Abstract
Objective
The salience network, an intrinsic brain network thought to modulate attention to internal versus external stimuli, has been consistently found to be atypical in autism spectrum disorders (ASD). However, little is known about how this altered resting-state connectivity relates to brain activity during information processing, which has important implications for understanding sensory over-responsivity (SOR), a common and impairing condition in ASD related to difficulty downregulating brain responses to sensory stimuli. This study examined how SOR in youth with ASD relates to atypical salience network connectivity and whether these atypicalities are associated with abnormal brain response to basic sensory information.
Method
Functional magnetic resonance imaging was used to examine how parent-rated SOR symptoms related to salience network connectivity in 61 youth (age 8–17; 28 ASD, 33 IQ-matched typically developing). Correlations between resting-state salience network connectivity and brain response to mildly aversive tactile and auditory stimuli were examined.
Results
SOR in youth with ASD was related to increased resting-state functional connectivity between salience network nodes and brain regions implicated in primary sensory processing and attention. Further, the strength of this connectivity at rest was related to extent of brain activity in response to auditory and tactile stimuli.
Conclusion
Results support an association between intrinsic brain connectivity and specific atypical brain responses during information processing. Additionally, findings suggest that basic sensory information is overly salient to individuals with SOR, leading to over-attribution of attention to this information. Implications for intervention include incorporating sensory coping strategies into social interventions for individuals with SOR.
Keywords: autism spectrum disorders, fMRI, resting-state, salience network, sensory over-responsivity
INTRODUCTION
Patterns of large-scale brain network organization during rest are thought to provide insight into intrinsic brain network abnormalities that underlie neuropsychiatric disorders, including autism spectrum disorders (ASD).1–4 Resting-state functional connectivity methods reveal co-activating brain regions during rest, and have been used to identify widely replicated large-scale neural networks in typical populations,5 as well as abnormalities in these networks in individuals with psychiatric disorders.6 ASD has been consistently linked to altered resting-state connectivity, particularly in the salience network,2 a brain network that is thought to be involved in an individual’s (conscious or unconscious) decisions about which of many internal and environmental stimuli are most important to attend to. As such, the salience network has been shown to play a role in switching between internally (e.g., default mode) and externally focused networks (e.g., central executive).7,8 Individuals with ASD have extremely high rates of sensory dysregulation problems, including sensory over-responsivity (SOR), which is an extreme negative reaction to or avoidance of sensory stimuli such as touch, sound, or busy visual environments. Recent neuroimaging research suggests that SOR may be related to an over-attribution of salience to extraneous sensory information.9,10 Thus, in the present study we sought to examine how intrinsic connectivity in the salience network relates to SOR by integrating resting-state functional magnetic resonance imaging (fMRI), task-based fMRI, and behavioral data, to test the assumption that atypical functional connectivity in ASD is directly related to altered brain activity during information processing, as well as to provide insight into SOR, an important source of heterogeneity and impairment in ASD that has as yet received little attention.
The salience network has been found to be hyperconnected in children and adolescents with ASD,2 and, compared to other resting-state networks, shows the highest accuracy in classifying participants with ASD from typically developing (TD) participants.2 The anterior insula, a region central to perception of emotionally salient information, serves as the hub of the salience network, with connections to many other brain regions, including anterior cingulate cortex, temporal poles, dorsolateral prefrontal cortex, amygdala, and other insular and opercular regions.11 Salience network abnormalities during resting state are thought to underlie some of the difficulties intrinsic to ASD, such as atypical allocation of attention to extraneous sensory stimuli rather than relevant social stimuli.2 Consistent with this hypothesis, the anterior insula has been found to be hypoactive in response to social cognition tasks in ASD but hyperactive in response to basic sensory information.10,12 However, to date there is little research on how differences in brain network organization in ASD relate to within-group heterogeneity in brain function during information processing, and none to our knowledge has focused on the salience network.
Atypical functional connectivity in the salience network may be particularly important to understanding altered sensory processing in ASD. At least 56–70% of children and adolescents with ASD meet criteria for SOR,13,14 which is associated with greater functional impairment, deficits in social and adaptive skills, and anxiety.15–17 Recent fMRI research suggests that a subset of individuals with ASD who have SOR have over-reactive responses to sensory input in primary sensory processing areas of the brain, as well as in brain areas related to affective valence, salience, and attention. These individuals also show decreased neural habituation to sensory stimuli and lack prefrontal inhibition of the amygdala during exposure to sensory stimuli.10 Taken together, these results suggest that SOR may be related to overattribution of salience to extraneous sensory information, as well as to an inability to down-regulate the brain’s responses to this information, thus leading to over-attention towards sensory information. Therefore, we hypothesized that within youth with ASD, individual differences in SOR would be related to extent of connectivity with the salience network, particularly that higher SOR would be related to greater connectivity within the salience network (i.e., with the amygdala), and between the salience network and areas related to primary sensory processing. We also predicted that this increased connectivity would be related to increased activation of these areas during exposure to sensory information.
The amygdala plays an important role in determining what is salient, detecting important environmental stimuli, and triggering an affective response to these stimuli.18–20 In one of the few studies of whole-brain amygdala connectivity in ASD, the amygdala was found to have significantly greater connectivity with salience network regions (e.g., insula, inferior frontal gyrus, pregenual cingulate) during attention to eye gaze that was incongruent with a simple attention task.21 The authors hypothesized that increased attribution of salience to socially incongruent or irrelevant information could contribute to deficits in social interaction. This is consistent with findings that in individuals with ASD and SOR, the amygdala shows relatively increased activation in response to basic sensory information and lacks prefrontal regulation.10 Given the role of the amygdala in SOR, we hypothesized that individuals with greater levels of SOR would have greater connectivity between the anterior insula and amygdala during resting state, and that this would relate to greater amygdala response to sensory input.
METHOD
Participants
Participants were 28 youth with ASD and 33 TD-matched controls aged 7.8–17.3 years (M=12.94; SD=2.6). All had full-scale, verbal, and performance IQs of 75 or greater based on the Wechsler Abbreviated Scales of Intelligence (WASI,22) or the Wechsler Intelligence Scale for Children–4th Edition (WISC-IV23). Participants with ASD had a prior diagnosis of autism spectrum disorder, confirmed using the Autism Diagnostic Interview–Revised (ADI-R24) and the Autism Diagnostic Observation Schedule–2nd Edition (ADOS-225). Of the original participants, 37 were TD and 31 had ASD. Three ASD and 4 TD participants were excluded due to insufficient motion-free data (>20 volumes scrubbed). Final groups did not differ significantly in age, IQ, or motion during fMRI (see Table 1). No participants reported loss of consciousness for longer than 5 minutes or any neurological (e.g., epilepsy), genetic (e.g., Fragile X), or severe psychiatric disorder (e.g., schizophrenia) other than autism. Additionally, no TD participants had comorbid psychiatric disorders (e.g., attention-deficit/hyperactivity disorder [ADHD], mood disorders, anxiety). All parents provided written informed consent, and children gave verbal assent. All study procedures were approved by the University of California, Los Angeles (UCLA) Institutional Review Board.
Table 1.
Descriptive Statistics
| ASDa Mean (SD) |
TDa Mean (SD) |
t or χ2 | ASD-funcb Mean (SD) |
|
|---|---|---|---|---|
| Age | 12.95 (1.98) | 12.93 (2.98) | −.04 | 13.84 (1.36) |
| Gender, n (% male) | 27 (96) | 28 (85) | 2.29 | 16 (94) |
| Handedness, n (% R) | 24 (86) | 30 (91) | 0.40 | 16 (94) |
| FSIQ | 103.68 (14.37) | 107.73 (12.25) | 1.19 | 101.76 (16.20) |
| VIQ | 102.18 (14.10) | 106.88 (11.63) | 1.43 | 101.47 (14.71) |
| PIQ | 108.50 (14.90) | 107.73 (12.25) | −0.41 | 108.47 (14.71) |
| Mean Absolute Motion | 0.31 (.13) | 0.31 (.25) | 0.09 | 0.30 (.16) |
| Max Absolute Motion | 0.95 (.57) | 0.76 (.57) | −1.32 | 0.83 (.40) |
| Mean Relative motion | 0.09 (.04) | 0.07 (.04) | −1.77+ | 0.08 (.03) |
| Max Relative Motion | 0.75 (.53) | 0.58 (68) | −1.06 | 0.72 (.45) |
| SensOR tactile count | 3.96 (4.15) | 1.70 (2.54) | −2.52** | 4.12 (4.77) |
| SensOR auditory count | 4.14 (5.71) | 1.24 (2.98) | −2.42* | 4.71 (6.50) |
| SensOR visual count | 0.82 (1.49) | 0.21 (.74) | −1.97+ | 0.82 (1.51) |
| SSP auditory/visual | 19.11 (5.10) | 23.52 (2.82) | 4.05*** | 19.76 (5.29) |
| SSP tactile sensitivity | 27.54 (6.36) | 32.23 (4.52) | 3.23** | 27.59 (6.64) |
| SOR composite | 0.37 (1.01) | −0.43 (.61) | −3.64** | 0.37 (0.97) |
| SCARED anxiety total | 13.54 (11.68) | 7.52 (6.94) | −2.39* | 12.35 (8.72) |
Note. ASD = autism spectrum disorder; FSIQ = Full-Scale IQ; PIQ = Performance IQ; R = right-handed; SCARED = Screen for Child Anxiety Related Emotional Disorders; SensOR = Sensory Over-Responsivity Inventory; SSP = Short Sensory Profile; TD = typically developing; VIQ = Verbal IQ.
n=28 ASD, 33 TD except for SSP analyses where n=28 ASD, 31 TD.
n=17 with ASD participating in sensory exposure scan.
p<.10;
p<.01;
p<.001.
Behavioral Measures
Diagnostic and cognitive measures were administered at a clinical assessment visit; child anxiety and sensory questionnaires were completed by parents (see Table 1). Handedness was measured using the Edinburgh Inventory.26 An SOR composite score was created by standardizing and averaging relevant subscales of the SOR measures (Short Sensory Profile [SSP] auditory/visual sensitivity, tactile sensitivity scales, and Sensory Over-Responsivity [SensOR] auditory, visual, and tactile scores) across all participants.
Screen for Child Anxiety Related Emotional Disorders (SCARED27)
The SCARED is a 41-item parent report form of child anxiety symptoms. The total score was used as a continuous measure of anxiety symptom severity. The SCARED has good internal consistency, test-retest reliability, and discriminative validity,27 and has been well validated with ASD populations.28,29
Short Sensory Profile.30
The SSP is a widely used, parent report measure of sensory dysregulation across modalities. We used the Auditory/Visual and Tactile Sensitivity subscales. Higher scores on the SSP indicate lower impairment. This measure has strong reliability and validity.31
Sensory Over-Responsivity Inventory.32
The SensOR Inventory is a parent checklist of sensory sensations that bother their child. For this study, the auditory, visual, and tactile subscales were used. The number of items parents rate as bothering their child has been shown to discriminate between children with and without SOR.32
SOR Composite
An SOR composite score was created by standardizing (creating Z-scores) and averaging each relevant subscale of the SOR measures (SSP reverse-scored auditory/visual sensitivity and tactile sensitivity scales and SensOR Inventory auditory, visual, and tactile scores).9,10 Two TD children did not have SSP scores; for these children, their SOR composite was made up of only SensOR scores.
MRI Data Acquisition
MRI data were acquired on a Siemens Trio 3 Tesla magnetic resonance imaging scanner. A high-resolution structural T2-weighted echo-planar imaging volume (spin-echo, TR=5000 ms, TE=33 ms, 128×128 matrix, 20cm FOV, 36 slices, 1.56mm in-plane resolution, 3mm thick) was acquired coplanar to the functional scans in order to ensure identical distortion characteristics to the fMRI scan. Resting-state runs involved the acquisition of 120 EPI volumes (TR=3000ms, TE=28ms, 64×64 matrix, 19.2cm FOV, 34 slices, 3.0mm in-plane resolution, 3mm thick). Each sensory paradigm run involved the acquisition of 137 EPI volumes (gradient-echo, TR=2500ms, TE=30ms, flip angle=90, 64×64 matrix, 20cm FOV, 33 slices, 3.125mm in-plane resolution, 3mm thick). Auditory stimuli were presented to the participant using magnet-compatible headphones under computer control (Resonance Technologies, Inc.). Participants wore earplugs and headphones to reduce interference of the auditory stimuli from the scanner noise.
FSL’s fMRI Expert Analysis Tool (FEAT), Version 5.98 was used for statistical analyses. Higher-level group analyses were carried out using FSL’s FLAME (FMRIB’s Local Analysis of Mixed Effects State) stage 1.33–35
fMRI Resting State Paradigm and Analysis
Participants were instructed to relax and keep their eyes open and focused on a fixation cross for 6 min. There is evidence that resting state connectivity is consistent whether participants have their eyes closed or open, but that there is greater reliability when a fixation cross is used36,37 with eyes open.
Preprocessing procedures are described in Supplement 1, available online.
To examine whole-brain connectivity with the salience network, we selected a 5-mm spherical seed in the right anterior insula (AI) based on Seeley et al.11 (Montreal Neurological Institute [MNI] coordinates 38, 26, −10). We extracted region-of-interest (ROI) time-series from each participant’s processed residuals in standard space and correlated them with every voxel in the brain to generate connectivity maps for each participant and ROI. Individual correlation maps were then converted into z-statistic maps using Fischer’s r-to-z transformation. At the group level, we modeled a paired-sample mixed-effects design (Z>2.3, corrected for multiple comparisons at the cluster level p<.05), and included SOR composite scores as a regressor to determine resting state connectivity as a function of SOR severity. Participants’ ages and parent-reported SCARED anxiety total scores were entered as covariates to determine the relationship between SOR and resting-state connectivity over and above age and anxiety. Anxiety was included as a covariate due to the high correlations between anxiety and SOR.38
While not a primary focus of this paper, we also examined group differences in salience network connectivity to confirm that the current sample was consistent with previous samples in basic salience network connectivity. Within-group maps were thresholded at Z>2.3, corrected for multiple comparisons at p<.05; between-group comparisons were thresholded at Z>1.7, corrected.
fMRI Sensory Paradigm
This paradigm was initially analyzed in Green et al.10 Participants were exposed to three stimulus conditions in a counterbalanced block design paradigm: an auditory, a tactile, and a “joint” condition where the auditory and tactile stimuli were presented simultaneously. The auditory stimuli consisted of traffic noises. The tactile stimulus was a scratchy wool fabric rubbed on participants’ inner arms at the rate of one stroke/sec. Stimuli were chosen that best differentiated ASD versus TD groups based on pilot testing with the SensOR Inventory. Participants were instructed to focus on a central fixation cross throughout the task. Each condition was presented 4 times, lasting 15 sec, with 12.5 sec of fixation between trials. Total scan length was 5 min, 42.5 sec including 12.5-sec initial and final fixations. See Green et al.10 for full details on this paradigm and data analysis. Seventeen participants with ASD and good resting state data (14 of which were included in the original Green et al. paper10) also had this sensory functional data and were included in the following analyses. For the purposes of this study, we extracted each participant’s parameter estimates of percent signal change in the amygdala and primary somatosensory and auditory cortices during the joint condition (as compared to fixation). The joint condition was chosen because youth with ASD and SOR were found to have the greatest differences from the comparison groups in this condition10. We used a functional ROI approach, extracting parameter estimates from all regions of the amygdala that were significantly activated in either group at a threshold of Z>1.7, p<.05 corrected for multiple comparisons. Due to the large size of the somatosensory and auditory cortices, we used a slightly different approach: we added together spheres (5-mm for somatosensory, 6-mm for auditory) around the peak coordinates in the postcentral gyrus and superior temporal gyrus, respectively, in each (ASD and TD) group, thus creating an ellipse around the area of peak activation for both groups.
RESULTS
Within- and Between-Group Salience Network Connectivity
Overall, both the TD and ASD groups showed salience network connectivity consistent with previous studies, with extensive anterior insula (AI) connectivity with anterior cingulate, fronto-insular, and limbic regions. Between-group comparisons showed that the group with ASD had greater connectivity with the AI compared to the TD group, particularly in sensory-motor regions (see Figure S1 and Table S1, available online). There were no significant areas where TD>ASD.
Salience Network Connectivity and SOR
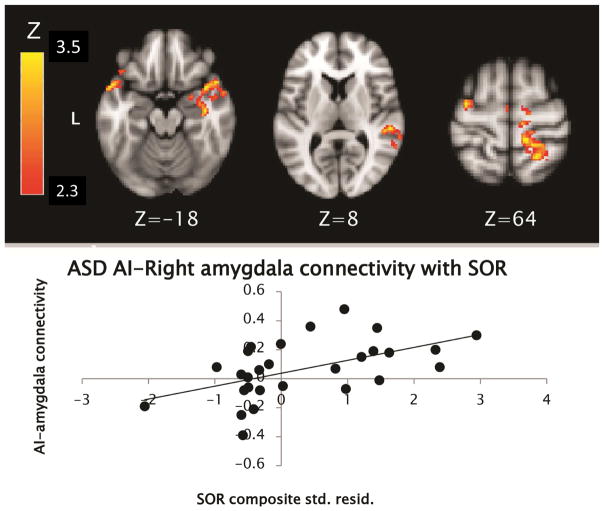
Within the group with ASD, higher levels of SOR were found to correlate with greater functional connectivity between right AI and sensory motor cortical areas (including bilateral precentral and right postcentral gyri and superior temporal gyrus), as well as greater within-network connectivity including right amygdala, insula, and bilateral temporal poles (see Table 2 and Figure 1).
Table 2.
Montreal Neurological Institute (MNI) Coordinates for Regions Where Greater Connectivity to Right Anterior Insula Was Correlated With Sensory Over-Responsivity (SOR) Including Positive Correlations in the Autism Spectrum Disorder Group (ASD+), Negative Correlations in the ASD Group (ASD−), and Negative Correlations in the Typically Developing Group (TD−)
| ASD+ | ASD− | TD− | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| MNI Peak (mm) | Max | MNI Peak (mm) | Max | MNI Peak (mm) | Max | |||||||
| x | y | z | Z | x | y | z | Z | x | y | z | Z | |
| Right superior parietal lobule | 22 | −50 | 66 | 3.79 | ||||||||
| Right precentral gyrus | 26 | −18 | 7 | 3.13 | ||||||||
| Right postcentral gyrus | 12 | −46 | 66 | 2.81 | ||||||||
| Right supramarginal gyrus | 48 | −40 | 14 | 2.77 | ||||||||
| Right superior temporal gyrus | 58 | −32 | 4 | 3.74 | ||||||||
| Right middle temporal gyrus | 62 | −50 | 10 | 3.31 | ||||||||
| Left superior frontal gyrus | −14 | −8 | 72 | 3.88 | ||||||||
| Left precentral gyrus | −34 | −8 | 64 | 3.51 | ||||||||
| Right superior frontal gyrus | 14 | 4 | 74 | 3.20 | ||||||||
| Right supplementary motor cortex | 4 | −8 | 52 | 2.91 | ||||||||
| Right temporal pole | 42 | 10 | −18 | 3.71 | ||||||||
| Right amygdala | 26 | 0 | −18 | 2.86 | ||||||||
| Right insula/planum polare | 44 | 2 | −10 | 2.76 | ||||||||
| Left temporal pole | −54 | 8 | −14 | 4.64 | ||||||||
| Left orbital frontal cortex | −44 | 24 | −18 | 2.74 | ||||||||
| Left cingulate gyrus | −6 | −46 | 2 | 3.63 | ||||||||
| Left lingual gyrus/fusiform | −6 | −80 | −8 | 3.39 | ||||||||
| Right hippocampus | 26 | −42 | −6 | 3.32 | ||||||||
| Right Lingual/Cingulate Gyrus/Hippocampus | 12 | −42 | −2 | 3.86 | ||||||||
| Left lateral occipital cortex | −50 | −80 | 4 | 4.35 | ||||||||
| Right precuneus | 4 | −72 | 54 | 3.74 | ||||||||
| V1 | 2 | −86 | 6 | 3.39 | −4 | −96 | −16 | 4.18 | ||||
| V1/lingual gyrus | 0 | −78 | −4 | 3.92 | ||||||||
| Left fusiform gyrus | −18 | −82 | −22 | 3.79 | ||||||||
| Right lingual gyrus | 16 | −50 | −6 | 3.60 | ||||||||
Note: x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; Z refers to the Z-score at those coordinates (local maxima in bold, submaxima underneath). Analyses are cluster corrected for multiple comparisons, Z>2.3, p<.05; Results show clusters with connectivity significantly correlated (either positively or negatively) with SOR composite, over and above age and anxiety symptoms. V1 denotes primary visual cortex.
Figure 1.
Areas in the group with autism spectrum disorder (ASD) where connectivity with right anterior insula (AI) was positively correlated with sensory over-responsivity (SOR). Note: SOR composite scores on X-axis are standardized residual (std. resid.) scores after controlling for anxiety and age.
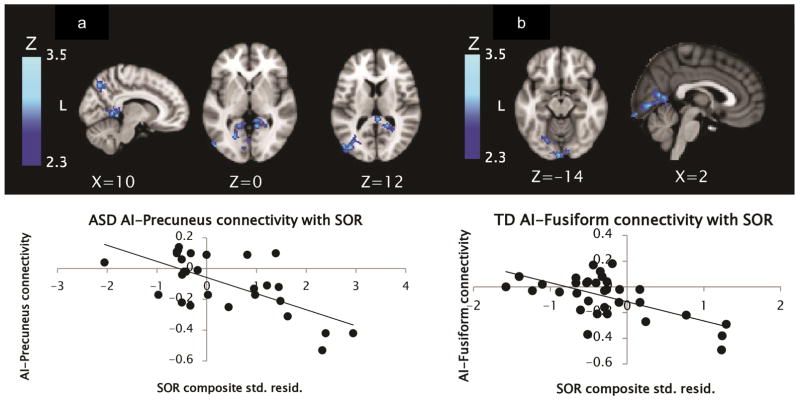
Within the group with ASD, SOR correlated negatively with connectivity between right AI and areas in the occipital cortex including primary visual cortex, left cingulate gyrus, and visual association areas (bilateral lingual gyrus and fusiform), as well as with the right precuneus (see Table 2 and Figure 2).
Figure 2.
Areas in the group with autism spectrum disorder (ASD) (a) and typically developing (TD) group (b) where connectivity with right anterior insula (AI) was negatively correlated with sensory over-responsivity (SOR). Note: SOR composite scores on X-axis are standardized residual (std. resid.) scores after controlling for anxiety and age.
In the TD group, connectivity with the right AI showed no significant positive correlations with SOR, but significant negative correlations were found with connectivity between right AI and an occipital cluster including primary visual cortex, lingual gyrus, and left fusiform gyrus.
Parameter estimates from areas where connectivity with the right AI was significantly correlated with SOR were extracted and plotted to ensure that correlations were not driven by outliers; an example scatterplot is included with each image to illustrate the distribution of data.
Association Between Resting State Connectivity and Sensory Exposure-Based Activation
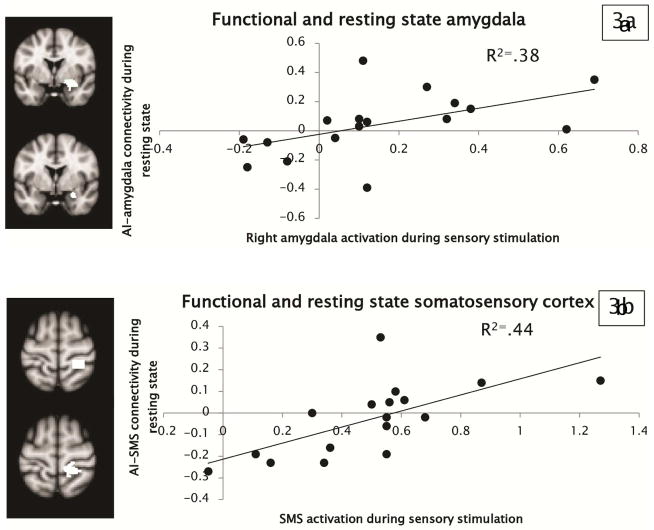
To demonstrate that these resting state abnormalities were indicative of atypical brain responses to sensory information, we then correlated the strength of functional connectivity during resting state with brain activity in the amygdala and somatosensory and auditory cortices during exposure to sensory stimuli (mildly aversive traffic sounds paired with a scratchy fabric rubbed on the inner wrist). A functional ROI approach was used in which we extracted parameter estimates of connectivity from the area of the right amygdala, right postcentral gyrus, and left superior temporal gyrus found to have greater connectivity with right AI in youth with ASD as a function of SOR. We then correlated these connectivity measures with parameter estimates of activation extracted from the right amygdala and postcentral gyrus and left superior temporal gyrus during exposure to the auditory and tactile stimuli (during a separate run; see Figure 3 for illustration of ROIs). Results demonstrated that resting-state salience network connectivity with amygdala and somatosensory cortex was significantly related to brain activity in response to the mildly aversive sensory stimuli. Specifically, the extent to which participants activated the right amygdala in response to the sensory stimuli was positively correlated with the strength of connectivity between right AI and amygdala during resting state (see Figure 3a). Similarly, somatosensory cortex activation during exposure to sensory stimuli was correlated with the strength of connectivity between right AI and somatosensory cortex during resting state (see Figure 3b). Correlations with the sensory exposure-based activation were specific to AI connectivity with the same area (e.g., amygdala activation during the task was related to AI-amygdala connectivity but was not correlated with AI-somatosensory connectivity, and vice versa). Correlations between auditory cortex activation and the strength of connectivity between AI and auditory cortex were not significant. As a confirmatory analysis, we also correlated parameter estimates from amygdala and somatosensory masks created from the resting-state and task-based ROIs added together (so that the mask used to extract parameter estimates was identical for the resting-state and task-based data); the correlations remained significant. There were no significant correlations between resting-state connectivity and task-based activation in the TD group.
Figure 3.
Scatterplots depicting the observed correlations between resting-state functional connectivity and task-based activation in (a) the amygdala and (b) the primary somatosensory cortex (SMS), within the group with autism spectrum disorder (ASD). Note: To the left of the scatterplots, task-based seed regions are shown above (area of activation in response to joint auditory+tactile stimuli in both ASD and typically developing participants), and resting-state seed regions are shown below (areas of connectivity with anterior insula [AI] significantly correlated with sensory over-responsivity).
DISCUSSION
Here, we demonstrated that specific patterns of resting-state connectivity are related to both brain and behavioral markers of SOR in ASD. Specifically, we showed that in children and adolescents with ASD, severity of SOR is associated with greater connectivity during resting state within the salience network (e.g., anterior insula, amygdala), as well as between the salience network and primary sensory processing areas. Importantly, we also showed that this increase in resting-state connectivity is associated with increased activity during exposure to sensory stimuli, which provides empirical support for the notion that intrinsic patterns of functional connectivity during resting state do meaningfully relate to how the brain responds during information processing.
Findings of increased resting-state connectivity between anterior insula, amygdala, and primary sensory processing regions are consistent with previous reports that these areas over-activate in response to sensory stimuli in youth with ASD and SOR.9,10 Further, the fact that the salience network is more connected with primary sensory processing regions at rest suggests that individuals with SOR have intrinsic patterns of brain connectivity that reflect over-attribution of salience and attention to basic sensory stimuli. Our findings of correlations between resting-state connectivity and task-based activation lend further support to this hypothesis: youth who had greater connectivity within the salience network (AI and amygdala) at rest also had greater amygdala activation to mildly aversive tactile and auditory sensory stimuli. Similarly, youth who had greater connectivity between the salience network (AI) and somatosensory cortex also had greater somatosensory activation to the tactile and auditory stimuli. Thus, continuous co-activation of salience and sensory-related brain regions throughout development may strengthen these connections and lead to these regions co-activating even during rest. Of course, the opposite could also be true: atypical intrinsic connectivity between salience and sensory-related regions could make it more likely that individuals with SOR activate salience-related brain regions in response to sensory stimuli. Further research taking a developmental approach will be necessary to address such questions of causality.
There was no significant correlation between auditory cortex activation during exposure to sensory stimuli and salience network connectivity with auditory cortex. This may be because the two regions were slightly different (the sensory stimuli activated a large area of auditory cortex, while the cluster significantly correlated with SOR during resting state was small and slightly outside of this area).
Interestingly, in the group with ASD, SOR was negatively correlated with connectivity between the salience network and visual association areas, perhaps reflecting a disassociation between these two networks in individuals with higher SOR. Many of these regions (e.g., precuneus, fusiform gyrus) are associated with social cognition.39,40 Thus, a pattern of increased connectivity with primary sensory processing regions and decreased connectivity with visual association areas is consistent with the idea that basic sensory stimuli are overly salient to individuals with SOR, leading them to attribute too much attention to this information and decreasing the salience of and attention to important social cues. It would be useful to compare resting-state salience network data to tasks that activate the visual cortex to better understand the functional basis of these negative correlations between AI and visual cortex. A similar pattern was found in the TD group, which also showed negative correlations between SOR and connectivity between the salience network and visual association areas. Thus, it is possible that these patterns of resting-state connectivity are more likely to reflect variability in sensory processing rather than characteristics specific to ASD; however, given the restricted range of SOR in the TD group, results in this group should be interpreted with caution. The lack of variability in SOR in the TD group is a limitation of this study, and future research should examine TD individuals with a broader range of SOR symptoms. An additional limitation of this study is the relatively small number of participants with both resting-state and task-based data, thus limiting our power to examine correlations between these two sets of data.
These findings have important implications for intervention. First, it is notable that individuals with ASD and SOR have increased co-activation of salience and sensory-related regions even during rest, suggesting that their brains may be “primed” to notice, attend to, and attribute salience to basic sensory information to the detriment of potentially important social information. Salience processing is a way in which individuals determine which of many competing stimuli is most relevant, and relies both on automatic bottom-up processing as well as on top-down attention and cognitive control.41 Thus, social interventions for youth with ASD and high SOR should occur in a context where all distracting sensory information is minimized, and it may be helpful to target social interventions specifically for youth with ASD and high SOR that can include sensory coping strategies to help decrease the salience of extraneous sensory input to allow them to increase attention to social stimuli. Secondly, not only is SOR extremely common and an important source of impairment for individuals with ASD,14,17 it is also present in individuals with other neuropsychiatric disorders such as ADHD and anxiety, those with histories of early life stress, and typically developing individuals.42,43 Thus, the findings of this study are likely to have broad relevance across these populations, which also often demonstrate related social deficits. Taken together, this research highlights the importance of continuing to study brain and behavioral markers of SOR across these groups, for whom SOR is not yet commonly identified or addressed in clinical settings. Here, we have shown that abnormalities in the salience network relate to heterogeneity within ASD both at the brain and behavioral level. Thus, future research should continue to examine the relationship between resting state network patterns and brain response to information processing in autism and other neuropsychiatric disorders, as this approach provides important insight into how abnormalities in intrinsic brain networks reflect and relate to brain and behavioral markers of disease symptomatology.
Supplementary Material
Figure S1. Areas where connectivity with right anterior insula (AI) was greater in the group with autism spectrum disorder (ASD) than in the typically developing group.
Table S1. Montreal Neurological Institute (MNI) Coordinates for Regions Where Autism (ASD) > Typically Developing (TD) for Connectivity to Right Anterior Insula
Clinical Guidance.
In children and adolescents with autism spectrum disorders, sensory over-responsivity is related to more connectivity between areas of the brain involved in salience and attention and areas involved in sensory processing, even when the brain is at rest.
The greater this connectivity at rest, the more over-active their brain response to mildly aversive sensory stimuli.
It is important to consider and minimize potential sensory distracters during intervention for individuals with ASD.
Even when sensory distracters are not present, individuals with higher levels of SOR may still be preparing to selectively notice and attend to such stimuli at the cost of attending to other important stimuli (such as social cues).
Acknowledgments
This work was supported by grants from the National Institute of Child Health and Human Development (grant number P50 HD055786) and the National Institute of Mental Health (grant number 1 R01 HD065280-01), as well as a National Institute of Health National Research Service Award predoctoral fellowship to S.G. (grant number F32MH105167-01). For generous support, the authors also wish to thank the Brain Mapping Medical Research Organization, the Brain Mapping Support Foundation, the Pierson-Lovelace Foundation, The Ahmanson Foundation, the William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, the Tamkin Foundation, the Jennifer Jones-Simon Foundation, the Capital Group Companies Charitable Foundation, the Robson Family, and the North Star Fund. The project described was supported by grant numbers RR12169, RR13642, and RR00865 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Supplemental material cited in this article is available online.
Disclosure: Drs. Green, Bookheimer, Dapretto, and Ms. Hernandez report no biomedical financial interests or potential conflicts of interest.
The funding sources and organizations listed above had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Monk CS, Peltier SJ, Wiggins JL, et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuro Image. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uddin LQ, Supekar K, Lynch CJ, et al. SAlience network–based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013;70:869–879. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wass S. Distortions and disconnections: Disrupted brain connectivity in autism. Brain Cogn. 2011;75:18–28. doi: 10.1016/j.bandc.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;24:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 5.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 6.Fox MD, Greicius M. Clinical Applications of Resting State Functional Connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green SA, Rudie JD, Colich NL, et al. Overreactive Brain Responses to Sensory Stimuli in Youth With Autism Spectrum Disorders. J Am Acad Child Adolesc Psychiatry. 2013;52:1158–72. doi: 10.1016/j.jaac.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green SA, Hernandez L, Tottenham N, Krasileva K, Bookheimer SY, Dapretto M. Neurobiology of sensory overresponsivity in youth with autism spectrum disorders. JAMA Psychiatry. 2015;72:778–86. doi: 10.1001/jamapsychiatry.2015.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional Brain Correlates of Social and Nonsocial Processes in Autism Spectrum Disorders: An Activation Likelihood Estimation Meta-Analysis. Biol Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory experience questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. J Child Psychol Psychiatry. 2006;47:591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Sasson A, Cermak SA, Orsmond GI, Carter AS, Kadlec MB, Dunn W. Extreme sensory modulation behaviors in toddlers with autism. Am J Occup Ther. 2007;61:584–592. doi: 10.5014/ajot.61.5.584. [DOI] [PubMed] [Google Scholar]
- 15.Liss M. Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006;10:155–72. doi: 10.1177/1362361306062021. [DOI] [PubMed] [Google Scholar]
- 16.Pfeiffer B, Kinnealey M, Reed C, Herzberg G. Sensory modulation and affective disorders in children and adolescents with Asperger’s disorder. Am J Occup Ther Off Publ Am Occup Ther Assoc. 2005;59:335–345. doi: 10.5014/ajot.59.3.335. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Sasson A, Cermak SA, Orsmond GI, Tager-Flusberg H, Kadlec MB, Carter AS. Sensory clusters of toddlers with autism spectrum disorders: Differences in affective symptoms. J Child Psychol Psychiatry. 2008;49:817–825. doi: 10.1111/j.1469-7610.2008.01899.x. [DOI] [PubMed] [Google Scholar]
- 18.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 19.Vuilleumier P. The role of the human amygdala in perception and attention. In: Whalen PJ, Phelps EA, editors. The Human Amygdala. New York: Guilford Press; 2009. pp. 220–249. [Google Scholar]
- 20.Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy ER, Foss-Feig J, Kenworthy L, Gaillard WD, Vaidya CJ. Atypical Functional Connectivity of the Amygdala in Childhood Autism Spectrum Disorders during Spontaneous Attention to Eye-Gaze. Autism Res Treat. 2012;2012:652408. doi: 10.1155/2012/652408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation: Harbourt Brace and Company; 1999. [Google Scholar]
- 23.Wechsler D. Wechsler Intelligence Scale for Children. 4. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- 24.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 25.Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- 26.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 27.Birmaher B, Khetarpal S, Brent D, et al. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Stern JA, Gadgil MS, Blakeley-Smith A, Reaven JA, Hepburn SL. Psychometric properties of the SCARED in youth with autism spectrum disorder. Res Autism Spectr Disord. 2014;8:1225–34. doi: 10.1016/j.rasd.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Steensel FJA, Deutschman AACG, Bögels SM. Examining the Screen for Child Anxiety-Related Emotional Disorder-71 as an assessment tool for anxiety in children with high-functioning autism spectrum disorders. Autism Int J Res Pract. 2013;17:681–692. doi: 10.1177/1362361312455875. [DOI] [PubMed] [Google Scholar]
- 30.Dunn W. The Sensory Profile: User’s Manual. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 31.McIntosh DN, Miller LJ. Evaluation of Sensory Processing. In: Dunn W, editor. The Sensory Profile: Examiner’s Manual. San Antonio, TX: The Psychological Corporation; 1999. pp. 59–73. [Google Scholar]
- 32.Schoen SA, Miller LJ, Green KE. Pilot study of the sensory over-responsivity scales: Assessment and inventory. Am J Occup Ther. 2008;62:393–406. doi: 10.5014/ajot.62.4.393. [DOI] [PubMed] [Google Scholar]
- 33.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuro Image. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 34.Woolrich M. Robust group analysis using outlier inference. Neuro Image. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 35.Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuro Image. 2004;21:1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 36.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patriat R, Molloy EK, Meier TB, et al. The effect of resting condition on resting-state fMRI reliability and consistency: A comparison between resting with eyes open, closed, and fixated. Neuro Image. 2013;78:463–473. doi: 10.1016/j.neuroimage.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green SA, Ben-Sasson A. Anxiety Disorders and Sensory Over-Responsivity in Children with Autism Spectrum Disorders: Is There a Causal Relationship? J Autism Dev Disord. 2010;40:1495–1504. doi: 10.1007/s10803-010-1007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz RT, Grelotti DJ, Klin A, et al. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos Trans R Soc B Biol Sci. 2003;358:415–27. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegal M, Varley R. Neural systems involved in “theory of mind”. Nat Rev Neurosci. 2002;3:463–71. doi: 10.1038/nrn844. [DOI] [PubMed] [Google Scholar]
- 41.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Sasson A, Carter AS, Briggs-Gowan MJ. Sensory Over-Responsivity in Elementary School: Prevalence and Social-Emotional Correlates. J Abnorm Child Psychol. 2009;37:705–16. doi: 10.1007/s10802-008-9295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carter AS, Ben-Sasson A, Briggs-Gowan MJ. Sensory Over-Responsivity, Psychopathology, and Family Impairment in School-Aged Children. J Am Acad Child Adolesc Psychiatry. 2011;50:1210–9. doi: 10.1016/j.jaac.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Areas where connectivity with right anterior insula (AI) was greater in the group with autism spectrum disorder (ASD) than in the typically developing group.
Table S1. Montreal Neurological Institute (MNI) Coordinates for Regions Where Autism (ASD) > Typically Developing (TD) for Connectivity to Right Anterior Insula