Abstract
The goal of the present study was to evaluate the potential use of slow release buprenorphine in sheep. Twelve adult female sheep (6 Dorset and 6 Suffolk, 12 months of age) were used for this project and were divided into 2 experimental groups (n = 6/group comprising 3 Dorset and 3 Suffolk sheep). Sustained release (SR) buprenorphine was administered subcutaneously in the scapular region at a concentration of 0.1 mg/kg body weight (BW) for group 1 and of 0.05 mg/kg BW for group 2. Following blood collections at selected time points, plasma concentrations of buprenorphine was performed by tandem liquid chromatograph-mass spectrometry. Mean buprenorphine concentration was above 0.1 ng/mL at 48 h up to 192 h post-injection for group 1 and it was above 0.1 ng/mL at 48 h up to 72 h post-injection for group 2. In conclusion, a long lasting potential analgesic plasma level of buprenorphine is attained following a single subcutaneous injection of 0.1 mg/kg BW of SR buprenorphine in sheep. However the effective analgesic plasma threshold still needs to be determined in sheep.
Résumé
L’objectif de la présente étude était d’évaluer l’utilisation potentielle de buprénorphine à relâchement lent (RL) chez le mouton. Douze brebis adultes (6 Dorset et 6 Suffolk, 12 mois d’âge) ont été utilisées pour ce projet et ont été réparties en deux groupes expérimentaux (n = 6/groupe, 3 Dorset et 3 Suffolk). De la buprénorphine à relâchement continu a été administrée par voie sous-cutanée dans la région scapulaire à une concentration de 0,1 mg/kg de poids corporel (PC) pour le groupe 1 et à 0,05 mg/kg de PC pour le groupe 2. Suite à des prélèvements sanguins à des moments sélectionnés, les concentrations plasmatiques de buprénorphine ont été déterminées par spectrométrie de masse en tandem avec la chromatographie en phase liquide. La concentration moyenne de buprénorphine était supérieure à 0,1 ng/mL après 48 h et jusqu’à 192 h post-injection pour le groupe 1, et était supérieure à 0,1 ng/mL après 48 et jusqu’à 72 h post-injection pour le groupe 2. En conclusion, un niveau plasmatique prolongé de buprénorphine avec un potentiel analgésique est atteint suite à une injection sous-cutanée unique de 0,1 mg/kg de PC de buprénorphine RL chez le mouton. Toutefois, le seuil plasmatique analgésique réel demeure encore à être déterminé chez le mouton.
(Traduit par Docteur Serge Messier)
Buprenorphine hydrochloride, a partial μ-opioid receptor agonist, is one of the most commonly used analgesics in laboratory animal medicine (1). Its simple formulation (Buprenorphine HCl) has an activity of approximately 8 h, which is due to its slow rate of drug disassociation from the receptor, making it a long-acting agent. It also has less respiratory and cardiovascular side effects than most opioids, which makes it a good therapeutic choice (2). Currently, only a few pharmaceutical opioid formulations with long-acting effects provide an appropriate analgesia for several days, seeing as their use prevents repeated injections and handling-associated stress. A common alternative to treat pain in a sustained manner is the use of transdermal systems such as fentanyl and buprenorphine patches (3–4). However, on many occasions, these patches do not properly adhere to the animals’ skin and therefore, animals do not receive an appropriate analgesia. Other problems may occur, such as fallen patches that are ingested by animals and represent a toxic risk (5–6).
The pharmacokinetics and pharmacodynamics of sustained release (SR) buprenorphine after a single subcutaneous injection has been evaluated in mice (7–9), rats (10,11), cats (12), and dogs (13). Effective buprenorphine blood levels are observed between 12 and 24 h post-injection in mice and up to 72 h post-injection in rats. In dogs, the effective buprenorphine plasma concentration is shown to be greater than 0.6 ng/mL after a single subcutaneous administration and this value is detected in the plasma for 72 h post-injection. In pigs, a threshold of 0.1 ng/mL buprenorphine plasma concentration is considered therapeutic and SR buprenorphine is allowed for an estimated 264 ± 32 h of analgesia (4). In macaques, a therapeutic plasma concentration of SR buprenorphine is detected for at least 5 d, with an effective analgesic plasma threshold of 0.1 ng/mL (14). These findings suggest a long duration of efficacy in all these species, when a single dose of SR buprenorphine is administered subcutaneously.
Buprenorphine is metabolized to its main metabolite, norbuprenophine, by the CYP3A liver enzyme. This metabolite is a μ-, δ-, and κ-opioid and nociceptin receptor agonist (15,16); however, it has poor brain penetrability and few analgesic properties in mice (17). In vitro, both buprenorphine and norbuprenorphine are metabolized by the same metabolic liver pathways, and they may each interfere with the degradation of the other molecule (18). The analysis of both buprenorphine and norbuprenorphine, therefore, is important in any pharmacokinetic study.
The goal of the present study was to measure the plasma concentrations of buprenorphine and its main metabolite, norbuprenorphine, following a single administration of a subcutaneous SR buprenorphine formulation in sheep. A theoretical effective concentration of 0.1 ng/mL was used for this analysis (13,14), which will need to be validated with a pharmacodynamics study in sheep. To our knowledge, no pharmacokinetic data relating to the administration of such a formulation to sheep is presently available. A longer effective duration — when compared to the simple formulation of buprenorphine hydrochloride — would have a beneficial effect on sheep welfare in experimental procedures such us orthopedic surgeries that induce long-lasting pain (19).
Twelve female sheep (6 Dorset and 6 Suffolk, 12 months of age) were used for this project. Animals were acclimated for 3 mo in the facilities of AccelLAB (AccelLAB Preclinical Research, Boisbriand, Quebec), where they were divided into 2 experimental groups (n = 6/group), each represented by 3 Dorsets and 3 Suffolk sheep. Sheep weighed 68.6 ± 22.8 kg and 63.2 ± 19.2 kg in groups 1 and 2, respectively. Animals were purchased from 2 different sources (Research Flock, University of Guelph, Ontario and Pozzi Ranch, California, USA) and were free of Q-fever, chlamydia, Johne’s disease, caseous lymphadenitis, Meadi Visna, and scrapie. The sheep were fed daily with Certified Diet (Harlan Teklad Ruminant diet 7060C; Harlan Laboratories, Madison, Wisconsin, USA) and had access to municipal water and certified hay ad libitum. Environmental conditions were maintained within accepted limits for temperature (16°C to 22°C) and relative humidity (40% to 70%). Lighting was maintained in a 12 h dark:12 h light cycle. Sheep were housed on a concrete floor, covered with wood chip bedding that was changed at least once every 3 wk and feces were removed daily. The experimental protocol was reviewed and approved by the AccelLAB’s Institutional Animal Care and Use Committee and was in compliance with Canadian Council on Animal Care regulations.
Sustained release buprenorphine (3 mg/mL; SR Veterinary Technologies, Windsor, Colorado, USA) was administered subcutaneously in the subscapular region. The area was shaved and cleaned with alcohol prior to injection. Group 1 received 0.1 mg/kg body weight (BW) and Group 2 received 0.05 mg/kg BW of SR buprenorphine. Blood samples were collected (1 mL/time point) in 2 mL K3EDTA tubes (Newton, North Carolina, USA) at selected time points (Predose, 1, 4, 8, 24, 48, 72, 96, 168, and 336 h) from the jugular vein. They were kept on ice pending centrifugation (3200 × g for 10 min, at room temperature) which was performed within 30 min of collection. Afterwards, plasma was harvested and transferred in identified plastic tubes which were placed on dry ice pending storage at −80°C.
Using protein precipitation as a sample preparation technique, buprenorphine and norbuprenorphine were extracted from sheep plasma. A 200 μL volume of internal standard solution (0.5 ng/mL of d4-buprenorphine and d3-norbuprenorphine in methanol) was added to an aliquot of 100 μL of plasma sample. The sample was vortexed for approximately 5 s and let still for a period of 10 min, then centrifuged at 12 000 × g for 10 min. The supernatant was transferred to an injection vial for online Solid-Phase Extraction (SPE) and HPLC-MS analysis. On-line SPE and HPLC were performed using Thermo UltiMate 3000 Rapid Separation UHPLC system, a Thermo Accela pump, a 6-port valve, and 2 HPLC columns. A 150 μL volume of the sample was injected onto the Thermo Hypersil GOLD 20 × 2.1 mm, 12 μm loading column in an aqueous mobile phase (e.g., 5:95:0.1; acetonitrile:water:formic acid). After 1 min, the 6-port valve was switched enabling the loading column to be backflushed onto the analytical column (Thermo BioBasic 50 × 2.1 mm, 5 μm). The chromatography was achieved using a linear gradient at a flow rate of 300 μL/min (i.e., 5:95:0.1 to 80:20:0.1 in 4 min) and maintained 1 min. The mobile phase composition ratio was reverted at the initial conditions (5:95:0.1) and the column was allowed to re-equilibrate for 5 min. Mass spectrometer detection was performed in positive ion mode and operating in full scan high-resolution/accurate-mass mode using a Thermo Q-Exactive Orbitrap Mass Spectrometer (San José, California, USA).
All pharmacokinetic parameters were calculated using WinNonLin 5.2 (Pharsight Corporation, Mountain View, California, USA) using noncompartmental methods (20). The elimination rate constant (kel) was calculated using the last 3 measured plasma concentrations for each animal [linear regression fit (R2) > 0.9] and a terminal elimination half-life (T1/2) was calculated using 0.693/kel. The area under the curve from time 0 to the last measurable concentration (AUC0−t) was calculated using the linear trapezoidal rule and with the last measured plasma concentration. The clearance was calculated by dividing the dose by the AUC0−t.
Motor and feeding behaviors appeared normal following the administration of SR buprenorphine. A slight dilation of the superficial vessels of the ears and sclera were observed in all animals from both groups at 48 h post-injection. This was still present in 66% of the animals at 72 h, while at 96 h post-injection, the vessels’ dilation was still present in 33% of the animals from group 2 but no longer present in group 1. A mild skin reaction (swelling and erythema) was seen in only one animal of each group at 24 h and 48 h post-injection.
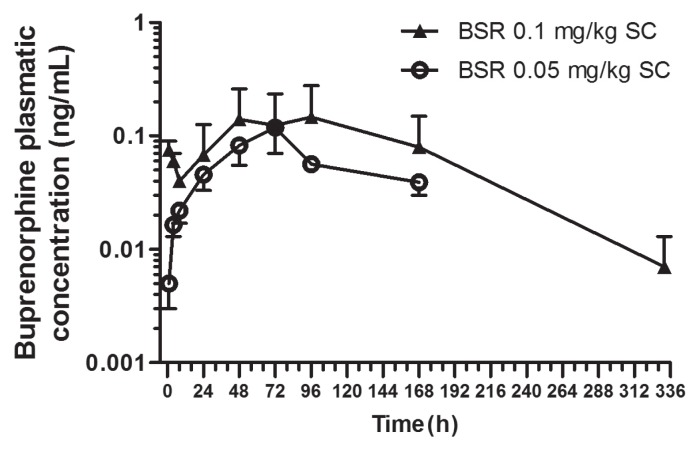
Buprenorphine plasma concentrations after a single subcutaneous injection of 0.1 mg/kg BW (group 1) and 0.05 mg/kg BW (group 2) SR Buprenorphine are shown in Figure 1. Mean buprenorphine concentrations were above the 0.1 ng/mL analgesic threshold starting at 48 h and up to 192 h post-injection for animals in group 1 and from 48 h up to 72 h for animals in group 2. For group 1, mean Cmax and Tmax were 0.28 ± 0.6 ng/mL and 96 h, respectively. The mean clearance and T1/2 were 0.05 ± 0.017 mL/h and 45.8 ± 23.4 h, respectively. For group 2, mean Cmax and Tmax were 0.14 ± 0.04 ng/mL and 72 h, respectively. The mean clearance and T1/2 were 0.014 ± 0.013 mL/h and 23.8 ± 13.6 h, respectively. Norbuprenorphine was not detected in any of the samples.
Figure 1.
Semi-logarithmic graph of the concentration-time profiles (mean ± SD) of plasma buprenorphine after a single subcutaneous administration of SR buprenorphine in sheep with either 0.1 mg/kg BW (n = 6) or 0.05 mg/kg BW (n = 6).
SC — subcutaneous; SD — standard deviation.
Buprenorphine’s wide safety margin and long duration of action are likely the most significant characteristics that determine its widespread use in veterinary medicine. Sheep are widely used in experimental surgical models and very often require adequate control of pain, especially following orthopedic procedures. There are several ways to manage post-surgical pain, depending on the research protocol and the animal model chosen; osmotic subcutaneous mini pumps may be an option to assure a constant and reliable drug delivery. One other option is repeated administration of analgesics. Moreover, the use of patches may constitute a good alternative to chronic injections; however, the toxicity that may be associated with the ingestion of such a formulation may represent a variable to be avoided, as reported in the literature (5–6). The simple formulation of buprenorphine hydrochloride — currently available and commonly used in sheep — has to be administered every 6 to 8 h to assure a good level of analgesia, thus requiring personnel efforts and potentially inducing significant stress as well as forcing the animals to move, which could be contraindicated in some protocols.
To predict the clinical management of pain, a theoretical effective buprenorphine concentration of 0.1 ng/kg BW was used, based on published data on different species (4,15). The subcutaneous administration of 0.1 mg/kg BW of SR buprenorphine provided mean plasma concentrations above the selected theoretical limit, starting at 48 h post-injection and up to 192 h post-injection. For our research objectives, the injection of 0.05 mg/kg BW did not provide sufficient effective drug exposure to be selected as a therapeutic option. This is justified by the longer delay to attain a 0.1 μg/kg BW plasma concentration (48 h post-administration) and the short effective exposure (48 to 72 h).
As for side effects at the injection site, mild skin reactions were previously observed in some species after a subcutaneous administration of SR buprenorphine, but resolved spontaneously with time and had no major effects on the animals’ health (4). In the literature, the presence of nonpainful subcutaneous nodules have also been reported and were described as not visually obvious; these resolved within 1 month of the initial injection. Furthermore, a nodule observed in the cervical region of a dog who received a single subcutaneous injection of SR buprenorphine, was removed and then analyzed for histopathology; the lesion was described as a pyogranuloma with central vacuoles containing sparse grey material and consistent with injection site material (13). The variability of the reactions reported may be explained in part by the lipophilic nature of buprenorphine and the diversity in adiposity in the subjects tested; such differences may alter the absorption and the metabolism of the compound, due to a possible accumulation in intracellular or subcutaneous fat.
The mass spectrometry method did not allow for any detection of norbuprenorphine, which is associated with potential clinical effects such as analgesia, respiratory depression, and sedation. Since no data are currently available on the pharmacokinetics of buprenorphine and its metabolites in sheep, it can only be speculated that the low concentrations of norbuprenorphine are most likely not contributing to the analgesia in this species.
In conclusion, this study shows a long-lasting potential analgesic plasma level of buprenorphine, after a single subcutaneous injection of 0.1 mg/kg BW of SR buprenorphine in sheep starting 2 d after the drug administration and lasting for 5 d. To make full use of SR buprenorphine and depending on the length of analgesia required, treatments could start 2 d before surgery to obtain an analgesic effect immediately after the surgery, or if SR buprenorphine is administered on the day of surgery, another analgesic drug could be given to provide analgesia for the first 2 d following surgery, providing a longer duration of analgesia post-operatively. However, before these results can be applied in a clinical setting, the effective analgesic plasma threshold — determined in other species as 0.1 ng/mL — still needs to be determined in sheep.
Acknowledgments
This study was supported by AccelLab and funded in by the Fond de recherche pour la médecine des animaux de laboratoire (Pascal Vachon) and the Fond du centenaire de la faculté de médecine vétérinaire. We thank Thermo Fisher Scientific for providing a generous access to a Q-Exactive Orbitrap Mass Spectrometer.
References
- 1.Roughan JV, Flecknell PA. Buprenorphine: A reappraisal of its antinociceptive effects and therapeutic use in alleviating post-operative pain in animals. Lab Anim. 2002;36:322–343. doi: 10.1258/002367702320162423. [DOI] [PubMed] [Google Scholar]
- 2.Fish RE, Brown MJ, Danneman PJ, Karas AZ. Anesthesia and Analgesia of Laboratory Animals. 2nd ed. San Diego, California: Academic Press; 2011. [Google Scholar]
- 3.Malavasi LM, Augustsson H, Jensen-Waern M, Nyman G. The effect of transdermal delivery of fentanyl on activity in growing pigs. Acta Vet Scand. 2005;46:149–157. doi: 10.1186/1751-0147-46-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiede AJ, Garcia KD, Stolarik DeAnne F, Ma J, Jenkins GJ, Nunamaker EA. Pharmacokinetics of sustained-relaase and transdermal buprenorphine in Göttingen minipigs (Sus scrofa domestica) J Am Assoc Lab Anim Sci. 2014;53:692–699. [PMC free article] [PubMed] [Google Scholar]
- 5.Schmiedt CW, Bjorling DE. Accidental prehension and suspected transmucosal or oral absorption of fentanyl from a transdermal patch in a dog. Vet Anaesth Analg. 2007;34:70–73. doi: 10.1111/j.1467-2995.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- 6.Deschamps JY, Gaulier JM, Podevin G, Cherel Y, Ferry N, Roux FA. Fatal overdose after ingestion of a transdermal fentanyl patch in two non-human primates. Vet Anaesth Analg. 2012;39:653–656. doi: 10.1111/j.1467-2995.2012.00749.x. [DOI] [PubMed] [Google Scholar]
- 7.Carbone ET, Lindstrom KE, Diep S, Carbone L. Duration of action of sustained-release buprenorphine in 2 strains of mice. J Am Assoc Lab Anim Sci. 2012;51:815–819. [PMC free article] [PubMed] [Google Scholar]
- 8.Kendall LV, Hansen RJ, Dorsey K, Kang S, Lunghofer PJ, Gustafson DL. Pharmacokinetics of sustained-release analgesics in mice. J Am Assoc Lab Anim Sci. 2014;53:478–484. [PMC free article] [PubMed] [Google Scholar]
- 9.Healy JR, Tonkin JL, Kamarec SR, et al. Evaluation of an improved sustained-release buprenorphine formulation for use in mice. Am J Vet Res. 2014;75:619–625. doi: 10.2460/ajvr.75.7.619. [DOI] [PubMed] [Google Scholar]
- 10.Foley PL, Liang H, Crichlow AR. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci. 2011;50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 11.Chum HH, Jampachairsri K, McKeon GP, Yeomans DC, Pacharinsak C, Felt SA. Antinociceptive effects of sustained-release buprenorphine in a model on incisional pain in rats (Rattus norvegicus) J Am Assoc Lab Anim Sci. 2014;53:193–197. [PMC free article] [PubMed] [Google Scholar]
- 12.Catbagan DL, Quimby JM, Mama KR, Rychel JK, Mich PM. Comparison of the efficacy and adverse effects of sustained-release buprenorphine hydrochloride following subcutaneous administration and buprenorphine hydrochloride following oral transmucosal administration in cats undergoing ovariohysterectomy. Am J Vet Res. 2011;72:461–466. doi: 10.2460/ajvr.72.4.461. [DOI] [PubMed] [Google Scholar]
- 13.Nunamaker EA, Stolarik DF, Ma J, Wilsey A, Jenkins GJ, Medina CL. Clinical efficacy of sustained-release buprenorphine with meloxicam for postoperative analgesia in Beagle dogs undergoing ovariohysterectomy. J Am Assoc Lab Anim Sci. 2014;53:494–501. [PMC free article] [PubMed] [Google Scholar]
- 14.Nunamaker EA, Halliday LC, Moody DE, Fang WB, Lindeblad M, Fortman JD. Pharmacokinetic of 2 formulations of buprenorphine in macaques (Macaca mulata and Macaca fascicularis) J Am Assoc Lab Anim Sci. 2013;52:48–56. [PMC free article] [PubMed] [Google Scholar]
- 15.Yassen A, Kan J, Olofsen E, Suidgeest E, Dahan A, Danhof M. Pharmacokinetic-pharmacodynamic modeling of the respiratory depressant effect of norbuprenorphine in rats. J Pharmacol Exp Ther. 2007;321:598–607. doi: 10.1124/jpet.106.115972. [DOI] [PubMed] [Google Scholar]
- 16.Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: Norbuprenorphine is a potent opioid agonist. J Pharmacol Exper Ther. 2001;297:688–695. [PubMed] [Google Scholar]
- 17.Brown SM, Campbell SD, Crafford A, Regina KJ, Holtzman MJ, Kharasch ED. P-glycoprotein is a major determinant of norbuprenorphine brain exposure and antinociception. J Pharmacol Exp Ther. 2012;343:53–61. doi: 10.1124/jpet.112.193433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown SM, Holtzman M, Kim T, Kharasch ED. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115:1251–1260. doi: 10.1097/ALN.0b013e318238fea0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martini L, Fini M, Giavaresi G, Giardino R. Sheep model in orthopedic research: A literature review. Comp Med. 2001;51:292–299. [PubMed] [Google Scholar]
- 20.Rowland M, Towzer TN. Clinical Pharmacokinetics: Concepts and Application. 4th ed. Philadelphia, Pennsylvania: Lippincott, Williams and Wilkins; 2010. pp. 367–389. [Google Scholar]