Abstract
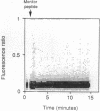
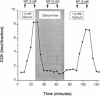
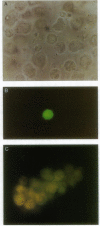
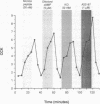
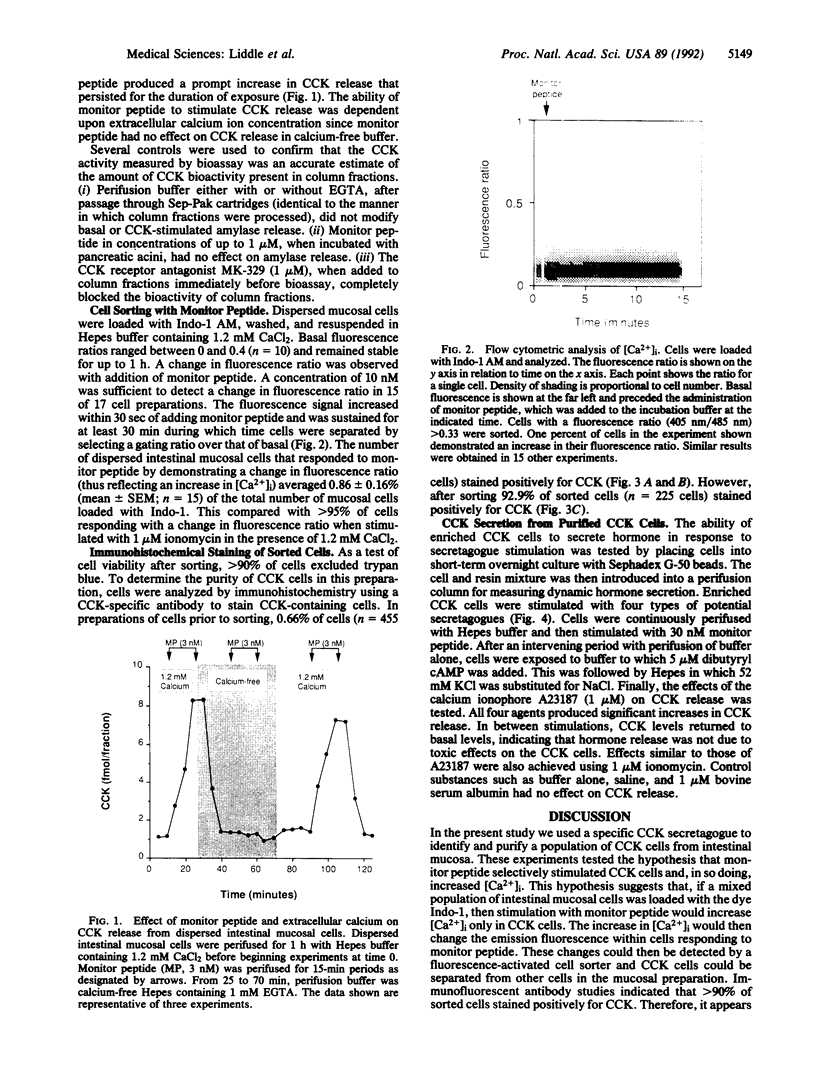
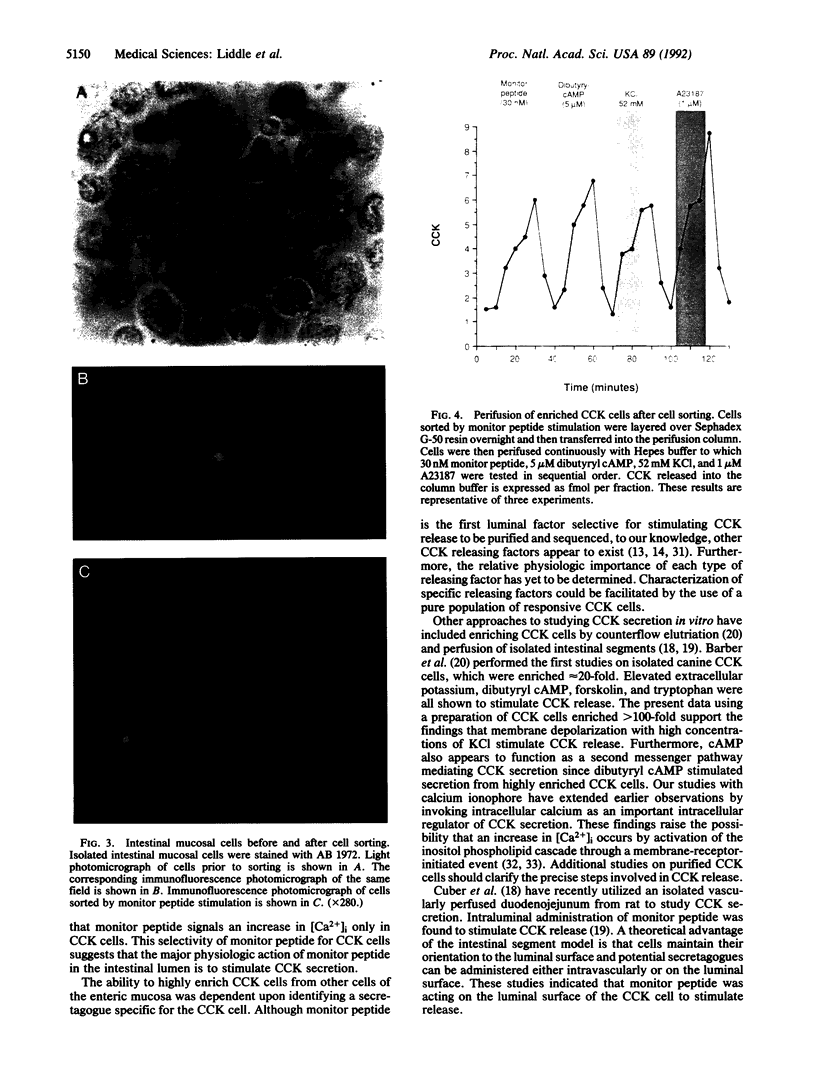
Cholecystokinin (CCK) is secreted from specific enteroendocrine cells of the upper small intestine upon ingestion of a meal. In addition to nutrients, endogenously produced factors appear to act within the gut lumen to stimulate CCK release. One such factor is a trypsin-sensitive CCK-releasing peptide found in pancreatic juice, known as monitor peptide. This peptide is active within the intestinal lumen and is hypothesized to stimulate CCK secretion by interacting directly with the CCK cell. We have found that monitor peptide releases CCK from isolated rat intestinal mucosal cells and that this effect is dependent upon extracellular calcium. In the present study, we used monitor peptide as a tool for isolating CCK cells from a population of small intestinal mucosal cells. Dispersed rat intestinal mucosal cells were loaded with the calcium-sensitive fluorochrome Indo-1, and CCK secretory cells were identified spectrofluorometrically by their change in fluorescence when stimulated with monitor peptide. Cells demonstrating a change in their emission fluorescence ratio were sorted using a fluorescence-activated cell sorter. More than 90% of the sorted cells stained positively for CCK with immunohistochemical staining. Furthermore, sorted cells secreted CCK when stimulated with membrane-depolarizing concentrations of potassium chloride, dibutyryl cAMP, calcium ionophore, and monitor peptide. These findings indicate that functional intestinal CCK cells can be highly enriched using fluorescence-activated cell sorting. Furthermore, monitor peptide appears to interact directly with CCK cells to signal CCK release through an increase in intracellular calcium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber D. L., Walsh J. H., Soll A. H. Release and characterization of cholecystokinin from isolated canine jejunal cells. Gastroenterology. 1986 Sep;91(3):627–636. doi: 10.1016/0016-5085(86)90632-3. [DOI] [PubMed] [Google Scholar]
- Buchan A. M., Polak J. M., Solcia E., Capella C., Hudson D., Pearse A. G. Electron immunohistochemical evidence for the human intestinal I cell as the source of CCK. Gut. 1978 May;19(5):403–407. doi: 10.1136/gut.19.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuber J. C., Bernard G., Fushiki T., Bernard C., Yamanishi R., Sugimoto E., Chayvialle J. A. Luminal CCK-releasing factors in the isolated vascularly perfused rat duodenojejunum. Am J Physiol. 1990 Aug;259(2 Pt 1):G191–G197. doi: 10.1152/ajpgi.1990.259.2.G191. [DOI] [PubMed] [Google Scholar]
- Cuber J. C., Vilas F., Charles N., Bernard C., Chayvialle J. A. Bombesin and nutrients stimulate release of CCK through distinct pathways in the rat. Am J Physiol. 1989 Jun;256(6 Pt 1):G989–G996. doi: 10.1152/ajpgi.1989.256.6.G989. [DOI] [PubMed] [Google Scholar]
- Evans W. S., Cronin M. J., Thorner M. O. Continuous perifusion of dispersed anterior pituitary cells: technical aspects. Methods Enzymol. 1983;103:294–305. doi: 10.1016/s0076-6879(83)03019-0. [DOI] [PubMed] [Google Scholar]
- Funakoshi A., Nakano I., Miyazaki K. Vasoactive intestinal polypeptide stimulates cholecystokinin secretion in perfused rat duodenum. Tohoku J Exp Med. 1989 Jan;157(1):55–59. doi: 10.1620/tjem.157.55. [DOI] [PubMed] [Google Scholar]
- Fushiki T., Iwai K. Two hypotheses on the feedback regulation of pancreatic enzyme secretion. FASEB J. 1989 Feb;3(2):121–126. doi: 10.1096/fasebj.3.2.2644146. [DOI] [PubMed] [Google Scholar]
- Green G. M., Lyman R. L. Feedback regulation of pancreatic enzyme secretion as a mechanism for trypsin inhibitor-induced hypersecretion in rats. Proc Soc Exp Biol Med. 1972 May;140(1):6–12. doi: 10.3181/00379727-140-36384. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Hopman W. P., Jansen J. B., Lamers C. B. Effect of atropine on the plasma cholecystokinin response to intraduodenal fat in man. Digestion. 1984;29(1):19–25. doi: 10.1159/000199003. [DOI] [PubMed] [Google Scholar]
- Iwai K., Fukuoka S., Fushiki T., Kodaira T., Ikei N. Elevation of plasma CCK concentration after intestinal administration of a pancreatic enzyme secretion-stimulating peptide purified from rat bile-pancreatic juice: analysis with N-terminal region specific radioimmunoassay. Biochem Biophys Res Commun. 1986 Apr 29;136(2):701–706. doi: 10.1016/0006-291x(86)90496-1. [DOI] [PubMed] [Google Scholar]
- Iwai K., Fukuoka S., Fushiki T., Tsujikawa M., Hirose M., Tsunasawa S., Sakiyama F. Purification and sequencing of a trypsin-sensitive cholecystokinin-releasing peptide from rat pancreatic juice. Its homology with pancreatic secretory trypsin inhibitor. J Biol Chem. 1987 Jul 5;262(19):8956–8959. [PubMed] [Google Scholar]
- Kanayama S., Liddle R. A. Somatostatin regulates duodenal cholecystokinin and somatostatin messenger RNA. Am J Physiol. 1990 Mar;258(3 Pt 1):G358–G364. doi: 10.1152/ajpgi.1990.258.3.G358. [DOI] [PubMed] [Google Scholar]
- Levan V. H., Green G. M. Effect of atropine on rat pancreatic secretory response to trypsin inhibitors and protein. Am J Physiol. 1986 Jul;251(1 Pt 1):G64–G69. doi: 10.1152/ajpgi.1986.251.1.G64. [DOI] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Rosen M. S., Taplitz R. A., Williams J. A. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985 Apr;75(4):1144–1152. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Williams J. A. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984 Sep;87(3):542–549. [PubMed] [Google Scholar]
- Lu L., Louie D., Owyang C. A cholecystokinin releasing peptide mediates feedback regulation of pancreatic secretion. Am J Physiol. 1989 Feb;256(2 Pt 1):G430–G435. doi: 10.1152/ajpgi.1989.256.2.G430. [DOI] [PubMed] [Google Scholar]
- Miyasaka K., Guan D. F., Liddle R. A., Green G. M. Feedback regulation by trypsin: evidence for intraluminal CCK-releasing peptide. Am J Physiol. 1989 Aug;257(2 Pt 1):G175–G181. doi: 10.1152/ajpgi.1989.257.2.G175. [DOI] [PubMed] [Google Scholar]
- Nakano I., Miyazaki K., Funakoshi A., Tateishi K., Hamaoka T., Yajima H. Gastrin-releasing peptide stimulates cholecystokinin secretion in perfused rat duodenum. Regul Pept. 1988 Nov;23(2):153–159. doi: 10.1016/0167-0115(88)90023-7. [DOI] [PubMed] [Google Scholar]
- Nakano I., Tawil T., Spannagel A. W., Liddle R. A., Green G. M. Atropine enhances food-stimulated CCK secretion in the rat. Pancreas. 1990 Sep;5(5):621–625. doi: 10.1097/00006676-199009000-00020. [DOI] [PubMed] [Google Scholar]
- Owyang C., Louie D. S., Tatum D. Feedback regulation of pancreatic enzyme secretion. Suppression of cholecystokinin release by trypsin. J Clin Invest. 1986 Jun;77(6):2042–2047. doi: 10.1172/JCI112534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. R., Herzenberg L. A. Fluorescence-activated cell sorting: theory, experimental optimization, and applications in lymphoid cell biology. Methods Enzymol. 1984;108:197–241. doi: 10.1016/s0076-6879(84)08086-1. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Richelsen B., Rehfeld J. F., Larsson L. I. Antral gland cell column: a method for studying release of gastric hormones. Am J Physiol. 1983 Oct;245(4):G463–G469. doi: 10.1152/ajpgi.1983.245.4.G463. [DOI] [PubMed] [Google Scholar]
- Tateishi K., Hamaoka T., Sugiura N., Yanaihara C., Yanaihara N. A novel immunization procedure for production of anti-cholecystokinin-specific antiserum of low cross-reactivity. J Immunol Methods. 1981;47(2):249–258. doi: 10.1016/0022-1759(81)90125-3. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescence measurement and photochemical manipulation of cytosolic free calcium. Trends Neurosci. 1988 Oct;11(10):419–424. doi: 10.1016/0166-2236(88)90192-0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescent probes of cell signaling. Annu Rev Neurosci. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- de Jong A. J., Klamer M., Jansen J. B., Lamers C. B. Effect of atropine and somatostatin on bombesin-stimulated plasma gastrin, cholecystokinin and pancreatic polypeptide in man. Regul Pept. 1987 May;17(5):285–293. doi: 10.1016/0167-0115(87)90286-2. [DOI] [PubMed] [Google Scholar]