Abstract
Synthesis of tubuphenylalanine and tubuvaline, α-substituted γ-amino acid building blocks for tubulysin family of antimitotic compounds, has been improved using a radical addition reaction in the presence of unprotected hydroxyl functionality. The key carbon–carbon bond construction entails stereoselective Mn-mediated photolytic additions of alkyl iodides to the C=N bond of chiral N-acylhydrazones, and generates the chiral amines in high yield with complete stereocontrol. Reductive N–N bond cleavage and alcohol oxidation converted these amino alcohols into the corresponding γ-amino acids. The route to tubuvaline proceeded via peptide coupling with serine methyl ester, followed by a high-yielding sequence to convert the serine amide to a thiazole. Finally, peptide bond construction established the tubulysin framework in the form of a C-terminal alcohol analog. Attempted oxidation to the C-terminal carboxylate was unsuccessful; control experiments with dipeptide 18 showed a cyclization interfered with the desired oxidation process.
1. Introduction
The tubulysins are a group of unusual peptides distinguished by two novel γ-amino acid residues, tubuphenylalanine and tubuvaline, both of which are α,γ-disubstituted γ-amino acids. Initially isolated from culture broths of the myxobacteria Archangium gephyra and Angiococcus disciformis, these are extraordinary antimitotic agents, with potency superior to vinblastine and dolastatin-10 (growth inhibition of human cervical carcinoma DSM ACC 158, tubulysin D: IC50 20 pg/mL).1,2 After further isolation efforts, the series now includes tubulysins A–I, U, and V, as well as pretubulysin (Scheme 1).3,4,5
Scheme 1.
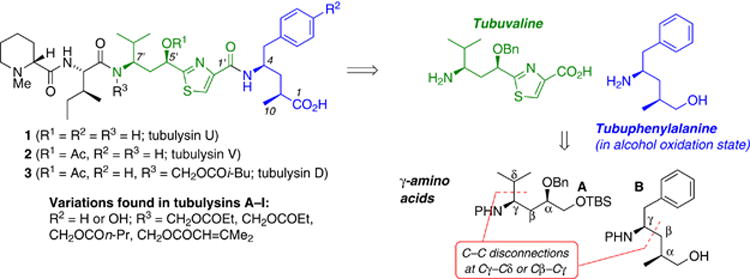
Retrosynthetic analysis of tubulysis, highlighting the γ-amino acids.
The extraordinary potency of tubulysins and their interesting impacts on tubulin biochemistry6 have inspired numerous efforts to probe their therapeutic potential through synthesis and medicinal chemistry. We disclosed our initial studies directed toward synthesis of the tubulysins in 2004,7 and the first total syntheses were achieved by Ellman (tubulysin D, 2006)8 and Zanda (tubulysins U and V, 2007).9 Despite numerous synthetic efforts (which have been reviewed10,11,12 and continue to emerge13,14,15), accessing both of the γ-amino acid subunits tubuvaline (Tuv, Scheme 1) and tubuphenylalanine (Tup) with excellent stereocontrol has proven to be a difficult challenge. Prior tubulysin syntheses have been hampered by limited stereoselectivity in alkene or ketone reductions to generate the requisite configuration at the α carbon of the γ-amino acids. In general, most approaches to γ-amino acids are limited by reliance upon homologation of the naturally abundant α-amino acids. These factors emphasize the need for new and versatile methodology for γ-amino acid synthesis,16,17 and serving this need became the cornerstone of our synthetic strategy.
Our plan for a more versatile C–C bond construction approach to synthesis of γ-amino acids would obviate the limitations of naturally occurring α-amino acid precursors, facilitating preparation of γ-amino acids bearing unusual functionality or substitution patterns. Taking guidance from the tubulysins, we required that the ideal method would be applicable to strategic construction of either the Cβ-Cγ or Cγ–Cδ bonds of γ-amino acids (as shown for Tup and Tuv, Scheme 1) and independent of substituents at the α-position. Stereoselective intermolecular additions of alkyl radicals to the C=N bonds are well-suited to this need; these reactions have been reviewed,18–26 and new ones continue to develop.27,28,29 We introduced a photolysis method30,31 for this reaction type which employs Mn2(CO)10 to generate alkyl radicals, leading to efficient additions of primary and secondary alkyl iodides to chiral N-acylhydrazones.32–35 The availability of efficient primary radical addition has opened many new synthetic applications; with earlier methods, primary alkyl radicals were prone to premature reduction or other side reactions (e.g., addition of ethyl radical under triethylborane or diethylzinc initiation).20–26 The functional group tolerance and non-basic conditions of this method show excellent potential for synthesis of multifunctional amines;36–41 the tubulysin γ-amino acids would test this potential. Indeed, our initial studies on tubulysins achieved preparation of Tuv precursor A and Tup itself (B) via these Mn-mediated radical addition reactions.7 Here we disclose improved preparations of A and B that demonstrate the functional group compatibility of a free hydroxyl during the Mn-mediated coupling, as well as tolerance of imino compounds that are prone to E1cb elimination. We further disclose a high-yielding sequence leading from A to tubuvaline, and the assembly of a C-terminal alcohol analog of the tubulysin tetrapeptide.
2. Results and Discussion
Preparation of γ-Amino Acids
Improvements to our previously reported γ-amino acid syntheses began with an effort to address a low yield (56%) in the Mn-mediated coupling of phenylacetaldehyde hydrazone 4 (Scheme 2) with iodide 5a, bearing a silyl-protected alcohol, en route to tubuphenylalanine.7 To this end, we examined alternate iodide partners to find that iodide 5b,42 bearing a free hydroxyl group, functioned more efficiently in the Mn-mediated radical addition. The reaction entailed photolysis (300 nm) of 4 and 5b with Mn2(CO)10 in the presence of InCl3 as a Lewis acid, and furnished 1,4-hydrazino alcohol 6b in 79% yield, a significant improvement over the previously reported coupling. The identical stereochemical outcomes in 6a and 6b were correlated via O-silylation of 6b in 98% yield, which established that the free hydroxyl group was not detrimental to stereoselectivity. Then, N–N bond cleavage afforded Tup precursor 7 with the C-terminus in the alcohol oxidation state.
Scheme 2.
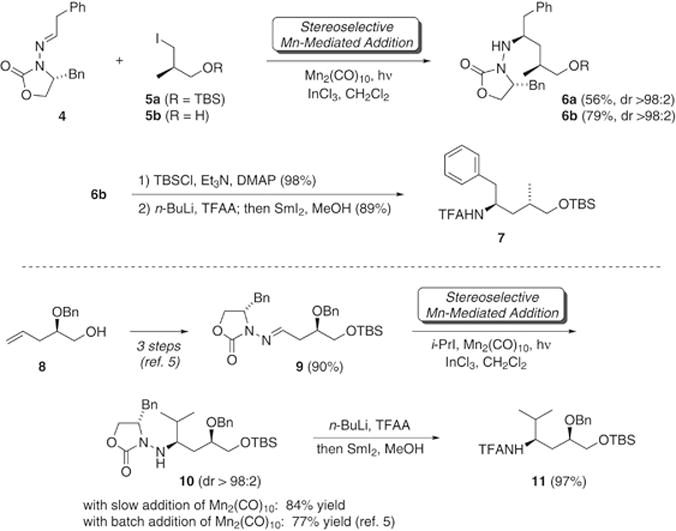
Mn-mediated radical additions in γ-amino acid synthesis.
Preparation of the γ-amino acid progenitor of tubuvaline followed the previously published route7 from known alcohol 843 (Scheme 2). Jin’s one-step Lemieux-Johnson oxidation44 shortened this sequence by one step, but did not improve the overall yield. In the key step, the Mn-mediated coupling method was carried out with slow addition (10 h) of a solution of Mn2(CO)10 in CH2Cl2 during the photolysis (254 nm, pyrex glassware). It should be noted that typical Mn-mediated radical addition reactions do not require 254 nm light nor slow addition for successful reaction, but in this case at least, it led to a small improvement in yield. Thus isopropyl iodide added to the C=N bond of hydrazone 9, affording 10 in 84% isolated yield as a single diastereomer (versus 77% reported previously7). Treatment of the hydrazone 9 with fluoride ion (TBAF) resulted in loss of the benzyloxy substituent, but this E1cb process did not occur during the radical addition reaction, underscoring its non-basic character. In another improvement upon our previously reported route, modified conditions for N–N bond cleavage45 furnished trifluoroacetamide 11 in 97% yield.
Elaboration of the Tubuvaline Thiazole
The thiazole portion of tubuvaline was envisioned to arise from serine via cyclization and oxidation. Desilylation and oxidation of 11 provided the γ-amino acid A (Scheme 3), to which serine methyl ester was attached in a peptide bond construction mediated by diethyl cyanophosphate (DECP). Silylation then afforded 12 in quantitative yield over the two steps. Alternatively, the serine could be installed with the silyl group already present, albeit with diminished coupling yield (83%). Next, conversion of the peptide bond to a thioamide was achieved with freshly prepared Belleau’s reagent46 added to a refluxing solution of 12 in THF; this afforded the thioamide 13 in dependably high yield. Desilylation of the serine hydroxyl group, then successive cyclization with diethylaminosulfur trifluoride (DAST)47 and mild oxidation from thiazoline to thiazole with BrCCl3 under Williams’ conditions48 completed the preparation of tubuvaline derivative 14 in excellent overall yield.
Scheme 3.
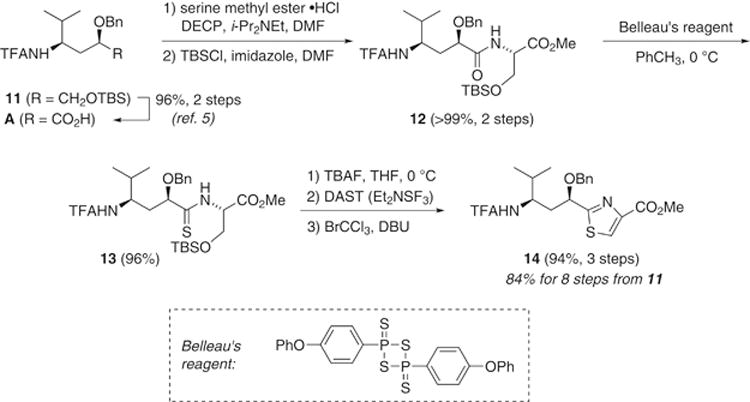
Installation of the Tuv thiazole.
Assembly of the Peptide Backbone
Next, assembly of the peptide was initiated via saponification of tubuvaline methyl ester 14 with LiOH in aqueous MeOH (Scheme 4). This proceeded selectively in 98% yield, preserving the N-trifluoroacetyl protection in 15. Meanwhile, Ba(OH)2 caused hydrolysis of the N-trifluoroacetamide of tubuphenylalaninol derivative 7, releasing free amine 16. The Tuv and Tup components then engaged in peptide coupling in the presence of DECP and Hunig’s base, leading to dipeptide 17 in quantitative yield. Another hydrolytic deprotection with Ba(OH)2 exposed the primary amine at the N-terminus, where the final two amino acids, N-methylpipecolic acid (Mep) and isoleucine (Ile) would next be attached.
Scheme 4.
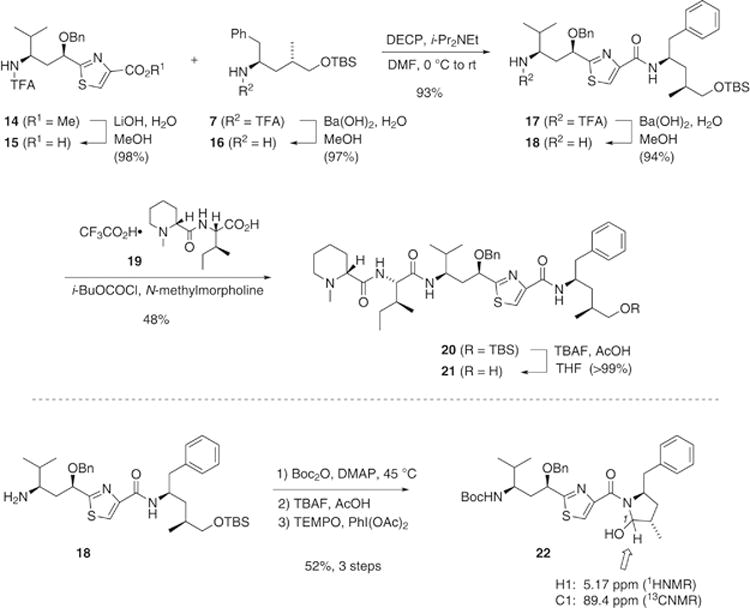
Assembly of di- and tetrapeptides.
We explored several alternatives for coupling the Mep and Ile units to the N-terminus of 18. One attractive approach was to introduce Ile as an azide, followed by hydrogenation of the azide with concomitant removal of the O-benzyl group in the Tuv fragment, with in situ coupling to an active ester form of Mep.8 This showed some promise, as the first coupling occurred smoothly (Equation 1). Unfortunately the azide reduction and coupling of 23 to Mep gave complex mixtures, due in part to incomplete removal of the O-benzyl group.
Coupling of 18 to a previously constructed Mep-Ile dipeptide9 emerged as a more reliable approach. The known dipeptide Mep-IleOH was prepared as its trifluoroacetate salt (19, Scheme 4) via acylation of isoleucine tert-butyl ester with MepOH49 and removal of the tert-butyl group with trifluoroacetic acid. An ethyl acetate solution of this salt was exposed to isobutyl chloroformate in the presence of N-methylpyrrolidine to form the mixed anhydride, and the Tuv-Tup dipeptide 18 was introduced. In several trials using this procedure, the tetrapeptide 20 was reliably obtained with yields ranging from 42–48%. Desilylation under typical conditions afforded primary alcohol 21 in quantitative yield.
To convert 21 to tubulysin V, all that remained was oxidation of the C-terminus to a carboxylic acid and debenzylation of the C5′ hydroxyl group. Toward this end, a number of oxidations were attempted, but useful oxidation products were not observed (neither aldehyde nor carboxylic acid). From examination of product mixtures in oxidations with PhI(OAc)2 and TEMPO, mass spectra and 1H NMR data suggested that the aldehyde was formed, then trapped intramolecularly by the γ-amido group. Although similar oxidations of related compounds have been reported,50,51 our attempts to purify a component of this material or make use of it for further transformations were unsuccessful. Toward a better understanding of the outcome, the simpler Tuv-Tup dipeptide 18 was used for a control experiment. After N-acylation with Boc2O and desilylation, oxidation under the PhI(OAc)2/TEMPO conditions gave cyclized hemiaminal 22 in 52% overall yield (Scheme 4). Although we were forced to consider an alternate path to access the tubulysins, this study placed the foundation for a modified route which is currently in progress.
3. Conclusion
In conclusion, a C-terminal alcohol analog in the tubulysin family has been assembled via a route which constructs both of the γ-amino acids with complete stereoselectivity (>98:2). The γ-amino acid synthesis expands the access to building blocks for bioorganic chemistry, including unusual peptide natural products52–58 and conformationally constrained peptide mimics.59,60 The key enabling methodology is a highly stereoselective Mn-mediated coupling of alkyl iodides with chiral N-acylhydrazones. Advances in this Mn-mediated radical addition chemistry attributed to this synthetic effort are (a) primary alkyl addition in the presence of unprotected hydroxyl functionality to access tubuphenylalanine in high yield, (b) avoidance of E1cb-type elimination of β-alkoxy functionality in a tubuvaline precursor by employing non-basic isopropyl radical addition to the C=N bond, and (c) an alternative procedure involving slow addition of Mn2(CO)10 to improve the yield of 10. Taken together with our previous Mn-mediated coupling studies,30,31,36–41 these advances broaden the scope and functional group compatibility of Mn-mediated radical additions to hydrazones, cementing the viability of this reaction for complex target-directed synthesis.
4. Experimental Section
Procedures and product characterization data for the Mn-mediated coupling steps are provided here; for complete experimental details, see Supporting Information.
Mn-Mediated Radical Addition: Hydrazino alcohol 6b
A solution of hydrazone 4 (360 mg, 1.22 mmol) in CH2Cl2 (30 mL) was added to InCl3 (541 mg, 2.45 mmol, dried under vacuum for ca. 12 h) in a pyrex Schlenk tube. The mixture was stirred for 2 h at room temperature. Then iodide 5b (degassed by bubbling argon for 15 min, 856 mg, 4.28 mmol) was added followed by addition of Mn2(CO)10 (524 mg, 1.35 mmol) as a solid. The reaction mixture was degassed by bubbling argon for 15 min, then irradiated for 15 h using a Rayonet photochemical reactor (300 nm, pyrex glassware); the ambient temperature inside the irradiation chamber reached ca 35 °C. The reaction mixture was diluted with diethyl ether, then triethylamine (2.0 mL, 16 mmol) was added. After stirring 1 h, concentration and flash chromatography (petroleum ether/ethyl acetate, 3:1 → 1:2) afforded hydrazino alcohol 6b (357 mg, 79% yield, dr >98:2) as a colorless oil; [α]D24 −26.6; IR νmax (NaCl, film) 3441, 2925, 1754, 1745, 1730, 1494, 1452, 1401, 1239, 1093, 1030 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.42–7.16 (m, 8H), 7.08–7.03 (m, 2H), 3.96–3.90 (m, 2H), 3.69–3.58 (m, 1H), 3.56–3.48 (m, 3H), 3.03 (dd, J = 13.4, 3.4 Hz, 1H), 2.80 (m, apparent d, J = 6.8 Hz, 2H), 2.45 (dd, J = 13.3, 10.1 Hz, 1H), 1.99–1.88 (m apparent octet, 1H), 1.58 (ddd, J = 14.3, 7.2, 5.5 Hz, 1H), 1.43 (ddd, J = 14.2, 6.5, 6.5 Hz, 1H), 0.93 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 158.5, 139.0, 135.6, 129.1, 129.1, 128.8, 128.5, 127.0, 126.4, 68.0, 65.7, 58.8, 58.5, 40.2, 37.3, 36.7, 32.5, 17.6; MS (ESI) m/e (rel. intensity) 391 ([M+Na]+, 100), 369 ([M + H]+, 57); Anal. Calcd for C22H28N2O3: C, 71.71; H, 7.66; N, 7.60. Found: C, 71.06; H, 7.47; N, 7.48.
Mn-Mediated Radical Addition with Slow Addition: Hydrazine 10
To a solution of hydrazone 9 (300 mg, 0.62 mmol) in CH2Cl2 (31 mL) was added InCl3 (275 mg, 1.24 mmol dried under vacuum for ca. 12 h), followed by isopropyl iodide (0.25 mL, 2.49 mmol, filtered through basic alumina). The mixture was stirred for 15 min at room temperature. Using a syringe pump, a solution of Mn2(CO)10 in CH2Cl2 (10 mL) was added over 10 h at a rate of 1 mL/h while the mixture was irradiated using a Rayonet photochemical reactor (254 nm, pyrex glassware). After the addition was complete, irradiation was continued for another 5 h; the ambient temperature inside the irradiation chamber reached ca 35 °C. Concentration and flash chromatography (hexanes → 3:1 hexanes/EtOAc) afforded hydrazine 10 as a colorless oil (276 mg, 84% yield, dr >98:2). Analytical data for this material were consistent with the prior report.7
Supplementary Material
Acknowledgments
We thank NIH (R01-GM67187) and NSF (CHE-0749850 and CHE-1362111) for generously supporting this work.
References
- 1.Sasse F, Steinmetz H, Heil J, Höfle G, Reichenbach H. Tubulysins, new cytostatic peptides from myxobacteria acting on microtubuli - Production, isolation, physico-chemical and biological properties. J Antibiot. 2000;53:879–885. doi: 10.7164/antibiotics.53.879. [DOI] [PubMed] [Google Scholar]
- 2.Höfle G, Glaser N, Leibold T, Karama U, Sasse F, Steinmetz H. Semisynthesis and degradation of the tubulin inhibitors epothilone and tubulysin. Pure Appl Chem. 2003;75:167–178. [Google Scholar]
- 3.Steinmetz H, Glaser N, Herdtweck E, Sasse F, Reichenbach H, Höfle G. Isolation, crystal and solution structure determination, and biosynthesis of tubulysins - Powerful inhibitors of tubulin polymerization from myxobacteria. Angew Chem Int Ed. 2004;43:4888–4892. doi: 10.1002/anie.200460147. [DOI] [PubMed] [Google Scholar]
- 4.Dömling A, Beck B, Eichelberger U, Sakamuri S, Menon S, Chen QZ, Lu YC, Wessjohann LA. Total synthesis of tubulysin U and V. Angew Chem, Int Ed. 2006;45:7235–7239. doi: 10.1002/anie.200601259. Corrigendum: ibid., 46, 2347–2348 (2007) [DOI] [PubMed] [Google Scholar]
- 5.Ullrich A, Chai Y, Pistorius D, Elnakady YA, Herrmann JE, Weissman KJ, Kazmaier U, Muller R. Pretubulysin, a Potent and Chemically Accessible Tubulysin Precursor from Angiococcus disciformis. Angew Chem, Int Ed. 2009;48:4422–4425. doi: 10.1002/anie.200900406. [DOI] [PubMed] [Google Scholar]
- 6.Khalil MW, Sasse F, Lünsdorf H, Elnakady YA, Reichenbach H. Mechanism of action of tubulysin, an antimitotic peptide from myxobacteria. ChemBioChem. 2006;7:678–683. doi: 10.1002/cbic.200500421. [DOI] [PubMed] [Google Scholar]
- 7.Friestad GK, Deveau AM, Marié J-C. Stereoselective Mn-mediated coupling of functionalized iodides and hydrazones: A synthetic entry to the tubulysin gamma-amino acids. Org Lett. 2004;6:3249–3252. doi: 10.1021/ol048986v. [DOI] [PubMed] [Google Scholar]
- 8.Peltier HM, McMahon JP, Patterson AW, Ellman JA. The total synthesis of tubulysin D. J Am Chem Soc. 2006;128:16018–16019. doi: 10.1021/ja067177z. [DOI] [PubMed] [Google Scholar]
- 9.Sani M, Fossati G, Huguenot F, Zanda M. Total synthesis of tubulysins U and V. Angew Chem, Int Ed. 2007;46:3526–3529. doi: 10.1002/anie.200604557. [DOI] [PubMed] [Google Scholar]
- 10.Neri D, Fossati G, Zanda M. Efforts toward the total synthesis of tubulysins: New hopes for a more effective targeted drug delivery to tumors. ChemMedChem. 2006;1:175–180. doi: 10.1002/cmdc.200500043. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Friestad GK, Yao L. Recent advances in the synthesis of tubulysins. Mini-Rev Med Chem. 2013;13:1572–1578. doi: 10.2174/13895575113139990063. [DOI] [PubMed] [Google Scholar]
- 12.Murray BC, Peterson MT, Fecik RA. Chemistry and biology of tubulysins: antimitotic tetrapeptides with activity against drug resistant cancers. Nat Prod Rep. 2015;32:654–662. doi: 10.1039/c4np00036f. [DOI] [PubMed] [Google Scholar]
- 13.Park Y, Lee JK, Ryu J-S. Synthesis of a cyclic analogue of Tuv N-methyl tubulysin. Synlett. 2015;26:1063–1068. [Google Scholar]
- 14.Hoffmann J, Gorges J, Junk L, Kazmaier U. Synthesis of pretubulysin-derivatives via the TubUgi-approach. Org Biomol Chem. 2015;13:6010–6020. doi: 10.1039/c5ob00587f. [DOI] [PubMed] [Google Scholar]
- 15.Paladhi S, Das J, Samanta M, Dash J. Asymmetric aldol reaction of thiazole-carbaldehydes: regio- and stereoselective synthesis of tubuvalin analogues. Adv Synth Cat. 2014;356:3370–3376. [Google Scholar]
- 16.Ordonez M, Cativiela C. Stereoselective synthesis of gamma-amino acids. Tetrahedron: Asymmetry. 2007;18:3–99. doi: 10.1016/j.tetasy.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trabocchi A, Guarna F, Guarna A. gamma- and delta-amino acids: Synthetic strategies and relevant applications. Curr Org Chem. 2005;9:1127–1153. [Google Scholar]
- 18.Friestad GK, Qin J. Highly stereoselective intermolecular radical addition to aldehyde hydrazones from a chiral 3-Amino-2-oxazolidinone. J Am Chem Soc. 2000;123:8329–8330. [Google Scholar]
- 19.Friestad GK, Draghici C, Soukri M, Qin J. Radical addition approach to asymmetric amine synthesis: Design, implementation, and comparison of new chiral N-acylhydrazones. J Org Chem. 2005;70:6330–6338. doi: 10.1021/jo050756m. [DOI] [PubMed] [Google Scholar]
- 20.Friestad GK. Addition of carbon-centered radicals to imines and related compounds. Tetrahedron. 2001;57:5461–5496. [Google Scholar]
- 21.Yamada K, Tomioka K. Copper-catalyzed asymmetric alkylation of imines with dialkylzinc and related reactions. Chem Rev. 2008;108:2874–2886. doi: 10.1021/cr078370u. [DOI] [PubMed] [Google Scholar]
- 22.Miyabe H, Yoshioka E, Kohtani S. Progress in intermolecular carbon radical addition to imine derivatives. Curr Org Chem. 2010;14:1254–1264. [Google Scholar]
- 23.Miyabe H. Inter- and Intramolecular Carbon-carbon bond-forming radical reactions. Synlett. 2012;23:1709–1724. [Google Scholar]
- 24.Friestad GK. Asymmetric radical addition to chiral hydrazones. In: Gansauer A, Heinrich M, editors. Topics In Current Chemistry: Radicals in Synthesis III. Vol. 320. Springer-Verlag; Berlin: 2012. pp. 61–92. [DOI] [PubMed] [Google Scholar]
- 25.Tauber J, Imbri D, Opatz T. Radical addition to iminium ions and cationic heterocycles. Molecules. 2014;19:16190–16222. doi: 10.3390/molecules191016190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friestad GK. Control of asymmetry in the radical addition approach to chiral amine synthesis. In: Li Wei, Zhang Xumu., editors. Topics In Current Chemistry: Stereoselective Formation of Amines. Vol. 343. Springer-Verlag; Berlin: 2014. pp. 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujii S, Konishi T, Matsumoto Y, Yamaoka Y, Takasu K, Yamada K. Radical aminomethylation of imines. J Org Chem. 2014;79:8128–8133. doi: 10.1021/jo501332j. [DOI] [PubMed] [Google Scholar]
- 28.Vo C-VT, Luescher MU, Bode JW. SnAP reagents for the one-step synthesis of medium-ring saturated N-heterocycles from aldehydes. Nature Chemistry. 2014;6:310–314. doi: 10.1038/nchem.1878. [DOI] [PubMed] [Google Scholar]
- 29.Rono LJ, Yayla HG, Wang DY, Armstrong MF, Knowles RR. Enantioselective photoredox catalysis enabled by Proton-coupled electron transfer: development of an asymmetric aza-pinacol cyclization. J Am Chem Soc. 2013;135:17735–17738. doi: 10.1021/ja4100595. [DOI] [PubMed] [Google Scholar]
- 30.Friestad GK, Qin J. Intermolecular alkyl radical addition to chiral N-acylhydrazones mediated by manganese carbonyl. J Am Chem Soc. 2001;123:9922–9923. doi: 10.1021/ja011312k. [DOI] [PubMed] [Google Scholar]
- 31.Friestad GK, Qin J, Suh Y, Marié J-C. Mn-mediated coupling of alkyl iodides and chiral N-acylhydrazones: optimization, scope, and evidence for a radical mechanism. J Org Chem. 2006;71:7016–7027. doi: 10.1021/jo061158q. [DOI] [PubMed] [Google Scholar]
- 32.Review:; Friestad GK. Chiral N-acylhydrazones: versatile imino acceptors for asymmetric amine synthesis. Eur J Org Chem. 2005:3157–3172. doi: 10.1021/jo050756m. [DOI] [PubMed] [Google Scholar]
- 33.Shen Y, Friestad GK. Comparison of electrophilic amination reagents for N-amination of 2-oxazolidinones and application to synthesis of chiral hydrazones. J Org Chem. 2002;67:6236–6239. doi: 10.1021/jo0259663. [DOI] [PubMed] [Google Scholar]
- 34.Friestad GK, Korapala CS, Ding H. Dual activation in asymmetric allylsilane addition to chiral N-acylhydrazones: method development, mechanistic studies, and elaboration of homoallylic amine adducts. J Org Chem. 2006;71:281–289. doi: 10.1021/jo052037d. [DOI] [PubMed] [Google Scholar]
- 35.Ding H, Friestad GK. Strecker reactions of chiral N-acylhydrazones. Heterocycles. 2006;70:185–199. [Google Scholar]
- 36.Korapala CS, Qin J, Friestad GK. Quinine synthesis studies: a radical–ionic annulation via Mn-mediated addition to chiral N-acylhydrazones. Org Lett. 2007;9:4246–4249. doi: 10.1021/ol7017938. [DOI] [PubMed] [Google Scholar]
- 37.Friestad GK, Ji A. Mn-mediated coupling of alkyl iodides and ketimines: a radical addition route to α,α-disubstituted α-aminoesters. Org Lett. 2008;10:2311–2313. doi: 10.1021/ol800733b. [DOI] [PubMed] [Google Scholar]
- 38.Friestad GK, Banerjee K. Synthesis of γ-amino esters via Mn-mediated radical addition to chiral γ-hydrazonoesters. Org Lett. 2009;11:1095–1098. doi: 10.1021/ol802932v. [DOI] [PubMed] [Google Scholar]
- 39.Friestad GK, Ji A, Korapala CS, Qin J. Intermolecular radical addition to N-acylhydrazones as a stereocontrol strategy for alkaloid synthesis: formal synthesis of quinine. Org Biomol Chem. 2011:4039–4043. doi: 10.1039/c1ob05219e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friestad GK, Ji A, Baltrusaitis J, Korapala CS, Qin J. Scope of stereoselective Mn-mediated radical addition to chiral hydrazones and application in a formal synthesis of quinine. J Org Chem. 2012;77:3159–3180. doi: 10.1021/jo2026349. [DOI] [PubMed] [Google Scholar]
- 41.Slater KA, Friestad GK. Mn-Mediated radical-ionic annulations of chiral N-acylhydrazones. J Org Chem. 2015;80:6432–6440. doi: 10.1021/acs.joc.5b00863. [DOI] [PubMed] [Google Scholar]
- 42.Chesis PL, Hwang DR, Welch MJ. N-(3-[18F]Fluoropropyl)-N-nordiprenorphine: synthesis and characterization of a new agent for imaging opioid receptors with positron emission tomography. J Med Chem. 1990;33:1482–1490. doi: 10.1021/jm00167a031. [DOI] [PubMed] [Google Scholar]
- 43.Crimmins MT, Emmitte KA, Katz JD. Diastereoselective alkylations of oxazolidinone glycolates: A useful extension of the Evans asymmetric alkylation. Org Lett. 2000;2:2165–2167. doi: 10.1021/ol006091m. [DOI] [PubMed] [Google Scholar]
- 44.Yu WS, Mei Y, Kang Y, Hua ZM, Jin Z. Improved procedure for the oxidative cleavage of olefins by OsO4-NaIO4. Org Lett. 2004;6:3217–3219. doi: 10.1021/ol0400342. [DOI] [PubMed] [Google Scholar]
- 45.Ding H, Friestad GK. Trifluoroacetyl-activated nitrogen-nitrogen bond cleavage of hydrazines by samarium (II) iodide. Org Lett. 2004;6:637–640. doi: 10.1021/ol036480r. [DOI] [PubMed] [Google Scholar]
- 46.Lajoie G, Lepine F, Maziak L, Belleau B. Facile regioselective formation of thiopeptide linkages from oligopeptides with new thionation reagents. Tetrahedron Lett. 1983;24:3815–3818. [Google Scholar]
- 47.Phillips AJ, Uto Y, Wipf P, Reno MJ, Williams DR. Synthesis of functionalized oxazolines and oxazoles with DAST and deoxo-fluor. Org Lett. 2000;2:1165–1168. doi: 10.1021/ol005777b. [DOI] [PubMed] [Google Scholar]
- 48.Williams DR, Lowder PD, Gu Y-G, Brooks DA. Studies of mild dehydrogenations in heterocyclic systems. Tetrahedron Lett. 1997;38:331–334. [Google Scholar]
- 49.Patrick KS, Singletary JL. Relative configuration of thioridazine enantiomers. Chirality. 1991;3:208–211. [Google Scholar]
- 50.Wipf P, Takada T, Rishel MJ. Synthesis of the tubuvaline-tubuphenylalanine (tuv-tup) fragment of tubulysin. Org Lett. 2004;6:4057–4060. doi: 10.1021/ol048252i. [DOI] [PubMed] [Google Scholar]
- 51.Chandrasekhar S, Mahipal B, Kavitha M. Toward tubulysin: gram-scale synthesis of tubuvaline-tubuphenylalanine fragment. J Org Chem. 2009;74:9531–9534. doi: 10.1021/jo9015503. [DOI] [PubMed] [Google Scholar]
- 52.Iwasaki A, Ohno O, Sumimoto S, Suda S, Suenaga K. Maedamide, a novel chymotrypsin inhibitor from a marine cyanobacterial assemblage of Lyngbya sp. Tetrahedron Lett. 2014;55:4126–4128. [Google Scholar]
- 53.Sun Y, Takada K, Nogi Y, Okada S, Matsunaga S. Lower homologues of ahpatinin, aspartic protease inhibitors, from a marine Streptomyces sp. J Nat Prod. 2014;77:1749–1752. doi: 10.1021/np500337m. [DOI] [PubMed] [Google Scholar]
- 54.Ross AC, Xu Y, Lu L, Kersten RD, Shao Z, Al-Suwailem AM, Dorrestein PC, Qian P-Y, Moore BS. Biosynthetic multitasking facilitates thalassospiramide structural diversity in marine bacteria. J Am Chem Soc. 2013;135:1155–1162. doi: 10.1021/ja3119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi H, Mevers E, Byrum T, Valeriote FA, Gerwick WH. Lyngbyabellins K-N from two Palmyra Atoll collections of the marine cyanobacterium Moorea bouillonii. Eur J Org Chem. 2012:5141–5150. doi: 10.1002/ejoc.201200691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malloy KL, Choi H, Fiorilla C, Valeriote FA, Matainaho T, Gerwick WH. Hoiamide D, a marine cyanobacteria-derived inhibitor of p53/MDM2 interaction. Bioorg Med Chem Lett. 2012;22:683–688. doi: 10.1016/j.bmcl.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nam S-J, Kauffman CA, Jensen PR, Fenical W. Isolation and characterization of actinoramides A-C, highly modified peptides from a marine Streptomyces sp. Tetrahedron. 2011;67:6707–6712. doi: 10.1016/j.tet.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Festa C, De Marino S, Sepe V, D’Auria MV, Bifulco G, Debitus C, Bucci M, Vellecco V, Zampella A. Solomonamides A and B, new anti-inflammatory peptides from Theonella swinhoei. Org Lett. 2011;13:1532–1535. doi: 10.1021/ol200221n. [DOI] [PubMed] [Google Scholar]
- 59.Bouillere F, Thetiot-Laurent S, Kouklovsky C, Alezra V. Foldamers containing gamma-amino acid residues or their analogues: structural features and applications. Amino Acids. 2011;41:687–707. doi: 10.1007/s00726-011-0893-3. [DOI] [PubMed] [Google Scholar]
- 60.Seebach D, Beck AK, Bierbaum DJ. The world of beta- and gamma-peptides comprised of homologated proteinogenic amino acids and other components. Chemistry & Biodiversity. 2004;1:1111–1239. doi: 10.1002/cbdv.200490087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


