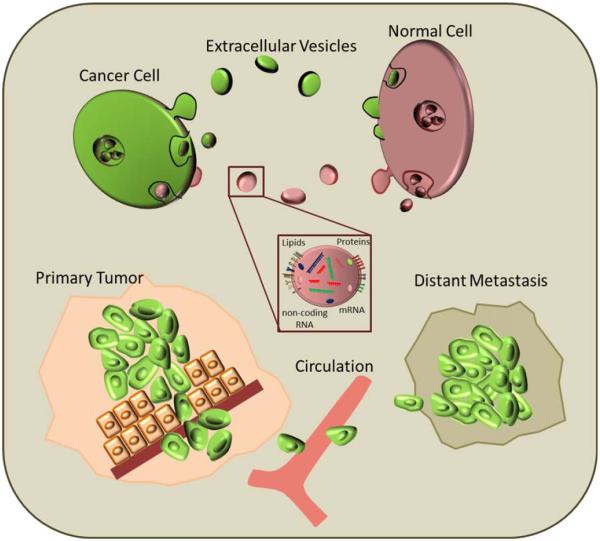
Figure 1.
Schematic representation of cellular cross talk in tumors: EVs are secreted by both cancer and normal cells either by budding directly from the plasma membrane or through invagination of the cellular membrane. This results in formation of EVs that contain cytoplasmic components like proteins, mRNA and other small noncoding RNA. Exchange of information between stem and cancer cells leads to proliferation of tumor at primary site but inhibits metastasis. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
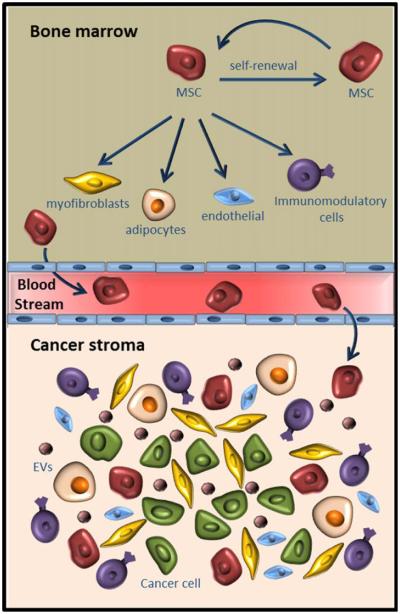
Figure 2.
Schematic representation of interactions between bone marrow niche (top) and cancer stroma (bottom). Bone marrow MSCs self-renew and also differentiate to various cell types that exhibit tumor supportive properties. MSCs are recruited from bone marrow to the tumor site through tumor derived soluble factors. MSCs secrete EVs containing various factors that support tumor progression. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Tumor Microenvironment
Stromal cells in the tumor microenvironment play a very important role in tumor progression. This microenvironment includes untransformed cells surrounding the tumor, which is composed of extracellular matrix (ECM) and numerous stromal cell types, including endothelial and inflammatory immune cells, fibroblasts, adipocytes and tumor-associated vasculature.26 Tumor malignancy is highly dependent on interactions between tumor cells and the tumor microenvironment.27 Studies on comprehensive gene expression and genomic profile study of epithelial, myoepithelial and stromal cells have revealed diverse microenvironments between normal breast tissue and breast carcinomas.28 Stromal elements secrete chemokines that act as paracrine factors that could induce ECM remodeling, enhance tumor cell proliferation and invasion.18,29 As an example of paracrine signaling in the tumor microenvironment, overexpression of the chemokines CXCL14 and CXCL12 in myoepithelial cells and myofibroblasts can enhance the proliferation, migration and invasion of breast cancer epithelial cells.18 Tumor stroma is also associated with therapeutic resistance and relapse—a main reason for breast cancer treatment failure.30,31
Mesenchymal Stem/Stromal Cells in Cancer
Mesenchymal stem/stromal cells (MSCs) are multipotent cells of nonhematopoietic origin and constitute a minor population (0.01%) of nucleated cells in bone marrow.32–34 MSCs are subsets of stromal cells and are known for their active mobilization from bone marrow and migration to sites of injury.35–37 Various reports suggested that bone marrowderived MSCs are preferentially recruited to the tumor surrounding stroma35 when compared to normal stroma,38 mainly by the inflammatory factors in the tumor microenvironment.39 These reports increased interest in understanding the potential role of MSCs in tumor progression. The MSCs recruited to the tumor microenvironment by various cytokines40–44 act as precursors for pericytes and cancer associated fibroblasts.44–46 MSCs promote tumor cell proliferation through their immunosuppressive properties and direct cell supportive properties.47,48 Earlier studies suggest that under nutrient-deprived conditions the MSCs associated with tumor stroma undergo autophagy, thereby facilitating tumor support through an anti-apoptotic secretome made of cytokines, growth factors and secreted vesicles such as EVs.49
Extracellular Vesicles—Definition
The word “extracellular vesicle” is actually a generic term that refers to a series of membrane-bound organelles, which are commonly distinguished by their size range. More specific nomenclature for EVs include exosomes (40–100 nm diameter), ectosomes (50–1,000 nm),50 argosomes51 and apoptotic bodies (50–5000 nm).52 There are problems in establishing a standard terminology in this field of research that have led to uncommon words such as “microparticles,”52 and even organ-specific classifications such as “prostasomes”53 used in literature. Any discrepancies between the characteristics of specific types of EVs is largely subject to debate, mainly due to the way these organelles are isolated (e.g., ultracentrifugation, use of a sucrose gradient, by biological markers),54 the precise context of study, or vesicle-specific properties.13 For the purposes of this review, EVs will be used for all organelles in this general category between 40 and 1,000 nm in diameter unless explicitly noted.
EVs are evolutionarily conserved, which suggests that they carry out important biological functions.52 Cells are known to secrete EVs due to factors such as environmental stress, cellular activation or apoptosis.2,13 The composition of EVs varies between cell types and environmental conditions, and a formal classification based on vesicles components is still being actively debated.36,55 As an example, exosomes can be characterized by membrane markers (CD63, CD81, CD9, TSG101 and Alix) though these markers are not exclusive to this type of EVs.11,56 Review article by van der Pol et al.57 elegantly describes other commonly used techniques to characterize exosomes, which include size distribution assays using optical microscopy and nonoptical microscopy based assays.58,59
Extracellular Vesicles—Genesis
At least three mechanisms for EVs generation have been proposed: (i) decay of dying cells into apoptotic bodies, (ii) cellular cell membrane blebbing ectosomes and (iii) inward budding of endosomal limiting membrane followed by emission of cell membrane into EVs.2,13,60–62 The result is outward budding and fission of vesicles from the tumor cell surface (Fig. 3). Some observations have also described a direct formation and release of EVs from cytoplasmic membrane budding of immune cells.63,64 The genesis of EVs that fall in the size of exosomes have been shown to occur both through inward budding of the endosomal limiting membrane to form multivesicular bodies (MVBs), which can fuse with the cell membrane and/or by budding off plasma membrane.63 The early stages of EVs synthesis starts with inward budding of the endosome membrane65; such enriched endosomes are referred to as MVBs. A fraction of such MVBs can fuse with the plasma membrane releasing EVs into the extracellular milieu as exosomes or alternatively the exosomes can be directly secreted into extracellular fluid.51
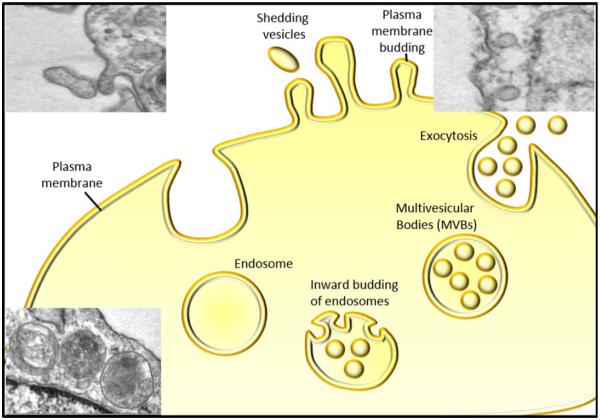
Figure 3.
Schematic representation of formation and release of EVs: in response to cell stimulus EVs are shed from cytoplasm by budding of plasma membrane of the cell. Inset 1 & 2 represents electron microscopy showing shedding and budding EVs, respectively. EVs generated through invagination of plasma membrane accumulated in the MVBs and are released by exocytosis. Inset 3 represent an electron microscopic image showing MVBs. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Extracellular Vesicles—Isolation
Several methods of isolation have been described ranging from ultracentrifugation, density gradient centrifugation, immunoaffinity capture using magnetic beads and commercially available precipitation methods. Comparative studies using these techniques demonstrated that the purity and the quality of preparations is dependent on the source of exosomes.66–69 Reports of EVs isolation, size, density and morphology should be interpreted with caution. Due to their small size and heterogeneity, conventional methods of classification for this type of biomolecule have proven to be difficult.11,55 EVs are hard to detect with basic light microscopy and flow cytometry because they are generally less than 200 nm in size. Several methods have been in use for isolation and purification of EVs, ranging from centrifugation techniques to antibody precipitation.16,70 Most commonly used is a differential ultracentrifugation including a sucrose density gradient.11,52,58 A recent study demonstrated that the g force and time of centrifugation significantly affect the quality of preparation.71 In addition, techniques such as these have been shown to change the size and morphology of EVs. For instance, while exosomes are frequently described as cupshaped in literature,58,72–74 Thery et al. demonstrated that this morphology was actually an artifact caused by the fixation process for transmission electron microscopy. In another study by Connor et al., repeated freeze-thaw cycles of plasma rich in platelets caused a considerable increase of annexin-V in EVs.75 The EVs count in a sample can vary with storage time, temperature, buffer composition and agitation. Moreover, the presence of fetal bovine serum (FBS) in culture media has many limitations with issues of contamination from EVs of foreign species.76 Such variability warrants a systematic and detailed methods section be supplemented with every publication.
Extracellular Vesicles—Cargo
Recent evidence shows that EVs can act as a unique vehicle for the release of soluble and insoluble molecules,2 including lipids, proteins and nucleic acids.10,77 EVs uptake by target cells may allow the exchange of these molecules from EVproducing cells. Such a mechanism would affect the target cell phenotype.2 EVs are enriched in lipids like ceramides, cholesterol and sphingomyelin, which promote vesicle release and play important roles in cell communication.78–82 More than 300 different proteins have been detected within EVs.83 The proteins reportedly belong, but are not limited, to families of surface receptors, signaling molecules and cell adhesion molecules.83,84 Nucleic acids such as DNA, mRNA and noncoding RNA (long noncoding RNA, tRNA and microRNA) have been reported in EVs.85–87 The mechanisms for EVs loading is unclear, however, a few types of proteins have been shown to be associated with MHC-II proteins that may play a role in protein sorting. For example, chaperones such as Hsc70, Hsp90, 14-3-3 epsilon and PKM2.88,89 Most proteins detected in exosomes are a class of proteins that lack secretory signal peptides, which are secreted through the ER-golgi pathway.90,91
MicroRNAs (miRNA) are noncoding small RNA (19–22 nucleotides in length) that play a wide spectrum of roles on both pre and post-transcriptional gene expression. miRNA are thought to regulate at least a third of the human genome by targeting mRNAs for degradation by the RISC complex, principally by targeting the 3’UTR coding mRNA92,93. Circulating miRNAs have been detected in various body fluids including serum, plasma, amniotic fluid, saliva, sweat, urine and milk.94 Data compilation shows that circulating miRNA are found both inside EVs secreted by all kind of cells or circulating miRNAs found bound to proteins. One example is miRNAs directly bind to Argonaute 2 (Ago2) proteins and form a very stable complex. Because of the high stability of the miRNA-Ago2 complex, it is challenging to trace the original source of these circulating miRNAs since these protein bound miRNA can be co-isolated in exosomal preparation making it difficult to interpret the data from plasma or serum derived exosomes.95
Single miRNAs have been identified to regulate the balance between normal and cancer cells. For example, the transfer of secreted miR-143 from normal prostate cells induces the growth inhibition of prostate cancer cells where miR-143 is downregulated.96 In another study, the EVs secreted by different cancer cell lines contain specific miRNAs (e.g., miR-9) that promote endothelial cell migration.97 A more recent study showed miR-23 inhibited metastasis and increased dormancy.98 The presence of miRNA into cancer cell-derived EVs seems to be driven selectively. For example, it has been shown that breast cancer cell lines secrete a variety of EVs containing more abundant and more diverse miRNA species compared to those secreted by normal epithelial cells.99,100 A recent study on quantitative analysis of miRNA content in exosomes suggest that most standard preparations of exosomes carry less than one miRNA molecule per exosome of any given miRNA. Therefore, standard preparations may not carry biologically significant numbers of miRNAs.101 Furthermore, Kalluri lab demonstrated that the exosomes from cancer cells are capable of synthesizing miRNA independent of originating cell.102
Horizontal transfer of mRNA and miRNA has been reported in numerous studies between normal cells,79,103–105 between embryonic stem cells,106,107 from MSCs to cancer cells,108 between cancer cells,25,109 from cancer to normal cells110 and from normal to cancer cells.25,109 This phenomenon indicates that transferred RNA may play a role in the regulation of gene expression in recipient cells. The ratio of RNA fragments found within EVs varies depending on the cell type from which the EVs originated.111
Evidence that the loading of miRNAs into EVs may not in fact be random, but instead controlled by specific proteins involved in the miRNA network, was demonstrated by Gibbings et al. in 2009, by demonstrating the presence of Ago2 protein and a noticeable enrichment of GW182 in purified EVs.112 A recent study also demonstrates that sumoylation of the ribonucleoprotein hnRNPA2B1 controls the sorting of miRNAs to exosomes.113
Extracellular Vesicles—Transfer
EVs secretion by most of the normal cell types is a regular physiological phenomenon and a mode of intercellular communication for cell growth and activation.52 The evidence that EVs were involved in cancer was first documented in patients with Hodgkin disease in the late 1970s.3 Since then, various studies have revealed the active involvement of EVs in different stages of cancer progression.114 In human breast cancer cell lines, there is a positive correlation between the amount of EVs released and the in vitro invasiveness of the cells.7 Similar results were observed in in vivo studies on ovarian cancer fluids.115 EVs secretion can provide either favorable or unfavorable features to cells depending on the contents of the EVs. Cancer cells can use EVs to evade protective mechanisms of the organism by inducing immune tolerance, expression of pro-apoptotic signals, extracellular matrix remodeling, drug resistance and in other various ways. EVs derived from antigen-presenting cells favor T cell activation.116 However, EVs secreted by cancer cells induce apoptosis in T cells, thereby promoting tumor cell survival.1,117 Cancer cells dispense caspase-3 through EVs, preventing its accumulation in cells that leads to apoptosis.118 EVs derived from cancer cells contain proteases and thereby increase the invasiveness of the cancer cells.119 Furthermore, EVs are shown to play a role in drug resistance in cancer cells through the transportation of multidrug resistant efflux pumps to other cancer cells in the surrounding environment, thus spreading drug resistance among cancer cells.120,121 In lung cancer models, an increased secretion of EVs containing VEGF and sphingomyelin under hypoxia conditions facilitates angiogenesis thereby rescuing the cancer cells from nutrient and oxygen deprivation.77
Extracellular Vesicles—Stromal Cell-Cancer Cell Crosstalk
Cancer cells actively interact with stromal cells through EVs. One study on invasive prostate cancer cell lines showed that cancer cells could not only activate fibroblasts in tumor stroma by secreting EVs, but also promote EVs release from these activated fibroblasts to advance their own migration and invasion.122 EVs contribute to the transformation of normal cells into cancer cells, as studies on breast carcinoma and glioma cells showed that EVs transfer tissue transglutaminase from cancer cells to both normal fibroblasts and epithelial cells.20 Similar to cancer cells, normal cells secrete EVs. Their function depends on the phenotype of the parent cells and the context. For example, EVs secreted by MSCs in breast cancers have been shown to be tumor supportive in primary tumor models and metastasis inhibitory in a metastatic model.123
Extracellular Vesicles—Regulatory Packages
Recent studies on miRNA sorting in EVs have indicated that the mature miRNA and its complementary miRNA are regulated. During active miRNA generation, the initial miRNA transcripts are processed by Drosha to produce miRNA hairpin precursors. Once exported from the nucleus by exportin-5, the primary miRNA hairpin precursors are cut by the endonuclease Dicer and released as short double stranded RNA molecules.124 Based on thermodynamic analysis of Watson-Crick terminal base pairs, one strand has generally been thought of as the active miRNA and the other strand is just a “passenger” (miRNA* also designated miRNA-3p or -5p depending on the context).125 Typically, the miRNA* strand is degraded, but recent analysis of nucleotide substitutions has implicated some miRNA* strands have a regulatory role.126 A recent publication demonstrating the presence of miRNA processing enzymes such as Dicer in exosomes further emphasizes the role of regulatory role of exosomes.102 Taken together, the evidence demonstrates a miRNA mediated regulatory role of EVs. However, other related studies demonstrate that functional properties vary significantly with the method of exosome preparations71 and the quantity of regulatory molecules loaded in exosomes should be factors of consideration.101
Further studies demonstrating the regulation induced by the uptake of secreted miRNA in the recipient cells have been reported. Rat gliosarcoma cells expressing an miRNA that lacks homology in rat cells were co-cultured with cells expressing a luciferase reporter encoding the target mRNA. The decrease in luciferase reporter activity that was observed was reversed with the addition of carbenoxolone, indicating that gap junction communication regulates intercellular transfer of miRNA.127 Other studies suggest the same miRNA transfer mechanism via gap junctions between cardiomyocytes in culture128 and between bone marrow stromal cells and a breast cancer cell line.21 Furthermore, transfer of functional miRNA from immune cells involving EVs was found to be a unidirectional and antigen-dependent driven mechanism. Targeting neutral sphingomyelinase-2 inhibited this transfer.79,129 miRNAs are well described to bind to the RISC protein complex130 but some miRNA have been reported to directly bind to other proteins, acting as a decoy and preventing the miRNA from blocking translation of mRNA.131 Fabbri et al. showed that EVs contain miRNAs that can reach and bind to Toll-like receptors (TLR)-containing endosomes in recipient cells, triggering a TLR-mediated prometastatic inflammatory response that may lead to tumor growth and metastasis.132 Thus, the role of transferred miRNAs secreted by donor cells can be not limited to post-transcriptional effects in the recipient cells but can also act as a paracrine signal.
Extracellular Vesicles—Metastasis
Metastasis is the leading cause of cancer death, yet it has been an enigma for researchers. It is considered a mechanistically inefficient process because of its dependence on very regulated and controlled systemic fueling. This premetastatic niche is presumed to play a role in dormancy, relapse and development of metastasis. An emerging role of EVs is formulating the premetastatic niche. Ghasemi et al. have termed these EVs “metastasomes” and hypothesized that they may aid foundation of the secondary lesions via a “malignant trait” spreading system that regulates the interactions between tumor tissue-specific RNA and the cell-type/tissuespecific RNA within the target organ, thus serving as tumororgan matchmakers.133 Recent studies have shown that these EVs are actually “customized” to the cancers. In studies comparing EVs from cancer cells and normal cells, the selectively exported miRNAs, whose release is increased in malignant cells, are packaged in structures that are different from those that carry neutrally released miRNAs.5,99
In closing, the recent discoveries on the study of tumor-derived EVs reveal new insights into the cellular basis of tumor stromal support. There is potential to translate this information into developing novel innovative approaches for cancer diagnostics and personalized therapy. The complexity and variety of the EV cargo implicates them in a multipronged approach toward tumor support, and hijacking their functions to engineer tumor-inhibitory EVs seems plausible. Most of the current knowledge is on the molecular profiling of the circulating EVs as biomarkers for cancers, which induces multiple platforms for personalized diagnostics. Recent literature demonstrates several possibilities of EVs as to whether the preparatory methods and studies performed are specific to the model system used. The debate is not easily resolved, but it stresses the importance of requiring in-process data for preparations and developing models to reconcile the differences in the observations related to the role of EVs in intercellular communication.
Footnotes
The authors disclose no potential conflicts of interest
References
- 1.Howlett AR, Bissell MJ. The influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium. Epithelial Cell Biol. 1993;2:79–89. [PubMed] [Google Scholar]
- 2.Lee TH, D’Asti E, Magnus N, et al. Microvesicles as mediators of intercellular communication in cancer–the emerging science of cellular ’debris’. Semin Immunopathol. 2011;33:455–67. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- 3.Friend C, Marovitz W, Henie G, et al. Observations on cell lines derived from a patient with Hodgkin’s disease. Cancer Res. 1978;38:2581–91. [PubMed] [Google Scholar]
- 4.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–39. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan BT, Teng K, Wu C, et al. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942–8. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trams EG, Lauter CJ, Salem N, Jr., et al. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 7.Ginestra A, La Placa MD, Saladino F, et al. The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res. 1998;18:3433–7. [PubMed] [Google Scholar]
- 8.Kim HK, Song KS, Park YS, et al. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer:possible role of a metastasis predictor. Eur J Cancer. 2003;39:184–91. doi: 10.1016/s0959-8049(02)00596-8. [DOI] [PubMed] [Google Scholar]
- 9.Zwicker JI, Liebman HA, Neuberg D, et al. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15:6830–40. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Souza-Schorey C, Clancy JW. Tumor-derived microvesicles:shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012;26:1287–99. doi: 10.1101/gad.192351.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Pol E, Boing AN, Harrison P, et al. classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 12.Kharaziha P, Ceder S, Li Q, Panaretakis T. Tumor cell-derived exosomes:a message in a bottle. Biochim Biophys Acta. 2012;1826:103–11. doi: 10.1016/j.bbcan.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Rak J. Microparticles in cancer. Semin Thromb Hemost. 2010;36:888–906. doi: 10.1055/s-0030-1267043. [DOI] [PubMed] [Google Scholar]
- 14.Kandouz M, Batist G. Gap junctions and connexins as therapeutic targets in cancer. Expert Opin Ther Targets. 2010;14:681–92. doi: 10.1517/14728222.2010.487866. [DOI] [PubMed] [Google Scholar]
- 15.Gilleron J, Carette D, Chevallier D, et al. Molecular connexin partner remodeling orchestrates connexin traffic:from physiology to pathophysiology. Crit Rev Biochem Mol Biol. 2012;47:407–23. doi: 10.3109/10409238.2012.683482. [DOI] [PubMed] [Google Scholar]
- 16.Mroue RM, El-Sabban ME, Talhouk RS. Connexins and the gap in context. Integr Biol (Camb) 2011;3:255–66. doi: 10.1039/c0ib00158a. [DOI] [PubMed] [Google Scholar]
- 17.Tsuyada A, Chow A, Wu J, et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. 2012;72:2768–79. doi: 10.1158/0008-5472.CAN-11-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Antonyak MA, Li B, Boroughs LK, et al. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci USA. 2011;108:4852–7. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim PK, Bliss SA, Patel SA, et al. Gap junctionmediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550–60. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 22.Luga V, Zhang L, Viloria-Petit AM, et al. Exosomes Mediate Stromal Mobilization of Auto-crine Wnt-PCP Signaling in Breast Cancer Cell Migration. Cell. 2012;151:1542–56. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Pap E. The role of microvesicles in malignancies. Adv Exp Med Biol. 2011;714:183–99. doi: 10.1007/978-94-007-0782-5_10. [DOI] [PubMed] [Google Scholar]
- 24.Janowska-Wieczorek A, Marquez-Curtis LA, Wysoczynski M, et al. Enhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cells. Transfusion. 2006;46:1199–209. doi: 10.1111/j.1537-2995.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang M, Chen J, Su F, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Artacho-Cordon A, Artacho-Cordon F, Rios-Arrabal S, et al. Tumor microenvironment and breast cancer progression:a complex scenario. Cancer Biol Ther. 2012;13:14–24. doi: 10.4161/cbt.13.1.18869. [DOI] [PubMed] [Google Scholar]
- 28.Louie E, Nik S, Chen JS, et al. Identification of a stem-like cell population by exposing metastatic breast cancer cell lines to repetitive cycles of hypoxia and reoxygenation. Breast Cancer Res. 2010;12:R94. doi: 10.1186/bcr2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 30.Cuiffo BG, Karnoub AE. Mesenchymal stem cells in tumor development:emerging roles and concepts. Cell Adh Migr. 2012;6:220–30. doi: 10.4161/cam.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao Y, Keller ET, Garfield DH, et al. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2012;32:303–15. doi: 10.1007/s10555-012-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pittenger AM, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 33.Smith JR, Pochampally R, Perry A, et al. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells. 2004;22:823–31. doi: 10.1634/stemcells.22-5-823. [DOI] [PubMed] [Google Scholar]
- 34.Wolfe M, Pochampally R, Swaney W, et al. Isolation and culture of bone marrow-derived human multipotent stromal cells (hMSCs) Methods Mol Biol. 2008;449:3–25. doi: 10.1007/978-1-60327-169-1_1. [DOI] [PubMed] [Google Scholar]
- 35.Hall B, Andreeff M, Marini F. The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. Handb Exp Pharmacol. 2007:263–83. doi: 10.1007/978-3-540-68976-8_12. [DOI] [PubMed] [Google Scholar]
- 36.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs):controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–46. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vallabhaneni KC, Tkachuk S, Kiyan Y, et al. Urokinase receptor mediates mobilization, migration, and differentiation of mesenchymal stem cells. Cardiovasc Res. 2011;90:113–21. doi: 10.1093/cvr/cvq362. [DOI] [PubMed] [Google Scholar]
- 38.Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–8. [PubMed] [Google Scholar]
- 39.Reddy BY, Lim PK, Silverio K, et al. The microenvironmental effect in the progression, metastasis, and dormancy of breast cancer:a model system within bone marrow. Int J Breast Cancer. 2012;2012:721659. doi: 10.1155/2012/721659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birnbaum T, Roider J, Schankin CJ, et al. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol. 2007;83:241–7. doi: 10.1007/s11060-007-9332-4. [DOI] [PubMed] [Google Scholar]
- 41.Dwyer RM, Potter-Beirne SM, Harrington KA, et al. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13:5020–7. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 42.Feng B, Chen L. Review of mesenchymal stem cells and tumors:executioner or coconspirator? Cancer Biother Radiopharm. 2009;24:717–21. doi: 10.1089/cbr.2009.0652. [DOI] [PubMed] [Google Scholar]
- 43.Schichor C, Birnbaum T, Etminan N, et al. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC) Exp Neurol. 2006;199:301–10. doi: 10.1016/j.expneurol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 44.Spaeth EL, Dembinski JL, Sasser AK, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PloS One. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hogan NM, Dwyer RM, Joyce MR, et al. Mesenchymal stem cells in the colorectal tumor microenvironment:recent progress and implications. Int J Cancer. 2012;131:1–7. doi: 10.1002/ijc.27458. [DOI] [PubMed] [Google Scholar]
- 46.Kidd S, Spaeth E, Klopp A, et al. The (in) auspicious role of mesenchymal stromal cells in cancer:be it friend or foe. Cytotherapy. 2008;10:657–67. doi: 10.1080/14653240802486517. [DOI] [PubMed] [Google Scholar]
- 47.Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–44. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 48.Zhu W, Xu W, Jiang R, et al. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80:267–74. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez CG, Penfornis P, Oskowitz AZ, et al. Activation of autophagy in mesenchymal stem cells provides tumor stromal support. Carcinogenesis. 2011;32:964–72. doi: 10.1093/carcin/bgr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cocucci E, Meldolesi J. Ectosomes. Curr Biol. 2011;21:R940–1. doi: 10.1016/j.cub.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Greco V, Hannus M, Eaton S. Argosomes:a potential vehicle for the spread of morphogens through epithelia. Cell. 2001;106:633–45. doi: 10.1016/s0092-8674(01)00484-6. [DOI] [PubMed] [Google Scholar]
- 52.Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane AR, current state-of-the-art:emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ronquist G. Prostasomes are mediators of intercellular communication:from basic research to clinical implications. J Intern Med. 2012;271:400–13. doi: 10.1111/j.1365-2796.2011.02487.x. [DOI] [PubMed] [Google Scholar]
- 54.Tauro BJ, Greening DW, Mathias RA, et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Mathivanan S, Ji H, Simpson RJ. Exosomes:extracellular organelles important in intercellular communication. J Proteom. 2010;73:1907–20. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Nabhan JF, Hu R, Oh RS, et al. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci USA. 2012;109:4146–51. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Pol E, Hoekstra AG, Sturk A, et al. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J Thromb Haemost. 2010;8:2596–607. doi: 10.1111/j.1538-7836.2010.04074.x. [DOI] [PubMed] [Google Scholar]
- 58.Thery C, Amigorena S, Raposo G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006:22. doi: 10.1002/0471143030.cb0322s30. Chapter 3:Unit 3. [DOI] [PubMed] [Google Scholar]
- 59.Vallabhaneni K PP, Dhule S, Guillonneau F, et al. Microvesicles from bone marrow mesenchymal stem/stromal cells transport tumor supportive microRNA, proteins and lipids. Oncotarget. 2014 doi: 10.18632/oncotarget.3211. Published December 31, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 61.Al-Nedawi K, Meehan B, Rak J. Microvesicles:messengers and mediators of tumor progression. Cell Cycle. 2009;8:2014–8. doi: 10.4161/cc.8.13.8988. [DOI] [PubMed] [Google Scholar]
- 62.Simpson RJ, Lim JW, Moritz RL, et al. Exosomes:proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267–83. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 63.Booth AM, Fang Y, Fallon JK, et al. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:923–35. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lenassi M, Cagney G, Liao M, et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD41 T cells. Traffic. 2010;11:110–22. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 66.Oksvold MP, Neurauter A, Pedersen KW. Magnetic bead-based isolation of exosomes. Methods Mol Biol. 2015;1218:465–81. doi: 10.1007/978-1-4939-1538-5_27. [DOI] [PubMed] [Google Scholar]
- 67.Van Deun J, Mestdagh P, Sormunen R, et al. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracellular Vesicles. 2014:3. doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lv LL, Cao Y, Liu D, et al. Isolation and quantification of microRNAs from urinary exosomes/ microvesicles for biomarker discovery. Int J Biol Sci. 2013;9:1021–31. doi: 10.7150/ijbs.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rekker K, Saare M, Roost AM, et al. Comparison of serum exosome isolation methods for microRNA profiling. Clin Biochem. 2014;47:135–8. doi: 10.1016/j.clinbiochem.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 70.Gyorgy B, Modos K, Pallinger E, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117:e39–48. doi: 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]
- 71.Jeppensen DK, Hvam ML, Primdahl-Bengtson B, et al. Comparative analysis odf discrete exosome fractions obtained by differential centrifugation. J Extracellular Vesicles. 2014;3 doi: 10.3402/jev.v3.25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hosseini-Beheshti E, Pham S, Adomat H, et al. Exosomes as biomarker enriched microvesicles:characterization of exosomal proteins derived from a panel of prostate cell lines with distinct AR phenotypes. Mol Cell Proteomics. 2012;11:863–85. doi: 10.1074/mcp.M111.014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kesimer M, Scull M, Brighton B, et al. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium:a possible role in innate defense. FASEB J. 2009;23:1858–68. doi: 10.1096/fj.08-119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lau CS, Wong DT. Breast cancer exosome-like microvesicles and salivary gland cells interplay alters salivary gland cell-derived exosome-like microvesicles in vitro. PloS One. 2012;7:e33037. doi: 10.1371/journal.pone.0033037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Connor DE, Exner T, Ma DD, et al. The majority of circulating platelet-derived microparticles fail to bind annexin AR, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost. 2010;103:1044–52. doi: 10.1160/TH09-09-0644. [DOI] [PubMed] [Google Scholar]
- 76.Shelke GV, Lasser C, Gho YS, et al. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracellular Vesicles. 2014;3 doi: 10.3402/jev.v3.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muralidharan-Chari V, Clancy JW, Sedgwick A, et al. Microvesicles:mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123:1603–11. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beloribi S, Ristorcelli E, Breuzard G, et al. Exosomal lipids impact notch signaling and induce death of human pancreatic tumoral SOJ-6 cells. PloS One. 2012;7:e47480. doi: 10.1371/journal.pone.0047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kosaka N, Iguchi H, Yoshioka Y, et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–52. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marsh M, van Meer G. Cell biology. No ESCRTs for exosomes. Science. 2008;319:1191–2. doi: 10.1126/science.1155750. [DOI] [PubMed] [Google Scholar]
- 81.Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–7. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 82.Wang G, Dinkins M, He Q, et al. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4):potential mechanism of apoptosis induction in Alzheimer disease (AD) J Biol Chem. 2012;287:21384–95. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mathivanan S, Fahner CJ, Reid GE, et al. ExoCarta 2012:database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–4. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim HS, Choi DY, Yun SJ, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11:839–49. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- 85.Khatua AK, Taylor HE, Hildreth JE, et al. Inhibition of LINE-1 and Alu retrotransposition by exosomes encapsidating APOBEC3G and APO- BEC3F. Virology. 2010;400:68–75. doi: 10.1016/j.virol.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peters DL, Pretorius PJ. Origin, translocation and destination of extracellular occurring DNA– a new paradigm in genetic behaviour. Clin Chim Acta. 2011;412:806–11. doi: 10.1016/j.cca.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 87.Vickers KC, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol. 2012;23:91–7. doi: 10.1097/MOL.0b013e328350a425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buschow ST, van Balkom BW, Aalberts M, et al. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol Cell Biol. 2010;88:851–6. doi: 10.1038/icb.2010.64. [DOI] [PubMed] [Google Scholar]
- 89.Geminard C, De Gassart A, Blanc L, et al. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic. 2004;5:181–93. doi: 10.1111/j.1600-0854.2004.0167.x. [DOI] [PubMed] [Google Scholar]
- 90.Rajendran L, Honsho M, Zahn TR, et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. ProcNatl Acad Sci USA. 2006;103:11172–7. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nickel W. Unconventional secretory routes:direct protein export across the plasma membrane of mammalian cells. Traffic. 2005;6:607–14. doi: 10.1111/j.1600-0854.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 92.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 93.He L, Hannon GJ. MicroRNAs:small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 94.Blondal S, Jensby Nielsen S, Baker A, et al. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods. 2012;59:S1–6. doi: 10.1016/j.ymeth.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 95.Eldh M, Lotvall J, Malmhall C, et al. Importance of RNA isolation methods for analysis of exosomal RNA:evaluation of different methods. Mol Immunol. 2012;50:278–86. doi: 10.1016/j.molimm.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 96.Kosaka N, Iguchi H, Yoshioka Y, et al. Competitive interactions of cancer cells and normal cells via secretory microRNAs. J Biol Chem. 2012;287:1397–405. doi: 10.1074/jbc.M111.288662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhuang G, Wu X, Jiang Z, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31:3513–23. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ono M, Kosaka N, Tominaga N, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Science Signal. 2014;7:ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 99.Palma J, Yaddanapudi SC, Pigati L, et al. MicroRNAs are exported from malignant cells in customized particles. Nucleic Acids Res. 2012;40:9125–38. doi: 10.1093/nar/gks656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pigati L, Yaddanapudi SC, Iyengar R, et al. Selective release of microRNA species from normal and malignant mammary epithelial cells. PloS One. 2010;5:e13515. doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chevillet JR, Kang Q, Ruf IK, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci USA. 2014;111:14888–93. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Melo SA, Sugimoto H, O’Connell JT, et al. Cancer Exosomes Perform Cell-Independent MicroRNA Biogenesis and Promote Tumorigenesis. Cancer Cell. 2014;26:707–21. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors:evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–56. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 104.Weber C, Schober A, Zernecke A. MicroRNAs in arterial remodelling, inflammation and atherosclerosis. Curr Drug Targets. 2010;11:950–6. doi: 10.2174/138945010791591377. [DOI] [PubMed] [Google Scholar]
- 105.Zernecke A, Bidzhekov K, Noels H, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 106.Koh W, Sheng CT, Tan B, et al. Analysis of deep sequencing microRNA expression profile from human embryonic stem cells derived mesenchymal stem cells reveals possible role of let-7 microRNA family in downstream targeting of hepatic nuclear factor 4 alpha. BMC Genom. 2010;11(Suppl1):S6. doi: 10.1186/1471-2164-11-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yuan A, Farber EL, Rapoport AL, et al. Transfer of microRNAs by embryonic stem cell microvesicles. PloS One. 2009;4:e4722. doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gregory LA, Ricart RA, Patel SA, et al. microRNAs, Gap Junctional Intercellular Communication and Mesenchymal Stem Cells in Breast Cancer Metastasis. Curr Cancer Ther Rev. 2011;7:176–83. doi: 10.2174/157339411796234915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Y, Liu D, Chen X, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–44. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 111.Chiba M, Kimura M, Asari S. Exosomes secreted from human colorectal cancer cell lines contain AR, microRNAs and natural antisense RNAs, that can transfer into the human hepatoma HepG2 and lung cancer A549 cell lines. Oncol Rep. 2012;28:1551–8. doi: 10.3892/or.2012.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gibbings DJ, Ciaudo C, Erhardt M, et al. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143–9. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 113.Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van Doormaal FF, Kleinjan A, Di Nisio M, et al. Cell-derived microvesicles and cancer. Neth J Med. 2009;67:266–73. [PubMed] [Google Scholar]
- 115.Ginestra A, Miceli D, Dolo V, et al. Membrane vesicles in ovarian cancer fluids:a new potential marker. Anticancer Res. 1999;19:3439–45. [PubMed] [Google Scholar]
- 116.van Niel G, Porto-Carreiro I, Simoes S, et al. Exosomes:a common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 117.Andreola G, Rivoltini L, Castelli C, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–16. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abid Hussein MN, Boing AN, Sturk A, et al. Inhibition of microparticle release triggers endothelial cell apoptosis and detachment. Thromb Haemost. 2007;98:1096–107. doi: 10.1160/th05-04-0231. [DOI] [PubMed] [Google Scholar]
- 119.Hotary KB, Allen ED, Brooks PC, et al. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the threedimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 120.Ambudkar SV, Sauna ZE, Gottesman MM, et al. A novel way to spread drug resistance in tumor cells:functional intercellular transfer of P-glycoprotein (ABCB1) Trends Pharmacol Sci. 2005;26:385–7. doi: 10.1016/j.tips.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Levchenko A, Mehta BM, Niu X, et al. Intercellular transfer of P-glycoprotein mediates acquired multidrug resistance in tumor cells. Proc Natl Acad Sci USA. 2005;102:1933–8. doi: 10.1073/pnas.0401851102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Castellana D, Zobairi F, Martinez MC, et al. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche:a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 2009;69:785–93. doi: 10.1158/0008-5472.CAN-08-1946. [DOI] [PubMed] [Google Scholar]
- 123.Katsuda T, Kosaka N, Takeshita F, et al. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13:1637–53. doi: 10.1002/pmic.201200373. [DOI] [PubMed] [Google Scholar]
- 124.Lund E, Guttinger S, Calado A, et al. Nuclear export of microRNA precursors. Science. 2004;303:95–8. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 125.O’Toole AS, Miller S, Haines N, et al. Comprehensive thermodynamic analysis of 3’ double-nucleotide overhangs neighboring Watson-Crick terminal base pairs. Nucleic Acids Res. 2006;34:3338–44. doi: 10.1093/nar/gkl428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo L, Lu Z. The fate of miRNA* strand through evolutionary analysis:implication for degradation as merely carrier strand or potential regulatory molecule? PloS One. 2010;5:e11387. doi: 10.1371/journal.pone.0011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Katakowski M, Buller B, Wang X, et al. Functional microRNA is transferred between glioma cells. Cancer Res. 2010;70:8259–63. doi: 10.1158/0008-5472.CAN-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kizana E, Cingolani E, Marban E. Non-cell-autonomous effects of vector-expressed regulatory RNAs in mammalian heart cells. Gene Ther. 2009;16:1163–8. doi: 10.1038/gt.2009.64. [DOI] [PubMed] [Google Scholar]
- 129.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fabian MR, Sonenberg N. The mechanics of miRNA-mediated gene silencing:a look under the hood of miRISC. Nat Struct Mol Biol. 2012;19:586–93. doi: 10.1038/nsmb.2296. [DOI] [PubMed] [Google Scholar]
- 131.Eiring AM, Harb JG, Neviani P, et al. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell. 2010;140:652–65. doi: 10.1016/j.cell.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fabbri M, Paone A, Calore F, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Nl Acad Sci USA. 2012;109:E2110–6. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ghasemi R, Grassadonia A, Tinari N, et al. Tumor-derived microvesicles:the metastasomes. Med Hypotheses. 2013;80:75–82. doi: 10.1016/j.mehy.2012.10.011. [DOI] [PubMed] [Google Scholar]