Abstract
PURPOSE
To investigate the effect of electro-acupuncture (EA) as a non-pharmacological intervention to prevent or reduce chemotherapy-induced peripheral neuropathy (CIPN) in breast cancer patients undergoing chemotherapy of taxane.
METHODS
Women with stage I-III breast cancer scheduled to receive taxane therapy were randomized to receive a standardized protocol of 12 true or sham EA (SEA) weekly treatments concurrent with taxane treatment. Subjects completed the Brief Pain Inventory-Short Form (BPI-SF), Functional Assessment of Cancer Therapy-Taxane neurotoxicity subscale (FACT-NTX), and other assessments at baseline and weeks 6, 12, and 16.
RESULTS
A total of 180 subjects were screened, 63 enrolled and 48 completed week 16 assessments. Mean age was 50 with 25% white, 25% black, and 43% Hispanic; 52% had no prior chemotherapy. At week 12, both groups reported an increase in mean BPI-SF worst pain score, but no mean differences were found between groups (SEA 2.8 vs. EA 2.6, p=.86). By week 16, the SEA group returned to baseline, while the EA group continued to worsen (mean=1.7 in SEA vs. 3.40 in EA, p=.03). The increase in BPI-SF worst pain score was 1.62 points higher in the EA group than in the SEA group at week 16 (p=.04).
CONCLUSIONS
In a randomized, sham-controlled trial of EA for prevention of taxane-induced CIPN, there were no differences in pain or neuropathy between groups at week 12. Of concern, subjects on EA had a slower recovery than SEA subjects. Future studies should focus on EA for treatment as opposed to prevention of CIPN.
Keywords: acupuncture, electro-acupuncture, breast cancer, chemotherapy-induced peripheral neuropathy, taxane
INTRODUCTION
Chemotherapy-induced peripheral neuropathy (CIPN) is a common and disabling toxicity of cytotoxic chemotherapy [1,2]. CIPN can cause treatment dose-reductions and discontinuation, leading to poor tumor control and worse prognosis [3–5]. Approximately 50% of breast cancer patients receive taxane therapy, including paclitaxel and docetaxel, as a large clinical trial has shown that adjuvant paclitaxel could effectively improve overall survival for women with node positive and high-risk node negative breast cancer [6–8]. Recent studies have shown that 60–70% of patients treated with taxanes report CIPN symptoms [2,9], and 25–40% have dose limiting CIPN [10,9]. CIPN can resolve with cessation of chemotherapy, but often persists long term.
There are currently no effective drugs or modalities to prevent CIPN. Current CIPN guidelines recommend duloxitene as the only effective treatment for painful CIPN [11,12]. Other medications prescribed to treat CIPN have shown limited efficacy, including tricyclic antidepressants [13,14], anticonvulsants [15,16], and a compounded topical gel containing baclofen, amitriptyline HCL, and ketamine [17]. Acupuncture is a therapeutic modality used to treat acute and chronic pain with a low side effect profile [18]. The physiologic mechanisms behind the analgesic effects of acupuncture are unclear, but likely involve the release of endogenous opiates and neurotransmitters [19]. In cancer patients, acupuncture and transcutaneous electrical nerve stimulation (TENS) have been used to treat cancer pain with demonstrated efficacy [20,21]. Electro-acupuncture (EA) involves using acupuncture needles attached to an electro-stimulator device that generates a low electrical current between the needles. Recently, studies have shown that acupuncture with and without electrical stimulation may effectively relieve CIPN symptoms [22,12]. Administering EA during chemotherapy to prevent CIPN symptoms has not been tested.
We conducted a randomized sham-controlled trial to investigate the effect of EA as a non-pharmacological intervention to prevent or reduce CIPN in women with stage I-III breast cancer receiving adjuvant or neo-adjuvant paclitaxel weekly for 12 cycles.
METHODS
Participants
Between February 2011 and October 2014, women age >21 years with a history of stage I–III breast cancer scheduled to receive 12 weeks of weekly adjuvant or neo-adjuvant paclitaxel were recruited from Columbia University Medical Center’s (CUMC) breast oncology clinic. Patients who reported prior treatment with acupuncture in the previous 12 months and/or a history of the following medical conditions were excluded: diabetic neuropathy or other neuropathic pain conditions; inflammatory, metabolic, or neuropathic arthropathies; current narcotic use; severe coagulopathy or bleeding disorder; dermatological disease within acupuncture needling area; and use of a pacemaker. Patients provided written informed consent. The study was approved by the institutional review board at CUMC and registered at ClinicalTrials.gov (NCT01163682).
Baseline data collection
At baseline, participants completed questionnaires on demographics and clinical characteristics. Breast cancer characteristics and chemotherapy treatment data were abstracted from the electronic medical record. Neuropathic symptoms were assessed using a range of subjective and objective assessment tools. The Brief Pain Inventory-Short Form (BPI-SF) used a 0–10 scale to rate the worst pain on the day of interview [23]. The Functional Assessment of Cancer Therapy Taxane (FACT-TAX) assessed quality of life domains (physical well-being, social well-being, emotional well-being, functional well-being) and neurotoxicity using an 11-item neurotoxicity score (FACT-NTX) [24]. The Neuropathic Pain Scale (NPS) assessed ten distinctive aspects of peripheral neuropathic pain conditions using a 0–10 scale [25]. We assessed neuropathy pain using the NPS-10 (includes all ten items in the NPS), NPS-8 (includes all items except for the “intense” and “unpleasant” pain), and NPS-4 (only includes the “sharp”, “hot”, “dull” and “deep” pain) scores. Development of sensory neuropathy was measured via changes in vibratory perception threshold using a handheld Model 2291L biothesiometer (Bio-Medical Instrument Company, Newbury, OH) [26]. Development of motor neurologic dysfunction was assessed via the Grooved Pegboard test Model 32025 (Lafayette Instrument Evaluation, Lafayette, IN), which measures hand eye coordination and fine motor skills [27].
Randomization and blinding
After baseline data collection was completed and prior to the initiation of taxane therapy, participants were randomized to receive either 12 weeks of weekly EA or 12 weeks of weekly sham EA (SEA). Participants, study staff, and clinicians were blind to study assignment; only the acupuncturist, study statistician and a study coordinator with no participant contact were aware of randomization assignment.
Electro-acupuncture and sham electro-acupuncture protocols
Weekly EA and SEA visits were scheduled within 2 days of the weekly chemotherapy infusion. All acupuncture sessions were conducted within the CUMC breast oncology clinic.
Electro-acupuncture protocol
The EA point protocol was developed based on a standard Traditional Chinese Medicine (TCM) protocol for qi and xue deficiency and stagnation and informal practitioner query. Three independent acupuncturists were queried about their standard practice for CIPN prevention and treatment and were asked to provide feedback on the proposed standardized study acupuncture protocol. Each practitioner had a minimum of 5 years of clinical experience treating oncology patients. The final point protocol for body, lower limb, and upper limb acupuncture points is shown in Table 1. All participants received all points. Stainless steel disposable acupuncture needle (MAC Co. Tian Jin Haing Lim Sou Wan Medical Instrument Co., Ltd. Distributed by Roslyn Heights, NY, USA, diameter 32G x 0.25 mm) were inserted into the skin to the appropriate depth (approximately 3–4 mm) needed to elicit de qi. De qi is the term used to describe a needle sensation characteristic of acupuncture needling described as a dull or achy sensation of soreness. Selected acupuncture points were attached to 2 leads connected to an electro-stimulator (KWD-808 Multi-Purpose Transcutaneous Simulator Device, Great Wall Brand, Distributed, NY, NY) that generated 2 Hz of mixed pulsatile intervals for a total of 30 minutes. Needles not attached to the electro-stimulator were manipulated manually to elicit de qi once during the treatment.
Table 1.
Acupuncture point protocol
| Electro-acupuncture | Sham electro-acupuncture | |||
|---|---|---|---|---|
|
|
||||
| Position | Points | Traditional name | Location | Location |
| General | GB34* | Yang ling quan | In a depression anterior and inferior to the head of the fibula | Sham 1: On the lateral side of the left forearm, near the elbow |
| ST36* | Zu san li | 3 cun** below ST 35, one finger width lateral from the anterior border of the tibia | Sham 2: On the lateral side of the right forearm, near the elbow, 3 cun** below the olecranon, 0.5 cun** toward the anterior of the small intestine meridian | |
| LI4* | Shou san li | In the middle of the 2nd metacarpal bone on the radial side | Sham 3: At the lower border of the medial condyle of the left tibia, 1 cun** anterior and superior to xi guan (Liv 7) of the liver meridian | |
| LI10* | He gu | 2 cun below LI 11 on the LI 5 to LI 11 line | Sham 4: At the lower border of the medial condyle of the right tibia, 1 cun anterior and superior to xi guan (Liv 7) point of the liver meridian | |
| Lower limb | L3, L5 | Huatuojiaji | 0.5 inches lateral to the spinous process of L3 and L4, and 0.5 inches lateral to the spinous process of L5 | 2 cun** above sham 3 or 4, respectively |
| Ba feng points | On the dorsum of the foot between the web and metatarsophalangeal joint (4 points on each foot) | |||
| Upper limb | C5, C7 | Huatuojiaji | 0.5 inches lateral to the spinous process of C5 at C5, and 0.5 inches lateral to the spinous process of C7 at C7 | On the lateral side of the left and right forearm, near the elbow, 5 cun** below the olecranon, 0.5 cun** toward the anterior of the small intestine meridian |
| Ba xie points | On the dorsum of the hand, at the webs between each finger (4 points on each hand) | |||
Abbreviations: C, cervical spine; GB, gallbladder meridian; L, lung meridian; LI, large intestine meridian; Liv, liver meridian, ST, stomach meridian,
These selected full body points were attached to the electrical stimulation unit.
A cun is a standard measurement used to locate acupuncture points. It varies by patient, and is equal to the width of the distal inter-phalangeal joint of the thumb.
Sham electro-acupuncture protocol
Patients in the control arm received weekly SEA using Park Sham collapsible needles (Dongbang AcuPrime, Exeter, United Kingdom) that touch but do not penetrate the skin [28]. The sham point prescription did not include any true acupuncture points and is shown in Table 1. The electro-stimulator was attached to the needles, the acupuncturist turned on the machine to a non-transmitting setting for 30 minutes, and the acupuncturist touched the collapsible acupuncture needs to simulate the manipulation of the needles.
Follow-up data collection
At weeks 6, 12, and 16, participants repeated in-person subjective and objective assessments of neuropathy. At week 16 participants were asked if they thought they received EA or SEA.
Study endpoints and statistical methods
Evaluable subjects were defined as those who signed consent, completed the baseline questionnaire and at least one of the follow-up questionnaires, and completed at least 3 weeks of acupuncture. The a priori primary endpoint was the difference in neuropathic pain between the two arms as measured by the Brief Pain Inventory-Short Form (BPI-SF) mean worst pain score at 12 weeks. The BPI-SF worst pain score uses a 0–10 scale for subject ratings. A priori reductions of ≥2 points on the BPI-SF worst pain score and ≥5-points on the FACT-NTX were considered to be a clinically meaningful change.
Sample size estimates were based on a two-arm normal design. Assuming a 2-point difference in BPI-SF worse pain score to be clinically significant and a standard deviation of the average difference to be 2, and using a two-sided test with α=.05, a sample size of 50 patients (25 per arm) was estimated to provide 94% power to detect a clinically meaningful change in BPI-SF worst pain score between arms. For the intention-to-treat analyses, two-sample t-tests were used to compare between-group differences and paired t-tests for within-group means for the EA and SEA groups in BPI-SF worst pain score. Generalized estimating equations (GEE) with the unstructured working correlation matrix and identity link function tested the interaction between treatment and visit on BPI-SF, adjusted for baseline scores and number of received taxane cycles. In multivariable analyses, logistic regression analyses of clinically meaningful changes in BPI-SF were adjusted for baseline BPI-SF worst pain score and number of received taxane cycles. Confidence intervals and P-values were estimated using the robust standard error. Secondary objectives were to determine differences in FACT-NTX, FACT-TAX, NPS, biothesiometer test scores, and grooved pegboard test scores. All analyses were conducted utilizing R statistical software (version 3.2.2, https://cran.r-project.org/).
RESULTS
Accrual, eligibility, and evaluability
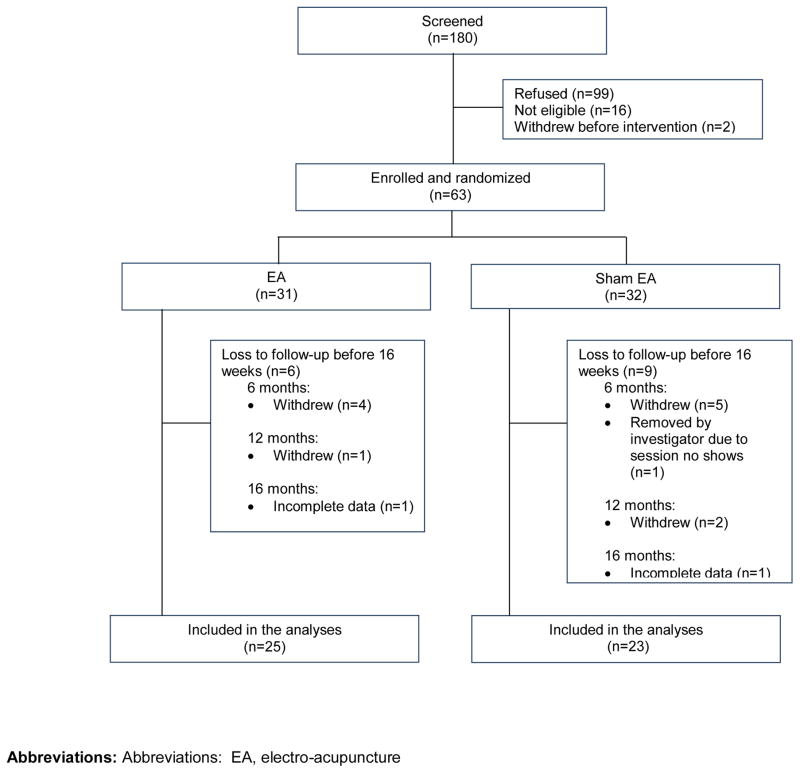
Between November 2009 and November 2012, a total of 180 subjects were screened and 63 were randomized (n=31, EA; n=32, SEA). Figure 1 shows a CONSORT diagram. A total of 48 patients were evaluable at week 16 (Figure 1).
Figure 1. CONSORT diagram of patient recruitment, randomization and follow-up.
Abbreviations: Abbreviations: EA, electro-acupuncture
Participant characteristics
The mean age of participants was 50 years (SD=11). Participants were diverse in race/ethnicity: 25.4% were non-Hispanic white, 25.4% were African American, and 42.9% were Hispanic. Nearly half (48%) had received doxorubicin and cyclophosphamide (AC) prior to taxane chemotherapy. There were no differences between groups in demographic or clinical characteristics (Table 2).
Table 2.
Baseline demographics and clinical characteristics by treatment group
| Electro-acupuncture (n = 31)
|
Sham electro-acupuncture (n = 32)
|
P value a | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| BASELINE CHARACTERISTICS | |||||
| Age, years | |||||
| Mean (SD) | 51.8 (10.7) | 48.3 (12.0) | .22 | ||
| Range | 36.0–75.0 | 27.0–79.0 | |||
| Body mass index, kg/m2 | |||||
| Mean (SD) | 27.6 (6.3) | 29.5 (7.1) | .29 | ||
| Range | 19.5–53.2 | 19.1–45.5 | |||
| Race | .52 | ||||
| Hispanic | 13 | 42 | 14 | 44 | |
| Non-Hispanic White | 7 | 23 | 9 | 28 | |
| African American | 10 | 32 | 6 | 19 | |
| Asian | 1 | 3 | 3 | 9 | |
| Breast cancer type | .86 | ||||
| Invasive ductal carcinoma | 25 | 81 | 27 | 84 | |
| Invasive lobular carcinoma | 5 | 16 | 5 | 16 | |
| Sarcomatoid | 1 | 3 | 0 | 0 | |
| ER/PR status | 1.00 | ||||
| Negative | 7 | 23 | 8 | 25 | |
| Positive | 24 | 77 | 24 | 75 | |
| HER2 status | .39 | ||||
| Negative | 25 | 81 | 22 | 69 | |
| Positive | 6 | 19 | 10 | 31 | |
| Stage | .09 | ||||
| I | 2 | 6 | 4 | 13 | |
| II | 26 | 84 | 19 | 59 | |
| III | 3 | 10 | 9 | 28 | |
| Tumor grade | .54 | ||||
| Well differentiated | 2 | 6 | 0 | 0 | |
| Moderately differentiated | 14 | 45 | 15 | 47 | |
| Poorly differentiated | 15 | 48 | 17 | 53 | |
| CHEMOTHERAPY INFORMATION | |||||
| Chemotherapy setting | .62 | ||||
| Adjuvant | 16 | 52 | 14 | 44 | |
| Neoadjuvant | 15 | 48 | 18 | 56 | |
| Type of taxane received | 1.00 | ||||
| Paclitaxel only | 30 | 97 | 31 | 97 | |
| Paclitaxel and Docetaxel b | 1 | 3 | 1 | 3 | |
| Received Paclitaxel cycles | .36 | ||||
| 12 cycles | 23 | 74 | 27 | 84 | |
| <12 cycles | 8 | 26 | 5 | 16 | |
| Taxane dose reduction c | 1.00 | ||||
| No | 21 | 68 | 21 | 66 | |
| Yes | 10 | 32 | 11 | 34 | |
| INTERVENTION INFORMATION | |||||
| Intervention adherence d | .30 | ||||
| Full attendance (12 sessions) | 13 | 45 | 16 | 59 | |
| Partial attendance (<12 sessions) | 16 | 55 | 11 | 41 | |
| Number of attended sessions | |||||
| Mean (SD) | 10 (3) | 10 (4) | .80 | ||
| Range | 1–12 | 1–12 | |||
| Blinding at week 16 e | .67 | ||||
| Thought received EA | 20 | 83 | 20 | 91 | |
| Thought received SEA | 4 | 17 | 2 | 9 | |
Abbreviations: SD, standard deviation; EA, electro-acupuncture; SEA, sham elector-acupuncture.
Two sample t-tests were used to compare the mean age and body mass index, and Fisher's exact test was used to compare all categorical variables.
Two participants had to switch to docetaxel due to drug supply issues.
Dose reduction is defined as any reduction in received taxane dose compared to the first cycle during the 12 weeks of chemotherapy.
Intervention attendance was tracked for 56 patients who received at least 1 EA or SEA session.
Effectiveness of blinding was assessed at 16 weeks by asking patients, "Do you think that you were receiving true acupuncture over the past twelve weeks?". A total of 46 responses were received.
Chemotherapy and intervention adherence
There were no differences in taxane treatment adherence between arms as assessed by cycles received or in dose limitations due to CIPN symptoms (Table 2). There were no differences between groups in acupuncture session attendance (Table 2). In the EA arm, participants received an average of 10 (SD=3) sessions, which was similar to the SEA arm, which received an average of 10 (SD=4) sessions (P=.80).
Blinding
The majority of patients (87%) believed that they received true electro-acupuncture regardless of the actual treatment received (Table 2). There was no between-group difference in effectiveness of blinding (SEA: 90% vs. EA: 83%, P=.67).
BPI-SF worst pain score
In between-group analyses, there were no differences in worst pain scores at 6 and 12 weeks. However, at 16 weeks, while the worst pain score reverted to baseline level in the SEA arm, it continued to worsen in the EA arm (P=.03; Table 3 and Figure 1A). In GEE analyses of interactions between treatment and visits, the increase in the worst pain score at 16 weeks was 1.62 points greater in the EA arm than in the SEA arm (P=.04; Table 4), suggesting that patients receiving EA experienced more increases in pain symptoms after the intervention.
Table 3.
Mean BPI-SF worst pain, FACT-NTX , and NPS score at 6, 12 and 16 week
| Sample size | Baseline
|
Follow-up
|
Within- group differences | Between- group differences | Change from baseline
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comparisons | Mean | SD | Mean | SD | P-value* | P-value* | Mean | SD | P-value* | |
| Brief Pain Inventory - Short Form a | ||||||||||
| BPI-SF worst pain score a | ||||||||||
| Week-6 | ||||||||||
| EA | 25 | 1.8 | 2.9 | 2.1 | 2.2 | .72 | .45 | 0.2 | 2.8 | .92 |
| SEA | 22 | 1.5 | 2.6 | 1.6 | 2.1 | .77 | 0.1 | 2.1 | ||
| Week-12 | ||||||||||
| EA | 25 | 1.8 | 2.9 | 2.6 | 3.3 | .32 | .86 | 0.8 | 3.8 | .41 |
| SEA | 21 | 1.1 | 2.1 | 2.8 | 3 | .03 | 1.7 | 3.3 | ||
| Week-16 | ||||||||||
| EA | 25 | 1.8 | 2.9 | 3.4 | 3 | .02 | .03** | 1.6 | 3 | .21 |
| SEA | 23 | 1 | 2 | 1.7 | 2.2 | .18 | 0.6 | 2.1 | ||
| Functional Assessment of Cancer Therapy- Taxane scales b | ||||||||||
| FACT-NTX score b | ||||||||||
| Week-6 | ||||||||||
| EA | 24 | 40.2 | 9.1 | 39.1 | 5.3 | .81 | .18 | −0.6 | 10.9 | .23 |
| SEA | 22 | 40.2 | 5.7 | 36.6 | 7.1 | .003 | −3.6 | 5 | ||
| Week-12 | ||||||||||
| EA | 25 | 41.5 | 3 | 33.4 | 8.8 | <.001 | .46 | −8 | 8.2 | .59 |
| SEA | 21 | 40.5 | 5.8 | 31.1 | 11.3 | <.001 | −9.4 | 9.1 | ||
| Week-16 | ||||||||||
| EA | 25 | 39.8 | 9 | 30.8 | 9.7 | <.001 | .61 | −9.4 | 11 | .76 |
| SEA | 23 | 40.7 | 5.6 | 32.3 | 10.4 | <.001 | −8.4 | 9.8 | ||
| FACT-TAX Total score b | ||||||||||
| Week-6 | ||||||||||
| EA | 24 | 135.4 | 24.1 | 128.4 | 17.4 | .22 | .54 | −6.3 | 24 | .50 |
| SEA | 22 | 134.6 | 23.4 | 124.3 | 26.4 | .005 | −10.3 | 15.6 | ||
| Week-12 | ||||||||||
| EA | 25 | 136.8 | 18 | 116.5 | 23.6 | <.001 | .72 | −19.7 | 21.4 | .91 |
| SEA | 21 | 132.4 | 23.2 | 113.5 | 31.8 | .001 | −19 | 23.1 | ||
| Week-16 | ||||||||||
| EA | 25 | 133.2 | 25 | 113.7 | 24.9 | .002 | .50 | −20.1 | 27.9 | .50 |
| SEA | 22 | 133.9 | 23.6 | 118.9 | 27.3 | .004 | −15 | 21.8 | ||
| Neuropathic Pain Scale c | ||||||||||
| NPS-4 score c | ||||||||||
| Week-6 | ||||||||||
| EA | 24 | 12.4 | 19.8 | 18.2 | 23 | .92 | .55 | −0.4 | 19 | .09 |
| SEA | 19 | 8 | 14.3 | 22.8 | 26.2 | .06 | 12.9 | 25.8 | ||
| Week-12 | ||||||||||
| EA | 24 | 16.8 | 25.4 | 29.3 | 30.7 | .22 | .57 | 9.2 | 31.8 | .51 |
| SEA | 20 | 8.8 | 17 | 24.2 | 28.6 | .009 | 15.2 | 22.5 | ||
| Week-16 | ||||||||||
| EA | 24 | 16.8 | 25.4 | 35.2 | 30.9 | .07 | .03** | 17.4 | 40.1 | .48 |
| SEA | 23 | 7.9 | 16.3 | 18 | 21.1 | .03 | 10.2 | 19.8 | ||
Abbreviations: BPI-SF, Brief Pain Inventory-Short Form; FACT-NTX, neurotoxicity component of Functional Assessment of Cancer Therapy Taxane scale; FACT-TAX, Functional Assessment of Cancer Therapy Taxane scale; NPS, Neuropathic Pain Scale; EA, electro-acupuncture; SEA, sham electro-acupuncture; SD, standard deviation.
Paired t-test was used to compare the mean scores at each follow-up and baseline within each treatment group. Two-sample t-test was used to compare the mean scores and mean change from baseline between treatment groups at each follow-up.
Statistically significant, P-value <.05
BPI-SF worst pain: Higher score indicates greater pain (range 0–10).
FACT-NTX subscale score (range 0–44) and FACT-TAX total score (range 0–172): Higher score indicates greater quality of life.
NPS: Higher score indicates greater neuropathy. The raw score ranges 0–10, the standardized scores were used for analysis. NPS-4 reflects non-peripheral pain mechanisms. Normalized to 0–100.
Table 4.
Multivariable analyses comparing BPI-SF worst pain and FACT-NTX scores between treatment groups at 6, 12, and 16 weeks
| 6 weeks
|
12 weeks
|
16 weeks
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| GEE of treatment and visit interaction a | Coefficient | 95% CI | P-value | Coefficient | 95% CI | P-value | Coefficient | 95% CI | P-value |
| BPI-SF worst pain score | |||||||||
| Treatment × visit | 0.34 | −1.05 to 1.74 | .63 | −0.31 | −2.34 to 1.72 | .77 | 1.62 | 0.04 to 3.20 | .04* |
| FACT-NTX score | |||||||||
| Treatment × visit | 3.33 | −1.48 to 8.14 | .17 | 3.09 | −3.02 to 9.20 | .32 | −0.71 | −6.61 to 5.18 | .81 |
| 6 weeks
|
12 weeks
|
16 weeks
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Logistic regression of clinical changes b | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P |
| Clinical change in BPI-SF worst pain score | |||||||||
| SEA | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |||
| EA | 1.07 | 0.82 to 1.39 | .63 | 0.92 | 0.70 to 1.22 | .57 | 1.16 | 0.90 to 1.50 | .25 |
| Clinical change in FACT- NTX score | |||||||||
| SEA | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |||
| EA | 0.82 | 0.62 to 1.08 | .16 | 0.82 | 0.60 to 1.10 | .19 | 1.25 | 0.97 to 1.62 | .09 |
Abbreviations: BPI-SF, Brief Pain Index-Short Form; FACT-NTX, neurotoxicity component of Functional Assessment of Cancer Therapy Taxane scale; EA, electro-acupuncture; SEA, sham electro-acupuncture; GEE, generalized estimating equation; SD, standard deviation.
Statistically significant, P-value <.05
GEE models adjusted for baseline values and number of taxane cycles received.
Logistic regression models adjusted for baseline values and number of taxane cycles received. An increase of 2 points in BPI-SF worst pain score was considered as clinically meaningful. A decrease of ≥5 points in FACT-NTX score was considered clinically meaningful.
FACT-NTX subscale score
In between-group analyses, there were no differences in FACT-NTX at 6, 12, and 16 weeks. In logistical regression analyses examining a clinically meaningful change in scores, there was a trend towards increased pain at week 16 (OR=1.25, 95% CI 0.97–1.62).
NPS, FACT-TAX, biothesiometer and grooved pegboard
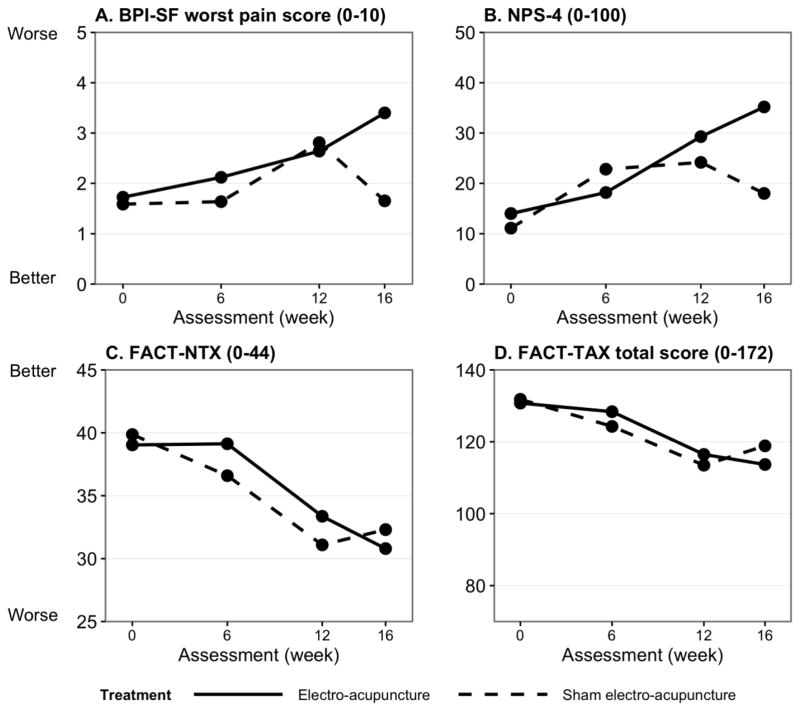
In between-group analyses, at week 16, participants in the EA arm reported worst pain in the NPS-4 scale (P=.03) (Table 3, Figure 2). There were no differences between groups in the FACT-TAX, biothesiometer or grooved pegboard tests (Online Figure 1).
Figure 2. Measurement of pain and neurotoxicity symptoms at baseline and week 6, 12 and 16.
Higher BPI-SF (A) and NPS-4 (B) scores indicate more pain symptoms; whereas higher FACT-NTX (C) and FACT-TAX (D) scores suggest less neurotoxicity. Over the course of receiving taxane chemotherapy, patients reported more pain and neurotoxicity symptoms in general. There was no difference in mean BPI-SF worst pain score (A), NPS-4 score (B), FACT-NTX score (C), and FACT-TAX score between treatment arms during the 12 weeks of chemotherapy. However, the electro-acupuncture (EA) arm reported significantly higher pain (p=0.03) (A) and more neuropathic pain (p=0.03) (B) compared to the sham electro-acupuncture (SEA) arm 4 weeks after chemotherapy was completed (16 weeks).
Adverse Events
One adverse event was reported, which was a grade 1 acupuncture needle site reaction with discomfort, minor swelling, and bruising after acupuncture needle withdrawal.
DISCUSSION
In this randomized sham controlled trial of EA to prevent CIPN in women receiving taxane-based chemotherapy for early stage breast cancer treatment, we did not observe differences in pain or neuropathy symptoms between treatment arms at 12 weeks. Unexpectedly, compared to SEA subjects, women on EA experienced greater increases in pain at 4 weeks after taxane completion. No differences were observed between groups with regard to taxane adherence.
A number of case reports suggested that acupuncture may be effective at reducing pain and improving nerve conduction studies in cancer patients with CIPN [29–31], however these studies suffer from lack of a control group. In a retrospective case series study (n=18), Donald et al. identified that 84% of patients attending 6 sessions of acupuncture reported improved CIPN symptoms [32]. In a recent single arm trial (n=27), Bao et al. reported that 10-weeks of acupuncture was significantly associated with reducing pain and improving neuropathy symptoms in multiple myeloma patients with bortezomib-induced peripheral neuropathy [33]. A randomized controlled trial among patients with paclitaxel or oxaliplatin-induced peripheral neuropathy (n=64) showed that sensory nerve disorder was improved in 67% of patients receiving acupuncture compared to 40% of patients who received a form of vitamin B12 [34]. These studies suggest acupuncture may serve as an effective treatment for CIPN symptoms, but have limited efficacy on pain in cancer patients. A number of ongoing clinical trials are currently investigating the role of acupuncture in the treatment (NCT02129686, NCT02615678) and prevention (NCT02615678) of taxane-induced peripheral neuropathy in breast cancer patients.
It is important to recognize that our findings are not directly comparable with previous reports, as our trial focused on the prevention of CIPN, whereas other studies focused on the management of post-chemotherapy CIPN. A trial by Lu et al. is somewhat comparable [35]. The trial investigated the effect of EA on quality of life outcomes in ovarian cancer patients (n=21) undergoing chemotherapy and found that patients in the EA arm reported worse peripheral neuropathy symptoms compared to the SEA arm. However, the study assessed peripheral neuropathy using the Quality of Life Questionnaire-Ovarian Module–28 Item (QLQ-OV28), which assesses tingling and numbness in the hands and feet via 2 items. This measure is less comprehensive compared to the 11-item FACT-NTX subscale used in our study. Furthermore, differences in cancer type, chemotherapy drugs and duration of both chemotherapy and acupuncture made it difficult to compare the two studies.
Another notable and puzzling finding of our trial was the post-intervention worsening of pain symptoms in the EA arm, which was similarly reported in Chen et al.’s RCT of EA vs. SEA in reducing pancreatic cancer pain [36]. The mechanism of such pain rebound is unknown. It is possible that the electrical stimulation component of the EA may have caused CIPN symptoms, and therefore it is possible that EA may not be as effective and safe as traditional acupuncture without electrical stimulation. In addition, patients without prior acupuncture treatment may experience stronger stimulation or even discomfort from EA compared to traditional acupuncture [37]. Therefore, other forms of acupuncture should be evaluated for the prevention of CIPN. It is also possible that there was higher response expectancy in the SEA arm, which led to better pain management. In our study, though it was not a statistically significant difference, a slightly larger percentage of patients in the SEA arm believed that they received true acupuncture, compared to the EA arm. A recent study suggested that higher response expectancy may moderate better acupuncture response in participants in patients receiving sham acupuncture, but not in patients receiving true acupuncture [38]. Therefore, it is possible that in our study a greater placebo effect may have led to better pain relief in the sham EA arm.
The mechanism by which acupuncture may affect CIPN is unclear. Acupuncture may relieve pain through the release of endogenous opiates and neurotransmitters [19]. An in vitro study showed that diabetic mice who received EA for four weeks had better nerve conduction studies and increased tactile threshold compared to the diabetic mice that did not receive EA [39]. In a retrospective study, patients with unspecified peripheral neuropathy who electively received acupuncture for pain reported reduced pain symptoms and improved nerve conduction studies [40]. Similarly, in a small non-randomized clinical trial in patients with chronic diabetic neuropathy, acupuncture relieved pain symptoms in 21% of patients, although the reduction in pain intensity was short term [41]. These studies suggest that acupuncture may not only relieve pain but may also be protective against the development of neuropathy. More recent studies suggest acupuncture mediates pain via inhibition of secretion of pro-inflammatory cytokines, including interleukin (IL)-1β, IL-6, IL-17, and tumor necrosis factor (TNF)-α [42,43].
The major strengths of our study are the use of a standardized EA and SEA protocol and the use of validated self-reported and objective outcome measures in a diverse population of breast cancer patients. We collected extensive data on patient information and chemotherapy dosage, allowing us to account for chemotherapy dosage in evaluating acupuncture effectiveness and examine chemotherapy adherence and dose-limiting between arms for any potential confounding. An additional strength was the heterogeneous race/ethnicity of the study population, which increases the generalizability of the findings. Though retention in the study was good (90% at 12 weeks), we did lose some patients to follow up, which could bias the results. Similarly, there was some non-adherence to the intervention with 48% of patients not attending at least 1 of the 12 sessions. Failure to attend the acupuncture sessions was primarily due to logistical issues with attending weekly acupuncture sessions in Manhattan. Future studies should test acupuncture protocols that are feasible given the study population.
Our RCT of electro-acupuncture vs. sham electro-acupuncture in preventing taxane-induced neuropathy among breast cancer patients did not identify a protective effect of acupuncture on CIPN symptoms over the course of chemotherapy. Unexpectedly, subjects receiving electro-acupuncture had a slower recovery in CIPN symptoms compared to subjects receiving sham electro-acupuncture. Future studies should focus on electroacupuncture or other forms of acupuncture for the treatment as opposed to prevention of CIPN. In addition, future studies should further investigate the worsening of pain in patients receiving EA and the possible role of outcome expectations in acupuncture studies.
Supplementary Material
Acknowledgments
Research Support: Breast Cancer Research Foundation (to DLH), National Cancer Institute NCI K23CA141052 (to HG)
Footnotes
Ethics: The experiments performed in this study comply with all relevant laws in the United States. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Disclaimers: The authors declare that they have no conflicts of interest.
Prior presentations: The 12th International Conference of the Society for Integrative Oncology
References
- 1.Cavaletti G, Zanna C. Current status and future prospects for the treatment of chemotherapy-induced peripheral neurotoxicity. Eur J Cancer. 2002;38:1832–1837. doi: 10.1016/S0959-8049(02)00229-0. [DOI] [PubMed] [Google Scholar]
- 2.Hershman DL, Weimer LH, Wang A, Kranwinkel G, Brafman L, Fuentes D, Awad D, Crew KD. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2011;125(3):767–774. doi: 10.1007/s10549-010-1278-0. [DOI] [PubMed] [Google Scholar]
- 3.Speck RM, Samuel MD, Farrar JT, Hennessy S, Mao JJ, Stineman MG, DeMichele A. Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J Oncol Pract. 2013 doi: 10.1200/jop.2012.000863. [DOI] [PubMed] [Google Scholar]
- 4.Carlson K, Ocean AJ. Peripheral neuropathy with microtubule-targeting agents: occurrence and management approach. Clin Breast Cancer. 2011;11(2):73–81. doi: 10.1016/j.clbc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Havrilesky LJ, Reiner M, Morrow PK, Watson H, Crawford J. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol. 2015;93(3):203–210. doi: 10.1016/j.critrevonc.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, Ingle JN, Cooper MR, Hayes DF, Tkaczuk KH, Fleming G, Holland JF, Duggan DB, Carpenter JT, Frei E, Schilsky RL, Wood WC, Muss HB, Norton L. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 7.Martin M, Ruiz A, Ruiz Borrego M, Barnadas A, Gonzalez S, Calvo L, Margeli Vila M, Anton A, Rodriguez-Lescure A, Segui-Palmer MA, Munoz-Mateu M, Dorca Ribugent J, Lopez-Vega JM, Jara C, Espinosa E, Mendiola Fernandez C, Andres R, Ribelles N, Plazaola A, Sanchez-Rovira P, Salvador Bofill J, Crespo C, Carabantes FJ, Servitja S, Chacon JI, Rodriguez CA, Hernando B, Alvarez I, Carrasco E, Lluch A. Fluorouracil, doxorubicin, and cyclophosphamide (FAC) versus FAC followed by weekly paclitaxel as adjuvant therapy for high-risk, node-negative breast cancer: results from the GEICAM/2003-02 study. J Clin Oncol. 2013;31(20):2593–2599. doi: 10.1200/JCO.2012.46.9841. [DOI] [PubMed] [Google Scholar]
- 8.Martin M, Segui MA, Anton A, Ruiz A, Ramos M, Adrover E, Aranda I, Rodriguez-Lescure A, Grosse R, Calvo L, Barnadas A, Isla D, Martinez del Prado P, Ruiz Borrego M, Zaluski J, Arcusa A, Munoz M, Lopez Vega JM, Mel JR, Munarriz B, Llorca C, Jara C, Alba E, Florian J, Li J, Lopez Garcia-Asenjo JA, Saez A, Rios MJ, Almenar S, Peiro G, Lluch A, Investigators G. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2010;363(23):2200–2210. doi: 10.1056/NEJMoa0910320. [DOI] [PubMed] [Google Scholar]
- 9.Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC, Sledge GW, Wood WC, Davidson NE. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JJ, Swain SM. Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol. 2006;24:1633–1642. doi: 10.1200/JCO.2005.04.0543. [DOI] [PubMed] [Google Scholar]
- 11.Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, Shapiro CL Alliance for Clinical Trials in O. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL. Prevention and management of chemotherapy- induced peripheral neuropathy in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 13.Hammack JE, Michalak JC, Loprinzi CL, Sloan JA, Novotny PJ, Soori GS, Tirona MT, Rowland KM, Stella PJ, Johnson JA. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain. 2002;98:195–203. doi: 10.1016/S0304-3959(02)00047-7. [DOI] [PubMed] [Google Scholar]
- 14.Kautio AL, Haanpaa M, Saarto T, Kalso E. Amitriptyline in the treatment of chemotherapy-induced neuropathic symptoms. J Pain Symptom Manag. 2008;35(1):31–39. doi: 10.1016/j.jpainsymman.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 15.Rao RD, Flynn PJ, Sloan JA, Wong GY, Novotny P, Johnson DB, Gross HM, Renno SI, Nashawaty M, Loprinzi CL. Efficacy of lamotrigine in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled trial, N01C3. Cancer. 2008;112(12):2802–2808. doi: 10.1002/cncr.23482. [DOI] [PubMed] [Google Scholar]
- 16.Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA, Warner DO, Novotny P, Kutteh LA, Wong GY North Central Cancer Treatment G. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007;110(9):2110–2118. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- 17.Barton DL, Wos EJ, Qin R, Mattar BI, Green NB, Lanier KS, Bearden JD, 3rd, Kugler JW, Hoff KL, Reddy PS, Rowland KM, Jr, Riepl M, Christensen B, Loprinzi CL. A double-blind, placebo-controlled trial of a topical treatment for chemotherapyinduced peripheral neuropathy: NCCTG trial N06CA. Support Care Cancer: Off J Multinatl Assoc Support Care Cancer. 2011;19(6):833–841. doi: 10.1007/s00520-010-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacPherson H, Thomas K, Walters S, Fitter M. The York acupuncture safety study: prospective survey of 34 000 treatments by traditional acupuncturists. BMJ. 2001;323:486–487. doi: 10.1136/bmj.323.7311.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361(1–3):258–261. doi: 10.1016/j.neulet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Paley CA, Johnson MI, Tashani OA, Bagnall A-M. Acupuncture for cancer pain in adults. Cochrane Database Syst Rev. 2011:CD007753. doi: 10.1002/14651858.CD007753.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Bennett MI, Johnson MI, Brown SR, Radford H, Brown JM, Searle RD. Feasibility study of transcutaneous electrical nerve stimulation (TENS) for cancer bone pain. J Pain: Off J Am Pain Soc. 2010;11(4):351–359. doi: 10.1016/j.jpain.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Brami C, Bao T, Deng G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: A systematic review. Crit Rev Oncol Hematol. 2016;98:325–334. doi: 10.1016/j.critrevonc.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Cella D, Peterman A, Hudgens S, Webster K, Socinski MA. Measuring the side effects of taxane therapy in oncology: The Functional Assessment of Cancer Therapy-Taxane (FACT-Taxane) Cancer. 2003;98:822–831. doi: 10.1002/cncr.11578. [DOI] [PubMed] [Google Scholar]
- 25.Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: The Neuropathic Pain Scale. Neurology. 1997;48:332–338. doi: 10.1212/WNL.48.2.332. [DOI] [PubMed] [Google Scholar]
- 26.Bloom S, Till S, Sonksen P, Smith S. Use of a biothesiometer to measure individual vibration thresholds and their variation in 519 non-diabetic subjects. BMJ. 1984;288:1793–1795. doi: 10.1136/bmj.288.6433.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Percept Mot Skills. 1993;76:1219–1230. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- 28.Park J, White A, Lee H, Ernst E. Development of a new sham needle. Acupunct Med. 1999;17(2):110–112. doi: 10.1136/aim.17.2.110. [DOI] [Google Scholar]
- 29.Wong R, Sagar S. Acupuncture treatment for chemotherapy-induced peripheral neuropathy--a case series. Acupunct Med. 2006;24:87–91. doi: 10.1136/aim.24.2.87. [DOI] [PubMed] [Google Scholar]
- 30.Bao T, Zhang R, Badros A, Lao L. Acupuncture treatment for bortezomib-induced peripheral neuropathy: a case report. Pain Res Treat. 2011;2011:920807. doi: 10.1155/2011/920807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schroeder S, Meyer-Hamme G, Epplée S. Acupuncture for chemotherapy-induced peripheral neuropathy (CIPN): a pilot study using neurography. Acupunct Med. 2012;30:4–7. doi: 10.1136/acupmed-2011-010034. [DOI] [PubMed] [Google Scholar]
- 32.Donald GK, Tobin I, Stringer J. Evaluation of acupuncture in the management of chemotherapy-induced peripheral neuropathy. Acupunct Med. 2011;29:230–233. doi: 10.1136/acupmed.2011.010025. [DOI] [PubMed] [Google Scholar]
- 33.Bao T, Goloubeva O, Pelser C, Porter N, Primrose J, Hester L, Sadowska M, Lapidus R, Medeiros M, Lao L, Dorsey SG, Badros AZ. A pilot study of acupuncture in treating bortezomib-induced peripheral neuropathy in patients with multiple myeloma. Integr Cancer Ther. 2014;13:396–404. doi: 10.1177/1534735414534729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu W-R, Hua B-J, Hou W, Bao Y-J. Clinical randomized controlled study on acupuncture for treatment of peripheral neuropathy induced by chemotherapeutic drugs. Zhongguo Zhen Jiu. 2010;30:457–460. [PubMed] [Google Scholar]
- 35.Lu W, Matulonis UA, Dunn JE, Lee H, Doherty-Gilman A, Dean-Clower E, Goodman A, Davis RB, Buring J, Wayne P, Rosenthal DS, Penson RT. The Feasibility and Effects of Acupuncture on Quality of Life Scores During Chemotherapy in Ovarian Cancer: Results from a Pilot, Randomized Sham-Controlled Trial. Acupunct Med. 2012;24:233–240. doi: 10.1089/acu.2012.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen H, Liu T-Y, Kuai L, Zhu J, Wu C-J, Liu L-M. Electroacupuncture treatment for pancreatic cancer pain: a randomized controlled trial. Pancreatol: Off J Int Assoc Pancreatol (IAP) [et al] 2013;13:594–597. doi: 10.1016/j.pan.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Dharmananda S. Electro-acupuncture. Subhuti Dharmananda; 2002. [Accessed 4 March 2016]. http://www.itmonline.org/arts/electro.htm. [Google Scholar]
- 38.Bauml J, Xie SX, Farrar JT, Bowman MA, Li SQ, Bruner D, DeMichele A, Mao JJ. Expectancy in real and sham electroacupuncture: Does believing make it so? J Natl Cancer Inst Monogr. 2014;2014:302–307. doi: 10.1093/jncimonographs/lgu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin CC, Chen MC, Yu SN, Ju MS. Chronic electrical stimulation of four acupuncture points on rat diabetic neuropathy. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2005;4:4271–4274. doi: 10.1109/iembs.2005.1615408. [DOI] [PubMed] [Google Scholar]
- 40.Schroder S, Liepert J, Remppis A, Greten JH. Acupuncture treatment improves nerve conduction in peripheral neuropathy. Eur J Neurol. 2007;14(3):276–281. doi: 10.1111/j.1468-1331.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 41.Abuaisha BB, Costanzi JB, Boulton AJ. Acupuncture for the treatment of chronic painful peripheral diabetic neuropathy: a long-term study. Diabetes Res Clin Pract. 1998;39(2):115–121. doi: 10.1016/S0168-8227(97)00123-X. [DOI] [PubMed] [Google Scholar]
- 42.Zijlstra FJ, van den Berg-de LI, Huygen FJ, Klein J. Antiinflammatory actions of acupuncture. Mediat Inflamm. 2003;12(2):59–69. doi: 10.1080/0962935031000114943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arrieta Ó, Hernández-Pedro N, Fernández-González-Aragón MC, Saavedra-Pérez D, Campos-Parra aD, Ríos-Trejo MÁ, Cerón-Lizárraga T, Martínez-Barrera L, Pineda B, Ordóñez G, Ortiz-Plata a, Granados-Soto V, Sotelo J. Retinoic acid reduces chemotherapy-induced neuropathy in an animal model and patients with lung cancer. Neurology. 2011;77:987–995. doi: 10.1212/WNL.0b013e31822e045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.