Abstract
The aim of this study was to determine the pharmacokinetics of amikacin and penicillin G sodium when administered in combination as an intravenous regional limb perfusion (IVRLP) to horses. Seven healthy adult horses underwent an IVRLP in the cephalic vein with 2 g of amikacin sulfate and 10 mill IU of penicillin G sodium diluted to 60 mL in 0.9% saline. A pneumatic tourniquet set at 450 mmHg was left in place for 30 min. Synovial fluid was collected from the metacarpophalangeal joint 35 min and 2, 6, 12, and 24 h after infusion of the antimicrobials. Concentrations of amikacin and penicillin in synovial fluid were quantitated by liquid chromatography tandem-mass spectrometry analysis. Therapeutic concentrations of amikacin and penicillin for equine-susceptible pathogens were achieved in the synovial fluid. Maximum synovial concentrations (Cmax) (mean ± SE) for amikacin and penicillin were 132 ± 33 μg/mL and 8474 ± 5710 ng/mL, respectively. Only 3 horses had detectable levels of penicillin at 6 h and 1 at the 12 h sample. The combination of amikacin with penicillin G sodium via IVDLP resulted in reported therapeutic concentrations of both antibiotics in the synovial fluid. The Cmax:MIC (minimum inhibitory concentration) ratio for amikacin was 8:1 and Time > MIC for penicillin was 6 h. At 24 h, the mean concentration of amikacin was still above 4 μg/mL. Terminal elimination rate constants (T1/2 lambdaz) were 13.6 h and 2.8 h for amikacin and penicillin, respectively. The use of IVDLP with penicillin may therefore not be practical as rapid clearance of penicillin from the synovial fluid requires frequent perfusions to maintain acceptable therapeutic concentrations.
Résumé
L’objectif de la présente étude était de déterminer la pharmacocinétique de l’amikacine et de la pénicilline G sodique lorsqu’administrées en combinaison par perfusion intraveineuse régionale d’un membre (PIVRM) à des chevaux. Sept chevaux adultes ont reçu une PIVRM dans la veine céphalique avec 2 g de sulfate d’amikacine et 10 millions d’UI de pénicilline G sodique dilués dans 60 mL de saline 0,9 %. Un tourniquet pneumatique réglé à 450 mmHg a été laissé en place pour 30 min. Du liquide synovial a été récolté de l’articulation métacarpo-phalangienne 35 min, 2, 6, 12, et 24 h après l’infusion des antimicrobiens. Les concentrations d’amikacine et de pénicilline dans le liquide synovial furent mesurées par spectrométrie de masse en tandem avec la chromatographie en phase liquide. Les concentrations thérapeutiques d’amikacine et de pénicilline pour des agents pathogènes équins sensibles ont été atteintes dans le liquide synovial. Les concentrations synoviales maximales (Cmax) [moyenne ± écart-type (EC)] pour l’amikacine et la pénicilline étaient de 132 ± 33 μg/mL et 8474 ± 5710 ng/mL, respectivement. Seulement 3 chevaux avaient des quantités détectables de pénicilline à 6 h et un seul pour l’échantillon de 12 h. La combinaison d’amikacine et de pénicilline G sodique via PIVRM a permis de rapporter des concentrations thérapeutiques des deux antibiotiques dans le liquide synovial. Le ratio Cmax-CMI (concentration minimale inhibitrice) pour l’amikacine était de 8:1 et la période de Temps > CMI pour la pénicilline était de 6 h. À 24 h, la concentration moyenne d’amikacine était toujours supérieure à 4 μg/mL. Les constantes du taux d’élimination terminal (T1/2 lambdaz) étaient 13,6 h et 2,8 h pour l’amikacine et la pénicilline, respectivement. L’utilisation de PIVRM avec la pénicilline ne serait ainsi pas pratique étant donné que la clairance rapide de la pénicilline à partir du liquide synovial requière des perfusions fréquentes pour maintenir des concentrations thérapeutiques acceptables.
(Traduit par Docteur Serge Messier)
Introduction
Orthopedic infections in horses are life-threatening emergencies that may affect the animal’s athletic future or require euthanasia. Intravenous regional limb perfusion (IVRLP) is commonly used to treat orthopedic infections because it delivers high antimicrobial concentrations above the minimum inhibitory concentration (MIC) for common equine bacterial isolates in the synovial, osseous, and soft-tissue structures of the distal limb, while limiting systemic side effects and cost (1–9). Compared with systemic antimicrobial administration, IVRLP results in higher regional concentration and reduces the risk of systemic toxicity that leads to renal and gastrointestinal side effects (1).
Common pathogens cultured from orthopedic infections in horses include Enterobacteriaceae, streptococci, staphylococci, and Pseudomonas spp. (10,11). Most of the studies on regional limb perfusion in horses have used aminoglycosides that provide good coverage for Gram-negative bacteria and Staphylococcus, but lack activity against other Gram-positive bacteria. Combinations of a beta-lactam with an aminoglycoside are commonly used systemically since they provide broad-spectrum coverage and have good drug synergy (4). At our hospital, a combination of systemic procaine penicillin and gentamicin is used as a first option for orthopedic infections until sensitivity results are obtained. Aminoglycosides used with intravenous regional limb perfusion (IVRLP), which has been reported in research studies, are the most common antimicrobials used regionally in clinical cases with orthopedic infections (1,4–7,12–15). The use of intraosseous or IVRLP with penicillin has only been reported in clinical cases (3,6,9,16,17).
When combining beta-lactams with aminoglycosides, both in-vitro and in-vivo drug interaction has been reported (15,18–20). The interaction depends on medium, temperature, time, pH, and concentration, which results in lower antimicrobial concentration of both antibiotics and can still occur in body fluids, such as urine. The reported mechanism of interaction involves a nucleophilic opening of the beta-lactam ring and a reaction with an amino group of the aminoglycoside to form an inactive amide (18,21). An in-vitro study comparing the interaction of 6 beta-lactam antibiotics with 5 aminoglycosides found that amikacin was the aminoglycoside least inactivated by all beta-lactams, retaining its activity with minor changes at 48 h. The combination of penicillin G and amikacin was the most stable of all combinations evaluated (21).
Aminoglycosides are commonly used to treat serious enterococcal, mycobacterial, staphylococcus, and Gram-negative bacterial infections. Among the aminoglycosides, amikacin is useful for gentamicin-resistant, Gram-negative pathogen infections and is one of the most commonly used antibiotics in IVRLP due to its concentration-dependent action (6). Penicillin is still one of the most commonly used beta-lactam antibiotics in veterinary medicine. Since penicillin is considered time-dependent in its activity, the time (T) of drug concentration above the MIC (T > MIC) is important to clinical success (22,23). Unlike aminoglucosides, beta-lactam antibiotics exhibit little concentration-dependent killing. Penicillin has only post-antibiotic effect for staphylococci and animal data suggest that levels need to exceed the MIC for 90% of the dosing interval against gram-negative bacilli and streptococci, but only 50% to 60% for staphylococci in neutropenic animals. In nonneutropenic animals, however, the T > MIC can be reduced to 25% to 30% (23).
Bacterial killing with penicillin depends on time and when administered via IVRLP is likely to result in local therapeutic concentrations of the antimicrobial for a longer time than is possible with systemic administration. It has been hypothetized that the use of time-dependent antimicrobials for IVRLP can be justified because it is likely to result in therapeutic concentrations of the antimicrobial in infected ischemic tissues for a longer time than is possible with systemic administration (6). In addition, after the tourniquet is released, the high antimicrobial concentrations in the surrounding tissues may serve as a depot, producing continuous diffusion from the surrounding tissues to the synovial structures (24,25).
The purpose of this study was to determine the pharmacokinetics of amikacin and penicillin G sodium when administered in combination as an IVRLP to healthy adult horses.
Materials and methods
Adult horses, 4 geldings and 3 females, with an average age of 16 y (5 to 18 y) and an average weight of 520 kg (500 to 603 kg) were used in the study. Breeds included 2 Thoroughbreds, 4 Quarter horses, and 1 Standardbred. Horses were healthy based on physical examination and had no signs of lameness or musculoskeletal injury.
The protocol was approved by the University of California Institutional Animal Care and Use Committee. Horses were sedated with detomidine hydrochloride [0.02 mg/kg body weight (BW)], intravenously (IV) and butorphanol tartrate (0.02 mg/kg BW, IV), administered via a 14-gauge (ga) IV catheter placed in a jugular vein. If horses required additional sedation, detomidine hydrochloride (0.004 mg/kg BW IV) was administered. Effort was made to maintain adequate sedation to prevent limb movement until tourniquet removal. A pneumatic tourniquet with a 10.5-cm cuff was placed at the mid-to-proximal antebrachium and insuflated at a pressure of 450 mmHg. On a randomly selected front limb, a 24-mm long, 20-ga IV catheter was aseptically placed into the cephalic vein approximately 10 cm proximal to the accessory carpal bone. Two grams of amikacin sulfate (Amikacin; Teva Pharmaceuticals, Sellersville, Pennsylvania, USA) and 10 mill IU of penicillin (Penicillin G Sodium; Sandoz, Princeton, New Jersey, USA) were diluted to a total volume of 60 mL with saline (0.9% Sodium Chloride; Baxter Healthcare, Deerfield, Illinois, USA) immediately before infusion and slowly injected into the cephalic vein over 1.5 min. The tourniquet was removed 30 min after injection.
Synovial fluid was collected from all 7 horses within 5 min of tourniquet removal (35 min) and 2, 6, 12, and 24 h after the antimicrobials were administered. Synoviocentesis was conducted using aseptic techniques from the metacarpophalangeal joint using a lateral approach through the collateral proximal sesamoidean ligament as previously described (26). Synovial fluid (1 mL) was collected and immediately centrifuged at 1700 × g for 5 min and frozen at −80°C until analysis. Horses were evaluated for lameness, joint swelling, and phlebitis every 24 h for 3 d after the IVRLP.
Measurement of amikacin and penicillin concentrations in synovial fluid
Concentrations of amikacin and penicillin were quantitated in horse synovial fluid by liquid-chromatography-tandem mass spectrometry analysis, using modifications of previously published methods (27,28). Tobramycin and penicillin V were used as the internal standard for amikacin and penicillin analyses, respectively. For amikacin analysis, plasma calibrators were prepared by diluting the working standard solutions with drug-free synovial fluid collected from horses to concentrations ranging from 1.0 to 600 μg/mL. Synovial fluid calibrators for penicillin analysis were similarly prepared to concentrations ranging from 10 to 100 000 ng/mL. Fresh calibration curves were prepared for each quantitative assay. In addition, quality control samples, prepared at concentrations within the standard curve, were included with each sample set as an additional check of accuracy.
The response for both amikacin and penicillin was linear and gave correlation coefficients (R2) of 0.99 or better. For amikacin analysis, the accuracy (percentage of nominal concentration) and precision (percentage relative to standard deviation) were 93% and 108% and 3% and 4% at 8.0 and 80.0 μg/mL, respectively. For penicillin analysis, accuracy and precision were 98%, 102%, and 98% and 3%, 3%, and 1% for 150, 8000, and 80 000 ng/mL, respectively. Accuracy and precision for both assays were considered acceptable based on US Food and Drug Administration (FDA) guidelines for bioanalytical method validation. The technique was optimized to provide a minimum limit of quantification (LOQ) of 1 μg/mL and a limit of detection (LOD) of 0.1 μg/mL for amikacin and an LOQ of 10 ng/mL and LOD of 0.5 ng/mL for penicillin.
Pharmacokinetic analysis
Nonlinear least square regression was carried out on the amikacin concentration in synovial fluid versus time data using commercially available software and non-compartmental analysis (Phoenix WinNonlin Version 6.2; Pharsight, Carey, North Carolina, USA). Due to the limited detection time, naïve pooling of datum points was used to combine data from different horses at each time point before pharmacokinetic analysis of penicillin concentration in synovial fluid. Non-compartmental analysis for sparse data was used to determine the pharmacokinetic parameters for penicillin. The area under the curve (AUC) for both amikacin and penicillin was calculated using the log-linear trapezoidal rule.
Results
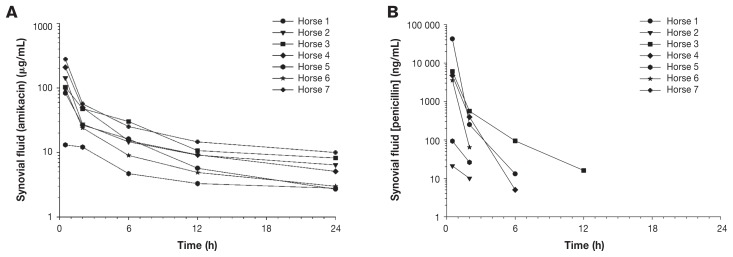
No lameness, joint effusion, or obvious phlebitis was observed in any of the horses. The concentrations of amikacin sulfate and penicillin G sodium in synovial fluid at different time points are presented in Figures 1A and 1B, respectively. Detectable concentrations of amikacin were obtained in synovial fluid of all horses at all time points. Penicillin was detected in the synovial fluid of 3 horses at 6 h, in only 1 horse at 12 h, and was not detected in any horses at 24 h. Pharmacokinetic variables are shown in Tables I and II. No clinically detectable adverse effects were identified.
Figure 1.
Concentration of amikacin (A) and penicillin (B) in synovial fluid of 7 horses after distal limb perfusion with 2 g of amikacin sulfate and 10 mill IU of penicillin G sodium. Samples were collected from the metacarpophalangeal joint at 5 min and 2, 6, 12, and 24 h after the tourniquet was removed.
Table I.
Individual pharmacokinetic parameters describing the disposition kinetics of amikacin in synovial fluid after intravenous perfusion of amikacin sulfate (2 g) and penicillin sodium (10 mill IU) in 7 horses. All parameters were generated using non-compartmental analysis
| Pharmacokinetic parameter | Horse 1 | Horse 2 | Horse 3 | Horse 4 | Horse 5 | Horse 6 | Horse 7 | Mean ± SE |
|---|---|---|---|---|---|---|---|---|
| Cmax (μg/mL)a | 83.0 | 142.7 | 102.4 | 208.8 | 13.1 | 95.2 | 279.4 | 132.1 ± 33.3 |
| Tmax (h)b | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 ± 0.0 |
| AUClast (h*μg/mL)c | 286.6 | 384.0 | 510.0 | 489.0 | 113.3 | 249.1 | 697.1 | 389.9 ± 73.3 |
| T1/2 lambdaz (h)d | 6.65 | 15.9 | 8.66 | 11.7 | 26.0 | 11.9 | 14.3 | 13.6 ± 2.4 |
Maximum synovial concentration.
Time of maximal synovial concentration.
Area under the curve to the last time point collected.
Terminal elimination rate constant.
Table II.
Average pharmacokinetic parameters describing the disposition kinetics of penicillin in synovial fluid after intravenous perfusion of penicillin G sodium (10 mill IU) and amikacin sulfate (2 g) in 7 horses. All parameters were generated using non-compartmental analysis for sparse data
| Pharmacokinetic parameter | Mean ± SE |
|---|---|
| Cmax (ng/mL)a | 8474 ± 5710 |
| Tmax (h)b | 0.58 |
| AUClast (h*ng/mL)c | 9247 ± 5738 |
| T1/2 lambdaz (h)d | 2.82 |
Maximum synovial concentration.
Time of maximal synovial concentration.
Area under the curve to the last time point collected.
Terminal elimination rate constant.
Discussion
It was found that, while a combination of amikacin sulfate and penicillin G sodium yield therapeutic concentrations of amikacin and penicillin in the synovial fluid, penicillin concentrations were maintained for only a short period of time (only 3/7 horses had detectable levels at 6 h and only 1 at 12 h). It was therefore concluded that IVRLP with this antibiotic combination may not be practical for treating clinical cases of sepsis.
As amikacin is a concentration-dependent antimicrobial, the rate and extent of bacterial killing is associated with high-peak concentration (Cmax) and MIC ratio. For the treatment of susceptible bacteria, the Cmax:MIC ratio should be 8:1 to 10:1 to maximize its effect (29,30). It has been reported that concentrations of amikacin from 1 to 4 μg/mL and from 2 to 16 μg/mL have an MIC90 for Escherichia coli and Pseudomonas aeruginosa respectively (31). Using the Cmax:MIC ratio of 8:1 synovial concentrations obtained in this study, 2 g of amikacin achieved therapeutic concentrations for E. coli and P. aeruginosa. In addition, at the 24-hour time point, the mean concentration of amikacin was still above 4 μg/mL MIC, which indicates that daily IVRLP with amikacin is adequate. In addition, aminoglycosides have a post-antibiotic effect (the continued suppression of bacterial growth after limited exposure of the organism to an antibiotic). The post-antibiotic effect of amikacin against staphylococci has been reported to be 5 to 10 h for clinically achievable concentrations (32). Another study (33) reported a mean post-antibiotic effect of amikacin of 3.43 h in equine isolates of methicillin-resistant Staphylococcus aureus.
Although the optimal dose and frequency of amikacin for IVRLP require further investigation, it seems that the dose used in this study provides antimicrobial coverage for at least 24 h. Reported synovial concentrations in studies using IVRLP with amikacin are highly variable, probably due in part to different methodologies, including dose of antimicrobial, type of tournique, volume and speed of infusion, vein and joints used, and amikacin determination assay. Similar to our results, however, other studies have also found wide ranges in concentration of amikacin in synovial fluids. One of the reasons we used 2 g of amikacin in this study was in part due to these wide ranges found in previous studies. Clinicians need to be aware that, even when the mean value from some studies showed good MIC concentrations, some horses may achieve low antimicrobial concentrations.
Some studies in horses have evaluated amikacin as an IVDLP using a methodology similar to the present study: horses standing under sedation, same amikacin dose, perfusion into the cephalic vein, tourniquet in the antebraquieium, and collecting fluid from the metacarpophalangeal joint (14,34). Maximum concentrations of amikacin in synovial fluid in those studies (277 and 50 μg/mL) compare with concentrations obtained in this study (132 μg/mL). One of these studies also found a very similar terminal elimination rate constant [12.9 h in the study by Kelmer et al (34) and 13.6 h in the present study].
Two IV penicillin G salts are commercially available, sodium and potassium. Due to more accessible price, penicillin G potassium is more commonly used at our hospital. As a million IU of penicillin G potassium contain 1.7 mEq of potassium ion, we therefore chose penicillin G sodium for this study as well as to prevent the possibility of hyperkalemia at the moment of tourniquet release. Doses of 1 to 10 mill IU of penicillin G potassium have been used in IVRLP in clinical cases in horses (6). Since no previous studies of IVRLP have been conducted with penicillin, we selected a high dose (10 mill IU) for this study because of the time-dependent characteristics of penicillin on the basis that a high dose could increase the half-life of the antibiotic.
Peak serum and synovial fluid concentration in healthy mares after an intramuscular (IM) injection of aqueous procaine penicillin G (22 000 IU/kg BW) was 1.42 μg/mL and 0.62 μg/mL, respectively (35). Furthermore, the mean concentration of penicillin in synovial fluid peaked at 4 h and decreased to 0.5 μg/mL and 0.23 μg/mL at 12 h and 24 h, respectively. The MIC of penicillin G in the horse for Streptococcus equui and Streptococcus zooepidemicus has been reported to be 0.002 to 0.08 μg/mL and 0.06 to 0.25 μg/mL for Corynebacterium pseudotuberculosis (35). In-vitro killing-curve studies of bacteria have shown that maximum killing is usually achieved at 3 to 4 times the MIC when using beta-lactam antibiotics (23).
Using the MIC from the previously mentioned studies, we achieved therapeutic levels of penicillin in the synovial fluid by using 10 mill IU in our IVRLP. Although our synovial fluid levels were higher than those achieved by parenteral administration of IM procaine penicillin, they dropped to 0.2 μg/mL by 2 h and were not detectable in 60% of our horses by 6 h. Concentrations of synovial and peritoneal penicillin parallel serum and penicillin leave the joint as readily as it enters in relation to serum concentrations (35). A previous study found that an intraperitoneal penicillin injection was absorbed and excreted less rapidly when supplemented by parenteral injections (36). It appears that intraarticular penicillin would equilibrate rapidly with serum, quickly depleting the synovial concentration because of the large volume of serum with which it is equilibrating. It is not known whether parenteral administration of penicillin in addition to the IVRLP would reduce the steep reduction of penicillin concentrations in the synovial fluid. The physiochemical property between aminoglycoside and beta-lactum has been reported (18,21). One of the horses in this study had a low concentration of amikacin and penicillin in the synovial fluid and a different horse with the lowest concentration of synovial penicillin had a very high concentration of amikacin.
A limitation of the study is that we did not collect blood to measure antibiotic concentrations before tourniquet removal to determine the effectiveness of the tourniquet. It is possible that the tourniquet failed in the horse with low concentrations of both antibiotics. The physical change of the penicillin molecule to form metabolites and decretive by-products occurs due to the exposure to plasma esterase present in the surrounding fluid. In addition, penicillins have a labile beta-lactam ring that has pronounced susceptibility to various nucleophiles, acid-base reagents, metal ions, oxidizing agents, or even solvents such as water and alcohol (37). Since we only measured penicillin and not its metabolites or breakdown products, it is not possible to determine whether the penicillin degraded in the horse with high amikacin and low penicillin levels.
We collected synovial fluid by arthrocentesis from the same joint on 5 occasions within a 24-hour period. Although the fluid was clear in all the initial samples, the fluid became serosanguinous in subsequent samples from some horses. It is possible that hemorrhage or inflammation as a result of repeated arthrocentesis may have decreased the concentration of antibiotics and potentially shortened its half-life. Although placing an in-dwelling small catheter could have prevented hemorrhage from repeated arthrocentesis, the inflammation induced by the catheter could also have affected concentrations of antimicrobials.
Although there are several studies showing positive results of synovial sepsis with the use of antimicrobials by IVRLP (1,4,6,8,10), all pharmacokinetic studies have been conducted in healthy animals (2,5,7,12,15,28,38). Septic arthritis can cause thrombosis of synovial vessels and necrosis of the synovial membrane, which may limit the delivery of systemically administered antibiotics. In addition, changes in vascular permeability induced by synovitis may increase the rate of antimicrobials entering or exiting the joint. A study of experimentally induced septic arthritis showed better outcome in horses receiving IVRLP with gentamicin than when the drug was administered intravenously (9). In that study, the perfused joints had lower nucleated cell counts and terminal bacterial cultures of synovial fluid and synovial membranes yielded negative results in 50% of joints, compared with 100% of joints treated only by systemic antimicrobials. A study of experimentally induced synovitis by lipo-polysacharide injection found a shorter Tmax and higher Cmax after IVRLP with amikacin compared to normal joints (38). Clinicians need to be aware, however, that the effect or the degree of antimicrobial diffusion and clearance in naturally inflamed and/or septic synovial structures when using IVRLP is not known.
Although we obtained acceptable levels of amikacin and penicillin in the synovial fluid, the levels of penicillin were present only for a short time. We used a high dose of penicillin in an effort to maintain antibiotic accumulation in the tissue outside the vascular space (depot phenomenon), but levels of antibiotics were detectable in only 40% of the horses in this study at 6 h. It is unknown if higher or long-lasting concentrations can be obtain if IVRLP is done using only penicillin as a single antibiotic. When penicillin G sodium is used by the IV route in horses, it is recommended that it be administered every 6 h (39). Our results indicate that if penicillin G sodium is used as IVRLP, it may also need to be administered at similar intervals. As the use of IVRLP in horses is painful and patients must be sedated, it is impractical to use IVRLP several times a day. Since the time that penicillin is above the MIC of the organism is the best predictor of bacterial killing and clinical efficacy, then the T > MIC can be maximized by administering the drug by continuous infusion (23). When high regional antibiotic concentration of amikacin and a beta-lactam antibiotic are desired, an alternative would be to carry out IVRLP with amikacin and administer the time-dependent antibiotic using a continuous delivery system (40).
Since the interaction between aminoglycosides and beta-lactam antibiotics is also concentration-dependent, it is not known if similar results would be obtained by using different concentrations of antibiotics than those used in this study. Regardless of the cause of the rapid reduction of penicillin from the synovial fluid (in-vivo interaction between amikacin and penicillin or rapid diffusion of penicillin from the synovial structure to systemic circulation), it is concluded that IVRLP with the combination used in this study is not recommended in clinical cases.
Acknowledgments
Supported by the University of California-Davis Comparative Gastrointestinal Laboratory by a gift from Mick and Sabrina Hellman.
References
- 1.Cruz AM, Rubio-Martinez L, Dowling T. New antimicrobials, systemic distribution, and local methods of antimicrobial delivery in horses. Vet Clin North Am Equine Pract. 2006;22:297–322. vii–viii. doi: 10.1016/j.cveq.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Errico JA, Trumble TN, Bueno AC, Davis JL, Brown MP. Comparison of two indirect techniques for local delivery of a high dose of an antimicrobial in the distal portion of forelimbs of horses. Am J Vet Res. 2008;69:334–342. doi: 10.2460/ajvr.69.3.334. [DOI] [PubMed] [Google Scholar]
- 3.Kettner NU, Parker JE, Watrous BJ. Intraosseous regional perfusion for treatment of septic physitis in a two-week-old foal. J Am Vet Med Assoc. 2003;222:346–350. 316. doi: 10.2460/javma.2003.222.346. [DOI] [PubMed] [Google Scholar]
- 4.Lugo J, Gaughan EM. Septic arthritis, tenosynovitis, and infections of hoof structures. Vet Clin North Am Equine Pract. 2006;22:363–388. viii. doi: 10.1016/j.cveq.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Murphey ED, Santschi EM, Papich MG. Regional intravenous perfusion of the distal limb of horses with amikacin sulfate. J Vet Pharmacol Ther. 1999;22:68–71. doi: 10.1046/j.1365-2885.1999.00180.x. [DOI] [PubMed] [Google Scholar]
- 6.Rubio-Martinez LM, Cruz AM. Antimicrobial regional limb perfusion in horses. J Am Vet Med Assoc. 2006;228:706–712. 655. doi: 10.2460/javma.228.5.706. [DOI] [PubMed] [Google Scholar]
- 7.Scheuch BC, Van Hoogmoed LM, Wilson WD, et al. Comparison of intraosseous or intravenous infusion for delivery of amikacin sulfate to the tibiotarsal joint of horses. Am J Vet Res. 2002;63:374–380. doi: 10.2460/ajvr.2002.63.374. [DOI] [PubMed] [Google Scholar]
- 8.Stewart AA, Goodrich LR, Byron CR, Evans RB, Stewart MC. Antimicrobial delivery by intrasynovial catheterisation with systemic administration for equine synovial trauma and sepsis. Aust Vet J. 2010;88:115–123. doi: 10.1111/j.1751-0813.2010.00553.x. [DOI] [PubMed] [Google Scholar]
- 9.Whithair KJ, Bowersock TL, Blevins WE, Fessler JF, White MR, Van Sickle DC. Regional limb perfusion for antibiotic treatment of experimentally induced septic arthritis. Vet Surg. 1992;21:367–373. doi: 10.1111/j.1532-950x.1992.tb01713.x. [DOI] [PubMed] [Google Scholar]
- 10.Schneider RK, Bramlage LR, Moore RM, Mecklenburg LM, Kohn CW, Gabel AA. A retrospective study of 192 horses affected with septic arthritis/tenosynovitis. Equine Vet J. 1992;24:436–442. doi: 10.1111/j.2042-3306.1992.tb02873.x. [DOI] [PubMed] [Google Scholar]
- 11.Snyder JR, Pascoe JR, Hirsh DC. Antimicrobial susceptibility of microorganisms isolated from equine orthopedic patients. Vet Surg. 1987;16:197–201. doi: 10.1111/j.1532-950x.1987.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 12.Alkabes SB, Adams SB, Moore GE, Alkabes KC. Comparison of two tourniquets and determination of amikacin sulfate concentrations after metacarpophalangeal joint lavage performed simultaneously with intravenous regional limb perfusion in horses. Am J Vet Res. 2011;72:613–619. doi: 10.2460/ajvr.72.5.613. [DOI] [PubMed] [Google Scholar]
- 13.Mattson S, Boure L, Pearce S, Hurtig M, Burger J, Black W. Intraosseous gentamicin perfusion of the distal metacarpus in standing horses. Vet Surg. 2004;33:180–186. doi: 10.1111/j.1532-950x.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 14.Sole A, Nieto JE, Aristizabal FA, Snyder JR. Effect of emptying the vasculature before performing regional limb perfusion with amikacin in horses. Equine Vet J. 2015 doi: 10.1111/evj.12501. [DOI] [PubMed] [Google Scholar]
- 15.Zantingh AJ, Schwark WS, Fubini SL, Watts AE. Accumulation of amikacin in synovial fluid after regional limb perfusion of amikacin sulfate alone and in combination with ticarcillin/clavulanate in horses. Vet Surg. 2014;43:282–288. doi: 10.1111/j.1532-950X.2014.12119.x. [DOI] [PubMed] [Google Scholar]
- 16.Palmer SE, Hogan PM. How to perform regional limb perfusion in the standing horse. Proceedings 45th Annu Conv Ann AAoc Equine Pract Year; pp. 124–127. [Google Scholar]
- 17.Santschi EM, Adams SB, Murphey ED. How to perform equine intravenous digital perfusion. 44th Annu Conv Am Assoc Equine Pract Year; pp. 198–201. [Google Scholar]
- 18.Wallace SM, Chan LY. In vitro interaction of aminoglycosides with beta-lactam penicillins. Antimicrob Agents Chemother. 1985;28:274–281. doi: 10.1128/aac.28.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tindula RJ, Ambrose PJ, Harralson AF. Aminoglycoside inactivation by penicillins and cephalosporins and its impact on drug-level monitoring. Drug Intell Clin Pharm. 1983;17:906–908. doi: 10.1177/106002808301701210. [DOI] [PubMed] [Google Scholar]
- 20.Holt HA, Broughall JM, McCarthy M, Reeves DS. Interactions between aminoglycoside antibiotics and carbenicillin or ticarillin. Infection. 1976;4:107–109. doi: 10.1007/BF01638726. [DOI] [PubMed] [Google Scholar]
- 21.Riff LJ, Thomason JL. Comparative aminoglycoside inactivation by beta-lactam antibiotics. Effects of a cephalosporin and six penicillins on five aminoglycosides. J Antibiot (Tokyo) 1982;35:850–857. doi: 10.7164/antibiotics.35.850. [DOI] [PubMed] [Google Scholar]
- 22.Papich MG, Riviere JE. B-lactam antibiotics: Penicillins, cephalosporins, and related drugs. In: Papich MG, Riviere JE, editors. Veterinary Pharmacology & Therapeutics. 9th ed. Vol. 1. United Sates: Wiley-Blackwell; 2009. pp. 865–893. [Google Scholar]
- 23.Turnidge JD. The pharmacodynamics of beta-lactams. Clin Infect Dis. 1998;27:10–22. doi: 10.1086/514622. [DOI] [PubMed] [Google Scholar]
- 24.Mattson SE, Pearce SG, Boure LP, Dobson H, Hurtig MB, Black WD. Comparison of intraosseous and intravenous infusion of technetium Tc 99m pertechnate in the distal portion of forelimbs in standing horses by use of scintigraphic imaging. Am J Vet Res. 2005;66:1267–1272. doi: 10.2460/ajvr.2005.66.1267. [DOI] [PubMed] [Google Scholar]
- 25.Finsterbusch A, Argaman M, Sacks T. Bone and joint perfusion with antibiotics in the treatment of experimental staphylococcal infection in rabbits. J Bone Joint Surg Am. 1970;52:1424–1432. [PubMed] [Google Scholar]
- 26.Moyer W, Schumacher J. A guide to equine joint injection and regional anesthesia. Yardley, OA: Veterinary Learning Systems; 2007. [Google Scholar]
- 27.Uboh CE, Soma LR, Luo Y, et al. Pharmacokinetics of penicillin G procaine versus penicillin G potassium and procaine hydrochloride in horses. Am J Vet Res. 2000;61:811–815. doi: 10.2460/ajvr.2000.61.811. [DOI] [PubMed] [Google Scholar]
- 28.Pinto N, Schumacher J, Taintor J, Degraves F, Duran S, Boothe D. Pharmacokinetics of amikacin in plasma and selected body fluids of healthy horses after a single intravenous dose. Equine Vet J. 2011;43:112–116. doi: 10.1111/j.2042-3306.2010.00144.x. [DOI] [PubMed] [Google Scholar]
- 29.Lacy MK, Nicolau DP, Nightingale CH, Quintiliani R. The pharmacodynamics of aminoglycosides. Clin Infect Dis. 1998;27:23–27. doi: 10.1086/514620. [DOI] [PubMed] [Google Scholar]
- 30.Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: Importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 31.Zhanel GG, DeCorby M, Nichol KA, et al. Antimicrobial susceptibility of 3931 organisms isolated from intensive care units in Canada: Canadian National Intensive Care Unit Study, 2005/2006. Diagn Microbiol Infect Dis. 2008;62:67–80. doi: 10.1016/j.diagmicrobio.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Isaksson B, Maller R, Nilsson LE, Nilsson M. Postantibiotic effect of aminoglycosides on staphylococci. J Antimicrob Chemother. 1993;32:215–222. doi: 10.1093/jac/32.2.215. [DOI] [PubMed] [Google Scholar]
- 33.Caron JP, Bolin CA, Hauptman JG, Johnston KA. Minimum inhibitory concentration and postantibiotic effect of amikacin for equine isolates of methicillin-resistant Staphylococcus aureus in vitro. Vet Surg. 2009;38:664–669. doi: 10.1111/j.1532-950X.2009.00551.x. [DOI] [PubMed] [Google Scholar]
- 34.Kelmer G, Bell GC, Martin-Jimenez T, et al. Evaluation of regional limb perfusion with amikacin using the saphenous, cephalic, and palmar digital veins in standing horses. J Vet Pharmacol Ther. 2013;36:236–240. doi: 10.1111/j.1365-2885.2012.01414.x. [DOI] [PubMed] [Google Scholar]
- 35.Stover SM, Brown MP, Kelly RH, Farver TB, Knight HD. Aqueous procaine penicillin G in the horse: Serum, synovial, peritoneal, and urine concentrations after single-dose intramuscular administration. Am J Vet Res. 1981;42:629–631. [PubMed] [Google Scholar]
- 36.Cooke JV, Goldering D. The concentrations of penicillin in various body fluids during penicillin therapy. J Am Med Assoc. 1945;127:80–87. [Google Scholar]
- 37.Deshpande AD, Baheti KG, Chatterjee NR. Degradation of Beta-lactam antibiotics. Current Science. 2004;87:1684–1695. [Google Scholar]
- 38.Beccar-Varela AM, Epstein KL, White CL. Effect of experimentally induced synovitis on amikacin concentrations after intravenous regional limb perfusion. Vet Surg. 2011;40:891–897. doi: 10.1111/j.1532-950X.2011.00875.x. [DOI] [PubMed] [Google Scholar]
- 39.Knottenbelt DC. Saunders Equine Frormulary. The Netherlands: Elsevier; 2006. [Google Scholar]
- 40.Lescun TB, Vasey JR, Ward MP, Adams SB. Treatment with continuous intrasynovial antimicrobial infusion for septic synovitis in horses: 31 cases (2000–2003) J Am Vet Med Assoc. 2006;228:1922–1929. doi: 10.2460/javma.228.12.1922. [DOI] [PubMed] [Google Scholar]