Abstract
Trichoderma species isolated from water-damaged buildings were screened for toxicity by using boar sperm cells as indicator cells. The crude methanolic cell extract from Trichoderma harzianum strain ES39 inhibited the boar sperm cell motility at a low exposure concentration (50% effective concentration, 1 to 5 μg [dry weight] ml of extended boar semen−1). The same exposure concentration depleted the boar sperm cells of NADH2. Inspection of the exposed boar sperm cells by transmission electron microscopy revealed damage to the plasma membrane. By using the black lipid membrane technique, it was shown that the semipurified metabolites (eluted from a SepPak C18 cartridge) of T. harzianum strain ES39 induced voltage-dependent conductivity. The high-performance liquid chromatography-purified metabolites of T. harzianum strain ES39 dissipated the mitochondrial membrane potential (Δψm) of human lung epithelial carcinoma cells (cell line A549). The semipurified metabolites (eluted from a SepPak C18 cartridge) of T. harzianum strain ES39 were analyzed by mass spectrometry (MS). Matrix-assisted laser desorption ionization and nanoflow electrospray ionization MS revealed five major peptaibols, each of which contained 18 residues and had a mass ranging from 1,719 to 1,775 Da. Their partial amino acid sequences were determined by collision-induced dissociation tandem MS.
Trichoderma species are common findings in water-damaged indoor environments (30). Trichoderma harzianum is also one of the fungal species most studied for biocontrol purposes (11, 35). It is known to produce volatile as well as nonvolatile compounds effective against phytopathogenic fungi, e.g., Rhizoctonia solani and the wood-rotting basidiomycete Lentinus lepideus (11). The biocontrol potential of T. harzianum is assumed to rely on the production of peptaibol molecules which, combined with the production of hydrolytic enzymes, strongly inhibit growth of fungal plant pathogens (31). Antibacterial and antiviral metabolites produced by T. harzianum have also been reported (13, 29).
Bioactivities of the low-molecular-weight compounds produced by T. harzianum on soilborne plant pathogens have long been studied (11). However, there are few reports on the effects of T. harzianum on mammalian cells (16) or humans, although the effects of peptaibols produced by other Trichoderma species have been widely investigated (9).
Human exposure to Trichoderma species may occur during the use of propagules of T. harzianum for biocontrol. Human exposure to Trichoderma species also occurs in water-damaged buildings, as this fungus is one of the indicator organisms of indoor air problems (30). We earlier reported on T. harzianum strain ES39 from a water-damaged building producing substances that inhibited boar sperm cell motility (26). The present study aims to explore the structure and biologically significant properties of these substances. We show that T. harzianum strain ES39 produces substances effective against mammalian cells. These substances belong to the peptaibol group of peptides. Peptaibols were found to damage the cell membrane barrier function of boar spermatozoa and dissipate the Δψm of A549 cells (a human lung epithelial carcinoma cell line).
MATERIALS AND METHODS
Description of the strain and source of isolation.
Trichoderma strain ES39 was isolated from insulation material of a private dwelling where the occupant exhibited multiple symptoms, including exacerbation of asthma, sinusitis, urticaria, blocked nose, rhinitis, otitis, hoarseness, ache in joints, myalgia, and tiredness (26).
Molecular identification of Trichoderma strain ES39.
The identity of strain ES39 was confirmed as T. harzianum by Laszlo Kredics (University of Szeged, Szeged, Hungary) based on the sequence analysis of the internal transcribed spacer 1 (ITS1)-5.8S rRNA-ITS2 region performed as described by Kredics et al. (19).
Analysis of cell toxicity.
T. harzianum strain ES39 was grown on 2% malt extract agar plates (Biokarr Diagnostics, Beauvais, France) or on dichloran agar plates with 18% glycerol (DG-18 agar; Oxoid, Unipath Ltd., Basingstoke, Hampshire, England) or in 2% malt extract broth (Biokarr Diagnostics) at 25°C for 3 to 10 days. The biomass was harvested, repeatedly frozen and thawed, and extracted into methanol as described by Andersson et al. (2, 3).
The methanol extract was tested for toxicity by a boar spermatozoan motility inhibition assay as described earlier (2, 26), except that the sperm cells were exposed to a temperature of 37°C for 5 min. The status of NAD reduction (NADH2/NAD+) of the boar spermatozoa was measured as the potential to reduce resazurin into resorufin, as described earlier (33).
Cells from the human lung epithelial carcinoma cell line A549 (ATCC CCL-185) were maintained in Dulbecco's Eagle's medium supplemented with penicillin (0.6 μg ml−1), streptomycin (60 μg ml−1), glutamine (2 mM), HEPES buffer (pH 7.4; 20 mM), and 10% fetal calf serum at 37°C. A549 cells were exposed to serial dilutions of methanolic metabolites produced by T. harzianum strain ES39 for 24 h at 37°C.
Damage to membrane permeability barriers of spermatozoa and A549 cells was inspected by differential staining with propidium iodide and SYBR-14 as described by Peltola et al. (26) or with propidium iodide and calcein-acetoxymethyleoter (AM) as described by Suominen et al. (33). The dissipation of mitochondrial membrane potential (Δψm) was determined by selective staining with JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolo carbocyanine iodide) as described by Peltola et al. (26) combined with staining with propidium iodide.
The exposed boar sperm cells were inspected by transmission electron microscopy (TEM) as described by Andersson et al. (5) with 2.5% glutaraldehyde used for fixation.
Alamethicin from Trichoderma viride was used as a control substance in toxicity assays, and it was obtained from Sigma (catalog no. A4665).
Fractionation steps of the sperm-toxic substances.
The crude methanolic extract of T. harzianum strain ES39 that was inhibitory in the boar sperm cell assay was dried and resolved in pentane. The pentane-insoluble residue was then extracted with water. The pentane and water extracts were tested for sperm motility-inhibiting activity and found to have little or no activity. The residue remaining after extraction with pentane and water was dissolved in 100% methanol and diluted 1:1 with water. The solution obtained contained the toxicity. The solution was loaded on a SepPak C18 cartridge (Waters Corp., Milford, Mass.) and eluted with a series of methanol-water mixtures (50:50, 60:40, 70:30, 80:20, 90:10, and 100:0) as described by Andersson et al. (4). All the fractions were tested for the inhibition of motility of boar sperm cells.
Sequence analysis of sperm-toxic substances.
Dried, purified fractions (eluted from SepPak C18 cartridges with 50 and 90% methanol) were dissolved in pure methanol and measured by matrix-assisted laser desorption ionization (MALDI) and single-stage nanoflow electrospray ionization (ESI) mass spectrometry (MS).
MALDI mass spectra were measured with a Voyager DE-Pro (Applied Biosystems, Foster City, Calif.) mass spectrometer with a reflector mode of operation. Freshly prepared α-cyano-4-hydroxycinnamic acid was used as the matrix at a 10-μg μl−1 concentration in 50% acetonitrile containing 0.1% tetrafluoroacetic acid. The following source parameters were applied: accelerating voltage, 24 kV; 76% grid voltage; and delay time, 180 ns. Spectra were acquired in a positive-ion mode over a mass range of m/z 500 to 2,200. The instrument was calibrated with a peptide mixture supplied with the instrument.
ESI mass spectra were acquired with a Q-Tof Micro hybrid, triple quadrupole-orthogonal acceleration time of flight (TOF) instrument (Waters Corp.) equipped with a nanoflow electrospray ion source. The sample was introduced with a syringe pump at a 1-μl min−1 flow rate. Spectrum acquisition was made in the positive-ion mode over a mass range of m/z 300 to 1,500 with the following ion source parameters: capillary voltage, 2,800 V; sample cone, 30 V; and extraction voltage, 3 V. The resolution of the instrument in single-stage TOF mode was measured at 5,400 at 50% valley definition with leucine enkephalin (Sigma L-9133). Calibration was performed for the entire mass range with cluster ions produced from a solution of acetonitrile containing 0.1% phosphoric acid.
Low-energy collision-induced dissociation (CID) product ion experiments were performed using the quadrupole section for precursor ion selection and the TOF analyzer for the product ion analysis. Argon was used as the collision gas, and the collision energy was set at 35 eV. Sequence analysis was performed as described by Pócsfalvi et al. (27, 28) and Suwan et al. (34), based on a comprehensive MS-MS analysis of both the molecular ions and characteristic complementary fragment ions produced in the ion source.
Conductance measurements in BLM.
Black lipid membrane (BLM) measurements were performed as described by Mikkola et al. (21). Briefly, the ionophoric activity of the semipurified sperm cell toxic fractions (eluted from SepPak C18 cartridges with 90% methanol) were analyzed using planar bilayer lipid membranes prepared from phosphatidylcholine (Sigma P-5638) at a concentration of 20 mg ml−1 in heptane, on an orifice (diameter, 0.3 mm) in a Teflon cylinder as described by Mueller and Rudin (23). The extract from T. harzianum strain ES39 was added as a methanolic solution during stirring to the cis side. The transmembrane current was fed to an OPA 2111 operational amplifier, used in a voltage clamp mode to amplify the currents and control the voltages across the BLM. The output signals were stored in the computer hard disk via analog input from an AT-MIO-16X card (National Instruments) and analyzed with WinEDR version 2.05 (Windows Electrophysiology Disk Recorder and Strathclyde Electrophysiology Software; Strathclyde Institute for Biomedical Sciences, University of Strathclyde, Glasgow, Scotland).
The number of peptaibol molecules needed for formation of a voltage-dependent channel was calculated according to the method of Hall et al. (15).
Purification steps of the sperm-toxic substances.
The crude methanol extract of T. harzianum strain ES39 was diluted to 90% (vol/vol) methanol by adding aqueous 0.1% HCOOH before fractionation by reversed-phase high-performance liquid chromatography (HPLC) (Smart System, Amersham Biosciences, Uppsala, Sweden). An Atlantic dC18 3-μm column (4.6 [inside diameter] by 150 mm; Waters Corp.) was used. The mobile phase was 0.1% formic acid in water and methanol. The elution gradient was 90 to 100% methanol in 17 min at a flow rate of 300 μl min−1, and detection wavelengths were 215, 254, and 280 nm. All the fractions were tested for the inhibition of motility of boar sperm cells.
Nucleotide sequence accession number.
The accession number in the GenBank database for the gene sequence of T. harzianum strain ES39 is AY585881.
RESULTS
Strains of Trichoderma species found in indoor environments inhibit the motility of boar spermatozoa.
Trichoderma species were isolated from water-damaged dwellings on several occasions. All tested isolates (n = 9) from three different dwellings inhibited the motility of boar spermatozoa. Four strains were identified at the species level on the basis of morphology: one strain was T. harzianum Rifai (26), and three were Trichoderma atroviride P. Karsten. The identity of T. harzianum Rifai (an anamorph of Hypocrea lixii) was confirmed based on sequence analysis of the ITS1-5.8S rRNA-ITS2 region (586 bp). The sperm-toxic substances emitted by strain ES39, identified as T. harzianum (26), were studied in detail.
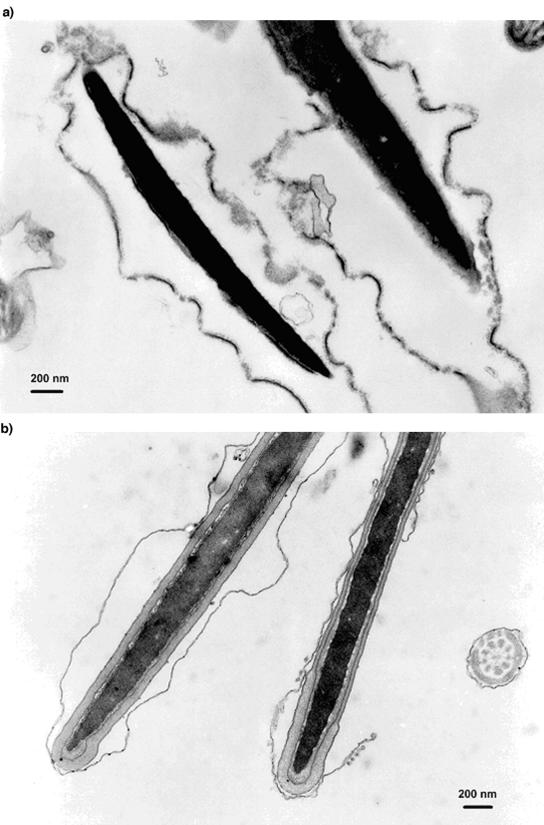
The crude cell extract prepared from T. harzianum strain ES39 found in indoor environments inhibited the motility of boar sperm cells at a low concentration; 50% effective concentration (EC50) was 1 to 5 μg (dry weight) ml of extended boar semen−1 . The same exposure concentration rendered the boar spermatozoa permeable to propidium iodide (26), indicating cell membrane damage, and suppressed the ability of boar sperm cells to reduce the resazurin dye into resorufin. The loss of motility and penetration of propidium iodide into boar spermatozoa were detected as early as 5 min after exposure. Damage to the plasma membrane of the exposed boar sperm cells was observed by TEM after exposure to 25 μg of crude cell extract (dry weight) ml of extended boar semen −1 (Fig. 1).
FIG. 1.
Effect of T. harzianum strain ES39 extract obtained from an indoor environment on boar spermatozoa. TEMs show thin sections of the middle piece of boar spermatozoa exposed for 3 days to T. harzianum strain ES39 extract (a) or to reagents only (1% methanol) (b). T. harzianum strain ES39 was used at 25 μg (dry weight) of crude extract ml−1 to expose spermatozoa. The demolished membranes of boar spermatozoan cell are visible in panel a.
The development of the boar sperm cell-toxic metabolites in a 2% malt extract plate culture of T. harzianum strain ES39 was monitored during 10 days of growth. Toxicity towards boar spermatozoa was first detected after 3 days of growth (EC50, 15 to 30 μg [dry weight] ml−1). It increased from day 3 to day 5 (EC50, <15 μg [dry weight] ml−1) and remained unchanged on day 8 and day 10, although the number of spores continued to increase threefold from day 5 to day 10. Thus, the production of sperm-toxic substance was not tightly coupled to the number of spores. The biomass of T. harzianum strain ES39 grown on a 2% malt agar plate was eightfold more toxic than biomass grown on 2% malt extract broth. The biomass grown on DG-18 agar plates exhibited no toxicity toward boar spermatozoa. T. harzianum strain ES39 retained toxicity when grown at temperatures as low as 11°C but not at 34°C. Of the media tested, 2% malt extract agar was the best medium for the production of toxin. Subculturing of strain ES39 on this medium preserved the toxin production capacity for more than 5 years.
Fractionation of the sperm-toxic methanol-soluble substance of T. harzianum strain ES39.
When the sperm-toxic T. harzianum extract was fractionated with solvents of different polarities, it was found that the toxic agent was insoluble in water and in pentane but soluble in methanol. Therefore, the crude extract was evaporated and washed with pentane and water. The residue was dissolved in methanol and fractionated for substances of different polarities with SepPak C18 column chromatography methanol as an eluent. All of the fractions obtained were tested for toxicity. The sperm-toxic substance was eluted with 90 and 100% methanol. Exposure of boar spermatozoa to these semipurified fractions from T. harzianum strain ES39 inhibited motility and relaxed the cell membrane permeability barrier to propidium iodide. Substances eluted with 50, 60, 70, and 80% methanol were not toxic in the boar spermatozoan motility assay; i.e., exposure to these fractions induced no loss of motility nor was any increased cell membrane permeability detected.
Structural analysis of the semipurified sperm-toxic substance of T. harzianum strain ES39.
Compounds eluted from SepPak C18 cartridges with 90% methanol were directly analyzed by nanoflow ESI and MALDI-TOF MS. Based on single-stage nanoflow ESI and MALDI mass spectra, the toxic fraction consisted of five main components.
MALDI-TOF MS resulted mainly in singly charged molecular ions with a sodium cation [(M + Na)+] at m/z 1,742.0 (40%), 1,756.0 (95%), 1,770.0 (100%), 1,784.0 (60%), and 1,798.0 (12%). ESI-TOF spectra yielded both singly and doubly charged sodiated [(M + Na)+ and (M + 2Na)2+], protonated [(M + H)+ and (M + 2H)2+], and mixed [(M + H + Na)2+] quasimolecular ions (Table 1). The distributions of the molecular ion peaks in the two spectra were quite similar, suggesting a similar distribution of the various molecular species in the mixture (supposing similar ionization efficiency for the homologues). The molecular mass of the most intense component was 1,747 Da (nominal molecular mass). The mass differences between the species were ±14 and ±28 Da, a typical characteristic of homologues of a certain class of compounds.
TABLE 1.
Structural analysis of the compounds present in the semipurified methanol extract of T. harzianum strain ES39a
| Ion species |
m/z (% relative peak intensity) for component:
|
||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| (M + H)+ | 1,719.9 (22) | 1,733.7 (71) | 1,747.8 (100) | 1,761.9 (70) | 1,775.7 (25) |
| (M + H + Na)2+ | 871.4 (27) | 878.4 (76) | 885.4 (100) | 892.4 (75) | 899.5 (25) |
| (b12/y"6) | 1,094.5, 626.4b | 1,108.5, 626.4b | 1122.6, 626.4b | 1,136.6, 626.4b | 1,150.7, 626.4b |
| b3 | — | 285.9 | 286.0 | 286.1 | 286.0 |
| b4 | 371.1 | 371.0 | 371.2 | 371.2 | 371.1 |
| b5 | 456.2 | 456.1 | 456.3 | 470.2 | 470.2 |
| b6 | — | 584.2 | 584.3 | 598.3 | 598.4 |
| b7 | 683.4 | 669.3 | 683.4 | 683.3 | 697.4 |
| b8 | 782.4 | 782.4 | 796.5 | 796.4 | 810.5 |
| b9 | 853.5 | 867.4 | 881.5 | 881.5 | 895.6 |
| b10 | 924.5 | 938.4 | 952.5 | 952.5 | 966.6 |
| b11 | 1,009.5 | 1,023.5 | 1,037.6 | 1,051.6 | 1,065.6 |
| b12 | 1,094.5 | 1,108.5 | 1,122.6 | 1,136.6 | 1,150.7 |
| b15 | 1,389.8 | 1,403.7 | 1,417.6 | 1,431.8 | 1,445.7 |
| b16 | 1,474.7 | 1,488.7 | 1,502.6 | 1,516.8 | 1,530.9 |
| b17 | 1,602.8 | 1,616.7 | 1,630.8 | 1,644.8 | 1,659.0 |
Singly [(M + H)+] and doubly [(M + H + Na)2+] charged quasimolecular ions (with relative peak intensities in parentheses) measured in single-stage nanoflow ESI MS. Characteristic (b12/y"6) complementary ion pairs were observed in the tandem mass spectra of the singly charged protonated molecular ions [(M + H)+]. Characteristic b3-b12 and b15-b17 ion series were observed in the MS-MS spectrum of doubly charged [(M + H + Na)2+] quasimolecular ions. —, not determined.
The first number indicates the m/z value for the b12 fragment; the second number indicates the m/z value for fragment y"6.
For the structural characterization of the main components observed in the single-stage mass spectra, CID tandem MS experiments were performed on selected singly and doubly charged molecular and fragment ions produced in the ESI source under low-energy collision conditions. Analysis of tandem mass spectra confirmed that the compounds belonged to the peptaibol class of peptides having 18 amino acid residues, including several 2-aminoisobutyric acid (Aib) compounds and a leucinol as the C-terminal amino-alcohol residue.
Cleavage of peptaibols at the Aib-Pro bond is a highly preferential fragmentation of this class of peptides and leads to two complementary ions of the b and y" type. These ions are usually also formed in source, and their subsequent collision-induced fragmentations are often used for sequence analysis of peptaibols. In this work, low-energy CID spectra were acquired on the protonated singly charged molecular ions of the five major components. All spectra showed a series of bn-type ions and a characteristic complementary ion pair identified as b12/y"6, suggesting a proline residue at position 13. Components I to V yielded b12 fragment at different m/z values, while fragment y"6 was observed at the same m/z value (626.4) in all the spectra (Table 1). In the case of component III [(MIII + H)+], for example, fragmentation of Aib-Pro led to complementary ion pairs at m/z 1,122.6 (82%) and 626.4 (3%) (Table 1). In fact, the sum of these masses yielded the mass of the intact peptide. Besides the characteristic (b12/y"6) ion pair, a series of bn-type fragment ions which enabled the identification of a partial sequence were also observed in the tandem mass spectra of (M + H)+. For example, fragmentation of (MIII + H)+ led to b ions at m/z 371.1, 456.2, 584.4, 683.4, 796.5, 881.5, 952.6, 1,037.6, and 1,122.6, starting from the fourth amino acid as-Aib4-Aib5-Gln6-Val/Iva7-Leu/Ileu8-Aib9-Ala10-Aib11-Aib12 (where Iva is isovaline and Ileu is isoleucine) directly from the MS-MS spectrum of the protonated molecular ion. The C-terminal amino acid (amino-alcohol, as is expected for a peptaibol) and a second partial-sequence tag were identified based on b15, b16, and b17 fragment ions present in the higher-molecular-mass region to be Aib16-Gln17-leucinol (Leuol)18. In a similar way, partial sequences were obtained for the other four components as well.
To confirm the partial-sequence identification obtained from CID fragmentation of (M + H)+ ions and to get more information, in-source-formed b12 and y"6 were selected for MS-MS analysis. Fragmentation of y"6 ion at m/z 626.4 led to intense b-type fragment ions at m/z 509.3 (y"6/b5), 381.2 (y"6/b4), 296.2 (y"6/b3), and 211.1 (y"6/b2) that allowed us to unambiguously determine the sequence of the C-terminal region (in the present case, positions 13 to 18) of the peptaibols as Pro13-(Leu/Ile14)-Aib15−Aib16-Gln17-Leuol18. Residue Leu/Ile14 was deduced by exclusion subtraction of the molecular weight of the Pro residue from the mass of fragment ion y"6/b2. On the other hand, fragmentation of the five b12 ions of different masses led to a more complex picture, due to the presence of various isobaric sequence analogues. Nevertheless, it was possible to identify the main sequence homologues based on the b12/bn secondary fragment ions.
Sequences of main components I to V were further confirmed by fragment ions observed in the tandem mass spectra of the doubly charged [(M + Na + H)2+] molecular ions. The advantage of using doubly charged ions instead of singlycharged ones is that doubly charged ions fragment at lower collisional energy with higher efficiency due to the coulomb repulsion force. The main characteristic of MS-MS spectra of (M + Na + H)2+ molecular ions was the presence of not one but a series of (bn/y"18 − n) complementary ion pairs, starting from n = 3 to n = 12 and permitting a more unambiguous sequence annotation. The proton was found to be allocated on the N-terminal bn ion, while sodium ion residues were allocated on the y"n C-terminal fragment. The observed (bn-y"18 − n) ions confirmed the partial sequences obtained by the fragmentation of (M + H)+. The first two missing amino acid residues were deduced from the measured molecular masses (Table 1) and from the fact that the N-terminal amino acid of peptaibols is most frequently acetyl-aminoisobutyric acid (AcAib); as a consequence, the second residue should be Ser for the species studied here. All the bn series observed in these mass spectra are reported in Table 1, and the sequences of major components I to V are shown in Table 2. The main variations in the sequence were found to be to Aib ↔ Leu/Ile at position 5, Aib ↔ Val/Iva at position 7, and Aib ↔ Leu/Ile at position 11.
TABLE 2.
Deduced partial sequences of the most abundant sequence variants of peptaibol components I to V
| Component | Amino acid at positiona:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| I | (AcAib) | (Ser) | (Ala) | Aib | Aib | (Gln) | Val/Iva | Val/Iva | Ala | Ala | Aib | Aib | Pro | (Leu/Ile) | Aib | Aib | Gln | Leuol |
| II | (AcAib) | (Ser) | Ala | Aib | Aib | Gln | Aib | Leu/Ile | Aib | Ala | Aib | Aib | Pro | (Leu/Ile) | Aib | Aib | Gln | Leuol |
| III | (AcAib) | (Ser) | Ala | Aib | Aib | Gln | Val/Iva | Leu/Ile | Aib | Ala | Aib | Aib | Pro | (Leu/Ile) | Aib | Aib | Gln | Leuol |
| IV | (AcAib) | (Ser) | Ala | Aib | Leu/Ile | Gln | Aib | Leu/Ile | Aib | Ala | Leu/Ile | Aib | Pro | (Leu/Ile) | Aib | Aib | Gln | Leuol |
| V | (AcAib) | (Ser) | Ala | Aib | Leu/Ile | Gln | Val/Iva | Leu/Ile | Aib | Ala | Leu/Ile | Aib | Pro | (Leu/Ile) | Aib | Aib | Gln | Leuol |
Amino acids shown in parentheses indicate the most probable amino acids.
An interesting feature of this MS-MS spectrum was the microheterogeneity: fragment ions were frequently accompanied by so-called satellite ions at a difference in mass of ±14 Da. This indicates that components with a given molecular weight were not single compounds but a mixture of at least two, but presumably more, sequence homologues.
Conductance measurements of semipurified sperm-toxic substance from T. harzianum strain ES39.
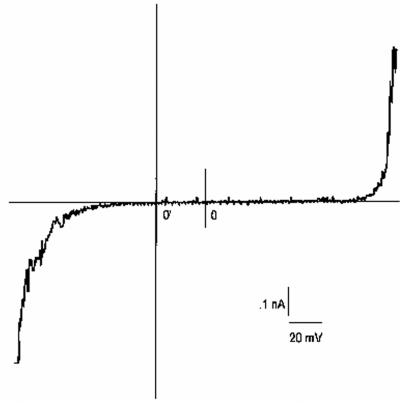
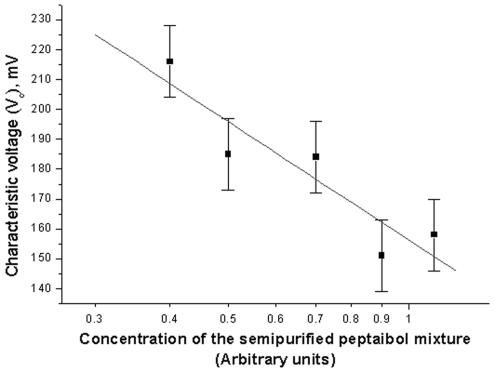
The semipurified fraction (eluted from a SepPak C18 cartridge with 90% methanol) of T. harzianum strain ES39 was shown to contain five major peptaibol homologues. This peptaibol mixture was investigated by the BLM technique. The peptaibols induced a steep development of membrane current that occurred above a voltage threshold (Fig. 2). Three parameters (Vc, Ve, and Va) were determined for the conductance induced by peptaibols from T. harzianum strain ES39. Vc is defined as the voltage for a given reference conductance (0.4 ns in the present study). The concentration dependence of Vc is shown in Fig. 3. The sensitivity of the macroscopic conductance to changes in aqueous dilutions of peptaibols is described by another parameter, Va, and can be calculated from the data shown in Fig. 3. In this study, Va was 57 mV (±7 mV); i.e., Vc was changed by 57 mV (±7 mV) for every e-fold change of concentration, i.e., a logarithmic change in which the base is e. The steepness of the current-voltage curve (Ve) was 7 ± 3 mV (Fig. 2). Ve indicates the voltage increment which results in e-fold increase of the current. The ratio Va/Ve allows one roughly to estimate the number of molecules of peptaibols required to form a conducting membrane aggregate, in this case, 8 ± 4. Thus, the sperm-toxic semipurified fraction contained peptaibols inducing voltage-sensitive conductivity composed of approximately eight monomers.
FIG. 2.
Current-voltage relationship for the BLM. The semipurified peptaibol mixture (eluted with 90% methanol from a SepPak C18 cartridge) of T. harzianum strain ES39 was present in the cis side. The peptaibol mixture was dispersed to the cis side of the BLM and allowed to equilibrate for 10 min. The bilayer was then subjected to a triangular waveform voltage (6 mV s−1). The potential of the cis chamber in reference versus the potential in the trans chamber was recorded in asymmetrical (840 and 200 mM) concentrations of KCl. The positive direction of the current is shown downward, and the positive direction of the voltage scale is shown from right to left. 0, zero current and zero voltage for asymmmetrical conditions, i.e., 200 and 840 mM KCl in the cis and trans chambers; 0′, zero current and zero voltage for symmetrical conditions, i.e., 200 and 200 mM KCl.
FIG. 3.
Characteristic voltage (Vc) of the BLM as a function of dilutions of the semipurified peptaibol mixture (eluted with 90% methanol from a SepPak C18 cartridge) of T. harzianum strain ES39. The semipurified fraction was dispersed to the cis side of the BLM and allowed to equilibrate for 10 min, and the bilayer was submitted to a triangular waveform voltage (6 mV s−1). Vc, voltage for a given reference conductance (4 ns).
To assess the ion selectivity of the peptaibols, we measured the shift of the current-voltage curve along the voltage axis in the BLM. This was done by measuring the differences between the zero-current voltages of the two current-voltage characteristics: one measured in asymmetrical concentrations (840 and 200 mM) (Fig. 2) and the other measured in symmetrical concentrations (200 and 200 mM) of KCl in cis and trans chambers. The difference between these is −36 mV (±5 mV) with the minus sign for the chamber with the higher KCl concentration. This value is close to the theoretical value of −33 mV. The results indicate that the ratio of cation-to-anion conductivity induced by the peptaibols was approximately 50. It is therefore possible that peptaibols form cation-selective channels.
Purification of the sperm-toxic methanol-soluble substance of T. harzianum strain ES39.
The sperm-toxic methanol-soluble crude extract of T. harzianum strain ES39 was purified by reversed-phase HPLC. All the obtained fractions were tested for sperm cell toxicity, and four sperm-toxic peaks were found absorbing at 215 nm. These sperm-toxic HPLC fractions were separately analyzed by ESI-ion trap MS (data not shown). Fraction 1 contained peptaibol components III and IV; fraction 2 contained components III, IV, and V; fraction 3 contained components IV and V; and fraction 4 contained component V. The sequences of peptaibol components I to V are listed in Table 2.
Biological properties of HPLC-purified sperm-toxic substances from T. harzianum strain ES39.
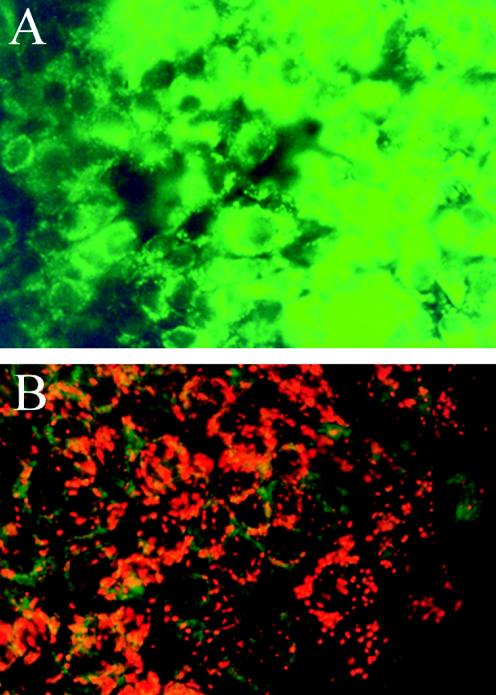
Four HPLC-purified fractions inhibited the motility of boar spermatozoa. Two of the HPLC-purified fractions (fractions 3 and 4 containing peptaibol components IV and V), where the yield was highest, were investigated for effects on human lung epithelial carcinoma cell line A549. It was found that these cells lost the Δψm when they were exposed to HPLC-purified fractions 3 (Fig. 4) and 4 at concentrations which excluded propidium iodide, showing that the plasma membrane barrier function was intact (Table 3). The results indicate that the subcellular target of the peptaibols purified from T. harzianum strain ES39 in A549 cells was mitochondrial. We also tested the effects of a reference compound, alamethicin (from T. viride) and found that it also dissipated the Δψm of A549 cells similarly to the sperm-toxic HPLC fraction 3 (Table 3).
FIG. 4.
Epifluorescence micrographs of A549 cells stained with JC-1 dye to visualize the ΔΨm. A549 cells were exposed to approximately 20 μg of HPLC-purified fraction 3 (containing peptaibol components IV and V) of T. harzianum ES39 per ml for 24 h (A) and to reagents only (3.3% methanol) (B). The preserved ΔΨm is visible as orange fluorescence, and the dissipation of Δψm changes the fluorescence to green. Excitation light, 390 to 490 nm.
TABLE 3.
Effects of exposing functionally stained human lung epithelial carcinoma A549 cells to T. harzianum strain ES39 and different concentrations of alamethicin
| Substance tested and concn | Observed effect
|
|
|---|---|---|
| Δψm detected with JC-1b | % Uptake of propidium iodide by calcein-AM- stained cellsc | |
| Alamethicin | ||
| 5 μg ml−1 | 100% Dissipated | <10 |
| 50 μg ml−1 | 100% Dissipated | 100 |
| T. harzianum strain ES39,a ∼20 μg ml−1 | 100% Dissipated | <10 |
| Methanol (negative control), 3.3% | 100% Preserved | <10 |
HPLC-purified fraction 3 (containing peptaibol components IV and V) from T. harzianum strain ES39 was used.
Staining with JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolo carbocyanine iodide) visualizes the membrane potential of mitochondria (Δψm). Preserved Δψm is visible as orange fluorescence, and the dissipation of mitochondrial membrane potential changes the fluorescence to green.
Dual staining with calcein-AM and propidium iodide stains the cells green unless the membrane damage allows the uptake of propidium iodide. Green fluorescence indicates no damage, and red fluorescence is due to uptake of propidium iodide.
DISCUSSION
T. harzianum strain ES39 found in an indoor environment was earlier shown to produce substances that inhibited the motility of boar sperm cells in vitro (26). Boar spermatozoa have been found to be sensitive indicators in detecting microbial toxins in indoor building materials (2, 25) as well as in detecting bacteria producing toxic depsipeptides in indoor environments (4) and in foods (3). Boar sperm cells were also shown to positively correlate with human cell toxicity (17). In this paper, the substances responsible for the inhibition of boar spermatozoan motility in the extract of T. harzianum strain ES39 were identified as a peptaibol group of peptides, based on the molecular mass of 1,600 to 2,000 Da, containing unusual amino acids (α-aminoisobutyric acid [Aib]), an acetylated N-terminal amino acid, and C-terminal amino alcohol residues. The single-stage mass spectrum also showed that the molecular masses of the five main components differed by multiples of 14 Da, typical of peptaibol homologues (27, 28). The peptaibols produced by T. harzianum strain ES39 consisted of 18 amino acids. The sequences of these peptaibols showed relatedness to the trichokindin family of peptaibols isolated from T. harzianum species and characterized by Iida et al. (16).
Peptaibols are linear hydrophobic peptides (11). In this paper, we examined peptaibols produced by T. harzianum strain ES39 that were dissolved in methanol. The methanol solubility of the substances indicates a certain level of hydrophobicity and that they may penetrate through the lipid bilayer into the eukaryotic cells. All peptaibols are reported to have membrane-modifying activity, which may lead to leakage of cytoplasmic material from an exposed cell (9). We showed earlier that crude cell extract from T. harzianum strain ES39 relaxed the membrane permeability barrier of boar spermatozoa to propidium iodide at exposures from 1 to 5 μg ml−1 (26). This exposure also inhibited the reduction of resazurin by boar sperm cells, indicating that the cells were depleted of NADH2. The membrane damage was also visible in TEMs (Fig. 1). The peptaibols were shown to be responsible for all the toxic properties exhibited by the crude extract towards the boar spermatozoa. The bioassay based on inhibition of boar sperm cell motility thus proved to be a useful and sensitive assay for peptaibol detection.
Peptaibols are known to be amphipathic in their nature and to form voltage-dependent ion channels in lipid bilayers (9). The antimicrobial activity of peptaibols is thought to result from the leakage of cytoplasmic material through such channels, leading to cell death (9, 32). The BLM results in this paper showed that the peptaibols from T. harzianum strain ES39 induced voltage-dependent conductivity. Steep development of a voltage-gated membrane current in the presence of peptaibols produced by T. harzianum strain ES39 resembled that described for other peptaibols, e.g., harzianins and alamethicin (8, 20, 32).
In this paper, we show an interesting feature of isolated peptaibols: the Δψm of human lung epithelial carcinoma A549 cells was dissipated, whereas at the same concentration, the cell membranes remained intact. Interestingly, the specific dissipation of Δψm was not observed in boar spermatozoa. The boar sperm cell membrane is low in sterols, rendering these cells highly permeable, and therefore sensitive, to hydrophobic molecules (1). Duval et al. (10) reported that trichorzin PA peptaibol embedment into a phospholipid bilayer was reduced when the amount of cholesterol in the bilayer was increased. Lucaciu et al. (20) also reported that harzianins increase permeability of the membranes made of egg phosphatidylcholine and cholesterol at higher concentrations and form voltage-dependent channels at lower concentrations. Alamethicin, which was used in this study as a reference compound, also showed membrane-modifying activity at a higher concentration (50 μg ml−1) and specific mitochondrial activity at a lower concentration (5 μg ml−1) (Table 3) when A549 cells were used as test cells. It was recently shown with the mammalian cell-toxic substance produced by Bacillus amyloliquefaciens that specific dissipation of Δψm was observed at exposure concentrations similar to those in BLM channel formation (22).
In earlier reports, peptaibol alamethicin was shown, for example, to lyse human erythrocytes, induce metabolic activity in bovine aorta endothelial cells, and release catecholamines from feline adrenal glands (reviewed in reference 9). Iida et al. (16) reported that trichokindins induced Ca2+-dependent catecholamine secretion from bovine adrenal medullary chromaffin cells at a concentration of 10 μM. To our knowledge, other activities of trichokindins have not been reported. The trichokindin-like peptaibols produced by T. harzianum strain ES39 in this study dissipated the Δψm of A549 cells at a concentration of approximately 10 μM. It is known that dissipation of the Δψm initiates programmed cell death in eukaryotic cells (reviewed in references 6 and 14).
T. harzianum strain ES39 was isolated from insulation material of a water-damaged dwelling (26). T. harzianum is known to be a heavily sporulating fungus with spores that easily become airborne. We showed here that the sperm-toxic substances identified as peptaibols were present in the mixture of mycelium and spores of T. harzianum strain ES39. It has been reported that fungal mycelial fragments are released together with spores from contaminated surfaces in water-damaged buildings (12). Kildesø et al. (18) measured the release of T. harzianum agglomerates from water-damaged building materials. The aerodynamic size of these agglomerates ranged from <1 to 10 μm, but most were around 4 μm in size. Particles of this size may reach the trachea and primary bronchi of the human lung (7). The indoor isolate of T. harzianum ES39 produced peptaibols when grown on 2% malt extract agar but not on DG-18 agar culture of lower water activity, consistent with the previously reported effect of low water activity of the growth substrate on toxin production by fungi (24). T. harzianum strain ES39 produced peptaibols at a temperature of 11°C, indicating its potential survival in toxic form in low-temperature environments such as basements. Thus, the pathogenic potential of T. harzianum deserves attention.
Acknowledgments
This research was supported by scholarships from the Finnish Graduate School of Applied Bioscience: Bioengineering, Food and Nutrition, Environment, University of Helsinki (J.P.), and from the Academy of Finland (grants 533305 and 50733). Support by the European Commission (EU COST Action 835) for our collaboration is also acknowledged.
We also thank R. A. Samson for identification of fungal strains, L. Kredics for molecular identification of strain ES39, I. Julkunen for donation of A549 cells, N.-E. Saris and R. Peltola for critical reading of the manuscript, and S. Hoffmann and S.-R. Hakasalo for technical assistance.
REFERENCES
- 1.Andersson, M. A. 1999. Bacterial diversity and toxicity in air, indoor environment and foods. Ph.D thesis. University of Helsinki, Helsinki, Finland.
- 2.Andersson, M. A., M. Nikulin, U. Kõljalg, M. C. Andersson, F. A. Rainey, K. Reijula, E.-L. Hintikka, and M. S. Salkinoja-Salonen. 1997. Bacteria, molds, and toxins in water-damaged building materials. Appl. Environ. Microbiol. 63:387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson, M. A., R. Mikkola, J. Helin, M. C. Andersson, and M. S. Salkinoja-Salonen. 1998. A novel sensitive bioassay for the detection of Bacillus cereus emetic toxin and related depsipeptide ionophores. Appl. Environ. Microbiol. 64:1338-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson, M. A., R. Mikkola, R. Kroppenstedt, F. A. Rainey, J. Peltola, J. Helin, K. Sivonen, and M. S. Salkinoja-Salonen. 1998b. Mitochondrial toxin produced by Streptomyces griseus strains isolated from indoor environment is valinomycin. Appl. Environ. Microbiol. 64:4767-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson, M. A., M. Laukkanen, E.-L. Nurmiaho-Lassila, F. A. Rainey, S. Niemelä, and M. S. Salkinoja-Salonen. 1995. Bacillus thermosphaericus sp. nov. A new thermophilic ureolytic Bacillus isolated from air. Syst. Appl. Microbiol. 18:203-220. [Google Scholar]
- 6.Bernardi, P., L. Scorrano, R. Colonna, V. Petronilli, and F. Di Lisa. 1999. Mitochondria and cell death (a review). Mechanistic aspects and methodological issues. Eur. J. Biochem. 264:687-701. [DOI] [PubMed] [Google Scholar]
- 7.Brooks, B. O., and W. F. Davis. 1992. Understanding indoor air quality, p. 199. CRC Press, Boca Raton, Fla.
- 8.Cafiso, D. S. 1994. Alamethicin: a peptide model for voltage gating and protein-membrane interactions. Annu. Rev. Biophys. Biomol. Struct. 23:141-165. [DOI] [PubMed] [Google Scholar]
- 9.Chung, J. K., and B. A. Wallace. 2001. Peptaibols: models for ion channels. Biochem. Soc. Trans. 29:565-570. [DOI] [PubMed] [Google Scholar]
- 10.Duval, D., P. Cosette, S. Rebuffat, H. Duclohier, B. Bodo, and G. Molle. 1998. Alamethicin-like behavior of new 18-residue peptaibols, trichorzins PA. Role of the C-terminal amino-alcohol in the ion channel forming activity. Biochim. Biophys. Acta 1369:309-319. [DOI] [PubMed] [Google Scholar]
- 11.Ghisalberti, E. L., and K. Sivasithamparam. 1991. Antifungal antibiotics produced by Trichoderma spp. Soil Biol. Biochem. 23:1011-1020. [Google Scholar]
- 12.Górny, R. L., T. Reponen, K. Willeke, D. Schmechel, E. Robine, M. Boissier, and S. A. Grinshpun. 2002. Fungal fragments as indoor air biocontaminants. Appl. Environ. Microbiol. 68:3522-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goulard, C., S. Hlimi, S. Rebuffat, and B. Bodo. 1995. Trichorzins HA and MA, antibiotic peptides from Trichoderma harzianum. I. Fermentation, isolation and biological properties. J. Antibiot. 48:1248-1253. [DOI] [PubMed] [Google Scholar]
- 14.Green, D., and J. Reed. 1998. Mitochondria and apoptosis. Science 281:309-312. [DOI] [PubMed] [Google Scholar]
- 15.Hall, J. E., I. Vodyanoy, T. M. Balasubramanian, and G. R. Marshall. 1984. Alamethicin—a rich model for channel behavior. Biophys. J. 45:233-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iida, A., M. Sanekata, T. Fujita, H. Tanaka, A. Enoki, G. Fuse, M. Kanai, P. J. Rudewicz, and E. Tachikawa. 1994. Fungal metabolites. XVI. Structures of new peptaibols, trichokindins I-VII, from the fungus Trichoderma harzianum. Chem. Pharm. Bull. 42:1070-1075. [DOI] [PubMed] [Google Scholar]
- 17.Jääskeläinen, E. L., V. Teplova, M. A. Andersson, L. C. Andersson, P. Tammela, M. C. Andersson, T. I. Pirhonen, N.-E. L. Saris, P. Vuorela, and M. S. Salkinoja-Salonen. 2003. In vitro assay for human toxicity of cereulide, the emetic mitochodrial toxin produced by food poisoning Bacillus cereus. Toxicol. In Vitro 17:737-744. [DOI] [PubMed] [Google Scholar]
- 18.Kildesø, J., H. Würtz, K. F. Nielsen, C. K. Wilkins, S. Gravesen, P. A. Nielsen, U. Thrane, and T. Schneider. 2000. The release of fungal spores from water damaged building materials, p. 313-318. In Proceedings of the International Conference of Healthy Buildings 2000, vol. 1. SIY Indoor Air Information Oy, Helsinki, Finland.
- 19.Kredics, L., Z. Antal, J. Varga, L. Manczinger, A. Szekeres, S. Kocsube, I. Doczi, M. Laday, F. Kevei, and E. Nagy. 2003. Genetic variability and antifungal susceptibilities of clinical Trichoderma isolates, p. 133-136. In Trends in medical mycology. Proceedings of the 9th Congress of the European Confederation of Medical Mycology and 7th Trends in Invasive Fungal Infections. Monduzzi Editore, Bologna, Italy.
- 20.Lucaciu, M., S. Rebuffat, C. Goulard, H. Duclohier, G. Molle, and B. Bodo. 1997. Interaction of the 14-residue peptaibols, harzianins HC, with lipid bilayers: permeability modifications and conductance properties. Biochim. Biophys. Acta 1323:85-96. [DOI] [PubMed] [Google Scholar]
- 21.Mikkola, R., N.-E. Saris, P. A. Grigoriev, M. A. Andersson, and M. S. Salkinoja-Salonen. 1999. Ionophoretic properties and mitochondrial effects of cereulide, the emetic toxin of B. cereus. Eur. J. Biochem. 263:112-117. [DOI] [PubMed] [Google Scholar]
- 22.Mikkola, R., M. A. Andersson, P. A. Grigoriev, V. V. Teplova, N.-E. L. Saris, F. A. Rainey, and M. S. Salkinoja-Salonen. 2004. Bacillus amyloliquefaciens strains isolated from moisture-damaged buildings produced surfactin and a substance toxic to mammalian cells. Arch. Microbiol. 181:314-323. [DOI] [PubMed] [Google Scholar]
- 23.Mueller, P., and D. O. Rudin. 1967. Action potential phenomena in experimental bimolecular lipid membranes. Nature 213:603-604. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen, K. F., P. A. Nielsen, and G. Holm. 2000. Growth of moulds on building materials under different humidities, p. 283-287. In Proceedings of the International Conference of Healthy Buildings 2000, vol. 3. SIY Indoor Air Information Oy, Helsinki, Finland.
- 25.Peltola, J., M. A. Andersson, R. Mikkola, H. Mussalo-Rauhamaa, and M. Salkinoja-Salonen. 1999. Membrane toxic substances in water-damaged construction materials and fungal pure cultures, p. 432-443. In E. Johanning (ed.), Bioaerosols, fungi and mycotoxins: health effects, assessment, prevention and control. Eastern New York Occupational and Environmental Health Center. Boyd Printing Company, Inc., Albany, N.Y.
- 26.Peltola, J., M. A. Andersson, T. Haahtela, H. Mussalo-Rauhamaa, F. A. Rainey, R. M. Kroppenstedt, R. A. Samson, and M. S. Salkinoja-Salonen. 2001. Toxic-metabolite-producing bacteria and fungus in an indoor environment. Appl. Environ. Microbiol. 67:3269-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pócsfalvi, G., A. Ritieni, P. Ferranti, G. Randazzo, K. Vékey, and A. Malorni. 1997. Microheterogeneity characterization of a paracelsin mixture from Trichoderma reesei using high-energy collision-induced dissociation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 11:922-930. [DOI] [PubMed] [Google Scholar]
- 28.Pócsfalvi, G., F. Scala, M. Lorito, A. Ritieni, G. Randazzo, P. Ferranti, K. Vékey, and A. Malorni. 1998. Microheterogeneity characterization of a trichorzianine—a mixture from Trichoderma harzianum. J. Mass Spectrom. 33:154-163. [DOI] [PubMed] [Google Scholar]
- 29.Qian-Cutrone, J., S. Huang, L. P. Chang, D. M. Pirnik, S. E. Klohr, R. A. Dalterio, R. Hugill, S. Lowe, M. Alam, and K. F. Kadow. 1996. Harziphilone and fleephilone, two new HIV REV/RRE binding inhibitors produced by Trichoderma harzianum. J. Antibiot. 49:990-997. [DOI] [PubMed] [Google Scholar]
- 30.Samson, R. A., B. Flannigan, M. E. Flannigan, A. P. Verhoeff, O. C. G. Adan, and E. S. Hoekstra, (ed.). 1994. Health implications of fungi in indoor environments, p. 531-538. Elsevier, Amsterdam, The Netherlands.
- 31.Schirmböck, M., M. Lorito, Y.-L. Wang, C. K. Hayes, I. Arisan-Atac, F. Scala, G. E. Harman, and C. P. Kubicek. 1994. Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Appl. Environ. Microbiol. 60:4364-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segalas, I., Y. Prigent, D. Davoust, B. Bodo, and S. Rebuffat. 1999. Characterization of a type of β-bend ribbon spiral generated by the repeating (Xaa-Yaa-Aib-Pro) motif: the solution structure of Harzianin HC IX, a 14-residue peptaibol forming voltage-dependent ion channels. Biopolymers 50:71-85. [Google Scholar]
- 33.Suominen, I., M. A. Andersson, M. C. Andersson, A.-L. Hallaksela, P. Kämpfer, F. A. Rainey, and M. S. Salkinoja-Salonen. 2001. Toxic Bacillus pumilus from indoor air, recycled paper pulp and Norway spruce, food poisoning outbreaks and clinical samples. Syst. Appl. Microbiol. 24:267-276. [DOI] [PubMed] [Google Scholar]
- 34.Suwan, S., M. Isobe, S. Kanokmedhakul, N. Lourit, K. Kanokmedhakul, K. Soytong, and K. Koga. 2000. Elucidation of high micro-heterogeneity of an acidic-neutral trichotoxin mixture from Trichoderma harzianum by electrospray ionization quadrupole time-of-flight mass spectrometry. J. Mass Spectrom. 35:1438-1451. [DOI] [PubMed] [Google Scholar]
- 35.Yedidia, I., N. Benhamou, and I. Chet. 1999. Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 65:1061-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]