Abstract
Background & Aims
Childhood obesity is increasing and is associated with adult obesity. Antibiotics have been used to promote weight gain in livestock for several decades. Antibiotics are commonly prescribed for children, but it is not clear how exposure to antibiotics early in life affects risk for obesity. We performed a population-based cohort study to assess the association between antibiotic exposure before age 2 years and obesity at age 4 years.
Methods
We performed a retrospective cohort study of 21,714 children in The Health Improvement Network —a population-representative dataset of more than 10 million individuals derived from electronic medical records from 1995 through 2013 in the United Kingdom. Eligible subjects were registered within 3 months of birth with complete follow-up and height and weight were recorded within 12 months of their 4th birthday. Antibiotic exposure was assessed before age 2 years, and classified based on anti-anaerobic activity. The primary outcome was obesity at age 4 years. We performed logistic regression analyses, adjusting for maternal and sibling obesity, maternal diabetes, mode of delivery, socioeconomic status, year and country of birth, and urban dwelling.
Results
In the cohort, 1306 of the children (6.4%) were obese at 4 years of age. Antibiotic exposure was associated with an increased risk of obesity at 4 years (odds ratio [OR]=1.21; 95% confidence interval [CI], 1.07–1.38). Odds ratios increased with repeated exposures: for 1–2 prescriptions, OR=1.07 (95% CI, 0.91–1.23); for 3–5 prescriptions, OR=1.41 (95% CI, 1.20–1.65); for 6 or more prescriptions, OR=1.47 (95% CI, 1.19–1.82). Antifungal agents were not associated with obesity (OR=0.81; 95% CI, 0.59–1.11).
Conclusions
Administration of 3 or more courses of antibiotics before children reach an age of 2 years is associated with an increased risk of early childhood obesity.
Keywords: THIN, microbiome, pediatrics, UK
Antibiotics have been used to promote weight gain in the agricultural industry for decades1,2. This effect has been hypothesized to be mediated via the gut microbiome, with studies demonstrating no effect on weight gain in germ-free animals2,3. The impact of antibiotics on animal weight is greater if given earlier in life4.
Childhood obesity is strongly associated with the risk of obesity and its complications in adulthood5. There are limited data addressing the effect of antibiotics on human weight gain, despite clear evidence of their overuse in pediatric populations6–8. Several studies have retrospectively examined antibiotic use by relying on parental recall to assess exposure to antibiotics. A recent UK study assessed antibiotic exposure in the first two years of life and body mass, appreciating an increased risk of obesity at 10 and 20 months, and a trend towards a sustained impact at 7 years, though this was not statistically significant after adjusting for parental obesity and tobacco use.8 Duration, dose, antibiotic class, and household factors such as sibling obesity were not assessed. A Danish registry examined antibiotic exposure in the first 6 months of life and demonstrated an increased risk of obesity in children of obese mothers, but noted a protective effect in children of mothers who were not obese. Recurrent exposures could not be assessed 7. An international survey also found an association between parental recall of antibiotic use in the first 12 months of life and subsequent obesity9.
Prior retrospective cohort studies using electronic health records have also suggested an association between antibiotics and obesity10–12. However, because of the limitations of the available data, substantial uncertainty remains about the importance of the age of exposure, cumulative frequency of exposure, and spectrum of antibiotics that may influence this association. Furthermore, there is remaining uncertainty about the potential confounding effects of environmental exposures.
In this study, we examined the association between antibiotic use before 2 years of age and obesity in a large population-representative cohort in the United Kingdom with complete follow-up from birth to 48 months, allowing for complete prescription information, adjusting for multiple confounders including sibling and maternal obesity, method of delivery, year of birth, socioeconomic status, and early obesity. We also assessed whether repeated antibiotic exposures and antibiotics with broader anaerobic coverage, and therefore potentially greater impact on the microbiome, were more strongly associated with obesity.
Methods
Study design
We performed a retrospective cohort study using data collected prospectively in the scope of routine care from 1995–2013 within The Health Improvement Network (THIN). THIN data are derived from general practitioners’ electronic medical records (EMR). THIN represents approximately 6% of the UK population13, includes information on age, sex, socioeconomic status, medication use, and has been validated for multiple medical diagnoses14–18. Height and weight data are recorded during clinical care. Mother-child pairings and siblings can be determined using a unique household code19.
Study population
We identified individuals who registered with a THIN practice within 3 months of birth who had complete follow-up to 48 months. Individuals were required to have a recorded height and weight within 12 months of their fourth birthday. Height and weight must have been recorded within 1 week of each other. To ensure recording completeness, all individuals born prior to a practice’s installation of the Vision electronic medical record were excluded, as were those who had died, transferred out of a practice, or did not reach their fourth birthday prior to the end of data recording. Given the potential for increased antibiotic or glucocorticoid exposures, we excluded individuals with diagnostic codes for reactive airway disease or asthma.
Study outcome
The primary outcome was obesity at 48 months, defined using BMI z-scores calculated from the UK World Health Organization Term growth reference table using both recorded height and weight as well as age at the date they were recored20. BMI can vary significantly based on age and gender among individuals. To account for this variation, we used z-scores, which convert BMIs into numerical values that represent how many standard deviations an individual’s BMI is away from the transformed age- and sex-specific population mean. Z-scores are employed in World Health Organization and Center for Disease Control growth charts. These values are particularly useful when examining extreme percentiles of the distribution, such as >99% or <1%21. Obesity was defined as a BMI z-score ≥ 2.37 for males and ≥ 2.25 for females based on previously published UK population-specific cut-offs22. The time point of 4 years was selected given the previously described association between obesity at this age and obesity in adulthood5.
Variables of interest
Antibiotic exposure was defined as any prescription for a systemic antibiotic occurring from enrollment in the practice (no later than 3 months after birth) to 2 years of age. We assessed the impact of repeated exposures via number of antibiotic prescriptions (none, 1–2, 3–5, or >5 prescriptions). We also assessed timing of the first antibiotic prescription and timing of first exposure to antibiotics (0–6 months, 6–12 months, or 12–24 months), as prior research had demonstrated a stronger association with exposure in the first year of life9. Specific classes of antibiotics were categorized as those with antianaerobic coverage (penicillins, imidazoles, lincosamides, tetracyclines) and without (cephalosporins, macrolides, sulfa-containing agents, isoniazid, rifampin, flouroquinolones, aminoglycosides). Systemic antifungal agents were assessed as a control.
We assessed those factors previously identified to be associated with obesity as potential confounders23. These included calendar year, geographic region, mode of delivery, socioeconomic status, obesity as an infant, maternal diabetes, and presence of obese family members in the household. A multi-step algorithm was employed to identify mothers within the THIN database, adapted from previously published methods within the same database19(see Supplemental Methods and Results). Average maternal BMI was calculated from available height and weight data from age 18 to 240 days prior to the child’s birth. A multilevel variable was then generated to indicate if the mother had an average BMI greater than 30, less than 30, or if we were unable to identify a mother.
Older siblings of the individuals of interest were identified using similar techniques (see Supplemental Methods and Results). As siblings, we included male and female individuals age ≤ 18 in the same household with complete follow-up during the 4 years of follow-up of the index individual’s time within THIN. BMI z-scores were calculated using the same criteria as the individuals in our primary cohort of interest. If any siblings met the criteria for obesity during the 4-year follow-up period of the individual within our cohort, they were categorized as obese. A multilevel categorical variable was then constructed, similar in structure to the maternal variable.
For each cohort member, we assessed the presence of obesity during the first year of life. To perform this analysis, we identified height and weight data within 14 days of each other within 12 months of their date of birth. Obesity was calculated for this infant data by calculating Z-Weight for length (zWFL) scores using the World Health Organization growth chart, with obesity defined as a Z-score >2 standard deviations above the mean (zWFL>1.96).
We assessed several additional factors that may influence the risk of obesity at age 4 years. Year of birth was measured to assess the potential influence of trends in obesity over time. Postal codes were used to determine if cohort members lived in an urban versus rural environment and to compute a Townsend score for socioeconomic status. The Townsend score categorizes socioeconomic status on a 5-point scale with 5 representing “most deprived”, and is derived from (1) percentage of households without access to a car, (2) percentage of households not in owner-occupied accommodations (3) percentage of households in overcrowded accommodations, and (4) the percentage of the economically active population aged 16–74 who are unemployed (See Supplemental Methods). Geographic region was categorized as Northern Ireland, Wales, Scotland, or England. Mode of delivery (caesarean or vaginal) was determined when diagnostic codes were present within 6 months after the birthdate of children of interest. In the subset of individuals who had codes for both vaginal delivery and caesarean delivery, delivery was categorized as caesarean. Maternal diabetes was assessed using diagnostic codes.
Analysis
Statistical analyses were conducted using Stata 13 (StataCorp, College Station, TX, USA). BMI z-scores were calculated using the zanthro package20. Univariable analyses were conducted using logistic regression. We included all potential confounding covariates in a fully adjusted model, without employing a predetermined step-wise backwards elimination strategy to avoid inappropriate over-fitting or oversimplification24. In a sensitivity analysis we also generated a more parsimonious model using a backwards elimination stepwise strategy to assess the impact on our results (see Supplemental Methods and Results).
Sensitivity analyses
We performed several sensitivity analyses related to antibiotic exposure. We assessed the impact of repeated antibiotic prescriptions, from 0–10 or more prescriptions, as a continuous variable in univariable and multivariable analyses25. We analyzed the impact of anti-anaerobic antibiotics, given their presumed greater impact on the predominantly anaerobic gut microbiome, in univariable analyses and in a combined model stratified on whether the agents were or were not antianaerobic26. We performed a sensitivity examining our classification of antibiotics with anaerobic coverage, classifying only those with definite anaerobic coverage as anaerobic, and also comparing amoxicillin, which has variable anaerobic coverage, to amoxicillin and clavulanic acid, which has more complete coverage (See Supplemental Methods). We identified households with more than one child within our cohort and assessed the impact of antibiotics among these pairs using conditional logistic regression. We repeated our primary analyses limited to those individuals with both identified mothers and siblings to assess any bias introduced by missing data on obesity among family members.
We assessed interaction between the number of prescriptions and age at first prescription while avoiding collinearity by combining both age of first antibiotic prescription and number of antibiotics over the first 2 years as a 5-level variable: No antibiotics (reference), 1–2 prescriptions with the first prescription in the first 12 months of life, 3 or greater prescriptions with the first prescription in the first 12 months of life, 1–2 prescriptions with the first prescription between 12 and 24 months of age, and 3 or greater prescriptions with the first prescription between 12 and 24 months of age (See Supplemental methods).
We performed analyses examining the effects of alternate definitions of obesity. For this sensitivity analysis, we used a BMI z-score cut-off of ≥3 for obesity at age 4 years. To assess the impact of categorizing obesity as a dichotomous variable, we performed linear regression to assess the impact of antibiotic exposures on BMI z-score at age 4.
To assess for possible selection bias, we examined the association of antibiotic prescriptions and recording of height and weight at age 4. To assess for the potential impact of unmeasured confounders, we determined the strength of association between an unmeasured confounder and the outcome required to explain our observed association between antibiotic exposure and obesity27. We modeled our unmeasured confounder after existing data on breastfeeding, as we were unable to measure this in our dataset (See Supplemental Methods). Breastfeeding is known to be associated with both a lower risk of infection and obesity. Approximately 80% of mothers in the UK breast-feed28. A recent meta-analyses demonstrated that breastfeeding is associated with a reduced risk of obesity (OR 0.78, 95% CI 0.74–0.81).29 Additionally, prior studies have demonstrated a protective effect of breast-feeding for respiratory tract infections, with an adjusted OR of 0.6530. We also performed an analysis examining the potential confounding effect of obesity during the first 6 months of life in a cohort of individuals with recorded height and weight in that time period using z-weight-for-length scores.
This study was considered exempt by the University of Pennsylvania IRB and approved by THIN’s scientific review committee.
Results
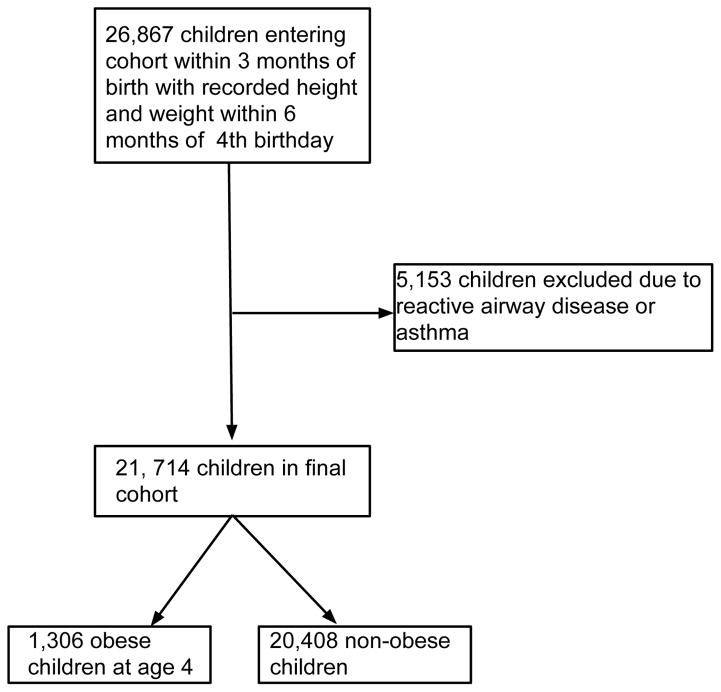
Among 533,238 children identified in THIN within 3 months of birth, 253,157 had 4 years of follow-up. 21,714 children with complete follow-up for four years met the inclusion and exclusion criteria (Figure 1); 64.1% were prescribed antibiotics prior to age 2 years. The median time from birth to cohort entry was 37 days (IQR 25–51). 1,306 (6.4%) were obese at age 4 years. Obesity at age 4 years was observed in 5.2% of children without antibiotic exposure and 6.4% of those with antibiotic exposure, with a risk difference of 1.2% (95% CI 0.1–1.9%). Baseline characteristics of obese and non-obese children are presented in Table 1.
Figure 1.
CONSORT Diagram: CONSORT diagram of children included in the study, demonstrating those with complete follow up and height and weight data that were then excluded based on exclusion criteria.
Table 1.
Characteristics of children within the cohort, stratified by the presence of obesity at 48 months
| Characteristics | Non-Obese (N=20,408) | Obese (N=1,306) |
|---|---|---|
| Born before 2000, No. (%) | 4,264 (20.9%) | 219 (16.8%) |
| Born after 2000, No. (%) | 16,144 (79.1%) | 1,087 (83.2%) |
| Sex | ||
| Male, No. (%) | 10,421 (51.1%) | 673 (51.5%) |
| Female, No. (%) | 9,987 (48.9%) | 633 (48.5%) |
| Area of Residence | ||
| Non-Urban environment, No. (%) | 3,360 (16.5%) | 193 (14.8%) |
| Urban environment, No. (%) | 12,319 (60.4%) | 847 (64.9%) |
| Residence data missing, No. (%) | 4,729 (23.2%) | 266 (20.4%) |
| Townsend Scorea | ||
| 1, No. (%) | 4,255 (20.9%) | 221 (16.9%) |
| 2, No. (%) | 4,024 (19.7%) | 227 (17.4%) |
| 3, No. (%) | 4,013 (19.6%) | 239 (18.3%) |
| 4, No. (%) | 4105 (20.1%) | 296 (22.7%) |
| 5, No. (%) | 3,172 (15.5%) | 275 (21.1%) |
| Missing data, No. (%) | 839 (4.1%) | 48 (3.7%) |
| Mother identified, No. (%) | 11,715 (57.4%) | 712 (54.5%) |
| Mother with documented obesity, No. (%) | 1,308 (6.4%) | 155 (11.9%) |
| Mother without documented obesity, No. (%) | 10,407 (51.0%) | 557 (42.7%) |
| Mother with diabetes, No, (%) | 92 (0.5%) | 11 (0.8%) |
| Sibling identified, No. (%) | 4,779 (23.4%) | 1,049 (19.7%) |
| Sibling with documented obesity, No. (%) | 325 (1.6%) | 49 (3.8%) |
| Sibling without documented obesity, No. (%) | 4,454 (21.8%) | 208 (15.9%) |
| Mode of delivery identified, No. (%) | 5,403 (26.5%) | 336 (25.7%) |
| Caesarean delivery, No. (%) | 946 (4.6%) | 75 (5.7%) |
| Vaginal delivery, No (%) | 4,457 (21.8%) | 261 (20.0%) |
| Antibiotic unexposed, No. (%) | 6,489 (31.8%) | 355 (27.2%) |
| Antibiotic exposed, No. (%) | 13,919 (68.2%) | 951 (72.8%) |
| 1–2 prescriptions, No. (%) | 8269 (40.5%) | 492 (37.7%) |
| 3–5 prescriptions, No. (%) | 4149 (20.3%) | 332 (25.4%) |
| >5 prescriptions, No. (%) | 1501 (7.4%) | 127 (9.7%) |
| Exposure by antibiotic class | ||
| Penicillins | 13,244 (64.9%) | 909 (69.6%) |
| Cephalosporin | 992 (4.9%) | 78 (6.0%) |
| Macrolides | 3,115 (15.3%) | 234 (17.9%) |
| Imidazoles | 44 (0.2%) | 2 (0.2%) |
| Isoniazid/Rifampin | 17 (0.1%) | 4 (0.3%) |
| Tetracyclines | 1(0.0%) | 0 (0.0%) |
| Flouroquinolones | 24 (0.1%) | 1 (0.1%) |
| Aminoglycosides | 4 (0.02%) | 0 (0.0%) |
| Sulfa-based | 769 (3.8%) | 63 (4.8%) |
Townsend score from least impoverished (1) to greatest level of poverty (5)
Increasing poverty, urban dwelling, year of birth, maternal and sibling obesity, maternal diabetes, and obesity in the first year of life were associated with an increased risk of obesity at age 4 years (Table 2). The risk of obesity increased with increasing number of courses of antibiotics and with antibiotic use in the first year. After adjusting for all covariates, any antibiotic prescription (aOR 1.21, [95%CI: 1.07–1.38]) and increasing number of antibiotic prescriptions were significantly associated with obesity, specifically for those receiving 3 or more prescriptions (1–2 prescriptions: aOR 1.07, [95% CI:0.93–1.23]; 3–5 prescriptions: aOR 1.41, [95% CI: 1.20–1.65]; >5 prescriptions: aOR 1.47, [95% CI: 1.19–1.82]). With each increase in antibiotic exposure category, there was an increased risk of obesity (p-for-trend p<0.001). Antibiotic use within the first year was also associated with increased risk: (0–6 mos: aOR, 1.33 [95% CI: 1.13–1.57], 6–12 mos: aOR, 1.27, [95% CI: 1.09–1.47]) (Table 3). After assessing for interaction between the number of prescriptions and age at first exposure, repeated exposures before age 2 had the greatest effect, regardless of whether these exposures started before or after 12 months (prior to 12 months: aOR 1.48 [95%CI 1.27–1.72]; after 12 months: aOR 1.60 [95%CI 1.22–2.10]; Table 5; See Supplemental Methods).
Table 2.
Univariable and multivariable models assessing the association of antibiotic exposure and all covariates of interest with the risk of obesity at age 4 years
| Exposure | Exposed and obese | Univariable Analysis | Most fully adjusted model assessing number of prescriptions | |
|---|---|---|---|---|
| Number exposed | Number obese (% of exposed) | OR, (95% CI) | OR, (95% CI) | |
| Number of antibiotic prescriptions: | 6,844 | 355 (5.2%) | 1.00 | 1.00 |
| 0 (ref) | ||||
| 1–2 | 8,761 | 492 (5.6%) | 1.09 (0.95–1.25) | 1.07 (0.93–1.23) |
| 3–5 | 4,481 | 332 (7.4%) | 1.46 (1.25–1.71) | 1.41 (1.20–1.65) |
| >5 | 1,628 | 127 (7.8%) | 1.55 (1.25–1.91) | 1.47 (1.19–1.82) |
| Maternal Obesity | 10,964 | 557 (5.1%) | 1.00 | 1.00 |
| Non-obese mother identified (ref) | ||||
| No mother identified | 9,287 | 594 (6.4%) | 1.27 (1.13–1.44) | 1.25 (1.08–1.43) |
| Obese mother identified | 1,463 | 155 (10.6%) | 2.21 (1.84–2.67) | 1.97 (1.62–2.38) |
| Maternal Diabetes | 12,324 | 701 (5.7%) | 1.00 | 1.00 |
| Not diabetic (ref) | ||||
| Diabetic mother | 103 | 11 (10.3%) | 1.98 (1.06–3.72) | 1.51 (0.79–2.90) |
| Sibling obesity | 4,662 | 208 (4.5%) | 1.00 | 1.00 |
| Siblings identified without obesity (ref) | ||||
| No Sibling identified | 16,678 | 1,049 (6.3%) | 1.44 (1.23–1.67) | 1.42 (1.23–1.68) |
| Obese sibling identified | 374 | 49 (13.1%) | 3.23 (2.32–4.50) | 2.64 (1.88–3.70) |
| Country | 16,231 | 981 (6.0%) | 1.00 | 1.00 |
| England (ref) | ||||
| N. Ireland | 896 | 48 (5.4%) | 0.88 (0.65–1.19) | 2.08 (1.07–4.11) |
| Scotland | 3,401 | 188 (5.5%) | 0.91 (0.77–1.07) | 2.18 (1.17–4.03) |
| Wales | 1,186 | 89 (7.5%) | 1.26 (1.01–1.58) | 1.09 (0.86–1.37) |
| Townsend Score | 4,476 | 221 (4.9%) | 1.00 | 1.00 |
| 1 (ref) | ||||
| 2 | 4,251 | 227 (5.3%) | 1.08 (0.90–1.31) | 1.07 (0.88–1.29) |
| 3 | 4,252 | 239 (5.6%) | 1.15 (0.95–1.38) | 1.10 (0.91–1.33) |
| 4 | 4,401 | 296 (6.7%) | 1.39 (1.16–1.66) | 1.30 (1.15–1.63) |
| 5 | 3,447 | 275 (8.0%) | 1.67 (1.39–2.00) | 1.52 (1.26–1.84) |
| Missing | 887 | 48 (5.4%) | 1.10 (0.80–1.52) | 2.19 (1.30–3.71) |
| Urban environment | 3,553 | 193 (5.4%) | 1.00 | 1.00 |
| Non-urban environment (ref) | ||||
| Urban environment | 13,166 | 847 (6.4%) | 1.20 (1.02–1.41) | 1.06 (0.90–1.25) |
| Missing t | 4,995 | 266 (5.3%) | 0.98 (0.81–1.18) | 0.39 (0.21–0.76) |
| Gender | 11,094 | 673 (6.1%) | 1.00 | 1.00 |
| Male (ref) | ||||
| Female | 10,620 | 633 (6.0%) | 0.98 (0.88–1.10) | 1.03 (0.92–1.15) |
| Year of birth | --- | --- | 1.04 (1.02–1.06) | 1.03 (1.02–1.05) |
| Obesity in first year | 8,928 | 471 (5.3%) | 1.00 | 1.00 |
| Non-Obese in first year (ref) | ||||
| Obese in first year | 617 | 93 (15.1%) | 3.19 (2.51–4.05) | 3.13 (2.46–4.00) |
| Missing | 12,169 | 742 (6.1%) | 1.17 (1.04–1.32) | 1.14 (1.00–1.29) |
| Method of delivery | 4,718 | 261 (5.5%) | 1.00 | 1.00 |
| Vaginal Delivery (ref) | ||||
| Caesarian section | 946 | 75 (7.4%) | 1.35 (1.04–1.77) | 1.20 (0.92–1.15) |
| Missing | 15,005 | 970 (6.1%) | 1.10 (0.96–1.27) | 0.97 (0.82–1.14) |
Table 3.
Univariable and fully adjusted models assessing association between number of antibiotic prescriptions, time of first prescription, and obesity
| Exposure | Univariable Analysis | Adjusted model assessing number of prescriptions | Adjusted model assessing age at first prescribed antibiotic | ||
|---|---|---|---|---|---|
| Number exposed | Number obese (% of exposed) | OR, (95% CI) | OR, (95% CI) | OR, (95% CI) | |
| Number of antibiotic prescriptions: | 6,844 | 355 (5.2%) | 1.00 | 1.00 | -- |
| 0 (ref) | |||||
| 1–2 | 8,761 | 492 (5.6%) | 1.09 (0.95–1.25) | 1.07 (0.93–1.23) | -- |
| 3–5 | 4,481 | 332 (7.4%) | 1.46 (1.25–1.71) | 1.41 (1.20–1.65) | -- |
| >5 | 1,628 | 127 (7.8%) | 1.55 (1.25–1.91) | 1.47 (1.19–1.82) | -- |
| Age at first prescription: | 6,489 | 355 (5.2%) | 1.00 | -- | 1.00 |
| None(ref) | |||||
| 0–6 months | 3,837 | 267 (7.0%) | 1.37 (1.16–1.61) | -- | 1.33 (1.13–1.57) |
| 6–12 months | 5,851 | 390 (6.7%) | 1.31 (1.13–1.51) | -- | 1.27 (1.09–1.47) |
| 12–24 months | 5,182 | 294 (5.7%) | 1.10 (0.94–1.29) | -- | 1.07 (0.91–1.26) |
Table 5.
Multivariable model assessing interaction variable of time of first antibiotic and number of antibiotic prescriptions with obesity
| Exposure | Multivariable Analysis | |
|---|---|---|
| No./Total(%) | OR, (95%CI) | |
| No antibiotic exposures | 345/6590 (5.2) | 1.00 |
| 1–2 prescriptions with first exposure between 0–12 months | 259/4279 (6.1) | 1.18 (0.995–1.39) |
| 3 or more prescriptions with first exposure between 0–12 months | 377/4989 (7.6) | 1.48 (1.27–1.72) |
| 1–2 prescriptions with first exposure between 12–24 months | 210/4141 (5.1) | 0.96 (0.81–1.15) |
| 3 or more prescriptions with first exposure between 12–24 months | 67/828 (8.1) | 1.60 (1.22–2.10) |
Multivariable model assessing the association of obesity at age 4 with time of first antibiotic exposure and number of exposures as a combined single interaction variable, adjusted for Townsend quintile (poverty scale), sibling and maternal obesity, and birth before or after 2000. In this model, there is a strong association between exposure to 3 or more antibiotics, regardless of first exposure before or after 12 months of age.
Anti-anaerobic antibiotics were associated with obesity in a dose-dependent manner (1–2 prescriptions: aOR, 1.09, [95% CI: 0.95–1.25]; 3–5 prescriptions: aOR, 1.45 [95% CI: 0.91–1.68]; >5 prescriptions: aOR, 1.46 [95% CI: 1.09–1.96]), while antibiotics without anti-anaerobic activity were not (Table 4). Antifungal agents were not associated with obesity (OR, 0.81, [95%CI, 0.59 – 1.11]).
Table 4.
Impact of anti-anaerobic activity on association with obesity
| Antibiotic Spectrum | 1 or more prescriptions (Unadjusted) | 1 or more prescriptions (Adjusted analysis) | Effect per number of prescriptions (adjusted OR, (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| No./Total (%) | (OR, 95%CI) | No./Total (%) | (OR, 95% CI) | 0 | 1–2 | 3–5 | >5 | |
| No antibiotic exposure | 355/6,489 (5.5%) | 1.00 | 345/6,245 (5.5) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Any antibiotic exposure | 951/13,919 (6.8%) | 1.24 (1.10–1.42) | 931/13,324 (6.9%) | 1.24 (1.09–1.41) | 1.00 | 1.07 (0.93–1.24) | 1.48 (1.27–1.74) | 1.52 (1.23–1.89) |
| Antibiotics with anaerobic coverage | 909/13,257 (6.9%) | 1.24 (1.09–1.39) | 872/12,683 (6.9%) | 1.23 (1.08–1.39) | 1.00 | 1.09 (0.95–1.25) | 1.45 (0.91–1.68) | 1.46 (1.09–1.96) |
| Antibiotics without anaerobic coverage | 316/4,248 (7.4%) | 1.21 (1.07–1.38) | 303/4,059 (7.5%) | 1.21 (1.06–1.38) | 1.00 | 1.09 (0.93–1.27) | 1.24 (0.91–1.68) | 1.00 (0.63–1.60) |
Impact of any antibiotic exposure and antibiotic exposure when stratified by anaerobic coverage. Exposure was assessed as any exposure, a continuous exposure with increasing antibiotic prescriptions, and as a categorical variable.
Multivariable models included year of birth, maternal and sibling obesity, maternal diabetes, mode of delivery, country of origin, urban environment, and Townsend score.
When assessing the subgroup of individuals for whom we had identified both mothers and siblings (n=3,296), antibiotic exposure was associated with an increased risk of obesity (any exposure: OR, 1.73 [95%CI, 1.16 to 2.57]). When examining families with multiple siblings with discordant outcomes (n=361), the risk with each antibiotic prescription (aOR, 1.08 [95%CI, 0.90 to 1.30]) and with number of prescriptions (1–2: aOR, 0.72 [95%CI, 0.43 to 1.24]; 3–5: aOR, 1.20 [95%CI, 0.65 to 2.21]; >5 aOR, 1.45 [95%CI, 0.54 to 4.06]) was similar, though not statistically significant.
Sensitivity Analyses
Results in a parsimonious model retaining only covariates that were significant in univariate analyses yielded similar results to our fully adjusted model (See Supplemental Methods and Results). When examining the impact of antibiotic exposures on the BMI z-score at age 4, in our fully adjusted model, any antibiotic exposure was associated with a 0.05 point (95% CI: 0.02–0.09) increase in z-score. Each antibiotic prescription was associated with a 0.014 point (95% CI:0.01 to 0.02) increase in Z-score. When using z-score ≥ 3 as an alternate definition of obesity, 542 children were classified as obese at age 4 years. In this fully adjusted model, any antibiotic prescription was associated with an increased risk of obesity (aOR: 1.22, 95% CI: 1.01–1.48). Increasing antibiotic use was also associated with an increased risk of obesity (1–2: aOR, 1.06 [95%CI, 0.86–1.32]; 3–5: aOR, 1.47 [95%CI 1.17–1.86]; >5 aOR, 1.39 [95%CI, 1.01–1.92]).
When using a more stringent definition of antibiotics with anaerobic coverage, there were 957 individuals exposed to anaerobic antibiotics. Any exposure was associated with an increased risk of obesity at age 4, although this was not statistically significant (OR 1.21, 95%CI: 0.95–1.55). When comparing those who received no antibiotics to those who had received amoxicillin, amoxicillin and clavulanic acid, or both, the effect estimates were similar for each exposure category (amoxicillin: OR 1.18 [95% CI 1.04–1.34]; amoxicillin with clavulanic acid: OR 1.20 [95% CI 0.70–2.09]; both: OR 1.33 [95% CI 0.99–1.80]) (see Supplemental Methods and Results).
Adjusting for documented obesity within the first 6 months of life did not meaningfully alter the association between repeated antibiotic use and obesity for those who had received 3 or more prescriptions (1–2: aOR, 1.08 [95%CI, 0.93–1.23]; 3–5: aOR, 1.41[95%CI 1.21–1.65]; >5 aOR, 1.48[95%CI, 1.19–1.83).
In our analyses of unmeasured confounders and their potential impact on these results, we estimate that failure to adjust for breastfeeding only biased our results for any antibiotic exposure by approximately 2%, with an externally adjusted odds ratio of 1.22 (See Supplemental Methods). If we were to assume that the association between breast-feeding and obesity was under-estimated in the meta-analysis by Yan et al and instead use the strongest effect estimate included in that analysis (OR 0.29 (95%CI 0.08–1.05), this would result in 13% bias, with an externally adjusted OR of 1.10. When considering those with 3 or more prescriptions for antibiotics, where we observed a greater risk of obesity, the adjusted association between obesity 3 or more antibiotic exposures would be 1.27.29–31 Thus, within the plausible range of effect estimates, external adjustment for breastfeeding was not able to fully explain the association between antibiotic exposure and obesity observed in this study.
Receipt of antibiotics was not associated with recording of height and weight among all 253,137 individuals within THIN who had 4 years of follow-up (OR 1.01, [95%CI 0.98–1.04]).
Discussion
With rising rates of childhood obesity worldwide, it is important to identify modifiable contributing factors32–35. Antibiotics are prescribed during an estimated 49 million pediatric outpatient visits per year in the United States; the majority are broad-spectrum agents6. Between 2006–2008, >10 million antibiotic prescriptions were written annually for children without clear indication, despite increased awareness of the societal risks of antibiotic resistance36,37. This study identified obesity as one of a growing list of more tangible risks associated with antibiotic utilization, including dermatologic, allergic, and infectious complications, inflammatory bowel disease, and autoimmune conditions38–44. Unlike other potential risks of antibiotic use, the risk of subsequent obesity is likely easily understandable by parents. The results of this study do not imply that antibiotics should not be used when indicated, but rather highlight a reason to avoid antibiotics in the absence of well-established indications. This may be particularly important if the child has been previously treated with antibiotics as the risk of subsequent obesity was greater in those children who had received 3 or more courses of antibiotics in the first 2 years of life.
These data are supported both in the agriculture industry and in murine models. Moore and colleagues first recognized the relationship between streptomycin and weight gain in chick models in 194645. Similar results were appreciated in 1949 in experiments where chickens fed fishmeal supplemented with cobalamin derived from the bacteria Streptomyces aureofaciens, which also produced the antibiotic streptomycin, outgrew chickens receiving fishmeal with a liver-derived B12 supplement4,46. Similar effects have been observed in other livestock with differing antibiotics, and the use of these agents rapidly became commonplace in the agricultural industry4. Several laboratory models have demonstrated that increases in weight induced by antibiotics are mediated via the drug’s impact on the microbiome, with no effect in germ free models2,47. This study is one of the first to demonstrate a similar impact in a human population, while adjusting for multiple factors previously demonstrated to be associated with obesity.
Strengths of the study are the large sample size, near complete capture of lifetime antibiotic exposure, and adjustment for multiple factors including both maternal and sibling obesity, maternal diabetes, obesity in the first year of life, mode of birth, and socioeconomic status. This design allowed us to disentangle the effects of age at first exposure to antibiotics and the number of courses, identifying repeated antibiotic exposure as the pivotal factor linking antibiotic exposure to childhood obesity. This supports the hypothesis that antibiotics may progressively alter the composition and function of the gut microbiome, thereby predisposing children to obesity as is seen in livestock and animal models.
There are several potential limitations of this research. We did not measure the indication for antibiotic use. However, recent data suggest no causal association between common antibiotic indications and obesity10. Children who receive multiple antibiotic prescriptions may differ from those with none or few in ways that relate to future risks of obesity. However, one would expect that in those children with more serious or repeated infections between ages 0 and 2, poor weight gain would be more common than excessive weight gain, thereby biasing our results towards the null. Employing an outpatient medical record, we were unable to capture inpatient medication administration. However, this represents a small minority of prescriptions and would also likely bias the results toward the null. Thus, our design may have slightly underestimated the association of antibiotic exposure and subsequent childhood obesity.
As with any retrospective study, it is possible that selection bias could influence our results. While growing children in the UK have routine measurements of their height and weight, these measurements are typically recorded via a paper record known as the Personal Child Health Record as opposed to in the EMR49. Inclusion in the EMR, and subsequently THIN, is at the discretion of the treating physician. Therefore, it is possible that selective recording of this information could influence our findings. However, when examining overall obesity rates in the United Kingdom, observed rates within THIN were only slightly lower than those previously published for the UK, and demonstrated similar temporal trends within our cohort (data not shown).50 For selection bias to have influenced the results of this study, recording of height and weight data would have to be associated with both obesity and receipt of antibiotics. As we observed no association between receipt of antibiotics and recording of height and weight in the overall cohort, selection bias is unlikely to explain the observed associations.
Despite adjusting for many potential confounders associated with obesity, there are a few potential confounders that we were not able to adjust for, including breast feeding, physical activity, and sleep23,29,30. However, it is unlikely that these factors fully explain the association between obesity and antibiotic exposure appreciated within our data given the results of the analyses of unmeasured confounders. The sensitivity analysis using external adjustment demonstrated that our estimate was robust to unmeasured confounders, even if they were strongly protective against future obesity. Furthermore, recent survey data demonstrate that mode of feeding does not usually differ within households51, and we appreciated similar effects of repeated antibiotic exposure among sibling pairs discordant for obesity. Although we were able to identify family members in order to adjust for environmental and genetic confounders, we were underpowered to perform a similar analysis examining only multiple births (i.e. twins or triplets), as obesity status and antibiotic exposure were nearly identical between these individuals. We also excluded children with asthma or reactive airway disease from our analyses, given the known association between these conditions and obesity and the potential for confounding due to glucocorticoid exposure.52. Therefore, one should use caution when interpreting the impact of our results in these populations. Further research is required to assess the durability of this response later into adolescence and young adulthood as well as later onset obesity.
Lastly, it is possible that our analyses examining antibiotic spectrum coverage could be underpowered and subject to misclassification bias. We appreciated an association between obesity and anti-anaerobic agents, with a dose-dependent response. As the majority of antibiotics prescribed were anti-anaerobic in our primary analyses, it is possible that the lack of association with the use of antibiotics without anti-anaerobic coverage was due in part to smaller numbers. In our sensitivity analysis, amoxicillin, an agent with minimal anti-anaerobic activity was significantly associated with obesity with an odds ratio comparable to that for amoxicillin with clavulanic acid. While our analysis of amoxicillin with clavulanic acid did not reach statistical significance, this may be due to limited numbers of individuals with this exposure in comparison to amoxicillin alone (see Supplemental Methods and results). As such, repeated courses of antibiotic exposure may be a more important risk factor than spectrum of activity. Further research is required to determine if specific classes of antibiotics are more strongly associated with subsequent obesity.
In summary, we have demonstrated that exposure to antibiotics in the first two years of life is associated with an approximately 1.2% absolute and 25% relative increase in the risk of early childhood obesity. This relationship is strongest when considering repeat exposures, particularly with 3 or more courses. While early antibiotic use has been associated with a number of rare long-term health consequences, these data link antibiotics to one of the most important and growing public health problems worldwide.
Supplementary Material
Acknowledgments
Grant Support: This research was supported in part by grants K08-DK095951 (Scott FI), K24-DK078228 (Lewis JD), and K08-DK098272 (Goldberg DS) from the National Institute of Diabetes and Digestive and Kidney Diseases, as well as grants K23-CA187185 (Mamtani R) from the National Cancer Institute and F32-AR066461 (Horton DB) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and by the University of Pennsylvania’s Center for Pharmacoepidemiology Research and Training and the Penn-CHOP Microbiome Program.
Abbreviations
- aOR
Adjusted odds ratio
- BMI
Body Mass Index
- CI
Confidence Interval
- EMR
Electronic Medical Record
- OR
Odds ratio
- THIN
The Health Improvement Network
- UK
United Kingdom
- zWFL
z-Weight for Length score
Footnotes
Transcript profiling: N/A
Writing Assistance: N/A
Disclosures: No authors have support from any company directly related to the submitted work. JDL has served as a consultant for the following antibiotic manufacturers (on matters completely unrelated to this manuscript): AbbVie, AstraZeneca, Janssen Pharmaceuticals, Medimmune, Merck, Takeda, Shire. He has served on a Data and Safety Monitoring Board for clinical trials (unrelated to antibiotics) sponsored by Pfizer and Gilead, other antibiotic manufacturers. He has also served as a consultant for Nestle Health Science and Rebiotix, companies studying therapies for intestinal health (on matters completely unrelated to this manuscript). FIS has received research support, unrelated to this research, from Takeda. DG has served as a consultant for Merck on matters completely unrelated to this manuscript. KH is currently employed by HealthCore, but contributed to this work outside of and prior to this employment. (3) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) FS, JDL, RM, DSG, DBH, and DYL have no non-financial interests that may be relevant to the submitted work.”
Author Contributions: Drs. Scott and Lewis had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design were performed by FIS and JDL. Acquisition, analysis, and interpretation of the data was performed by FIS, JDL, DSG, DYL, DBH, and RM. Drafting of the manuscript was performed by FIS and JDL. Critical revision of the manuscript for important intellectual content was performed by all authors. Statistical analyses were conducted by FIS, JDL, RM, and DSG. Administrative, technical, or material support was provided by JDL. The study was supervised by JDL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jukes TH. Antibiotics in Animal Feeds and Animal Production. BioScience. 1972;22(9):526–534. [Google Scholar]
- 2.Gaskins HR, Collier CT, Anderson DB. Antibiotics as growth promotants: mode of action. Animal biotechnology. 2002;13(1):29–42. doi: 10.1081/ABIO-120005768. [DOI] [PubMed] [Google Scholar]
- 3.Coates ME, Fuller R, Harrison GF, Lev M, Suffolk SF. A comparison of the growth of chicks in the Gustafsson germ-free apparatus and in a conventional environment, with and without dietary supplements of penicillin. The British journal of nutrition. 1963;17:141–150. doi: 10.1079/bjn19630015. [DOI] [PubMed] [Google Scholar]
- 4.Lassiter CA. Antibiotics as Growth Stimulants for Dairy Cattle: A Review1. Journal of dairy science. 1955;38(10):1102–1138. [Google Scholar]
- 5.Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature. Preventive medicine. 1993;22(2):167–177. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- 6.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(6):1053–1061. doi: 10.1542/peds.2011-1337. [DOI] [PubMed] [Google Scholar]
- 7.Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond) 2011;35(4):522–529. doi: 10.1038/ijo.2011.27. [DOI] [PubMed] [Google Scholar]
- 8.Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond) 2013;37(1):16–23. doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy R, Stewart AW, Braithwaite I, et al. Antibiotic treatment during infancy and increased body mass index in boys: an international crosssectional study. Int J Obes (Lond) 2014;38(8):1115–1119. doi: 10.1038/ijo.2013.218. [DOI] [PubMed] [Google Scholar]
- 10.Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA pediatrics. 2014;168(11):1063–1069. doi: 10.1001/jamapediatrics.2014.1539. [DOI] [PubMed] [Google Scholar]
- 11.Azad MB, Bridgman SL, Becker AB, Kozyrskyj AL. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes (Lond) 2014;38(10):1290–1298. doi: 10.1038/ijo.2014.119. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz BS, Pollak J, Bailey-Davis L, et al. Antibiotic use and childhood body mass index trajectory. Int J Obes (Lond) 2015 doi: 10.1038/ijo.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Informatics in primary care. 2011;19(4):251–255. doi: 10.14236/jhi.v19i4.820. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JD, Brensinger C, Bilker WB, Strom BL. Validity and completeness of the General Practice Research Database for studies of inflammatory bowel disease. Pharmacoepidemiology and drug safety. 2002;11(3):211–218. doi: 10.1002/pds.698. [DOI] [PubMed] [Google Scholar]
- 15.Ruigomez A, Martin-Merino E, Rodriguez LA. Validation of ischemic cerebrovascular diagnoses in the health improvement network (THIN) Pharmacoepidemiology and drug safety. 2010;19(6):579–585. doi: 10.1002/pds.1919. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiology and drug safety. 2007;16(4):393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 17.Lo Re V, 3rd, Haynes K, Forde KA, Localio AR, Schinnar R, Lewis JD. Validity of The Health Improvement Network (THIN) for epidemiologic studies of hepatitis C virus infection. Pharmacoepidemiology and drug safety. 2009;18(9):807–814. doi: 10.1002/pds.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haynes K, Forde KA, Schinnar R, Wong P, Strom BL, Lewis JD. Cancer incidence in The Health Improvement Network. Pharmacoepidemiology and drug safety. 2009;18(8):730–736. doi: 10.1002/pds.1774. [DOI] [PubMed] [Google Scholar]
- 19.Ban L, Gibson JE, West J, Tata LJ. Association between perinatal depression in mothers and the risk of childhood infections in offspring: a population-based cohort study. BMC public health. 2010;10:799. doi: 10.1186/1471-2458-10-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. WHO child growth standards: Head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: Methods and development. Geneva: World Health Organization; 2007. [Google Scholar]
- 21.Flegal KM, Ogden CL. Childhood obesity: are we all speaking the same language? Adv Nutr. 2011;2(2):159S–166S. doi: 10.3945/an.111.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. Bmj. 2000;320(7244):1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reilly JJ, Armstrong J, Dorosty AR, et al. Early life risk factors for obesity in childhood: cohort study. Bmj. 2005;330(7504):1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sainani KL. Multivariate regression: the pitfalls of automated variable selection. PM R. 2013;5(9):791–794. doi: 10.1016/j.pmrj.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Gamma A. LINCHECK: Stata module to graphically assess the linearity of a continuous variable in a regression model. Boston College Department of Economics. 2005 [Google Scholar]
- 26.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361(9356):512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 27.Orsini N, Bellocco R, Bottai M, Wolk A, Greenland S. A tool for deterministic and probabilistic sensitivity analysis of epidemiologic studies. Stata Journal. 2008;8(1):29–48. [Google Scholar]
- 28.Renfrew M, Pokhrel S, MQ [Accessed 9/24/2015];Preventing disease and saving resources: the potential contribution of increasing breastfeeding rates in the UK. 2012 http://www.unicef.org.uk/Documents/Baby_Friendly/Research/Preventing_disease_saving_resources.pdf.
- 29.Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: a meta-analysis. BMC public health. 2014;14:1267. doi: 10.1186/1471-2458-14-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duijts L, Jaddoe VW, Hofman A, Moll HA. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126(1):e18–25. doi: 10.1542/peds.2008-3256. [DOI] [PubMed] [Google Scholar]
- 31.van Rossem L, Taveras EM, Gillman MW, et al. Is the association of breastfeeding with child obesity explained by infant weight change? Int J Pediatr Obes. 2011;6(2–2):e415–422. doi: 10.3109/17477166.2010.524700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA : the journal of the American Medical Association. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lioret S, Touvier M, Dubuisson C, et al. Trends in child overweight rates and energy intake in France from 1999 to 2007: relationships with socioeconomic status. Obesity. 2009;17(5):1092–1100. doi: 10.1038/oby.2008.619. [DOI] [PubMed] [Google Scholar]
- 34.Orsi CM, Hale DE, Lynch JL. Pediatric obesity epidemiology. Current opinion in endocrinology, diabetes, and obesity. 2011;18(1):14–22. doi: 10.1097/MED.0b013e3283423de1. [DOI] [PubMed] [Google Scholar]
- 35.Stamatakis E, Wardle J, Cole TJ. Childhood obesity and overweight prevalence trends in England: evidence for growing socioeconomic disparities. Int J Obes (Lond) 2010;34(1):41–47. doi: 10.1038/ijo.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson RL, Dowell SF, Jayaraman M, Keyserling H, Kolczak M, Schwartz B. Antimicrobial use for pediatric upper respiratory infections: reported practice, actual practice, and parent beliefs. Pediatrics. 1999;104(6):1251–1257. doi: 10.1542/peds.104.6.1251. [DOI] [PubMed] [Google Scholar]
- 37.Dowell SF, Marcy SM, Phillips WR, Gerber MA, Schwartz B. Principles of Judicious Use of Antimicrobial Agents for Pediatric Upper Respiratory Tract Infections. Pediatrics. 1998;101(Supplement 1):163–165. [Google Scholar]
- 38.Lessa FC, Gould CV, McDonald LC. Current Status of Clostridium difficile Infection Epidemiology. Clinical Infectious Diseases. 2012;55(suppl 2):S65–S70. doi: 10.1093/cid/cis319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic Exposure and IBD Development Among Children: A Population-Based Cohort Study. Pediatrics. 2012;130(4):e794–e803. doi: 10.1542/peds.2011-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldman JL, Jackson MA, Herigon JC, Hersh AL, Shapiro DJ, Leeder JS. Trends in Adverse Reactions to Trimethoprim-Sulfamethoxazole. Pediatrics. 2013;131(1):e103–e108. doi: 10.1542/peds.2012-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsakok T, McKeever TM, Yeo L, Flohr C. Does early life exposure to antibiotics increase the risk of eczema? A systematic review. British Journal of Dermatology. 2013;169(5):983–991. doi: 10.1111/bjd.12476. [DOI] [PubMed] [Google Scholar]
- 42.Tahtinen PA, Laine MK, Huovinen P, Jalava J, Ruuskanen O, Ruohola A. A Placebo-Controlled Trial of Antimicrobial Treatment for Acute Otitis Media. New England Journal of Medicine. 2011;364(2):116–126. doi: 10.1056/NEJMoa1007174. [DOI] [PubMed] [Google Scholar]
- 43.Hoberman A, Paradise JL, Rockette HE, et al. Treatment of Acute Otitis Media in Children under 2 Years of Age. New England Journal of Medicine. 2011;364(2):105–115. doi: 10.1056/NEJMoa0912254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horton DB, Scott FI, Haynes K, et al. Antibiotic Exposure and Juvenile Idiopathic Arthritis: A Case-Control Study. Pediatrics. 2015;136(2):e333–343. doi: 10.1542/peds.2015-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore P, Evenson A, Luckey T, McCoy E, Elvehjem E, Hart E. Use of sulphasuccidine, streptothricin and streptomycin in nutrition studies with the chick. J Biol Chem. 1946;165:437–441. [PubMed] [Google Scholar]
- 46.Stokstad EL, Jukes TH, et al. The multiple nature of the animal protein factor. J Biol Chem. 1949;180(2):647–654. [PubMed] [Google Scholar]
- 47.Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Royal College of Paediatrics and Child Health. [Accessed January 21, 2016, 2016];Early years - UK-WHO growth charts and resources. 2015 http://www.rcpch.ac.uk/child-health/researchprojects/uk-who-growth-charts/uk-who-growth-chart-resources-0-4-years/uk-who-0.
- 50.van Jaarsveld CH, Gulliford MC. Childhood obesity trends from primary care electronic health records in England between 1994 and 2013: population-based cohort study. Archives of disease in childhood. 2015;100(3):214–219. doi: 10.1136/archdischild-2014-307151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McAndrew F, Thompson J, Fellows Lydia, Large A, Speed M, Renfrew MJ. Infant Feeding Survey 2010. Information Centre for health and social care, NHS; 2012. [Google Scholar]
- 52.Black MH, Smith N, Porter AH, Jacobsen SJ, Koebnick C. Higher prevalence of obesity among children with asthma. Obesity. 2012;20(5):1041–1047. doi: 10.1038/oby.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.