Abstract
Lying at the intersection between neurobiology and epigenetics, Rett syndrome (RTT) has garnered intense interest in recent years, not only from a broad range of academic scientists, but also from the pharmaceutical and biotechnology industries. In addition to the critical need for treatments for this devastating disorder, optimism for developing RTT treatments derives from a unique convergence of factors, including a known monogenic cause, reversibility of symptoms in preclinical models, a strong clinical research infrastructure highlighted by an NIH-funded natural history study and well-established clinics with significant patient populations. Here, we review recent advances in understanding the biology of RTT, particularly promising preclinical findings, lessons from past clinical trials, and critical elements of trial design for rare disorders.
Progress in Identifying Potential RTT Therapeutics
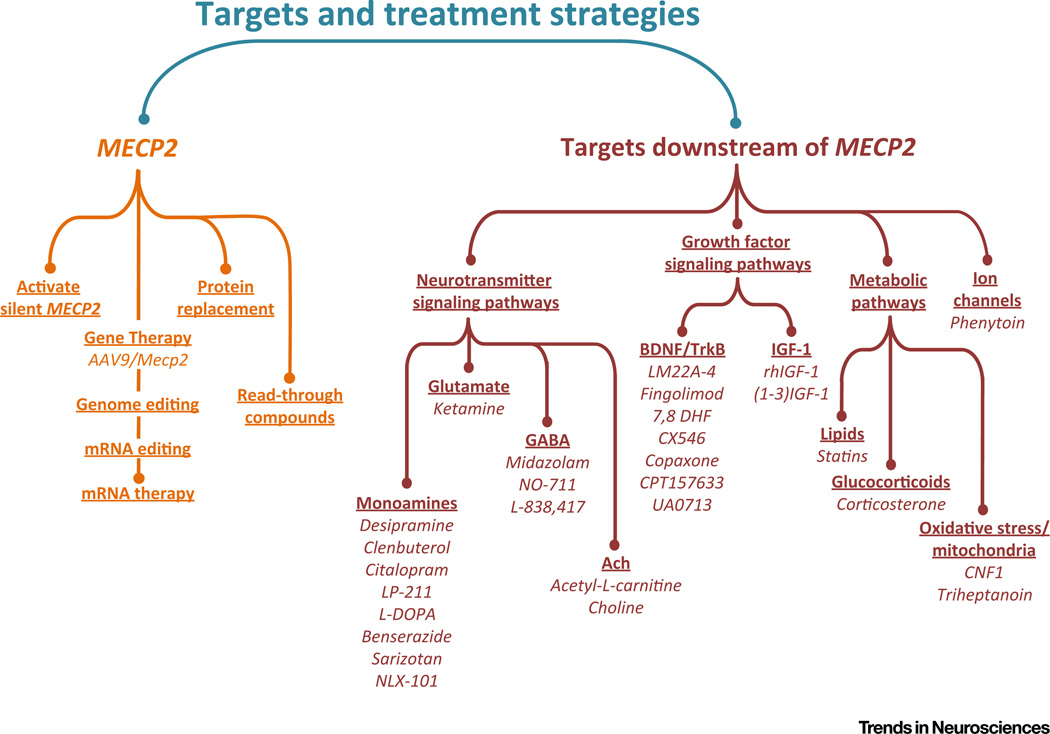
RTT is a severe neurodevelopmental disorder resulting from mutations in the X-linked gene encoding methyl-CpG-binding protein 2 (MeCP2) [1]. Progress in understanding the pathophysiology of RTT and in identifying potential therapies has outpaced that in many other neurodevelopmental disorders due, in part, to the availability of rodent models with good construct and face validity [2–4]. These include strains of mice carrying either Mecp2-null or hypomorphic alleles or human disease-causing mutations [2, 4], as well as an Mecp2-null rat model (SAGE Labs). In addition, some of the core symptoms of RTT, such as abnormal breathing, are more readily quantifiable and translate more directly from mice to humans compared with the complex behavioral abnormalities that define more prevalent disorders, such as nonsyndromic autism. Over the past few years, studies of the biology of MeCP2 (Box 1) and the consequences of MeCP2 loss for neural circuit function and behavior have led to the identification of potential therapeutic strategies [3–8], including: (i) molecular genetic approaches that target MECP2 itself, ranging from gene and protein replacement therapy to development of novel tools for activating the wild-type allele on the inactive X chromosome; (ii) pharmacologic approaches that target mechanisms downstream of MECP2 to restore excitatory–inhibitory synaptic balance in specific neural circuits, including some drugs that are now in early-stage clinical trials in patients with RTT (Figure 1; see Table S1 in the supplemental information online for the figure references).
Box 1. Function of MeCP2.
MeCP2 is a basic nuclear protein that is highly expressed in the brain [89]. Its amino acid sequence is conserved in vertebrate evolution, being 95% identical between humans and mice. Functional studies have identified a DNA-binding domain (MBD) as the major determinant of chromosome binding through its affinity for short sequences in the genome that contain 5-methylcytosine (mC) [90]. Methylation of the cytosine pyrimidine ring follows DNA synthesis and primarily affects the two base-pair sequence CG, which becomes a major target of MeCP2 binding [91, 92]. However, other methylated sites are now known and some of these also bind MeCP2. In particular, the sequence mCA, which is abundant in neurons but rare in other cell types, is established as a target for MeCP2 [93, 94]. In addition, the oxidized derivative of mC, hydroxymethylcytosine (hmC), is also abundant at CG sites in the brain and is elevated at transcriptionally active genes and their regulatory regions [95]. MeCP2 does not bind to hmCG, suggesting that this chemical change switches the mCG site to a form that cannot interact with the protein [94, 96]. In the genome, both mCG and mCA are broadly distributed, but are absent at CpG islands, which surround the promoters of most genes [97]. Accordingly, MeCP2 binding to the brain genome is relatively uniform, but dips sharply at CpG islands [91, 98].
Binding to DNA is evidently an essential part of MeCP2 function, because mutations that compromise MBD function cause RTT [99]. MeCP2 interacts with other partner macromolecules, but so far only one such protein–protein interaction has been experimentally linked to RTT. A discrete domain within the C-terminal half of the protein binds to the two closely related co-repressor complexes NCoR and SMRT (hence ‘NCoR/SMRT Interaction Domain’ or NID) [100] and mutations that disrupt binding cause RTT. The importance of DNA and co-repressor interactions is highlighted by the mutational spectrum underlying RTT. Of the many documented disease-causing mutations, missense mutations are particularly informative because they accurately pinpoint important functional domains. The distribution of RTT missense mutations is strikingly nonrandom, being largely confined to regions of the gene that encode the MBD and the NID [101]. A simplistic explanation for this observation is that MeCP2 forms a bridge between methylated DNA and the co-repressor complexes, and disruption of the bridge at either end results in RTT [100].
While there is a depth of biochemical and genetic evidence favoring the idea that MeCP2 represses transcription [100, 102, 103], analysis of gene expression in MeCP2-deficient brains does not reveal simple derepression of genes [104, 105]. Instead, large numbers of modest transcriptional changes are observed, both positive and negative. Analysis of multiple published and novel gene expression data sets uncovered a subtle but consistent upregulation of long genes in the MeCP2-deficient brain [94]. Given that many brain-specific genes are long, it is possible that modestly deregulated expression of thousands of such genes compromises brain function. By contrast, a separate study suggests that genes with more bound MeCP2 are either up- or downregulated in its absence [98]. However, both studies agree that non-CG methylation (e.g., mCA) makes a disproportionately large contribution to this effect.
Several other hypotheses have been advanced to explain MeCP2 function. For example, it has been proposed, based on functional studies, that MeCP2 is an activator of transcription [105–107], a regulator of miRNA processing or splicing [108, 109], a facilitator of chromosome looping or compaction [110, 111], or a regulator of several other aspects of cellular metabolism. A way of unifying these disparate potential functions is to propose that MeCP2, similar to some other relatively unstructured protein molecules, serves as a coordinating platform for multiple different interactions. In other words, MeCP2 might be an important ‘multifunctional hub’ for many pathways that support brain function [112].
Figure 1. Therapeutic Targets and Potential Pharmacological Strategies Currently Being Explored in Animal Models for the Treatment of Rett Syndrome.
Underlined headings indicate therapeutic targets; compounds that have been reported in the literature to be effective in improving behavioral outcome measures or physiological function in vivo are shown in italics (see Table S1 in the supplemental information online for the figure references).
Genetics and Clinical Features of RTT
The MECP2 gene is X linked and RTT mutations arise predominantly in the paternal germ line. Given that the gene is subject to X chromosome inactivation, most affected individuals are female heterozygotes who are somatic mosaics for normal and mutant MECP2. In rare cases, males can be born with an MECP2 mutation derived from the mother who either has favorable X chromosome inactivation patterns or gonadal mosaicism. However, because males have only one X chromosome, many such individuals are more severely affected than females and die, often early [9]. The prevalence of RTT is estimated at 1 in 10 000 live female births [10], corresponding to approximately 15 000 affected children and women in the USA and 350 000 worldwide. The disorder is diagnosed based on history and clinical presentation, and approximately 95% of individuals with a RTT diagnosis have a confirmed mutation in MECP2 [10]. While hundreds of mutations in MECP2 have been identified, eight hotspot mutations account for more than 60% of all cases [11].
Girls affected with RTT exhibit apparently typical early postnatal development followed by stagnation of developmental milestones and regression of skills, usually during the second year of life [12]. The hallmark symptoms of RTT include significant verbal and nonverbal communication deficits and the loss of motor skills, including purposeful hand use, which is replaced by almost constant stereotypical movements. Approximately half of affected individuals cannot walk and those who do have a wide-based and unsteady gait that becomes more pronounced with age. Particularly challenging, especially for families, is loss of speech. Autonomic and respiratory problems are frequent and include dysregulation of breathing with periods of hyperventilation, breath-holding, and abnormal cardiorespiratory coupling, gastrointestinal dysfunction, including severe constipation, and cardiac electrical problems, such as a prolonged QT interval. Seizures, anxiety, and orthopedic problems, such as scoliosis, contractures, and fractures, are common. Most individuals affected with RTT live well into adulthood and require total, round-the-clock care.
During the period of neurological regression, it is not uncommon for girls with RTT to exhibit autistic-like behaviors, such as social withdrawal [13]. However, as they get older, they often become very social and interactive. In fact, as noted by Andreas Rett, when he first described the disorder in 1966 [14], many girls with RTT have a penetrating gaze that they use effectively for communication purposes. Despite the fact that RTT is no longer classified as an autism spectrum disorder (ASD) in the latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders, an individual with RTT can also receive a diagnosis of ASD if she meets the behavioral criteria.
Although RTT has historically been described as a cognitive disorder, recent data suggest that the girls have strong receptive language [15]. Without the ability to speak or to adeptly use their hands for pointing, typing, or sign language, expressive language is difficult. Evolving strategies in teaching and augmentative communication technologies have resulted in fresh perspectives and attitudes about what individuals with RTT can achieve [16].
Neural Circuit Defects Resulting from Loss of MeCP2
Despite ongoing questions about the normal function of MeCP2, the effects of MeCP2 deficiency on many aspects of brain structure and function are now clear. Histopathological evidence from patients with RTT and Mecp2 mutant mice shows that loss of MeCP2 does not result in neuronal cell death, axonal degeneration, or other irreversible deficits [17], consistent with the finding that neurological dysfunction in conditional Mecp2 mutants is largely reversible upon reactivation of silent Mecp2 alleles [18]. By contrast, numerous structural and functional abnormalities have been identified at the level of brain microcircuits, all of which are potentially reversible. For example, reduced dendritic complexity and spine density are consistent findings in Mecp2 mutant mice and in postmortem material from patients with RTT [2, 19–21]. Mecp2 mutants also exhibit decreased expression of multiple neurotransmitters, neuromodulators, transmitter receptors, and transporters required for normal synaptic function [2, 3, 6, 9]. To the degree that these endpoints have been analyzed in human samples, similar deficits have been found, including decreased levels of brain monoamines and their metabolites, decreased cholinergic markers and abnormal patterns of NMDA receptor expression [22–25] (also see references in [9]). These changes may arise in large measure from the failure of activity-dependent mechanisms that depend on intact MeCP2 function and are required to maintain fully differentiated neuronal and synaptic phenotypes [26]; this view is supported by the fact that loss of MeCP2 at any stage of life is deleterious [27, 28]. In addition, abnormal glial function may also have a role [29]. As a result of these molecular and cellular abnormalities, brain microcircuits in Mecp2 mutants exhibit shifts in excitatory–inhibitory synaptic balance [19], defects in homeostatic synaptic scaling [30], excitatory or inhibitory connectivity [31], and/or changes in intrinsic neuronal excitability compared with controls [32, 33].
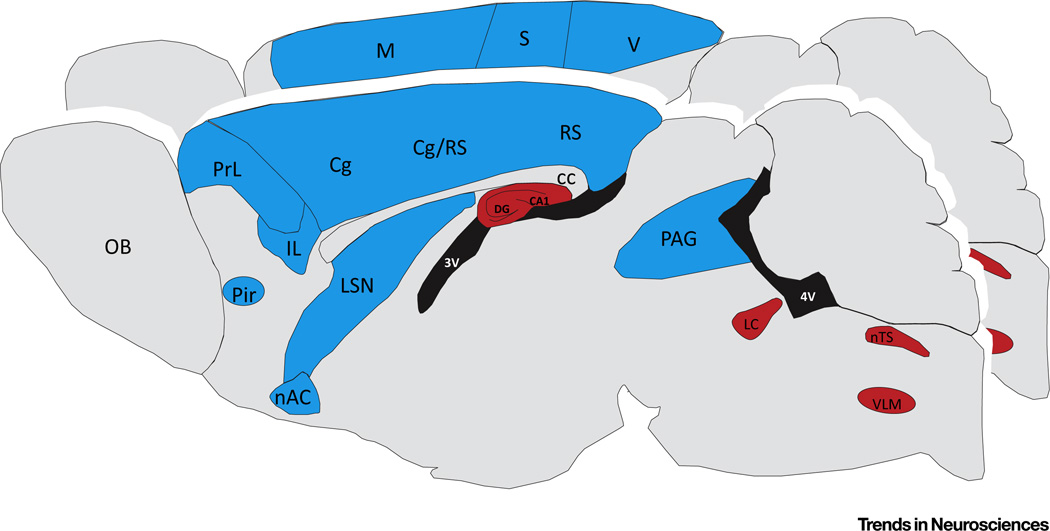
Of particular interest is the topology of changes in neural circuit function in the MeCP2-deficient brain. Studies in Mecp2-null and heterozygous mice demonstrated that loss of MeCP2 results in a regional pattern of dysfunction characterized by excitatory hypoconnectivity in many forebrain structures and hyperconnectivity in the caudal brainstem compared with wild-type mice [34] (Figure 2). For example, shifts in excitatory–inhibitory synaptic balance towards reduced excitation and/or increased inhibition have been documented in all cortices examined thus far, including somatosensory, visual, motor-frontal, and medial prefrontal (mPFC) [35–38]. These regions also exhibit marked reductions in the expression of the immediate early gene product Fos, a surrogate marker of neuronal activity [34]. By contrast, brainstem structures, including the locus coeruleus, nucleus tractus solitarius, and ventrolateral medulla, exhibit shifts towards synaptic or intrinsic hyperexcitability [32, 39], increased Fos expression [34], and enhanced excitatory activity in respiratory motor nerves [40]. An exception to this dichotomy between forebrain and brainstem is the hippocampus, which is hyperexcitable in Mecp2 mutants due, at least in part, to a loss of excitatory synaptic drive to inhibitory interneurons [41] and increased network synchrony [42].
Figure 2. Neural Circuit Dysfunction in the Methyl-CpG-Binding Protein 2 (Mecp2) Mutant Brain.
Colors indicate brain regions in which Mecp2 mutant mice exhibit a shift in either neuronal or synaptic activity towards decreased (blue) or increased (red) excitation compared with wild-type controls. This schematic summarizes findings from numerous laboratories and is based on electrophysiological recordings of intrinsic neuronal activity, synaptic activity, and/or population activity, as well as Fos mapping of neuronal activity. Abbreviations: 3 V, third ventricle; 4 V, fourth ventricle; CA1, cornu ammonis; cc, corpus callosum; Cg, cingulate; DG, dentate gyrus; IL, infralimbic cortex; LC, locus coeruleus; LSN, lateral septal nuclei; M, motor cortex; nAC, nucleus accumbens; nTS, nucleus of the solitary tract; OB, olfactory bulb; PAG, periaqueductal gray; Pir, piriform nucleus; PrL, prelimbic cortex; RS, retrosplenial cortex; S, somatosensory cortex; V, visual cortex; VLM, ventrolateral medulla.
This regional pattern of functional hypo- and hyperconnectivity accords well with the clinical picture of RTT; that is, cognitive and behavioral deficits consistent with cortical hypofunction coupled with paroxysmal events in brainstem control of respiratory and autonomic outflow. However, the prevalence of seizures in RTT seems at odds with the fact that excitatory synaptic drive onto pyramidal neurons is reduced in cortical circuits in MeCP2-deficient mice. On the other hand, increased network synchrony, even in the face of reduced excitatory connectivity may be a key factor driving epileptiform discharges in RTT [42]. Given the importance of the forebrain in regulating brainstem autonomic, respiratory and somatomotor outputs, the interplay between cortical hypofunction and brainstem hyperactivity likely has a key role in the pathophysiology of RTT. Thus, a major goal, and challenge, in therapy development for RTT is to redress excitatory–inhibitory imbalance not only in particular neuronal cell groups, but also across the neuraxis as a whole.
MECP2 and MeCP2 as Therapeutic Targets
Gene Dosage Concerns
The ultimate goal of strategies that target MECP2 directly would be to normalize expression without affecting the levels of other genes. However, these treatment approaches must carefully consider the consequences of MeCP2 dosage. An excess of MeCP2 in both humans and mice impairs neuronal development and causes severe neurological dysfunction. For example, mice overexpressing MeCP2 display seizures and hypoactivity [42, 43], and boys with MECP2 duplication syndrome exhibit some phenotypes that are similar to RTT [44–46]. Recent investigations in mice have shown that the syndrome associated with MeCP2 doubling requires two functional gene copies [47]. Accordingly, both gene therapy and small-molecule strategies to normalize MECP2 gene expression levels must take care to provide enough MeCP2 per cell to impart a therapeutic benefit, while limiting MeCP2 overexpression.
Activating MECP2 on the Inactive X Chromosome by Small-Molecule Approaches
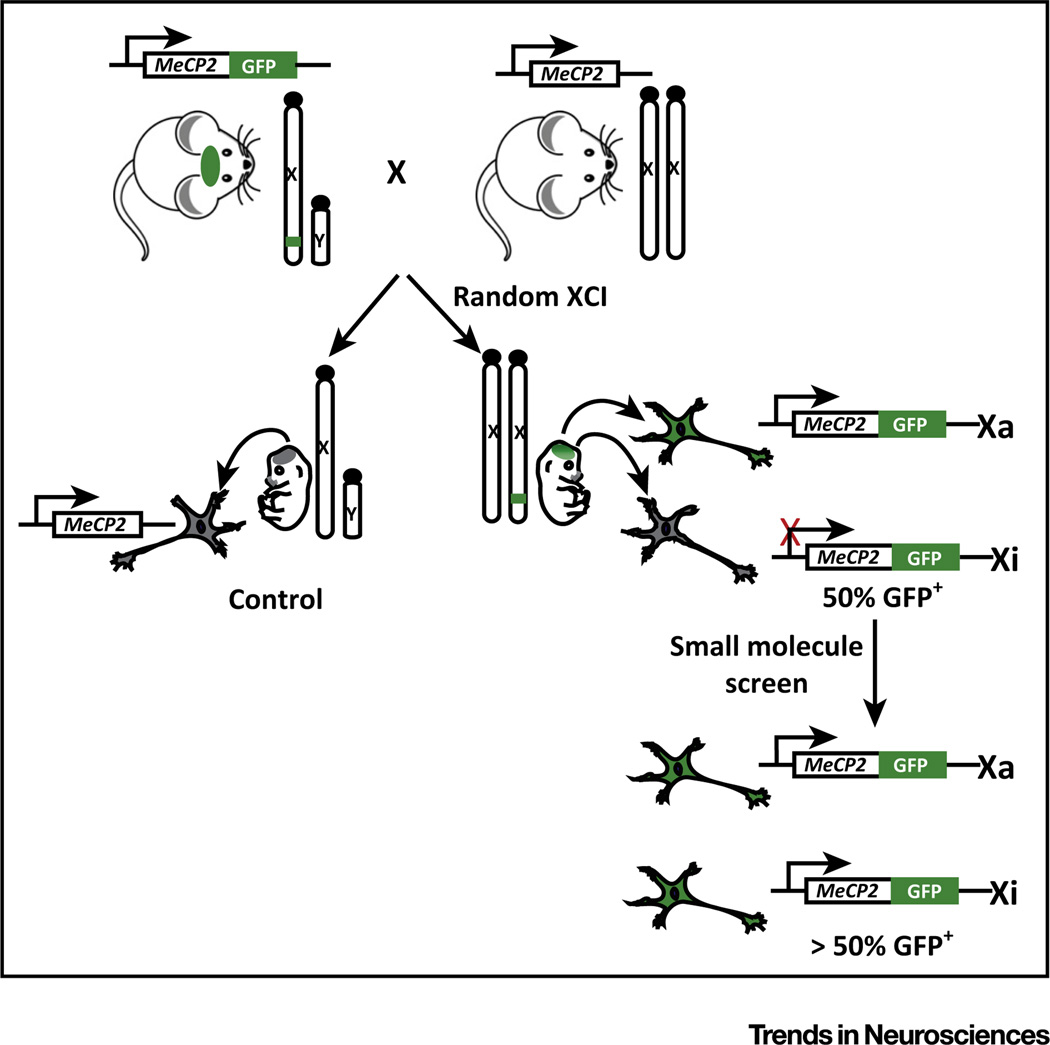
Most mutations in MECP2 prevent production of functional MeCP2 protein, rather than producing a partially functional or dominant-negative protein [47], suggesting that reactivating the wild-type copy of MECP2 on the inactive X (Xi) may be a viable approach for treating most forms of RTT. The therapeutic value of reactivating disease genes has been previously demonstrated in the case of the neurodevelopmental disorder Angelman syndrome, in that a dormant but intact copy of the Ube3a gene can be pharmacologically activated to replace the mutated active copy of Ube3a in a mouse model [48, 49]. Thus, the technology and procedures for identifying gene unsilencing agents are already established. Towards this goal, Mecp2-GFP fluorescent reporter mice offer a valuable tool for assessing allelic activation of Mecp2 (Figure 3). One can use high-content imaging of neurons from these mice to assess changes in GFP expression in a high-throughput, small-molecule screen. This approach is unbiased and is only limited by cost and the availability of drug-screening libraries. This screening approach cannot discriminate on first pass between compounds that are specific to de-inactivating Mecp2 or that produce global X de-inactivation, but these possibilities could be easily distinguished with secondary screens and experiments. It will be essential to validate activities in patient iPSC-derived neurons to verify their applicability to humans.
Figure 3. High-Content Small-Molecule Screening Strategy to Detect Methyl-CpG-Binding Protein 2 (Mecp2) Reactivation.
Neurons harvested from Embryonic day (E)15.5 embryos produced in matings between hemizygous Mecp2-GFP males and wild-type females are used to screen for Mecp2 de-inactivating compounds. Of the neurons derived from female embryos, approximately 50% will be GFP+ due to random X chromosome inactivation (XCI). Positive hits result in an increase in the proportion of GFP-labeled neurons. Neurons derived from nontransgenic male embryos serve as negative controls. GFP reporter mice are available from Jackson Laboratories (Mecp2tm3.Bird/J; Reference #014610).
Rather than specifically targeting MECP2, some therapeutic approaches might involve reactivating the entire inactive X (Xi). While this approach may seem intuitively less attractive, recent work has shown that the loss of a protein hormone, Stanniocalcin 1 (Stc1) [50], perturbs silencing at a handful of X-linked genes, including Mecp2, and produces X reactivation without grossly affecting chromosome-wide gene expression [51]. Thus, approaches that specifically reactivate MECP2 or that produce more widespread X chromosome reactivation might help to restore normal MeCP2 protein levels and treat RTT. Successful translation from screening to the clinic will depend on whether active compounds are safe, can be easily administered, are diffusible across the blood–brain barrier, and achieve relatively stable MeCP2 restoration broadly across relevant cell types.
Gene Therapy and/or Genome Editing
Another possible therapeutic approach to restoring MeCP2 function is a gene replacement or gene-editing strategy. The recent discovery of adeno-associated virus (AAV) vector designs, such as AAV9, that can achieve widespread gene transfer across the nervous system has opened up the possibility of a translatable gene therapy approach for RTT [52–54]. Two groups have independently demonstrated the potential of gene replacement therapy in RTT model mice, showing that intravenous delivery of an AAV9/MeCP2 vector prolonged the lifespan of MeCP2 knockout mice as well as partially normalized behavioral phenotypes of male and female RTT mice [55, 56]. The challenge of gene therapy is to deliver and express MeCP2 within a narrow range of expression that is therapeutic without resulting in detrimental overexpression. For example, although the AAV9 vector can deliver the MECP2 gene across the blood–brain barrier to the brain, approximately 100-fold higher gene transfer occurs to the liver, resulting in some liver toxicity [55]. Thus, the greatest challenge for gene therapy is the ability to homogenously deliver the MECP2 gene, but to avoid overexpression in the context of a mosaic mixture of wild-type and affected cells in RTT females.
In theory, gene or mRNA editing could circumvent problems associated with MeCP2 dosage, since only the mutant MECP2 would be targeted and MeCP2 would retain all of its endogenous regulation [57, 58]. However, development of a translatable gene-editing approach is confounded by the following unresolved issues: (i) the ability to deliver the nuclease and editing template broadly to all cells; (ii) the relatively low efficiency of gene editing in vivo in postmitotic cells; (iii) potential nonspecific nuclease cleavage elsewhere in the genome, especially upon chronic expression of the editing nuclease; and (iv) potential immune responses against the editing nuclease, which would be a nonhuman protein.
Protein replacement is another approach that could conceptually offer the ability to titrate appropriate MeCP2 levels, but this would need to overcome the following obstacles: (i) ensuring the proper post-translational modifications are present; (ii) homogenous and ongoing delivery of the appropriate levels across the blood–brain barrier; and (iii) adequate cell penetration and localization of the supplied MeCP2 to the nucleus. In addition, pharmaceutical compounds have been developed that allow the read through of premature stop codons [59]. Conceptually, this could provide functional MeCP2 from the endogenous active allele, retaining native regulatory elements and circumventing any risk of overexpression-related toxicity. This treatment would only apply to disease-causing MECP2 mutations that introduce in-frame premature stop codons, representing approximately 35% of patients. Such a strategy was able to provide some full-length MeCP2 in cultured R168X mouse fibroblasts [60]; however, this has not yet been shown to be effective in in vivo models.
Therapeutic Targets Downstream of MECP2
By using clinically relevant outcome measures, preclinical studies of potential RTT therapeutics have, in a relatively short period of time, produced compelling evidence that signaling pathways well downstream of Mecp2 can be effectively targeted to ameliorate specific disease symptoms. In general, the pathways that have been targeted fall into three categories: (i) classical neurotransmitter and neuromodulator systems, including noradrenergic, serotonergic, glutamatergic, GABAergic, and cholinergic signaling; (ii) growth factor signaling, including brain-derived neurotrophic factor (BDNF) and insulin-like growth factor 1 (IGF-1); and (iii) metabolic signaling, including the cholesterol biosynthesis pathway and mitochondrial function [3, 4, 6, 61].
Given that loss of MeCP2 results, to varying degrees, in dysregulation of all of these pathways, it is generally thought that pharmacological strategies focused on targets downstream of Mecp2 will likely require multiple drugs to effectively treat the full spectrum of RTT symptoms. By contrast, patients’ quality of life (QoL) would be significantly improved by treatments that ameliorate or reverse even one of the core symptoms of the disease. In this regard, pharmacological improvement of breathing abnormalities is a particularly good example of preclinical findings with high translational potential. Dysregulation of breathing is a core feature of RTT in up to 93% of patients, significantly impacts QoL, and is thought to contribute to early mortality in some patients [62]. In human RTT and mouse models, respiratory dysfunction is characterized by periods of hyperventilation and prolonged respiratory pauses, including breath holds and apneas [62], which can be rigorously quantified using noninvasive plethysmography. Several laboratories have now shown that respiratory abnormalities in RTT mice can be significantly improved by manipulating diverse transmitter or neuromodulatory systems, including glutamatergic, GABAergic, noradrenergic, serotonergic, and neurotrophin signaling, either with experimental molecules or drugs already approved by the US FDA for other indications [62, 63].
Of particular interest are drugs that improve function across multiple symptom domains. One such example is the nonselective NMDAR antagonist ketamine (2-O-chlorophenyl-2-methyl-amino cyclohexanone), which has been independently validated in two different laboratories in two different strains of Mecp2 mice and is now in a clinical trial with RTT patients (Table 2). The therapeutic potential of ketamine for treating RTT was first demonstrated by Katz and colleagues, who found that treatment of heterozygous female Mecp2 mutant mice with a subanesthetic dose of ketamine (8 mg/kg) acutely reversed abnormalities in Fos expression and sensorimotor function [34]. More recently, chronic administration of ketamine was also found to improve symptoms and extend lifespan in null male Mecp2 mutants [64]. The ability of low-dose ketamine to improve function across a broad range of symptoms may be related to its ability to increase cortical network activity, presumably by selective inhibition of GABAergic interneurons [65], as well as to decrease synaptic excitability in brainstem networks important for respiratory and autonomic control [66]. Thus, ketamine and related molecules may be ideally suited to redress the imbalance between cortical and brainstem activity that characterizes the MeCP2-deficient brain (Figure 2). Moreover, in addition to its acute effects on circuit function, work in other disease models has shown that ketamine also rapidly stimulates dendritic growth, BDNF translation, and expression of key synaptic proteins [67, 68], at least in part through activation of mTOR signaling, which is deficient in Mecp2 mutants [69]. These findings suggest that, in addition to acute rescue of neurological function, ketamine also has the potential to effect long-term synaptic repair in RTT by enhancing structural and functional connectivity, as previously shown in animal models of depression and stress [70].
Table 2.
| Intervention | Proposed Mechanism | N | Status | Design | Outcome Measures |
|---|---|---|---|---|---|
| IGF-1 | Enhance growth factor signaling pathways |
30 | Recruiting | Double-blind, placebo- controlled, cross- over |
Kerr, EEG, RSBQ, ADAMS, ABC, CGI, VAS, Vineland |
| Glatiramer acetate (Israel) |
Increase BDNF | 10 | Stopped | Open label | EEG, seizure frequency, sleep diary, height, weight, respiratory regulation, Kerr and Naidu severity scores |
| Glatiramer acetate (New York) |
Increase BDNF | 20 | Active, not recruiting (4/20/2015) |
Open label | EEG, gait, autonomic, visual attention, behavior, QOL |
| Dextromethorphanb | NMDA receptor antagonist |
60 | Recruiting | Double-blind | Mullen, Vineland, Screen for Social Interaction |
| Desipramine | Inhibit norepinephrine reuptake |
36 | Completed (8/6/2015) |
Unbalanced (high- dose, low-dose, placebo), double- blind, placebo controlled, parallel |
Respiratory regulation |
| Fingolimod | Increase BDNF | 6 | Recruiting | Phase I | Serum and CSF BDNF levels |
| Triheptanoin | Increase metabolic substrate |
10 | Pending | Open label, challenge- dechallenge |
Seizure frequency, dystonia |
| Ketamine | NMDA receptor antagonist |
30 | Recruiting | Double-blind, placebo-controlled |
Respiratory regulation, cardio-respiratory coupling, EEG, auditory evoked potentials, RSBQ, RBSR |
| Lovastatin | Modulate cholesterol synthesis |
20 | Recruiting | Open label | Gait velocity, visual attention and memory, visual pursuit, respiratory regulation, EEG, QOL |
Abbreviations: ABC, Aberrant Behavior Checklist; ADAMS, Anxiety, Depression, and Mood Scale; BDNF, brain-derived neurotrophic factor; CGI, Clinical Global Impression; CSF, cerebrospinal fluid; EEG, electroencephalogram; RBSR, Repetitive Behavior Scale-Revised; RSBQ, Rett Syndrome Behavior Questionnaire; VAS, Visual Analog Scale; ω-3 PUFAs, ω-3 polyunsaturated fatty acids.
One stage of this study is completed; no published data available.
Clinical Trials: Resources, Possibilities and Challenges
The United States RTT Natural History Study
Clinical trials in rare diseases are confounded by the limited, often heterogeneous, pool of affected individuals, and difficulty selecting endpoints with a large effect size [71–75]. However, observational natural-history studies have been useful to understand the range of manifestations and progression of other rare diseases, and to establish valid and reliable short-term and long-term outcome measures or biomarkers [76, 77]. Therefore, the United States RTT Natural History Study (USNHS) was conceived in 2003 to acquire longitudinal baseline data in preparation for clinical trials. The objectives of the USNHS are to evaluate the current RTT diagnostic criteria, to examine phenotype–genotype correlation, and to understand the evolution of developmental milestones and associated features, such as seizures, scoliosis, gastrointestinal issues, and breathing dysfunction. At each visit, physicians administer two commonly used RTT instruments (the Clinical Severity Score, and the Motor-Behavioral Assessment [78, 79]) and health-related QoL measures for both caregivers and, by proxy, RTT participants. Several lessons have been learned from the USNHS that can now help inform clinical trial design. For example, the study revealed some phenotype–genotype correlations [11, 80] that could aid in stratifying patients into more homogenous subgroups for clinical trials. The study also showed that most RTT sequelae are not static over time [81, 82], presenting a significant challenge for crossover trials conducted over several months.
Lessons from Past Trials
Over the past three decades, 16 studies of treatment effect have been conducted in RTT (Table 1). Nine others are either underway or in prerecruitment status (Table 2). Many of the completed studies were handicapped by critical design flaws, which can serve as lessons and warnings for future trial design. Remarkably, only three were parallel, randomized, double-blind, placebo-controlled trials (RCT). Five others were crossover studies, a design in which subjects initially receive either the active drug or a placebo, and then switch to the opposite group. Although most studies reported improvement in some outcome measures (Table 1), these have not been independently validated and none have resulted in the use of these treatments in clinical practice.
Table 1.
| Intervention | Proposed Mechanism |
N | Year | Design |  |
Refs |
|---|---|---|---|---|---|---|
| Ketogenic diet | Induce ketosis | 7 | 1986 | Open label, uncontrolled |
 |
[113] |
| Bromocriptine | Stimulate dopamine receptors |
10 | 1990 | Double-blind, randomized, placebo- controlled, partial cross-over |
 |
[114] |
| Naltrexone | Opiate receptor antagonist |
25 | 1994 | Double-blind, randomized, placebo- controlled, cross-over |
 |
[84] |
| L-Carnitine | Increase metabolic substrate |
35 | 1999 | Double-blind, randomized, placebo- controlled, cross-over |
 |
[115] |
| L-Carnitine | Increase metabolic substrate |
21 | 2001 | Open label |  |
[116] |
| Folate/Betaine | Enhance MeCP2 binding |
73 | 2009 | Double-blind, placebo- controlled, parallel |
 |
[84] |
| Folinic acid | Increase CSF folate |
25 | 2009 | Open label, allocated based on low CSF folate |
Seizure frequency, clinical exam |
[117] |
| Folinic acid | Increase CSF folate |
12 | 2011 | Double-blind, placebo- controlled, cross-over |
EEG, Hagberg Stage, Motor-Behavioral Assessment, Hand Apraxia Scale, Modified Symptom Severity Score, Overall Well-Being Index, Dependency Scale, CSF 5- MTHF, seizure frequency, clinical exam |
[86,118] |
| Creatine | Increase metabolic substrate |
18 | 2011 | Double-blind, placebo- controlled, cross-over |
Motor-Behavioral Assessment, clinical lab values |
[119] |
| ω-3 PUFAs | Antioxidant | 42 | 2011 | Open label | CSS, oxidative stress markers |
[120,121] |
| IGF-1 (Boston) | Enhance growth factor signaling pathways |
12 | 2012 | Phase I, open label |
 |
[85] |
| IGF-1 (Italy) | Enhance growth factor signaling pathways |
6 | 2012 | Open label | [122] | |
| ω-3 PUFAs | Antioxidant | 20 | 2012 | Single-blind, placebo- controlled |
CSS, oxidative stress markers |
[123] |
| NNZ-2566c | Unclear; possibly enhance growth factor signaling pathways |
60 | 2014 | Unbalanced (high-dose, low- dose, placebo), double-blind, placebo- controlled, parallel |
 |
|
| EPI-743c | Augment glutathione biosynthesis |
24 | 2014 | Double-blind, placebo- controlled, parallel |
 |
|
| ω-3 PUFAs | Antioxidant | 66 | 2014 | Single-blind, placebo- controlled |
CSS, myocardial function, oxidative stress markers |
[124] |
Outcome measures in blue were reported as improving, those in red were reported as worsening and those in black were reported as not changing. Single case reports and retrospective case series are not included in this table.
Abbreviations: ADAMS, Anxiety, Depression, and Mood Scale; CGI, Clinical Global Impression; CSF, cerebrospinal fluid; CSS, Clinical Severity Score; EEG, electroencephalogram; PedsQL, Pediatric Quality of Life Inventory; RSBQ, Rett Syndrome Behavioral Questionnaire; RS, SSI, Rett Syndrome, Symptom Severity Index; SF-36, Short-form 36 items; VAS, Visual Analog Scale; ω-3 PUFAs, ω-3 polyunsaturated fatty acids.
Studies completed; no published data available.
The crossover design can be problematic in RTT, as highlighted by the naltrexone study [83]. The researchers tested the hypothesis that a period-by-treatment interaction existed before and after the 30-day washout period. Although the half-life of naltrexone is less than 1 day, the researchers found a carryover effect that confounded analysis. Changes, particularly in behavioral outcome measures, may outlast the drug in an unpredictable manner. The same authors conducted the folate-betaine study, a balanced allocation, parallel RCT, which is the largest and longest RCT to date in RTT [84]. The authors recognized methodological issues in their naltrexone study; accordingly, they altered the design to exclude young participants, who are in a period of rapid change, and selected a longer, parallel design, as opposed to a crossover design. Also recognizing the strong placebo effect in parent reporting of outcome measures, they implemented numerous objective measures, including laboratory, polygraphic, neurophysiological, anthropometric, nutritional, and clinical assessments. The only objective finding was improved head growth in the treatment group; however, this effect apparently reflected the overrepresentation of a ‘mild’ MECP2 mutation in the active treatment group, highlighting the need for balanced allocation based on genetic characteristics.
In an attempt to shorten the clinical trials process, a recent IGF-1 study in patients with RTT [85] used the highest dose of IGF-1 already approved for other indications, rather than applying to the FDA for permission to use higher doses. However, this study concluded that, due to the complex pharmacokinetics of IGF-1, the FDA-approved maximum dosing regimen was inadequate for a Phase II study [85], highlighting the need for rigorous dose exploration in both preclinical and clinical trials.
Trial Designs
Given that the standard clinical trial process requires thousands of subjects and many years to complete, rare disease researchers have attempted to streamline this process. Open-label designs require fewer participants, all of whom are guaranteed to receive the medication, and have been used for most studies in RTT. However, this model is confounded by multiple sources of bias, most notably the placebo effect, which was 63% in one RTT clinical trial [86]. Consequently, the results of these studies can be uninterpretable. In rare diseases, historical controls have been sufficient for FDA approval of an investigational drug. Since the placebo effect can be large, an objective historical control with good reliability must be chosen if it is to be used in lieu of a placebo group. Another strategy, the adaptive design, incorporates participant covariate values and prior responses to treatment. Response-adaptive trials and sequential designs offer the ability to recruit fewer participants overall, and minimize the number who receive placebo [87]. Crossover designs necessitate longer trials and pose the risk of carryover effect; as an alternative, Bayesian statistics can help incorporate previous information (e.g., from natural history studies) into the clinical trial design, improving statistical power and limiting the number of subjects needed for a trial [88]. Alternative trial designs can increase the possibility of type 1 error and, thereby, the approval of medications that are not in fact safe and efficacious. However, systematic postmarketing studies (i.e., additional clinical trials of safety and effectiveness after a drug has been approved for use) can attenuate this risk in a rare disease.
Concluding Remarks and Future Directions
This is a promising time for the RTT field as researchers move closer to understanding the basic biology of MeCP2 and there are more and more examples of interventions that improve or reverse symptoms in mouse models. By definition, therefore, this is also a time for caution, because the expectations of families affected by RTT must be managed appropriately (see Outstanding Questions). Based on a wealth of experience with other disorders, the chance that any particular treatment will translate from preclinical RTT models to humans is predicted to be low. However, with increased attention to rigorous preclinical trial design in RTT [2], it is hoped that success in translation will improve. Although streamlining the clinical trials process could result in more drugs being brought to market, the great risk is that many will lack true efficacy unless adequate safeguards are in place. The RTT field will also face the challenge of which trials to run, given a relatively small patient pool and funding limitations. Fortunately, with recent incentives from the FDA for companies to invest in drug development for orphan indications, there is new hope that the RTT field will be able to attract the kind of large-scale funding that is required to run clinical trials. Nonetheless, as preclinical studies generate more and more promising results, the need to prioritize clinical trials will become increasingly important and will require a high degree of coordination within the RTT community and with industry partners. If a concerted global effort can be made in optimizing preclinical research, clinical trial design, and prioritization goals, pooled resources and shared methodology could result in efficacious treatments for RTT in the near future.
Outstanding Questions.
Can the apparent imbalance between cortical hypoconnectivity and brainstem hyperexcitability in the MeCP2 deficient brain be addressed by pharmacological strategies aimed at restoring the excitatory–inhibitory synaptic balance? Will this require combination therapies targeting multiple neurotransmitter and/or neuromodulator signaling pathways?
Can gene replacement or MECP2 reactivation strategies be developed that are effective and safe for human translation? In particular, will it be possible to titrate MeCP2 levels within the relatively narrow range required for healthy brain function?
What criteria will be used to prioritize the selection of candidate therapeutics that advance to clinical trials in patients with RTT? Presently, candidate molecules are being proposed at a rate that exceeds the patient population and resources needed to study them using the traditional clinical trial model.
What is the optimal clinical trial design to study a rare neurological disease? An optimal design would incorporate expected delays between improved neuronal function and measurable changes in clinical symptoms or behavior, the likelihood of effect outlasting treatment cessation, and patient-specific data, such as genotype.
Supplementary Material
Trends.
Studies of RTT mouse models have convincingly demonstrated that neurological disability caused by loss of methyl-CpG-binding protein 2 (MeCP2) function is reversible to a significant degree.
Recent insights into the biology of MeCP2 and its role in regulating interactions between DNA and repressor protein complexes seemed poised to resolve longstanding controversies about the role of MeCP2 in transcriptional control.
The knowledge that reintroduction of Mecp2 can restore circuit functionality in mouse models of RTT has spurred the investigation of gene replacement and gene reactivation strategies as comprehensive and potentially transformative treatment approaches for RTT.
Pharmacologic strategies targeting neurotransmitter and neuronal growth factor signaling pathways have proven highly effective at improving neurological function in mouse models of RTT.
The natural history of RTT is becoming increasingly well defined, facilitating the identification of clinically measurable endpoints for therapeutic trials.
Acknowledgments
This work was supported by grants awarded to D.M.K. from NINDS (RO1NS057398) and the Rett Syndrome Research Trust (RSRT); to A.B. from The Wellcome Trust (grants 091580 and 092076) and RSRT; to B.D.P. from the Simons Foundation (SFARI Award 274426), NINDS (R01NS085093), NIMH (R01MH093372), and RSRT; to S.J.G. from Rettsyndrome.org, RSRT and Research to Prevent Blindness through the UNC Department of Ophthalmology; to D.U.M. from NICHD (RO1HD036655; T. Magnuson, PI). The authors thank Dr. James Eubanks for helpful discussions and Erica Kimmick for help with citations. Authorship: Authors’ names are listed in alphabetic order after D.M.K.
Footnotes
Supplementary information
Supplementary information associated with this article can be found, in the online version, at doi:10.1016/j.tins.2015.12.008.
References
- 1.Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 2.Katz DM, et al. Preclinical research in Rett syndrome: setting the foundation for translational success. Dis. Model. Mech. 2012;5:733–745. doi: 10.1242/dmm.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gadalla KK, et al. MeCP2 and Rett syndrome: reversibility and potential avenues for therapy. Biochem. J. 2011;439:1–14. doi: 10.1042/BJ20110648. [DOI] [PubMed] [Google Scholar]
- 4.Lombardi LM, et al. MECP2 disorders: from the clinic to mice and back. J. Clin. Invest. 2015;125:2914–2923. doi: 10.1172/JCI78167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weng SM, et al. Rett syndrome: from bed to bench. Pediatr. Neonatol. 2011;52:309–316. doi: 10.1016/j.pedneo.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Pozzo-Miller L, et al. Rett Syndrome: reaching for clinical trials. Neurotherapeutics. 2015;12:631–640. doi: 10.1007/s13311-015-0353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricceri L, et al. Rett syndrome treatment in mouse models: searching for effective targets and strategies. Neuropharmacology. 2013;68:106–115. doi: 10.1016/j.neuropharm.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Chapleau CA, et al. Recent progress in Rett Syndrome and MeCP2 dysfunction: assessment of potential treatment options. Future Neurol. 2013 doi: 10.2217/fnl.12.79. Published online January 1, 2013. http://dx.doi.org/10.2217/fnl.12.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Laurvick CL, et al. Rett syndrome in Australia: a review of the epidemiology. J. Pediatr. 2006;148:347–352. doi: 10.1016/j.jpeds.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 11.Neul JL, et al. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology. 2008;70:1313–1321. doi: 10.1212/01.wnl.0000291011.54508.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagberg B. Clinical manifestations and stages of Rett syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2002;8:61–65. doi: 10.1002/mrdd.10020. [DOI] [PubMed] [Google Scholar]
- 13.Neul JL. The relationship of Rett syndrome and MECP2 disorders to autism. Dialogues Clin. Neurosci. 2012;14:253–262. doi: 10.31887/DCNS.2012.14.3/jneul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rett A. On a unusual brain atrophy syndrome in hyperammonemia in childhood. Wien. Med. Wochenschr. 1966;116:723–726. [PubMed] [Google Scholar]
- 15.Neul JL, et al. Developmental delay in Rett syndrome: data from the natural history study. J. Neurodev. Disord. 2014;6:20. doi: 10.1186/1866-1955-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wandin H, et al. Communication intervention in Rett syndrome: a survey of speech language pathologists in Swedish health services. Disabil. Rehabil. 2015;37:1324–1333. doi: 10.3109/09638288.2014.962109. [DOI] [PubMed] [Google Scholar]
- 17.Akbarian S. The neurobiology of Rett syndrome. Neuroscientist. 2003;9:57–63. doi: 10.1177/1073858402239591. [DOI] [PubMed] [Google Scholar]
- 18.Guy J, et al. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shepherd GM, Katz DM. Synaptic microcircuit dysfunction in genetic models of neurodevelopmental disorders: focus on Mecp2 and Met. Curr. Opin. Neurobiol. 2011;21:827–833. doi: 10.1016/j.conb.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong DD. Neuropathology of Rett syndrome. J. Child Neurol. 2005;20:747–753. doi: 10.1177/08830738050200090901. [DOI] [PubMed] [Google Scholar]
- 21.Chapleau CA, et al. Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiol. Dis. 2009;35:219–233. doi: 10.1016/j.nbd.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samaco RC, et al. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc. Natl. Acad. Sci. U.S.A. 2009;106:21966–21971. doi: 10.1073/pnas.0912257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston MV, et al. Neurobiology of Rett syndrome. Neuropediatrics. 1995;26:119–122. doi: 10.1055/s-2007-979740. [DOI] [PubMed] [Google Scholar]
- 24.Blue ME, et al. Development of amino acid receptors in frontal cortex from girls with Rett syndrome. Ann. Neurol. 1999;45:541–545. doi: 10.1002/1531-8249(199904)45:4<541::aid-ana21>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Wenk GL, et al. Altered neurochemical markers in Rett's syndrome. Neurology. 1991;41:1753–1756. doi: 10.1212/wnl.41.11.1753. [DOI] [PubMed] [Google Scholar]
- 26.Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu. Rev. Cell Dev. Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheval H, et al. Postnatal inactivation reveals enhanced requirement for MeCP2 at distinct age windows. Hum. Mol. Genet. 2012;21:3806–3814. doi: 10.1093/hmg/dds208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGraw CM, et al. Adult neural function requires MeCP2. Science. 2011;333:186. doi: 10.1126/science.1206593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGann JC, et al. Astrocytes conspire with neurons during progression of neurological disease. Curr. Opin. Neurobiol. 2012;22:850–858. doi: 10.1016/j.conb.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackman MP, et al. A critical and cell-autonomous role for MeCP2 in synaptic scaling up. J. Neurosci. 2012;32:13529–13536. doi: 10.1523/JNEUROSCI.3077-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dani VS, Nelson SB. Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett syndrome. J. Neurosci. 2009;29:11263–11270. doi: 10.1523/JNEUROSCI.1019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taneja P, et al. Pathophysiology of locus ceruleus neurons in a mouse model of Rett syndrome. J. Neurosci. 2009;29:12187–12195. doi: 10.1523/JNEUROSCI.3156-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He LJ, et al. Conditional deletion of Mecp2 in parvalbumin-expressing GABAergic cells results in the absence of critical period plasticity. Nat. Commun. 2014;5:5036. doi: 10.1038/ncomms6036. [DOI] [PubMed] [Google Scholar]
- 34.Kron M, et al. Brain activity mapping in Mecp2 mutant mice reveals functional deficits in forebrain circuits, including key nodes in the default mode network, that are reversed with ketamine treatment. J. Neurosci. 2012;32:13860–13872. doi: 10.1523/JNEUROSCI.2159-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dani VS, et al. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U.S.A. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durand S, et al. NMDA receptor regulation prevents regression of visual cortical function in the absence of Mecp2. Neuron. 2012;76:1078–1090. doi: 10.1016/j.neuron.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood L, Shepherd GM. Synaptic circuit abnormalities of motor-frontal layer 2/3 pyramidal neurons in a mutant mouse model of Rett syndrome. Neurobiol. Dis. 2010;38:281–287. doi: 10.1016/j.nbd.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sceniak MP, et al. Mechanisms of functional hypoconnectivity in the medial prefrontal cortex of Mecp2 null mice. Cereb. Cortex. 2015 doi: 10.1093/cercor/bhv002. Published online February 7, 2015. http://dx.doi.org/10.1093/cercor/bhv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kline DD, et al. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J. Neurosci. 2010;30:5303–5310. doi: 10.1523/JNEUROSCI.5503-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdala AP, et al. Correction of respiratory disorders in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U.S.A. 2010;107:18208–18213. doi: 10.1073/pnas.1012104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calfa G, et al. Excitation/inhibition imbalance and impaired synaptic inhibition in hippocampal area CA3 of Mecp2 knockout mice. Hippocampus. 2015;25:159–168. doi: 10.1002/hipo.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, et al. The MeCP2-null mouse hippocampus displays altered basal inhibitory rhythms and is prone to hyperexcitability. Hippocampus. 2008;18:294–309. doi: 10.1002/hipo.20389. [DOI] [PubMed] [Google Scholar]
- 43.Luikenhuis S, et al. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6033–6038. doi: 10.1073/pnas.0401626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Esch H, et al. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am. J. Hum. Genet. 2005;77:442–453. doi: 10.1086/444549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.del Gaudio D, et al. Increased MECP2 gene copy number as the result of genomic duplication in neurodevelopmentally delayed males. Genet. Med. 2006;8:784–792. doi: 10.1097/01.gim.0000250502.28516.3c. [DOI] [PubMed] [Google Scholar]
- 46.Meins M, et al. Submicroscopic duplication in Xq28 causes increased expression of the MECP2 gene in a boy with severe mental retardation and features of Rett syndrome. J. Med. Genet. 2005;42:e12. doi: 10.1136/jmg.2004.023804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heckman LD, et al. Rett-causing mutations reveal two domains critical for MeCP2 function and for toxicity in MECP2 duplication syndrome mice. Elife. 2014;3:02676. doi: 10.7554/eLife.02676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang HS, et al. Topoisomerase inhibitors unsilence the dormant allele of Ube3a in neurons. Nature. 2012;481:185–189. doi: 10.1038/nature10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng L, et al. Towards a therapy for Angelman syndrome by targeting a long non-coding RNA. Nature. 2015;518:409–412. doi: 10.1038/nature13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeung BH, et al. Evolution and roles of stanniocalcin. Mol. Cell. Endocrinol. 2012;349:272–280. doi: 10.1016/j.mce.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Bhatnagar S, et al. Genetic and pharmacological reactivation of the mammalian inactive X chromosome. Proc. Natl. Acad. Sci. U.S.A. 2014;111:12591–12598. doi: 10.1073/pnas.1413620111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gray SJ, et al. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol. Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duque S, et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foust KD, et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gadalla KK, et al. Improved survival and reduced phenotypic severity following AAV9/MECP2 gene transfer to neonatal and juvenile male Mecp2 knockout mice. Mol. Ther. 2013;21:18–30. doi: 10.1038/mt.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garg SK, et al. Systemic delivery of MeCP2 rescues behavioral and cellular deficits in female mouse models of Rett syndrome. J. Neurosci. 2013;33:13612–13620. doi: 10.1523/JNEUROSCI.1854-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savic N, Schwank G. Advances in therapeutic CRISPR/Cas9 genome editing. Transl. Res. 2015 doi: 10.1016/j.trsl.2015.09.008. Published online September 26, 2015. http://dx.doi.org/10.1016/j.trsl.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Deffit SN, Hundley HA. To edit or not to edit: regulation of ADAR editing specificity and efficiency. RNA. 2015 doi: 10.1002/wrna.1319. Published online November 26, 2015. http://dx.doi.org/10.1002/wrna.1319. [DOI] [PubMed] [Google Scholar]
- 59.Keeling KM, et al. Therapeutics based on stop codon readthrough. Annu. Rev. Genomics Hum. Genet. 2014;15:371–394. doi: 10.1146/annurev-genom-091212-153527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brendel C, et al. Readthrough of nonsense mutations in Rett syndrome: evaluation of novel aminoglycosides and generation of a new mouse model. J. Mol. Med. (Berl) 2011;89:389–398. doi: 10.1007/s00109-010-0704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ricceri L, et al. Mouse models of Rett syndrome: from behavioural phenotyping to preclinical evaluation of new therapeutic approaches. Behav. Pharmacol. 2008;19:501–517. doi: 10.1097/FBP.0b013e32830c3645. [DOI] [PubMed] [Google Scholar]
- 62.Ramirez JM, et al. Breathing challenges in Rett Syndrome: lessons learned from humans and animal models. Respir. Physiol. Neurobiol. 2013;189:280–287. doi: 10.1016/j.resp.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katz DM, et al. Breathing disorders in Rett syndrome: progressive neurochemical dysfunction in the respiratory network after birth. Respir. Physiol. Neurobiol. 2009;168:101–108. doi: 10.1016/j.resp.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patrizi A, et al. Chronic administration of the N-methyl-D-aspartate receptor antagonist ketamine improves Rett Syndrome phenotype. Biol. Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.08.018. Published online August 24, 2015. http://dx.doi.org/10.1016/j.biopsych.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seamans J. Losing inhibition with ketamine. Nat. Chem. Biol. 2008;4:91–93. doi: 10.1038/nchembio0208-91. [DOI] [PubMed] [Google Scholar]
- 66.Jin YH, et al. Ketamine differentially blocks sensory afferent synaptic transmission in medial nucleus tractus solitarius (mNTS) Anesthesiology. 2003;98:121–132. doi: 10.1097/00000542-200301000-00021. [DOI] [PubMed] [Google Scholar]
- 67.Lepack AE, et al. BDNF release is required for the behavioral actions of ketamine. Int. J. Neuropsychopharmacol. 2015 doi: 10.1093/ijnp/pyu033. Published online October 31, 2014. http://dx.doi.org/10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am. J. Psychiatry. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- 69.Ricciardi S, et al. Reduced AKT/mTOR signaling and protein synthesis dysregulation in a Rett syndrome animal model. Hum. Mol. Genet. 2011;20:1182–1196. doi: 10.1093/hmg/ddq563. [DOI] [PubMed] [Google Scholar]
- 70.Dwyer JM, Duman RS. Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid-acting antidepressants. Biol. Psychiatry. 2013;73:1189–1198. doi: 10.1016/j.biopsych.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bogaerts J, et al. Clinical trial designs for rare diseases: studies developed and discussed by the International Rare Cancers Initiative. Eur. J. Cancer. 2015;51:271–281. doi: 10.1016/j.ejca.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tudur Smith C, et al. Methodology of clinical trials for rare diseases. Best Pract. Res. Clin. Rheumatol. 2014;28:247–262. doi: 10.1016/j.berh.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 73.van der Lee JH, et al. Efficient ways exist to obtain the optimal sample size in clinical trials in rare diseases. J. Clin. Epidemiol. 2008;61:324–330. doi: 10.1016/j.jclinepi.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 74.Bauer P, Brannath W. The advantages and disadvantages of adaptive designs for clinical trials. Drug Discov. Today. 2004;9:351–357. doi: 10.1016/S1359-6446(04)03023-5. [DOI] [PubMed] [Google Scholar]
- 75.Gerss JW, Kopcke W. Clinical trials and rare diseases. Adv. Exp. Med. Biol. 2010;686:173–190. doi: 10.1007/978-90-481-9485-8_11. [DOI] [PubMed] [Google Scholar]
- 76.Takeuchi F, et al. Prednisolone improves walking in Japanese Duchenne muscular dystrophy patients. J. Neurol. 2013;260:3023–3029. doi: 10.1007/s00415-013-7104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blat Y, Blat S. Drug discovery of therapies for Duchenne muscular dystrophy. J. Biomol. Screen. 2015;20:1189–1203. doi: 10.1177/1087057115586535. [DOI] [PubMed] [Google Scholar]
- 78.Monros E, et al. Rett syndrome in Spain: mutation analysis and clinical correlations. Brain Dev. 2001;23(Suppl. 1):S251–S253. doi: 10.1016/s0387-7604(01)00374-6. [DOI] [PubMed] [Google Scholar]
- 79.FitzGerald PM, et al. Rett syndrome and associated movement disorders. Mov. Disord. 1990;5:195–202. doi: 10.1002/mds.870050303. [DOI] [PubMed] [Google Scholar]
- 80.Cuddapah VA, et al. Methyl-CpG-binding protein 2 (MECP2) mutation type is associated with disease severity in Rett syndrome. J. Med. Genet. 2014;51:152–158. doi: 10.1136/jmedgenet-2013-102113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Motil KJ, et al. Gastrointestinal and nutritional problems occur frequently throughout life in girls and women with Rett syndrome. J. Pediatr. Gastroenterol. Nutr. 2012;55:292–298. doi: 10.1097/MPG.0b013e31824b6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glaze DG, et al. Epilepsy and the natural history of Rett syndrome. Neurology. 2010;74:909–912. doi: 10.1212/WNL.0b013e3181d6b852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Percy AK, et al. Rett syndrome: controlled study of an oral opiate antagonist, naltrexone. Ann. Neurol. 1994;35:464–470. doi: 10.1002/ana.410350415. [DOI] [PubMed] [Google Scholar]
- 84.Glaze DG, et al. A study of the treatment of Rett syndrome with folate and betaine. J. Child Neurol. 2009;24:551–556. doi: 10.1177/0883073808327827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khwaja OS, et al. Safety, pharmacokinetics, and preliminary assessment of efficacy of mecasermin (recombinant human IGF-1) for the treatment of Rett syndrome. Proc. Natl. Acad. Sci. U.S.A. 2014;111:4596–4601. doi: 10.1073/pnas.1311141111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hagebeuk EE, et al. Folinic acid supplementation in Rett syndrome patients does not influence the course of the disease: a randomized study. J. Child Neurol. 2012;27:304–309. doi: 10.1177/0883073811417184. [DOI] [PubMed] [Google Scholar]
- 87.Kesselheim AS, Gagne JJ. Strategies for post-marketing surveillance of drugs for rare diseases. Clin. Pharmacol. Ther. 2014;95:265–268. doi: 10.1038/clpt.2013.218. [DOI] [PubMed] [Google Scholar]
- 88.Hampson LV, et al. Elicitation of expert prior opinion: application to the MYPAN trial in childhood polyarteritis nodosa. PLoS ONE. 2015;10:e0120981. doi: 10.1371/journal.pone.0120981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lewis JD, et al. Purification, sequence and cellular localisation of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69:905–914. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- 90.Nan X, et al. Dissection of the methyl-CpG binding domain from the chromosomal protein MeCP2. Nucleic Acids Res. 1993;21:4886–4892. doi: 10.1093/nar/21.21.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skene PJ, et al. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baubec T, et al. Methylation-dependent and -independent genomic targeting principles of the MBD protein family. Cell. 2013;153:480–492. doi: 10.1016/j.cell.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 93.Guo JU, et al. Distribution, recognition and regulation of non-CpG methylation in the adult mammalian brain. Nat. Neurosci. 2014;17:215–222. doi: 10.1038/nn.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gabel HW, et al. Disruption of DNA-methylation-dependent long gene repression in Rett syndrome. Nature. 2015;522:89–93. doi: 10.1038/nature14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Valinluck V, et al. Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2) Nucleic Acids Res. 2004;32:4100–4108. doi: 10.1093/nar/gkh739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen L, et al. MeCP2 binds to non-CG methylated DNA as neurons mature, influencing transcription and the timing of onset for Rett syndrome. Proc. Natl. Acad. Sci. U.S.A. 2015;112:5509–5514. doi: 10.1073/pnas.1505909112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ballestar E, et al. Effects of Rett syndrome mutations of the methyl-CpG binding domain of the transcriptional repressor MeCP2 on selectivity for association with methylated DNA. Biochemistry. 2000;39:7100–7106. doi: 10.1021/bi0001271. [DOI] [PubMed] [Google Scholar]
- 100.Lyst MJ, et al. Rett syndrome mutations abolish the interaction of MeCP2 with the NCoR/SMRT co-repressor. Nat. Neurosci. 2013;16:898–902. doi: 10.1038/nn.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lyst MJ, Bird A. Rett syndrome: a complex disorder with simple roots. Nat. Rev. Genet. 2015;16:261–275. doi: 10.1038/nrg3897. [DOI] [PubMed] [Google Scholar]
- 102.Nan X, et al. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell. 1997;88:471–481. doi: 10.1016/s0092-8674(00)81887-5. [DOI] [PubMed] [Google Scholar]
- 103.Nan X, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 104.Ben-Shachar S, et al. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum. Mol. Genet. 2009;18:2431–2442. doi: 10.1093/hmg/ddp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chahrour M, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Y, et al. Global transcriptional and translational repression in human-embryonic-stem-cell-derived Rett syndrome neurons. Cell Stem Cell. 2013;13:446–458. doi: 10.1016/j.stem.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yazdani M, et al. Disease modeling using embryonic stem cells: MeCP2 regulates nuclear size and RNA synthesis in neurons. Stem Cells. 2012;30:2128–2139. doi: 10.1002/stem.1180. [DOI] [PubMed] [Google Scholar]
- 108.Cheng TL, et al. MeCP2 suppresses nuclear microRNA processing and dendritic growth by regulating the DGCR8/Drosha complex. Dev. Cell. 2014;28:547–560. doi: 10.1016/j.devcel.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 109.Maunakea AK, et al. Intragenic DNA methylation modulates alternative splicing by recruiting MeCP2 to promote exon recognition. Cell Res. 2013;23:1256–1269. doi: 10.1038/cr.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Horike S, et al. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nat. Genet. 2005;37:31–40. doi: 10.1038/ng1491. [DOI] [PubMed] [Google Scholar]
- 111.Georgel PT, et al. Chromatin compaction by human MeCP2: Assembly of novel secondary chromatin structures in the absence of DNA methylation. J. Biol. Chem. 2003;278:32181–32188. doi: 10.1074/jbc.M305308200. [DOI] [PubMed] [Google Scholar]
- 112.Ausio J, et al. MeCP2: the long trip from a chromatin protein to neurological disorders. Trends Mol. Med. 2014;20:487–498. doi: 10.1016/j.molmed.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 113.Haas RH, et al. Therapeutic effects of a ketogenic diet in rett syndrome. Am. J. Med. Genet. 1986;25:225–246. doi: 10.1002/ajmg.1320250525. [DOI] [PubMed] [Google Scholar]
- 114.Zappella M. A double blind trial of bromocriptine in the Rett syndrome. Brain Dev. 1990;12:148–150. doi: 10.1016/s0387-7604(12)80198-7. [DOI] [PubMed] [Google Scholar]
- 115.Ellaway C, et al. Rett syndrome: randomized controlled trial of L-carnitine. J. Child Neurol. 1999;14:162–167. doi: 10.1177/088307389901400306. [DOI] [PubMed] [Google Scholar]
- 116.Ellaway CJ, et al. Medium-term open label trial of L-carnitine in Rett syndrome. Brain Dev. 2001;23(Suppl. 1):S85–S89. doi: 10.1016/s0387-7604(01)00346-1. [DOI] [PubMed] [Google Scholar]
- 117.Temudo T, et al. Evaluation of CSF neurotransmitters and folate in 25 patients with Rett disorder and effects of treatment. Brain Dev. 2009;31:46–51. doi: 10.1016/j.braindev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 118.Hagebeuk EE, et al. Clinical and electroencephalographic effects of folinic acid treatment in Rett syndrome patients. J. Child Neurol. 2011;26:718–723. doi: 10.1177/0883073810390037. [DOI] [PubMed] [Google Scholar]
- 119.Freilinger M, et al. Effects of creatine supplementation in Rett syndrome: a randomized, placebo-controlled trial. J. Dev. Behav. Pediatr. 2011;32:454–460. doi: 10.1097/DBP.0b013e31822177a8. [DOI] [PubMed] [Google Scholar]
- 120.Leoncini S, et al. Oxidative stress in Rett syndrome: natural history, genotype, and variants. Redox Rep. 2011;16:145–153. doi: 10.1179/1351000211Y.0000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Signorini C, et al. F(4)-neuroprostanes mediate neurological severity in Rett syndrome. Clin. Chim. Acta. 2011;412:1399–1406. doi: 10.1016/j.cca.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 122.Pini G, et al. IGF1 as a potential treatment for Rett Syndrome: safety assessment in six Rett patients. Autism Res. Treat. 2012;2012:679801. doi: 10.1155/2012/679801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.De Felice C, et al. Partial rescue of Rett syndrome by omega-3 polyunsaturated fatty acids (PUFAs) oil. Genes Nutr. 2012;7:447–458. doi: 10.1007/s12263-012-0285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maffei S, et al. Effects of omega-3 PUFAs supplementation on myocardial function and oxidative stress markers in typical Rett syndrome. Mediators of Inflamm. 2014;2014:983178. doi: 10.1155/2014/983178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.