Abstract
Tumor necrosis factor alpha (TNF-α) plays an important role in the immune system. In this study, TNF-α expression was analyzed in 11 tissues of 8 piglets resistant to enterotoxigenic Escherichia coli (ETEC) F18 and 8 ETEC F18-susceptible piglets from the Large White breed. The expression levels of TNF-α were high in immune organs (spleen, lung, thymus, and lymph nodes). The levels were higher in ETEC F18-resistant piglets than in ETEC F18-susceptible piglets, with significant differences in spleen, kidney, thymus, lymph node, and duodenum (P < 0.05). The mutation TNF-α −791(C→T) and 3 genotypes (CC, CT, and TT) were identified. The TNF-α expression levels in the spleen, kidney, lymph nodes, and duodenum were significantly higher in the TT pigs than in the CC pigs (P < 0.05). Thus, TNF-α −791(C→T) has significant effects on mRNA expression and may regulate ETEC F18 resistance of weaning piglets. Therefore, the −791(C→T) mutation of the TNF-α gene could be considered an important potential genetic marker of ETEC F18 resistance.
Résumé
Le facteur alpha nécrosant des tumeurs (TNF-α) joue un rôle important dans le système immunitaire. Dans la présente étude, l’expression de TNF-α a été analysée dans 11 tissus provenant de huit porcelets résistants à une souche d’Escherichia coli entérotoxinogène (ETEC) F18 et huit porcelets sensibles à une souche ETEC F18 de race Large White. Les degrés d’expression de TNF-α étaient élevés dans les organes immunitaires (rate, poumon, thymus, et noeuds lymphatiques). Les niveaux étaient plus élevés chez les porcelets résistants à la souche ETEC F18 que chez les porcelets sensibles à la souche ETEC F18, avec des différences significatives dans la rate, rein, thymus, noeud lymphatique, et duodénum (P < 0,05). La mutation TNF-α –791(C→T) et 3 génotypes (CC, CT, et TT) ont été identifiés. Les niveaux d’expression de TNF-α dans la rate, rein, noeuds lymphatiques, et duodénum étaient significativement plus élevés dans les porcs TT que dans les porcs CC (P < 0,05). Ainsi, TNF-α −791(C→T) avait des effets significatifs sur l’expression d’ARNm et pourrait réguler la résistance envers ETEC F18 chez des porcelets au sevrage. Ainsi, la mutation −791(C→T) du gène du TNF-α pourrait être considérée comme un important marqueur génétique potentiel de la résistance envers les ETEC F18.
(Traduit par Docteur Serge Messier)
Introduction
Tumor necrosis factor alpha (TNF-α), a cellular immune factor that is mainly secreted by macrophages, monocytes, and lymphocytes, stimulates T-cells to release cytokines [e.g., interleukin 1β (IL-1β), IL-2, and IL-6], which are involved in immune reactions (1,2). In the medical field, the study of TNF-α has been focused mostly on the promoter region, where point mutations are associated with either resistance or susceptibility to disease. For example, TNF-α 308(G→A) is associated with increased risk of premature birth, vitiligo, and duodenal ulcers (3–5), TNF-α 238(G→A) with increased risk of hepatitis C (6), and TNF-α 204(C→T) with a reduced risk of severe acute respiratory syndrome (7). However, in studies about mutations in the promoter region of porcine TNF-α, the mutation g.6464(C→T) was found to be associated with back fat accumulation in Large White pigs (8), and the polymorphic loci were 791 base pairs upstream from the transcription initiation site. In addition, TNF-α is a cytokine that plays opposing roles in the context of infectious disease pathogenesis. Many studies have found that upregulated expression of TNF-α contributes to improving immune response and resistance to infection in pigs (9–11). The promoter, an integral upstream regulatory region of a gene, controls the initiation of gene transcription as well as the level of gene expression.
In this study TNF-α mRNA expression in 11 tissues (heart, liver, spleen, lung, kidney, stomach, muscle, thymus, lymph node, duodenum, and jejunum) was analyzed in 8 piglets resistant to enterotoxigenic Escherichia coli (ETEC) F18 and 8 ETEC F18-susceptible piglets of the Large White breed. The technique of polymerase chain reaction single-strand conformation polymorphism (PCR-SSCP) was used to detect −791(C→T) mutations in the TNF-α promoter and to determine the mRNA expression levels for TNF-α in order to assess the feasibility of using the −791(C > T) mutation as a genetic marker and provide some theoretical and experimental basis for disease-resistance breeding based on the TNF-α gene.
Materials and methods
Experimental materials and sample collection
The animal study proposal was approved by the Institutional Animal Care and Use Committee (IACUC) of the Yangzhou University Animal Experiments Ethics Committee with the permit number SYXK(Su) IACUC 2012-0029. All piglet experimental procedures were done in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of the People’s Republic of China.
In previous studies we developed a V-secretion system and a receptor-binding assay for identifying resistance and sensitivity to ETEC F18 (12–14). In the present study we conducted the F18-adhesion test in 50 Large White piglets from 8 families at Kangle Farming Company (Changzhou, Jiangsu) that had almost the same weight at birth and at 28 d of age, around the time of weaning, when they are most vulnerable to ETEC F18 infection. The piglets had been allowed access to food and water ad libitum and kept in the same conditions until humanely euthanized at age 28 d. The adhesion test was done according to the method by Liu et al (12). When a large amount of adherence by F18ab-expressing fimbriae of the standard ETEC strain to intestinal epithelial cells was displayed the piglets were identified as E. coli F18-susceptible (Figure 1A), whereas when no adherence was displayed the piglets were identified as E. coli F18-resistant (Figure 1B).
Figure 1.
A — large numbers of enterotoxigenic Escherichia coli (ETEC) F18 adhering to an intestinal epithelial cell of a susceptible Large White piglet. B — almost no bacterial adhesion in an ETEC F18-resistant piglet. Oil immersion lens; scale bar = 20 μm.
For 8 pairs of ETEC F18-susceptible and ETEC F18-resistant were full-sib piglets, tissue samples from 11 organs (heart, liver, spleen, lung, kidney, stomach, muscle, thymus, lymph node, duodenum, and jejunum) were collected, immersed in liquid nitrogen, and stored at −70°C.
Approximately 1.0 g of ear tissue was collected from 201 Large White sows from the 8 families and placed in 1.5-mL Eppendorf tubes. Genomic DNA was extracted by means of a modified phenol and chloroform protocol (15). The purity and concentration of the genomic DNA were assessed in a NanoDrop-1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, Delaware, USA). The genomic DNA was diluted to 100 ng/μL and stored at −20°C.
Primer design
Primers for PCR and real-time PCR were designed according to the TNF-α gene sequence obtained from the GenBank database (accession no. X54859; National Center for Biotechnology Information, Bethesda, Maryland, USA). The primers (Table I) were synthesized by Shanghai Invitrogen Biotechnology (Shanghai, China). The genes for glyceraldehyde 3-phosphate dehydrogenase, TATA box-binding protein 1, and β-actin were used as internal controls.
Table I.
Primers for polymerase chain reaction single-strand conformation polymorphism (PCR-SSCP) and real-time PCR
| Gene | Method | Sequence (5′→3′) |
|---|---|---|
| TNF-α | PCR-SSCP | F: GCCCGCCATGGTGGGTTTGT R: TGATTTCCGAACAGGGCTCAGGTA |
| TNF-α | Real-time PCR | F: CGCCCACGTTGTAGCCAATGT R: CAGATAGTCGGGCAGGTTGATCTC |
| GAPDH | Real-time PCR | F: ACATCATCCCTGCTTCTACTGG R: CTCGGACGCCTGCTTCAC |
| TBP1 | Real-time PCR | F: ACATCATCCCTGCTTCTACCGG R: CTCGGACGCCTGCTTCAC |
| ACTB | Real-time PCR | F: TGGCGCCCAGCACGATGAAG R: GATGGAGGGGCCGGACTCGT |
TNF-α — gene for tumor necrosis factor alpha; GAPDH, TBP1, and ACTB — genes for glyceraldehyde 3-phosphate dehydrogenase, TATA box-binding protein 1, and β-actin, used as internal controls; F — forward; R — reverse.
Analysis by PCR-SSCP
The reaction mixture (20 μL) contained 100 ng of DNA, 5 pmoL of each primer, 10 μL of PCR Master Mix (Tiangen Biotech, Beijing, China), and sterilized distilled water. The PCR protocol consisted of 1 cycle at 95°C for 5 min, followed by 30 cycles at 94°C for 40 s, 63°C for 40 s, and 72°C for 45 s, and an extension step at 72°C for 10 min. The PCR products were confirmed by electrophoresis in 1% agarose gel stained with ethidium bromide. Then the SSCP of the PCR products was detected by polyacrylamide gel electrophoresis. Loading buffer (7 μL) was mixed with 15 μL of PCR product, which was denatured at 98°C for 15 min and incubated in ice for 10 min. The denatured ice-cold mixtures were loaded onto a 12% non-denaturing polyacrylamide gel (Acr:Bis = 29:1). Electrophoresis was done at 130 V overnight, and then the products were silver-stained. According to the PCR-SSCP results, PCR products of different homozygotes were sequenced in an ABI PRISM 377 automatic DNA sequencer (Applied Biosystems, Foster City, California, USA). The PCR products from genotypes CC and TT were sequenced by Sangon Biotech Company, Shanghai, China, and the results were analyzed with the use of DNAMAN 5.0 software (Lynnon Biosoft, USA).
Total RNA extraction and real-time PCR
Total RNA was extracted from the tissue samples (50 to 100 mg) with the use of Trizol (TaKaRa Biotechnology, Dalian, China). Precipitated RNA was dissolved in 20 μL of RNase-free water. Qualitative and quantitative measurements of RNA were assessed by agarose gel electrophoresis and the ChemiDoc XRS+ Bio-imaging system (Bio-Rad Laboratories, Hercules, California, USA), respectively. The concentration of total RNA was measured by the NanoDrop-1000 spectrophotometer.
The 10-μL reaction mixture for cDNA synthesis contained 2 μL of 5× PrimerScript buffer, 0.5 μL of PrimerScript RT Enzyme Mix I, 0.5 μL of oligo-dT (a short sequence of deoxythymine nucleotides), 0.5 μL of random hexamers, 500 ng of total RNA, and RNase-free H2O. The reaction was done at 37°C for 15 min and then 5 s at 85°C.
Real-time PCR amplification was done with a 20-μL reaction mixture containing 1 μL of cDNA (100 to 500 ng), 0.4 μL of each forward and reverse primer (10 μM each), 0.4 μL of 50× ROX Reference Dye II, 10 μL of 2× SYBR Green real-time PCR Master Mix, and 7.8 μL of double-distilled water. The PCR protocol included 1 cycle at 95°C for 15 s, followed by 40 cycles at 95°C for 5 s and 62°C for 34 s. The dissociation curve was analyzed after amplification. A melting temperature (Tm) peak at 85 ± 0.8°C was used to determine the specificity of the PCR amplification. Each sample was analyzed 3 times.
The mRNA expression of TNF-α in different tissues was analyzed by real-time PCR. We first analyzed the expression levels of TNF-α mRNA in 11 tissues from Large White piglets resistant or susceptible to enterotoxigenic Escherichia coli (ETEC) F18. Then other 201 Large White sows were analyzed by PCR-SSCP, and grouped according to genotype, we chose 6 pigs from each genotype to analyze the expression levels of TNF-α mRNA in the 11 tissues according to piglet genotype.
Statistical analyses
Gene frequencies and genotype were calculated by the Hardy–Weinberg equilibrium principle: p = P + H/2, q = Q + H/2, x2 = ∑d2/e; d = e − o, which is the difference between the predicted and obtained values. The variables p and q represent allele frequencies at certain positions.
The 2−ΔΔCt method (16) was used to analyze the real-time PCR results:
Statistical analyses were done with SPSS software, version 15.0 (SPSS, Chicago, Illinois, USA). Data were expressed as mean ± standard deviation. Student’s t-test was used to determine differences in mRNA expression, or immune index.
Results
The TNF-α mRNA expression levels in the spleen, lung, thymus, and lymph node tissues were high in both the ETEC F18-resistant and the ETEC F18-susceptible animals (Table II). The expression levels in the duodenum, immune tissues (spleen, thymus, and lymph node) and kidney were significantly higher (P < 0.05) in the resistant piglets than in the susceptible piglets.
Table II.
Expression levels of TNF-α mRNA in 11 tissues from Large White piglets resistant or susceptible to enterotoxigenic Escherichia coli (ETEC) F18
| Tissue | Expression level (mean ± standard deviation) | Difference multiples | |
|---|---|---|---|
|
| |||
| Resistant (n = 8) | Susceptible (n = 8) | ||
| Heart | 1.076 ± 0.435 | 0.970 ± 0.284 | 1.109 |
| Liver | 9.266 ± 2.590 | 8.602 ± 2.489 | 1.077 |
| Spleen | 146.270 ± 20.99a | 85.808 ± 18.127b | 1.709 |
| Lung | 72.300 ± 21.083 | 63.981 ± 18.791 | 1.130 |
| Kidney | 17.521 ± 3.718a | 8.650 ± 3.029b | 2.025 |
| Stomach | 11.920 ± 3.338 | 6.405 ± 2.458 | 1.861 |
| Muscle | 0.246 ± 0.040 | 0.197 ± 0.068 | 1.253 |
| Thymus | 76.252 ± 8.353a | 36.127 ± 10.276b | 2.111 |
| Lymph node | 24.811 ± 4.313a | 12.806 ± 5.657b | 1.937 |
| Duodenum | 8.882 ± 2.015a | 5.241 ± 0.878b | 1.695 |
| Jejunum | 7.154 ± 1.835 | 5.217 ± 1.547 | 1.371 |
ΔΔCt = [average cycle threshold (Ct) value of the target gene in the tested group − average Ct value of the housekeeping gene in the tested group] − (average Ct value of the control gene in the control group − average Ct value of the housekeeping gene in the control group).
Within a row, the means without a common superscript differ significantly (P < 0.05).
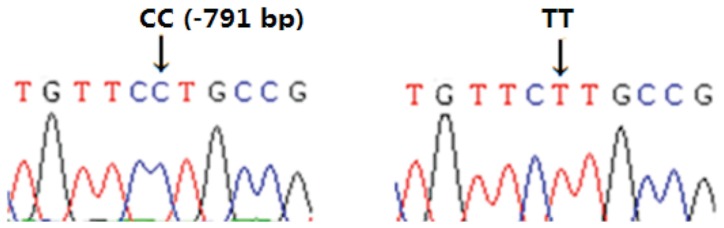
According to the results of the 1% agarose gel electrophoresis the length of the amplified fragment was in agreement with the predicted fragment length. The SSCP analysis revealed 3 genotypes: CC, CT, and TT (Figure 2). With a sequence for TNF-α identical to that in GenBank (accession no. X54859), the CC genotype was defined as the wild type. In comparison, the TT genotype had a C/T substitution mutation at nucleotide −791, located in the promoter (Figure 3).
Figure 2.
Result of polymerase chain reaction single-strand conformation polymorphism of the gene fragment of TNF-α. Lane 1 represents genotype CC, lane 2 genotype CT, and lane 3 genotype TT.
Figure 3.
Sequencing peak chart of the CC and TT genotypes. Because the sequence was identical to that in GenBank (accession no. X54859), the CC genotype was defined as the wild type. In comparison, the TT genotype had a C/T substitution mutation at nucleotide −791, located in the promoter.
The frequencies of genotypes CC, CT, and TT were 0.0857, 0.5571, and 0.3571, respectively; T was the dominant allele. The results revealed that TNF-α −791(C→T) was consistent with the Hardy–Weinberg equilibrium (Table III).
Table III.
Genotypic and allelic frequencies of the TNF-α −791(C→T) mutation in the piglets
| Sample size | Frequency (number of samples) | χ2 valuea | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Genotype | Allele | |||||
|
|
|
|||||
| CC | CT | TT | C | T | ||
| 201 | 0.254 (51) | 0.522 (105) | 0.224 (45) | 0.515 | 0.485 | 0.420 |
χ20.05 (1) = 3.84; χ20.01 (1) = 6.63.
When grouped according to genotype (Table IV), the TNF-α expression levels in the spleen, kidney, lymph nodes, and duodenum were significantly lower in the CC pigs than in the TT pigs (P < 0.05), and the trend in expression level was CC < CT < TT.
Table IV.
Expression levels of TNF-α mRNA in the 11 tissues according to piglet genotype
| Expression level (mean ± standard deviation), genotype (number of piglets) | |||
|---|---|---|---|
|
|
|||
| Tissue | CC (6) | CT (6) | TT (6) |
| Heart | 1.295 ± 0.443 | 0.903 ± 0.299 | 0.975 ± 0.353 |
| Liver | 9.610 ± 1.630 | 8.631 ± 3.302 | 9.186 ± 3.154 |
| Spleen | 79.424 ± 8.548a | 127.241 ± 48.479a,b | 141.208 ± 10.077b |
| Lung | 72.835 ± 20.146 | 66.006 ± 22.501 | 69.802 ± 24.143 |
| Kidney | 7.770 ± 1.856a | 14.653 ± 7.830a,b | 16.398 ± 2.531b |
| Stomach | 7.447 ± 2.274 | 8.321 ± 5.696 | 11.535 ± 3.819 |
| Muscle | 0.243 ± 0.051 | 0.184 ± 0.080 | 0.247 ± 0.048 |
| Thymus | 15.380 ± 6.988 | 15.190 ± 6.297 | 25.360 ± 5.419 |
| Lymph node | 40.819 ± 5.283a | 58.688 ± 21.960a,b | 86.849 ± 19.840b |
| Duodenum | 5.373 ± 1.275a | 6.122 ± 1.240a,b | 9.6535 ± 2.185b |
| Jejunum | 6.205 ± 1.542 | 5.674 ± 2.738 | 6.858 ± 1.967 |
Within a row, the means without a common superscript differ significantly (P < 0.05).
Discussion
The expression of TNF-α, which depends on environmental stressors, triggers inflammation and apoptosis, and it prevents bacterial proliferation (17). The real-time PCR results in this study revealed high expression levels of TNF-α mRNA in the immune system (in spleen, thymus, and lymph nodes) and in the lungs. The immune system confers resistance against infections; immune cells generate several factors that are distributed to various organs of the body. As a vital organ in the respiratory system, the lungs frequently communicate with the outside environment. Neutrophils and T-cells produce high levels of immune factors in response to various external stimuli; however, this does not occur in the lungs (18). The high levels of TNF-α mRNA expression in the immune system were attributed to the biofunctions of TNF-α.
It has been reported that TNF-α can promote dendritic cell differentiation (19,20). In piglets infected with porcine reproductive and respiratory syndrome virus (PRRSV), TNF-α inhibited the replication of PRRSV (21). Furthermore, lipopolysaccharides enhance TNF-α transcription and translation (22). In our study, the TNF-α expression levels in the ETEC F18-resistant piglets were higher than those in the ETEC F18-susceptible piglets. Additionally, there were significant differences in the TNF-α expression levels in the spleen, kidney, lymph nodes, and duodenum between piglets with the TT and CC genotypes. The duodenum, which is the first intestinal segment, is vulnerable to bacterial infections. In addition to its role in digestion and absorption of nutrients, the intestine has immune and endocrine functions (23,24). However, the intestinal immune function depends on the structural integrity and function of the intestinal epithelial cells. With infection by pathogenic bacteria, the intestinal levels of IL-8, IL-1, and TNF-α increase; thus, both the immune system and the absorption of nutrients are affected (25). Therefore, intestinal TNF-α expression levels are closely linked to health: high expression levels improve the resistance to several pathogenic bacteria.
In addition to its role in the immune system, TNF-α plays an important role in the development of skeletal muscle via proteolytic digestion of the protein ubiquitin and the mitogen-activated protein kinase signal transduction pathway (26,27). As well, TNF-α may be involved in pregnancy via follicle development and ovulation, corpus luteum formation and regression, cyclic endometrium function through the secretion of prostaglandin (PG) F2 alpha and PGE2 (28), and embryo implantation and immune suppression (29).
This study showed that the TT genotype of TNF-α −791(C→T) may contribute to higher levels of TNF-α expression in intestinal tissue (duodenum) and immune tissues (spleen and lymph nodes) than the CT and CC genotypes, and these higher levels could play an important role in conferring ETEC F18 resistance. Therefore, this mutation could be considered as a potential genetic marker for ETEC F18 resistance in piglets of the Large White breed, and we should further assess this possibility in larger populations and several generations.
Acknowledgments
This study was funded by the National Natural Science Funds (grants 31172183 and 31372285), the Genetically Modified Organisms Technology Major Project (2014ZX0800601B), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- 1.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB. Signaling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 3.Menon R, Merialdi M, Betrán AP, et al. Analysis of association between maternal tumor necrosis factor-α promoter polymorphism (−308), tumor necrosis factor concentration, and preterm birth. Am J Obstet Gynecol. 2006;195:1240–1248. doi: 10.1016/j.ajog.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 4.Namian AM, Shahbaz S, Salmanpoor R. Association of interferon-gamma and tumor necrosis factor alpha polymorphisms with susceptibility to vitiligo in Iranian patients. Arch Dermatol Res. 2009;301:21–25. doi: 10.1007/s00403-008-0904-8. [DOI] [PubMed] [Google Scholar]
- 5.Lanas A, Garcia-González MA, Santolaria S, et al. TNF and LTA gene polymorphisms reveal different risk in gastric and duodenal ulcer patients. Genes Immun. 2001;2:415–421. doi: 10.1038/sj.gene.6363798. [DOI] [PubMed] [Google Scholar]
- 6.Höhler T, Kruger A, Gerken G, Schneider PM, Meyer zum Büschenfelde KH, Rittner C. Tumor necrosis factor alpha promoter polymorphism at position −238 is associated with chronic active hepatitis C infection. J Med Virol. 1998;54:173–177. doi: 10.1002/(sici)1096-9071(199803)54:3<173::aid-jmv5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Wei MT, Han YY, He L, et al. Study on the roles of TNF-α gene polymorphism at the promoter region in the susceptibility and symptoms of SARS coronavirus infection [in Chinese] Acta Acad Med CPAF. 2009;18:169–174. [Google Scholar]
- 8.Szydlowski M, Buszka A, Mackowski M, Lechniak D, Switonski M. Polymorphism of genes encoding cytokines IL6 and TNF is associated with pig fatness. Livest Sci. 2011;136:150–156. [Google Scholar]
- 9.Pomorska-Mól M, Markowska-Daniel I, Kwit K, et al. Immune and inflammatory response in pigs during acute influenza caused by H1N1 swine influenza virus. Arch Virol. 2014;159:2605–2614. doi: 10.1007/s00705-014-2116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabner S, Worliczek HL, Witter K, Meyer FR, Gerner W, Joachim A. Immune response to Cystoisospora suis in piglets: Local and systemic changes in T-cell subsets and selected mRNA transcripts in the small intestine. Parasite Immunol. 2014;36:277–291. doi: 10.1111/pim.12116. [DOI] [PubMed] [Google Scholar]
- 11.Vicenova M, Nechvatalova K, Chlebova K, et al. Evaluation of in vitro and in vivo anti-inflammatory activity of biologically active phospholipids with anti-neoplastic potential in porcine model. BMC Complement Altern Med. 2014;14:339. doi: 10.1186/1472-6882-14-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Wang J, Zhao QH, et al. Genetic variation in exon 10 of the BPI gene is associated with Escherichia coli F18 susceptibility in Sutai piglets. Gene. 2013;523:70–75. doi: 10.1016/j.gene.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 13.Bao WB, Ye L, Pan ZY, et al. Microarray analysis of differential gene expression in sensitive and resistant pig to Escherichia coli F18. Anim Genet. 2012;43:525–534. doi: 10.1111/j.1365-2052.2011.02287.x. Epub 2011 Nov 7. [DOI] [PubMed] [Google Scholar]
- 14.Wu SL, Yuan ZW, Ju HP, et al. Study on genetically susceptible piglets of small intestinal epithelium receptors to pathogenic F18 fimbrial Escherichia coli adhesion in vitro. Chin J Vet Sci. 2006;26:622–625. [Google Scholar]
- 15.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Chapter 6. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2001. Protocol 1. [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz H, Fromm M, Bentzel CJ, et al. Tumor necrosis factor-alpha (TNF-α) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112:137–146. doi: 10.1242/jcs.112.1.137. [DOI] [PubMed] [Google Scholar]
- 18.Raz E. Organ-specific regulation of innate immunity. Nat Immunol. 2007;8:3–4. doi: 10.1038/ni0107-3. [DOI] [PubMed] [Google Scholar]
- 19.Chomarat P, Dantin C, Bennett L, Banchereau J, Palucka AK. TNF skews monocyte differentiation from macrophages to dendritic cells. J Immunol. 2003;171:2262–2269. doi: 10.4049/jimmunol.171.5.2262. [DOI] [PubMed] [Google Scholar]
- 20.Clay H, Volkman HE, Ramakrishnan L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity. 2008;29:283–294. doi: 10.1016/j.immuni.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng WH, Du HL, Lin ZQ, Shan Y, Wang XN. Differentially expressed genes and gene function enrichment analysis in the process of porcine alveolar macrophages infected with PRRSV [in Chinese] Genomics Appl Biol. 2011;29:1039–1046. [Google Scholar]
- 22.Feng LL, Zhang LP, Zhang GP, et al. In vitro effects of lipopolysaccharide on the expression of IL-1 and TNF-α in porcine alveolar macrophage [in Chinese] J Henan Agric Sci. 2009;3:106–109. [Google Scholar]
- 23.Harari Y, Weisbrodt NW, Moody FG. Ileal mucosal response to bacterial toxin challenge. J Trauma. 2000;49:306–313. doi: 10.1097/00005373-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Kiyono H, Kweon MN, Hiroi T, Takahashi I. The mucosal immune system: From specialized immune defense to inflammation and allergy. Acta Odontol Scand. 2001;59:145–153. doi: 10.1080/000163501750266738. [DOI] [PubMed] [Google Scholar]
- 25.McKay DM, Baird AW. Cytokine regulation of epithelial permeability and ion transport. Gut. 1999;44:283–289. doi: 10.1136/gut.44.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li YP, Lecker SH, Chen Y, Waddell ID, Goldberg AL, Reid MB. TNF-α increases ubiquitin-conjugating activity in skeletal muscle by up-regulating UbcH2/E220k. FASEB J. 2003;17:1048–1057. doi: 10.1096/fj.02-0759com. [DOI] [PubMed] [Google Scholar]
- 27.Saini A, Al-Shanti N, Stewart CEH. Waste management — Cytokines, growth factors and cachexia. Cytokine Growth Factor Rev. 2006;17:475–486. doi: 10.1016/j.cytogfr.2006.09.006. Epub 2006 Nov 22. Erratum in: Cytokine Growth Factor Rev 2007;18:345. [DOI] [PubMed] [Google Scholar]
- 28.Calleja-Agius J, Muttukrishna S, Jauniaux E. Role of TNF-α in human female reproduction. Exp Rev Endocrinol Metab. 2009;4:273–282. doi: 10.1586/eem.09.4. [DOI] [PubMed] [Google Scholar]
- 29.Jana B, Koszykowska M, Andronowska A. The influence of interleukin (IL)-1β and IL-6 and tumour necrosis factor-α on prostaglandin secretion from porcine myometrium during the first third of pregnancy. Acta Vet Brno. 2010;79:559–569. [Google Scholar]