Abstract
Nucleic acid sequence-based amplification (NASBA) is an isothermal method of RNA amplification that has been previously used in clinical diagnostic testing. A real-time NASBA assay has been developed for the detection of rbcL mRNA from the red tide dinoflagellate Karenia brevis. This assay is sensitive to one K. brevis cell and 1.0 fg of in vitro transcript, with occasional detection of lower concentrations of transcript. The assay did not detect rbcL mRNA from a wide range of nontarget organisms and environmental clones, while 10 strains (all tested) of K. brevis were detected. By the use of standard curves based on time to positivity, concentrations of K. brevis in environmental samples were predicted by NASBA and classified into different levels of blooms per the Florida Fish and Wildlife Conservation Commission (FWC) system. NASBA classification matched FWC classification (based on cell counts) 72% of the time. Those samples that did not match were off by only one class. NASBA is sensitive, rapid, and effective and may be used as an additional or alternative method to detect and quantify K. brevis in the marine environment.
By conservative estimates, harmful algal blooms (HABs) cost the United States $50 million per year (7). Such estimates are based upon direct economic impacts on tourism, fisheries, etc., and do not account for irremediable costs such as those caused by mass marine mammal mortalities (8, 9). Worldwide, algal toxins of all types may be responsible for as many as 60,000 human intoxication events per year (20).
Nearly all coastal regions of the United States are impacted by HABs for various intervals in time and intensity. Perhaps no coastal environment has a frequency of HABs equal to that of the Florida Gulf Coast, caused by the nonperidinin dinoflagellate Karenia brevis (Davis) cf. Hansen and Moestrup (= Gymnodinium breve). Although red tides have been observed in the Gulf of Mexico since the Spanish Conquests and reports of catastrophic fish mortalities go back to 1844, the identity of K. brevis, initially named G. breve, as the causative agent was not determined until the bloom of 1946 to 1947 (6). In certain years red tides have occurred during 12 months of the year, although they are most often encountered in the late summer and early fall, correlating with heavy rainfall (8).
There is a need for monitoring and prediction of HABs, and those of K. brevis are of particular concern. A myriad of approaches have been taken to this problem, including satellite ocean color sensing (17), photopigment analysis (12, 13, 14), and toxin analysis (16). Additionally, molecular methods are being developed to detect a variety of HAB species, including Alexandrium sp. (1, 4), Gymnodinium sp. (4, 15), Pseudonitzschia sp. (15), Pfiesteria sp., and Pfiesteria-like organisms (10) as well as K. brevis (5, 11). All of these methods must be calibrated with microscopy-derived cell counts, and yet cell counts are also prone to errors (2).
Using nucleic acid sequence-based amplification (NASBA), we have developed a novel molecular assay to detect and quantify K. brevis organisms via the ribulose-1,5-bisphosphate carboxylase-oxygenase (RuBisCO) large-subunit gene (rbcL). The rbcL mRNA was selected as our target because cellular levels of mRNA are typically high and RNA degrades quickly in the environment, resulting in detection of viable K. brevis populations only. NASBA is an isothermal method for RNA amplification that occurs at 41°C (3). RNA is amplified by use of an enzyme cocktail consisting of T7 RNA polymerase, avian myeloblastosis virus reverse transcriptase, and RNaseH and two target-specific oligonucleotide primers.
Real-time detection of the amplicon is accomplished by use of a molecular beacon, a single-stranded oligonucleotide that forms a stem-loop structure (19). The molecular beacon is labeled with 6-carboxy fluorescein (6-FAM) at its 5′ end and quencher DABCYL at its 3′ end. When the beacon is in the closed (hairpin loop) configuration the fluorophore is quenched. Upon binding to the amplicon, the quencher is separated from the fluorophore and the probe fluoresces. During the amplification reactions, the fluorescent signal is measured. The time at which the signal reaches exponential growth is defined as the time to positivity (TTP), which is analogous to the threshold cycle value in PCR. The TTP value is a function of how much initial target RNA is in the sample. We have used this strategy to successfully detect and quantify K. brevis in cultures and field samples collected from the coastal waters of southwest Florida.
MATERIALS AND METHODS
Primer and beacon design.
Sequence information for the rbcL genes from K. brevis and K. mikimotoi was obtained from GenBank and from prior sequencing efforts in our lab (5). Sequences were aligned using the ClustalW algorithm (18) and Kodon version 1.0 software (Applied Maths Inc., Austin, Tex.). Primers were designed manually to target an 87-bp region internal to the K. brevis rbcL gene that was distinct from that of K. mikimotoi (Table 1). Primers were checked for self-annealing by the use of an Oligo tool kit. The molecular beacon was designed internal to the two primers. The hairpin folding of the beacon was checked using Mfold software (http://bioweb.pasteur.fr/seqanal/interfaces/mfold-simple.html), and the free energy of the hairpin structure was determined to be between −2.5 and −3.5 kcal/mol.
TABLE 1.
K. brevis NASBA primer set and beacon
| Primer or beacon | Sequence |
|---|---|
| Forward primer | ACGTTATTGGGTCTGTGTA |
| Reverse primer | AATTCTAATACGACTCACTATAGGGAGAAGGTACACACTTTCGTAAACTA |
| Molecular beacon | [6-FAM]CGATCGCTTAGTCTCGGGTTATTTTTTCGATCG-[DABCYL] |
Sensitivity and specificity.
Cultured K. brevis cells (Piney Island B4 isolate) obtained from the FWC Florida Marine Research Institute (FMRI; St. Petersburg, Fla.) were used to determine the sensitivity of the assay. Cells were concentrated by filtration onto 0.22-μm-pore-size black polycarbonate filters (Osmonics, Inc., Minnetonka, Minn.) and counted by epifluorescence microscopy using an Olympus BX-60 microscope and blue excitation (filter set U-MNIB) with ×200 magnification. Once a concentration was determined, the culture was diluted appropriately to result in a specific number of cells per reaction. To determine whether environmental stressors or conditions might elicit various levels of rbcL RNA/cell, cultures were exposed to low-salinity (25 ppt), low-nutrient (50 and 75% less than normal concentrations), low-light (3 μmol s−1 m−2), and high-light (80 μmol s−1 m−2) treatments. Cells were exposed to the low-light and low-nutrient treatments for 72 h prior to RNA extraction. Since 24 h of exposure to low-salinity and high-light treatments resulted in culture death, exposure times for these treatments were reduced to 4 h. Following exposure, cellular RNA from each treatment was extracted using an RNeasy Mini kit (QIAGEN, Valencia, Calif.) and tested against known amounts of in vitro transcript to determine whether there were differences in the amounts of RNA/cell for the stress treatments.
To determine the specificity of the assay, the primers and probe were tested on 10 different K. brevis strains isolated from locations along the Florida coast as well as on a variety of nontarget organisms (including K. mikimotoi) and environmental clones. All K. brevis strains and several of the nontarget organisms were obtained from FMRI. Additional nontarget organisms were obtained from the Provasoli-Guillard National Center for Culture of Marine Phytoplankton (West Boothbay Harbor, Maine). All cultures were incubated using a 12 h of light-12 h of dark cycle at 26 μmol s−1 m−2 in appropriate medium and temperature conditions according to Provasoli-Guillard National Center for Culture of Marine Phytoplankton guidelines. Nontarget environmental rbcL clones were obtained from the Gulf of Mexico as part of a previous study by B. Wawrik (University of South Florida, St. Petersburg, Fla.).
K. brevis and nontarget environmental clones were constructed as described by Gray et al. (5). The 554-bp K. brevis rbcL insert was sequenced at the DNA Sequencing Core laboratory at the University of Florida. One of the clones carrying the K. brevis insert was used to make transcript by in vitro transcription with a Riboprobe combination system-SP6/T7 RNA polymerase kit (Promega Corp., Madison, Wis.), according to the manufacturer's instructions. Transcript was also made from the nontarget environmental clones. Following transcription, the reaction mixtures were purified with an RNeasy Mini Kit and quantified with a Ribogreen RNA quantification kit (Molecular Probes, Eugene, Oreg.). Transcripts were then diluted 1:1 in RNA storage buffer (8 M guanidinium isothiocyanate, 80 mM Tris-HCl [pH 8.5], 24 mM MgCl2, 140 mM KCl), aliquoted, and stored at −80°C.
To determine whether competitive inhibition might occur from closely related strains, NASBA reaction mixtures containing 100 fg of K. brevis rbcL mRNA and 1,000 fg of K. mikimotoi rbcL mRNA were set up as described above.
Sequence information for rbcL of clones and nontarget organisms was obtained from GenBank or from in-house sequencing efforts. Phylogenetic information on these clones appears in a report by Gray et al. (5).
Environmental samples.
Environmental K. brevis samples were collected by several agencies from sites along coastal Florida as part of a regular monitoring program, and K. brevis cells were enumerated by FMRI staff. Samples were typically obtained from FMRI and processed for NASBA approximately 1 day after collection. RNA from both environmental and cultured cell samples was extracted via either an RNeasy Mini kit from QIAGEN or an Absolutely RNA Microprep kit from Stratagene (La Jolla, Calif.). Cells were filtered onto sterile Millipore Durapore 0.45-μm-pore-size filters, and the filters were incubated in the supplied lysis buffer for 10 min at room temperature. After incubation the buffer was recovered and the appropriate extraction protocol was followed. Early experiments generated standard curves from one RNA extract that was diluted to appropriate concentrations. Later standard curves, however, were generated from individual extractions of RNA for each concentration.
NASBA assay.
NASBA was performed using a Nuclisens basic kit (bioMérieux, Durham, N.C.) and an ABI 7700 sequence detection system (Applied Biosystems, Foster City, Calif.). Samples in each NASBA run included cell standards, environmental samples, and a negative control. Cell standards typically spanned 4 orders of magnitude and were run in duplicate or triplicate reactions at each concentration, while all environmental samples were run in triplicate. Standard curves were created using TTP, the time at which a significant increase in fluorescence occurred. Each sample was run in a 10-μl NASBA reaction mixture (half the volume recommended by the bioMérieux protocol), consisting of 5 μl of NASBA reagent-primer mix, 2.5 μl of RNA template, and 2.5 μl of enzyme. Primers and beacon were obtained from QIAGEN and were diluted to final concentrations per NASBA reaction mixture of 400 nM for primers and 100 nM for the beacon. The KCl concentration was 80 mM in each reaction mixture, and 6-carboxy-X-rhodamine (6-ROX) was diluted to 0.5 μM in each reaction mixture.
RESULTS
Sensitivity and specificity.
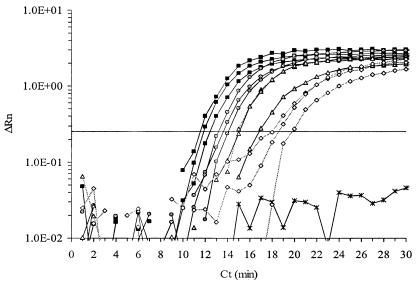
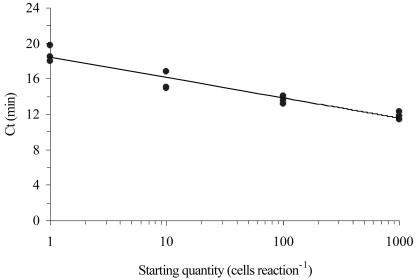
All K. brevis strains were successfully amplified and were detected by an ABI 7700 sequence detection system, while none of the nontarget organisms or environmental clones were detected (Table 2). The assay was sensitive to one K. brevis cell, consistently detected 1.0 fg of in vitro transcript, and intermittently detected 0.1 fg of transcript (data not shown). Amplification and detection occurred rapidly: 1,000 cells were detected in approximately 11 min, and 1 cell was routinely detected in approximately 17 min (Fig. 1). There was a negative logarithmic relationship between initial cell number and TTP over at least 4 orders of magnitude, from 1 cell to 1,000 cells (Fig. 2). There was no inhibition of NASBA when a closely related competitor rbcL mRNA (from K. mikimotoi) was added at 10-fold excess to the target K. brevis rbcL mRNA (data not shown).
TABLE 2.
Specificity of K. brevis NASBA amplification assay
| Species and category | Isolate | Detection resulta |
|---|---|---|
| K. brevis | Apalachicola B5 | + |
| K. brevis | Apalachicola C6 | + |
| K. brevis | Charlotte Harbor A3 | + |
| K. brevis | Charlotte Harbor C2 | + |
| K. brevis | Mexico Beach B3 | + |
| K. brevis | Mexico Beach A5 | + |
| K. brevis | Jacksonville C3 | + |
| K. brevis | Piney Island A3 | + |
| K. brevis | Piney Island B4 | + |
| K. brevis | Wilson | + |
| Negative controls | ||
| Dinoflagellates | ||
| K. mikimotoi | CCMP430 | − |
| Amphidinium carterae | CCMP1314 | − |
| Akashiwo sanguinea | CCMP1321 | − |
| Alexandrium tamarense | CCMP1493 | − |
| Glenodinium foliacrum | NAb | − |
| Gymnodinium catenatum | CCMP1937 | − |
| Gyrodinium sp. | NA | − |
| Kryptoperidinium folicrum | NA | − |
| Lingulodinium polyedra | CCMP1738 | − |
| Prorocentrum micans | NA | − |
| Scrippsiella trochoidea | NA | − |
| Scrippsiella precaria | NA | − |
| Diatoms | ||
| Phaeodactylum tricornutum | CCMP1327 | − |
| Cylindrotheca sp. | ST6CH2 clone | − |
| Skeletonema sp. | ST4CH31 clone | − |
| Skeletonema sp. | ST4CH14 clone | − |
| Raphidophyte | ||
| Heterosigma akashiwo | NA | − |
| Praisinophytes | ||
| Tetraselmis sp. | 850001 | − |
| Tetraselmis sp. | CCMP961 | − |
| Unidentified sp. | CCMP1536 | − |
| Prymnesiophytes | ||
| Isochrysis sp. | 3C | − |
| Pavlova lutheri | CCMP1325 | − |
| Prymnesium parvum | NA | − |
| Unidentified sp. | ST8CH26 clone | − |
| Unidentified sp. | ST1CH3 clone | − |
| Chlorophytes | ||
| Chlamydomonas euryale | CCMP219 | − |
| Unidentified sp. | ST5SY7 clone | − |
| Chlamydomonas sp. | ST2SY2 clone | − |
| Pycnococcus sp. | ST6SY8 clone | − |
| Cyanophytes | ||
| Synechococcus sp. | CCMP836 | − |
| Synechococcus sp. | WH7803 | − |
| Synechococcus sp. | ST2SY26 clone | − |
| Prochlorococcus sp. | ST2SY33 clone | − |
| Trichodesmium sp. | ST8SY15 clone | − |
| Trebouxiophyte | ||
| Chlorella autotrophica | CCMP243 | − |
| Coscinodicsophyte | ||
| Thalassiosira pseudonana | CCMP 1335 | − |
| Eustigmatophyte | ||
| Nannochloropsis sp. | ST3CH27 clone | − |
| Nannochloropsis sp. | ST1CH4 clone | − |
| Xanthophyte | ||
| Heterococcus sp. | ST6CH33 clone | − |
+, detected; −, not detected.
NA, organisms obtained from FMRI.
FIG. 1.
Typical amplification plot of K. brevis cell standards (squares, 1,000 cells; circles, 100 cells; triangles, 10 cells; diamonds, 1 cell; ×, no cells). Samples were run in triplicate for each concentration. The horizontal line on the figure indicates the threshold fluorescence used to calculate the TTP value. ΔRn, relative fluorescence.
FIG. 2.
Typical standard curve generated from K. brevis cells (r2 = 0.92). Points at each concentration represent a single RNA extraction, run in triplicate NASBA reactions. Ct, threshold cycle.
For the stress treatments, all conditions resulted in equivalent levels of cellular rbcL RNA except the high-light treatment, which resulted in significantly higher rbcL levels (14 fg/cell compared to an average of 2.6 fg/cell, or a 5.4-fold increase) (Table 3).
TABLE 3.
Effect of culture stress on cellular rbcL transcript levels
| Treatment | Cellular rbcL transcript levels +/− SD (fg/cell) |
|---|---|
| None (control) | 2.8 ± 2.6 |
| Low nutrient (1:2 dilution) | 2.7 ± 1.3 |
| Low nutrient (1:4 dilution) | 2.5 ± 1.2 |
| Low salinity | 3.2 ± 3.0 |
| Low light | 1.7 ± 0.7 |
| High light | 14 ± 7.8a |
Significantly different (P = 0.0106).
Environmental samples.
Cultures of K. brevis were used to construct cell standard curves for each experiment. Studies with these cultures indicated that cellular levels of rbcL mRNA were relatively constant over a diel period (M. Gray, unpublished data). The resulting regression lines were used to calculate concentrations of cells in 18 different environmental samples. The Florida Fish and Wildlife Conservation Commission (FWC) employs the following eight classes for bloom levels: none detected, present (<1,001 cells liter−1), very low (a) (1,001 to <5,000 cells liter−1), very low (b) (5,000 to <10,000 cells liter−1), low (a) (10,000 to <50,000 cells liter−1), low (b) (50,000 to <100,000 cells liter−1), medium (<100,000 to 1,000,000 cells liter−1), and high (≥1,000,000 cells liter−1)(http://www.floridamarine.org/features/view_article.asp?id=9670). We have modified this classification system by combining the classes none detected and present into normal, very low (a) and (b) into very low, and low (a) and (b) into low to form five classes as shown in Table 4. Of the 18 environmental samples, 8 were classified by FWC as at least low-level blooms, indicating concentrations that were above 1,000 cells liter−1. On the basis of FWC cell counts and using our modified classification system, two of the eight bloom samples were considered very low, four were classified as low, and two were medium-level blooms. In the remaining 10 samples no K. brevis cells were detected by FWC (the minimum detection limit for FWC is 333 cells liter−1). NASBA matched FWC classification in 8 of the 10 nonbloom samples, in 0 of the 2 very low samples, in 3 of the 4 low samples, and in both medium samples. Overall, NASBA classification matched FWC classification 72% of the time (Table 4). Those samples that did not match differed by, at most, one class. The cell concentrations in the environmental samples calculated by NASBA are compared to FWC counts in Table 4.
TABLE 4.
Comparison of FWC and NASBA bloom sample cell counts
| Site | Date of collection (mo/day/yr) | Cell count (cells/liter)a | NASBA (cells/liter)a | NASBA SE |
|---|---|---|---|---|
| Barefoot Beach | 3/26/2003 | 333,000 (M) | 418,222 (M) | 47,904 |
| Clam Pass | 12,300 (L) | 15,543 (L) | 8,376 | |
| Naples Pier | 0 (N) | 514 (N) | 269 | |
| Naples Pier | 4/2/2003 | 190,000 (M) | 107,406 (M) | 36,286 |
| Launch Ramp | 0 (N) | 843 (N) | 27 | |
| Marco Pass | 0 (N) | 85,208 (L) | 43,259 | |
| Goodland | 0 (N) | 25,604 (L) | 6585 | |
| Levy Barefoot Beach | 10/16/2003 | 4,670 (VL) | 283 (N) | 21 |
| Naples Pier | 0 (N) | 15 (N) | 15 | |
| Seagate | 27,300 (L) | 3,115 (VL) | 1,977 | |
| S. Marco Beach | 18,300 (L) | 47,058 (L) | 17,215 | |
| Vanderbilt Beach | 19,000 (L) | 50,692 (L) | 22,749 | |
| Seagate | 10/27/2003 | 667 (N) | 445 (N) | 377 |
| Naples Pier | 3,670 (VL) | 0 (N) | 0 | |
| Levy Barefoot Beach | 11/11/2003 | 0 (N) | 4 (N) | NAb |
| Naples Pier | 0 (N) | 363 (N) | NA | |
| Seagate | 0 (N) | 45 (N) | NA | |
| Vanderbilt | 0 (N) | 79 (N) | NA |
Letters in parentheses next to cell numbers refer to the modified FWC bloom classification system employed. N, normal concentrations (0 to 1,000 cells liter−1); VL, very low (>1,000 to <10,000 cells liter−1); L, low (10,000 to <100,000 cells liter−1); M, medium (100,000 to <1,000,000 cells liter−1); H, high (≥1,000,000 cells liter−1).
NA, not available.
DISCUSSION
NASBA is a rapid and effective method for the detection of K. brevis rbcL mRNA. To date, we have been able to detect as little as one cell and are able to create cell standard curves based on TTP over at least 4 orders of magnitude. Using these curves to predict concentrations of K. brevis cells from environmental samples we have been able to classify samples into different levels of bloom (according to cell counts from FWC).
In several of the environmental samples, NASBA detected K. brevis mRNA in samples that were classified by FWC as not present. With the exception of two of these samples, NASBA predicted cell counts to be less than 1,000 cells liter−1, which is considered to be normal background level and poses no threat to human health or shellfish contamination. Of the two nonbloom samples for which NASBA cell counts were greater than 1,000 cells liter−1, one of the samples (Marco Pass, 2 April 2003) was classified by FWC 1 week later as a low-level bloom. This pattern was noted for Naples Pier as well in the 26 March 2003 sampling, which yielded a count of 0 by microscopy yet a count of 514 by real-time NASBA. At 1 week later (2 April 2003) both microscopic and NASBA counts for Naples Pier yielded >100,000 cells liter−1. Therefore, the NASBA method may be able to detect blooms in an early stage, thus permitting predictions of later blooms. The other positive result may have been due to contamination of samples or potentially the detection of another Karenia sp. by our assay. Since sequence information for Karenia species other than K. mikimotoi is not yet available, we are unable to determine whether our primers will detect other Karenia species. Finally, we are uncertain of the error associated with the counts obtained by the FWC for field samples or changes that may have occurred over the day period between counts and RNA analysis. A recent study has shown that consensus between determinations by individuals with an expert taxonomic labeling task can be as low as 43% (2).
A criticism of an earlier version of this work suggested that cellular levels of rbcL mRNA may change in the face of environmental stress. Our finding of general agreement of measurements of K. brevis abundance in natural samples with microscopic cell counts (i.e., within an order of magnitude) argues against this. Additionally, cellular levels of rbcL mRNA were not shown to vary with time of day in diel studies (M. Gray, personal communication). A range of stresses resulted in no difference in cellular rbcL mRNA levels except for high-light stress, which was lethal to the cells within 24 h. The average increase (5.4-fold) is within the precision (basically 1 order of magnitude) of the assay. Additionally, no inhibition of NASBA occurred in the presence of a closely related rbcL mRNA.
This assay calculates concentrations of K. brevis in environmental samples by the use of standard curves generated from TTP values of known cell numbers. While this method has proved to be relatively successful in terms of classification of unknown samples into different levels of blooms, there is still room to improve on the precision of the NASBA assay. Some of the variability may be a result of using TTP. There is evidence to suggest that TTP is not the best method for quantifying with NASBA, since the three enzymes have different kinetics and all reactions occur continuously and simultaneously (21). As an alternative to using TTP, Weusten et al. (21) suggest the use of an internal calibrator RNA. The calibrator is a synthetic RNA molecule of the same sequence as the target RNA that has a modified beacon binding site. By modeling the growth curves of both wild-type (target) RNA and calibrator RNA, a more precise quantification of the wild type can be achieved. We are presently investigating the use of internal calibrator RNA in the K. brevis NASBA assay.
This NASBA assay, however, is an effective and rapid method for the detection and quantification of K. brevis from environmental samples. Furthermore, NASBA detection can more easily be adapted to the field environment than traditional microscopy due to its speed and simplicity. Therefore, NASBA may be used in combination with, or as an alternative to, traditional techniques for quantification of K. brevis by delivering rapid results and information about the status of red tides in the coastal Gulf of Mexico.
Acknowledgments
This work was supported in part by a National Oceanic and Atmospheric Administration Florida ECOHAB grant and a National Science Foundation Biocomplexity Award.
We are indebted to Karen Steidinger, Bill Richardson, and Earnest Truby of the FWC Florida Marine Research Institute for providing us with the cultures of K. brevis and other algae and bloom samples from around Florida and for sharing data on K. brevis abundance in these bloom samples. We are indebted to Pierre Van Aarle, Lynell Grosso, Teri Kelley, and BioMerieux, Inc., for information concerning and reagents for NASBA.
REFERENCES
- 1.Adachi, M., Y. Sako, and Y. Ishida. 1996. Identification of the toxic dinoflagellates Alexandrium catenella and A. tamarense (Dinophyceae) using DNA probes and whole-cell hybridization. J. Phycol. 32:1049-1052. [Google Scholar]
- 2.Culverhouse, P. F., R. Williams, B. Reguera, V. Henry, and S. Gonzalez-Gil. 2003. Do experts make mistakes? A comparison of human and machine identification of dinoflagellates. Mar. Ecol. Prog. Ser. 247:17-25. [Google Scholar]
- 3.Davey, C., and L. T. Malek. 1989. Nucleic acid amplification process. European patent EP 0329822.
- 4.Godhe, A., S. K. Otta, A.-S. Rehnstam-Holm, I. Karunasagar, and I. Karunasagar. 2001. Polymerase chain reaction in detection of Gymnodinium mikimotoi and Alexandrium minutum in field samples from southwest India. Mar. Biotechnol. 3:152-162. [DOI] [PubMed] [Google Scholar]
- 5.Gray, M., B. Wawrik, J. Paul, and E. Casper. 2003. Molecular detection of the red tide dinoflagellate Karenia brevis in the marine environment. Appl. Environ. Microbiol. 69:5726-5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunther, G., R. H. Williams, C. C. Davis, and F. G. W. Smith. 1948. Catastrophic mass mortality of marine animals and coincident phytoplankton bloom on the west coast of Florida, November 1946 to August 1947. Ecol. Monogr. 18:311-324. [Google Scholar]
- 7.Hoagland, P., D. M. Anderson, Y. Kaoru, and A. W. White. 2002. The economic effects of harmful algal blooms in the United States: estimates, assessment issues, and information needs. Estuaries 25:819-837. [Google Scholar]
- 8.Landsberg, J. H. 2002. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 10:113-390. [Google Scholar]
- 9.Landsberg, J. H., and K. A. Steidinger. 1998. A historical review of Gymnodinium breve red tides implicated in mass mortalities of the manatee (Trichechus mantus latirostris) in Florida, USA, p. 97-100. In B. Reguera, J. Blanco, M. L. Fernandez, and T. Wyatt (ed.), Proceedings of the 8th International Conference on Harmful Algae. Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO, Vigo, Spain.
- 10.Litaker, R. W., M. W. Vandersea, S. R. Kibler, K. S. Reece, N. A. Stokes, K. A. Steidinger, D. F. Millie, B. J. Bendis, R. J. Pigg, and P. A. Tester. 2003. Identification of Pfiesteria piscicida (Dinophyceae) and Pfiesteria-like organisms using internal transcribed spacer-specific PCR assays. J. Phycol. 39:754-761. [Google Scholar]
- 11.Loret, P., T. Tengs, T. A. Villareal, H. Singler, B. Richardson, P. McGuire, S. Morton, M. Busman, and L. Campbell. 2002. No difference found in ribosomal DNA sequences from physiologically diverse clones of Karenia brevis (Dinophyceae) from the Gulf of Mexico. J. Plankton Res. 24:735-739. [Google Scholar]
- 12.Millie, D. F., O. M. Schofield, G. J. Kirkpatrick, G. Johnsen, P. A. Tester, and B. T. Vinyard. 1997. Detection of harmful algal blooms using photopigments and absorption signatures: a case study of the Florida red tide dinoflagellate, Gymnodinium breve. Limnol. Oceanogr. 42:1240-1251. [Google Scholar]
- 13.Millie, D. F., G. J. Kirkpatrick, G. Johnsen, O. M. E. Achofield, and T. J. Evens. 2001. Using absorbance and fluorescence spectra to discriminate microalgal phylogenetic groups and taxa. J. Phycol. 37:35. [Google Scholar]
- 14.Oernolfsdottir, E. B., J. L. Pinckney, and P. A. Tester. 2003. Quantification of the relative abundance of the toxic dinoflagellate, Karenia brevis (Dinophyta) using unique photopigments. J. Phycol. 39:449-457. [Google Scholar]
- 15.Peperzak, L., B. Sandee, C. Scholin, P. Miller, and L. van Nieuwerburgh. 2000. Application and flow cytometric detection of antibody and rRNA probes to Gymnodinium mikimotoi (Dinophyceae) and Pseudo-nitzschia multiseries (Bacillariophyceae), p. 206-209. In G. M. Hallegraff, S. I. Blackburn, C. J. Bolch, and R. J. Lewis (ed.), Harmful algal blooms 2000. IOC-UNESCO, Paris, France.
- 16.Pierce, R. H., and G. J. Kirkpatrick. 2001. Innovative techniques for harmful algal toxin analysis. Environ. Toxicol. Chem. 20:107-114. [DOI] [PubMed] [Google Scholar]
- 17.Stumpf, R. P. 2001. Applications of satellite ocean color sensors for monitoring and predicting harmful algal blooms. Hum. Ecol. Risk Assess. 7:1363-1368. [Google Scholar]
- 18.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyagi, S., and F. R. Kramer. 1996. Molecular beacons: probes that fluoresce upon hybridization. Nat. Biotechnol. 14:303-308. [DOI] [PubMed] [Google Scholar]
- 20.Van Dolah, F. M., D. Roelke, and R. M. Greene. 2001. Health and ecological impacts of harmful algal blooms: risk assessment needs. Hum. Ecol. Risk Assess. 7:1329-1345. [Google Scholar]
- 21.Weusten, J. J. A. M., W. M. Carpay, T. A. M. Oosterlaken, M. C. A. van Zuijlen, and P. A. van de Wiel. 2002. Principles of quantitation of viral loads using nucleic acid sequence-based amplification in combination with homogenous detection using molecular beacons. Nucleic Acids Res. 30:e26. [Online.] [DOI] [PMC free article] [PubMed] [Google Scholar]