Abstract
Bacterial and viral abundances were measured in 24 lakes with dissolved organic carbon (DOC) concentrations ranging from 3 to 19 mg of C liter−1. In addition, a laboratory experiment was performed to test the effects of different sources of carbon (i.e., glucose and fulvic acids) and nutrients on the dynamics of viruses and bacteria. In the lake survey, no correlation was found between virus abundance and DOC concentration, yet there was a significant positive correlation between bacterial abundance and DOC concentration. A negative correlation was found between the virus-to-bacteria ratio and DOC level. These results are in agreement with our findings in the laboratory, where virus counts were significantly lower in treatments with fulvic acid additions than in a control (mean, 67.4% ± 6.5% of the control). Virus counts did not differ significantly among the control and treatments with glucose, indicating that it was the type of organic carbon and not quantity which had an impact on viruses. Results from this study suggest that the way viruses control bacterial assemblages in humic lakes is different from the mechanism in clear water systems.
Since the utilization of the direct counting of viruses in aquatic ecosystems was introduced (1, 12, 21, 27), there has been a growing body of evidence indicating that viruses are the most abundant and dynamic members of aquatic microbial communities (3, 19, 20). Viral lysis plays a significant role in the cycling of nutrients and organic matter (reference 7 and references therein) and can match grazing by protists as a source of mortality for bacteria (8, 36). The distribution of viruses has been found to be determined by factors that affect the activity and density of the host populations, mainly bacteria (5, 6, 14, 30, 36). Several studies suggest that bacterial mortality caused by viruses is higher in more productive environments (22, 36). Most studies have been conducted in marine environments, and consequently the effects of dissolved organic carbon (DOC) on virus distribution have seldom been discussed.
Lakes with high concentrations of humic substances, also known as brown-water lakes, bog lakes, or humic lakes, occur all over the world. Contrary to what was previously assumed, bacteria can sustain growth on humic substrates (31, 32). Indeed, humic substances, many of which are of allochthonous origin, represent the main energy source for microbial food webs in humic lakes (15, 16). High-molecular-weight dissolved organic matter (HMW DOM) may influence the interactions between viruses and bacteria in several ways. As viral abundance has been reported to correlate to bacterial abundance (40), factors that influence bacteria should also have an impact on viruses. One would predict that the positive effects of humic substances on bacteria would reflect positively on the abundance of viruses. However, there is evidence that HMW DOM may negatively affect virus infection of bacterial cells in the aquatic environment (23).
Comparisons of viral and bacterial dynamics between humic and clear water lakes are rare, and differing conclusions on the interactions between viruses and DOC have been described. In a recent study, Vrede et al. (35) investigated a humic lake and a clear water lake, and despite finding higher bacterial abundances in the humic lake, they found no difference in viral abundances between the two lakes. Maranger and Bird (20), who surveyed 22 lakes in Québec, examined correlations between viral abundance and chlorophyll (Chl) a, bacterial abundance, DOC, and total phosphorus, and found that Chl a was the best predictor of viral abundance, while the concentration of DOC did not correlate to virus abundance. However, Maranger and Bird (20) warned that the range of variation in DOC levels in their study area was small in comparison to areas where humic lakes are present. Laybourn-Parry et al. (17) found a strong correlation between viruses and DOC in nine Antarctic lakes, with DOC concentrations varying between 0.8 and 30.7 mg of C liter−1. The lakes studied by Laybourn-Parry et al. (17) were not humic lakes but spanned clear freshwater to saline lakes.
In this study, bacterial and viral abundances were measured in 24 lakes with DOC concentrations ranging from 3 to 19 mg of C liter−1. Our objective was to assess relationships between viral abundance and the major variables that have been shown to affect bacterial abundance in freshwaters. In addition, a laboratory experiment was performed to test the effects of different sources of carbon (i.e., glucose and fulvic acids) and nutrients on the abundance of viruses and bacteria. To the best of our knowledge, this study is the first systematic report of the effects of humic substances on the interactions between viruses and bacteria in freshwaters.
MATERIALS AND METHODS
Field sampling and lake survey.
Samples were collected on 6 to 7 May 2003 from the upper 1 m of the water column in 24 lakes by using a 1-m-long Plexiglas tube. The tube was emptied in a 5-liter acid (10% HCl)- and Milli-Q water-rinsed polycarbonate bottle. The temperature in the surface ranged between 8.7 and 12.7°C. The lakes were located within the boreal region of southern Sweden (ca. 57°10′N and 14°35′E) within a 30-km radius. The lakes were chosen to represent a DOC gradient from clear water to humic water. The purpose of the lake survey was to identify any relationships between viruses, bacteria, and DOC concentrations. This was the same approach as used by Maranger and Bird (20), who surveyed 22 Canadian lakes. Our lakes presented a large variation in DOC levels, while the variation in levels of Chl a was smaller than the one found by Maranger and Bird (20).
Duplicate 5-ml samples for viruses and bacteria enumeration were immediately fixed with prefiltered (0.02-μm-pore-size filter) glutaraldehyde (2% final concentration). Samples for total phosphorus and nitrogen were taken directly from the 5-liter polycarbonate bottle, and 20-ml DOC samples were prefiltered in 0.2-μm-pore-size polycarbonate filters. All samples were stored at 4°C and processed within 1 week for bacterial and viral abundances and DOC concentrations and within 2 weeks for total nitrogen and phosphorus. Samples for Chl a between 0.5 and 1 liter were filtered through GF/C filters immediately upon arrival at the lab and kept frozen until processing within 1 week.
The determination of lysogeny (defined here as the percentage of bacteria in the community containing a dormant phage) was conducted in samples from five lakes by prophage induction with mitomycin C (a potent inducing agent of lysis) treatment, according to the method described by Paul and Jiang (25). In brief, triplicate 1-ml samples with and without the addition of mitomycin C (final concentration, 1 μg ml−1) were incubated for 18 h at ambient temperature (7.5 to 12.0°C) and then preserved with glutaraldehyde. Triplicate initial samples were immediately fixed with glutaraldehyde. The percentage of lysogenic bacteria was calculated as follows: % of lysogeny = {[(VLPT − VLPC)/BZ]/BI} × 100, where VLPT and VLPC are the viral abundances in samples treated with mitomycin C and control, respectively, BZ is the average burst size, and BI is the bacterial abundance at the start of incubations. We used 30 as the average burst size, which is within the range for oligotrophic and more productive systems found by Weinbauer and Suttle (37).
Effect of carbon and nutrient amendments on the dynamics of bacteria and viruses.
A laboratory experiment was conducted to test the effects of different sources of carbon (i.e., glucose and fulvic acids) and nutrients on the dynamics of viruses and bacteria. Approximately 40 liters from the upper 1 m of the water column was collected from Lake Skärlen, which has one of the lowest DOC concentrations among the lakes surveyed in this study (3.3 mg of C liter−1). One week after the sampling, the water was filtered through 142-mm Gelman A/E filters (nominal pore size, 0.7 μm) in order to remove particulate matter and grazers. Thereafter, the filtered water was stored for 96 h in the dark at 20°C before incubations were begun. The initial sampling of bacterial and viral abundances, lysogeny incidence, and bacterial and viral production were done just before the additions of glucose, nutrients, and humic substances. The water was distributed in 1-liter triplicate acid (10% HCl)- and Milli-Q water-rinsed glass bottles. Eight treatments were established in the experiment: no amendments (control), additions of humic substances in two different concentrations (H1 and H2 with final concentrations of 5 and 11 mg of C liter−1, respectively), addition of glucose (final concentration of 11 mg of C liter−1), addition of nitrogen and phosphorus (NP treatment; final concentrations of 10 and 1 μM, respectively), addition of humic substances and nutrients (H1NP and H2NP), and addition of glucose and nutrients (GNP treatment). The chemical characteristics and DOM absorptivity at 320 nm of the water in the different treatments are shown in Table 1. Samples for determining bacterial and viral abundances were taken 4, 10, 72, and 360 h after the beginning of the experiment and fixed with glutaraldehyde. Bacterial production was measured after 24, 72, 216, and 360 h. The incidence of lysogeny was measured on a subset of samples from 72 and 360 h with carbon addition and in the control. The determination of the incidence of lysogeny was done as described above.
TABLE 1.
Chemical and absorptivity characteristics of Lake Skärlen water and treatments in the laboratory experimenta
| Treatment | DOC concn (mg of C/liter) | Absorptivity at 320 nm (m−1) | DOC-normalized absorptivity (liters/m/mg of C) |
|---|---|---|---|
| Control | 3.0 | 5.6 | 1.8 |
| H1 | 8.3 | 32.7 | 3.9 |
| H2 | 13.9 | 59.3 | 4.3 |
| G | 13.9 | 6.1 | 0.4 |
| NP | 3.0 | 5.5 | 1.9 |
| H1NP | 8.5 | 33.0 | 3.8 |
| H2NP | 13.9 | 59.2 | 4.2 |
| GNP | 13.9 | 5.7 | 0.4 |
Results given are the mean initial values from four samples.
Nutrients and carbon source solutions were freshly made with filtered (0.2-μm-pore-size filter) Milli-Q water. Solutions of NH4NO3 and KH2PO4 were used as sources of N and P, respectively. Humic substances were added in the form of fulvic acid extracted from Laurentian soils (Fredriks Research Products, Amsterdam, The Netherlands). A partial elemental composition of the fulvic acid, as given by the supplier, was C (43.2%), H (4%), N (0.83%), Na (0.27%), Fe (0.013%), and ash (<1.0%).
Bacterial and viral abundances.
Bacteria and viruses were counted by epifluorescence microscopy with SYBR Gold stain (10,000× original dilution; Molecular Probes) and an Olympus microscope, according to the method of Chen et al. (4). Samples of 10 to 750 μl were filtered on 0.02-μm-pore-size Anodisc membrane filters (Whatman), with a 0.45-μm-pore-size backing membrane filter. The filter was then laid, sample side up, on a drop of SYBR Gold working solution (25 μl of 100× diluted SYBR Gold solution and 75 μl of filtered [0.02-μm-pore-size filter] Milli-Q water), for 15 min in the dark. After drying, the filter was mounted on a glass slide with a drop of Molecular Probe Slow Fade antifade solution. For each filter, >200 viruses and >150 bacteria were counted on 10 to 15 fields of view selected randomly.
Additions of 4, 8, and 14 mg of fulvic acid liter−1 (final concentration) were made to duplicate glutaraldehyde-fixed samples from a clear water lake (3 mg of C liter−1), in order to ensure that the fulvic acid addition did not interfere with the microscope counts or cause underestimations due to a possible deterioration of fluorescence. Viruses were counted as above. Results from the test revealed a nonsignificant reduction of 15% in viral counts of fixed samples with fulvic acid additions of 8 and 14 mg of C liter−1 (P = 0.083, as determined by analysis of variance [ANOVA]). Further, we measured the speed of fading of the viruses in each treatment, according to a modification of the method described by Bettarel et al. (2). Briefly, viruses in the same microscope field were counted every 30 s for 5 min. Four replicates were counted for each treatment. Data were fitted in a negative linear regression between viruses and time (all r2 > 0.90; number of samples, 11). On average, it took approximately 200 s for the virus counts to be reduced to 50% of the initial values, and no significant differences between samples with and without fulvic acid additions were found (P = 0.751, as determined by an analysis of covariance).
Bacterial production.
Bacterial production was estimated by the thymidine incorporation method (9), as modified by Smith and Azam (29). Triplicate 1.7-ml samples were collected into 2-ml microcentrifuge tubes, and [methyl-3H]thymidine (84.0 Ci mmol−1) was added to a final concentration of 30 nM. Duplicate control samples were inactivated with 90 μl of 100% trichloroacetic acid (TCA) and thymidine label was added. The samples were then incubated for 1 h. After incubation, the samples were cooled to 4°C, and 90 μl of 100% TCA was added to all samples except for the controls. Thereafter, the tubes were centrifuged at 16,000 × g at 4°C for 10 min, and the supernatant was aspirated. Then, 1.7 ml of ice-cold 5% TCA was added to each tube and vortex mixed, and another centrifugation step was performed. The TCA was aspirated, and 1.7 ml of ice-cold 80% ethanol was added, followed by another centrifugation step. The final supernatant was aspirated, 1 ml of scintillation cocktail was added, and the samples were counted by liquid scintillation.
Analytical methods.
Samples (∼20-ml) for DOC analysis in the laboratory experiment were filtered through 0.2-μm-pore-size polycarbonate filters and immediately transferred into acid-rinsed, precombusted (+500°C, overnight) glass vials with Teflon-lined screw caps. Samples from the lake survey were kept in a refrigerator and analyzed after a few days. DOC concentration was analyzed by the Pt-catalyzed high-temperature combustion method and a Shimadzu TOC-5000 total carbon analyzer equipped with an ASI-5000 auto sampler (10). Inorganic carbon was purged for 5 min from acidified samples (HCl, pH ∼2). A minimum of three replicate injections were made on the carbon analyzer, resulting in a coefficient of variation of less than 2% for each analysis. Chl a was extracted by using ethanol (13) after subsamples were filtered through GF/C (47-mm) filters and stored at −20°C. Filters were extracted in 5 ml of 95% ethanol at 4°C overnight. The samples were then refiltered through Whatman GF/C filters (25-mm) and measured spectrophotometrically at 665 and 750 nm. Corrections for turbidity were made by subtracting the absorbance at 750 nm from the 665-nm measurements. Total phosphorus and nitrogen were analyzed spectrophotometrically at 880 and 550 nm, respectively, after potassium peroxide disulfate digestion.
Statistics.
Statistical analyses were performed in Genstat (version 6.1 for Windows). For the field survey, Pearson correlations were examined between bacterial and viral abundances, virus-to-bacteria ratios (VBRs), inorganic nutrients, DOC concentration, absorbance at 320 nm, and Chl a. Data from the laboratory experiment, after log transformation, were normally distributed and analyzed by using repeated-measures ANOVA. Post hoc testing was performed by using Dunnett's method to compare each treatment to the control. Multivariate ANOVA (MANOVA) was further performed for tests where interactions between treatment and time were found. Initial values were not taken into account in the repeated-measures ANOVA.
RESULTS
Lake survey.
Chemical characteristics and the DOM absorptivity at 320 nm of the lakes surveyed are presented in Table 2. DOC concentrations ranged from 3.2 to 19.3 mg of C liter−1 among the 24 lakes surveyed. The DOC concentration had a significant positive correlation with total nitrogen and phosphorus and absorptivity characteristics (r2 = 0.70, 0.60, and 0.97, respectively; P < 0.01). The maximum Chl a value observed was 12.4 μg of Chl a liter−1, with an average of 6.0 ± 3.2 μg of Chl a liter−1 (Table 2). Bacterial numbers ranged from 0.2 × 106 to 1.1 × 106 cells ml−1 (mean, 0.56 × 106± 0.21 × 106 cells ml−1) and were positively related to DOC and total nitrogen and phosphorus concentrations (Table 3). The number of virus-like-particles (VLP) varied less than the number of bacteria, between 1.2 × 107 and 2.4 × 107 VLP ml−1 (mean, 1.71 × 107± 0.36 × 107 VLP ml−1) and did not correlate to any of the chemical characteristics or the DOM absorptivity analyzed in this study. VBRs varied widely from 13 to 73 (mean, 34.8 ± 14.3) and correlated negatively with DOC concentration (Table 3). The application of multiple-regression analysis did not improve the predictability of either bacterial or viral abundance.
TABLE 2.
Chemical and absorptivity characteristics of the studied lakes
| Lake | DOC concn (mg of C/liter) | Absorptivity at 320 nm (m−1) | DOC-normalized absorptivity (liters/m/mg of C) | P (μg/liter) | N (μg/liter) | Chl a (μg/liter) |
|---|---|---|---|---|---|---|
| Näversjön | 3.2 | 4.1 | 1.3 | 1 | 250 | 2.1 |
| Skärlen | 3.3 | 4.1 | 1.2 | 8 | 200 | 3.2 |
| Klintsjön | 3.3 | 6.9 | 2.1 | 1 | 260 | 2.5 |
| Lundasjön | 4.5 | 7.0 | 1.6 | 10 | 400 | 9.4 |
| Hindsen | 5.0 | 10.2 | 2.0 | 1 | 370 | 4.9 |
| Feresjön | 5.6 | 16.7 | 3.0 | 5 | 300 | 3.2 |
| Hacksjön | 6.4 | 17.4 | 2.7 | 6 | 360 | 5.4 |
| Homehultsjön | 8.0 | 25.1 | 3.1 | 6 | 560 | 7.6 |
| Lången I | 8.6 | 24.8 | 2.9 | 7 | 500 | 8.3 |
| Allgunnen | 8.7 | 24.0 | 2.8 | 8 | 550 | 6.1 |
| Kalvsjön | 8.9 | 32.6 | 3.6 | 7 | 500 | 3.6 |
| Hisshultasjön | 9.0 | 27.0 | 3.0 | 10 | 660 | 11.4 |
| Asasjön | 9.1 | 32.7 | 3.6 | 10 | 520 | 4.8 |
| Lången II | 9.2 | 33.0 | 3.6 | 9 | 540 | 2.4 |
| Lyen | 10.1 | 35.9 | 3.5 | 14 | 720 | 12.3 |
| Åbodasjön | 10.4 | 40.2 | 3.9 | 9 | 660 | 7.4 |
| Stråken | 10.8 | 41.5 | 3.9 | 13 | 590 | 12.4 |
| Gyslättasjön | 11.2 | 42.7 | 3.8 | 10 | 530 | 7.5 |
| Bråtasjön | 11.5 | 45.2 | 3.9 | 8 | 550 | 3.8 |
| Skärshultsjön | 14.6 | 66.3 | 4.6 | 12 | 660 | 2.9 |
| Grunnen | 16.5 | 76.7 | 4.7 | 14 | 760 | 3.9 |
| Skärsjön | 17.3 | 80.6 | 4.7 | 10 | 660 | 3.4 |
| Fräjen | 18.3 | 93.8 | 5.1 | 19 | 740 | 10.3 |
| N. Trehörning | 19.3 | 110.3 | 5.7 | 13 | 610 | 4.1 |
TABLE 3.
Pearson correlation matrix between bacteria, viruses, and VBRs and study parametersa
| Parameter | Bacteria | Virus | VBR |
|---|---|---|---|
| DOC | 0.58** | −0.07 NS | −0.54** |
| Absorptivity | 0.58** | −0.05 NS | −0.51* |
| Abs DOC | 0.55** | −0.06 NS | −0.55** |
| P | 0.62** | 0.07 NS | −0.43* |
| N | 0.56** | 0.06 NS | −0.53** |
| Chl a | 0.33 NS | 0.31 NS | −0.23 NS |
Abs DOC, DOC-normalized absorptivity; NS, not significant; *, 0.01 < P < 0.05; **, P < 0.01 (n = 24).
Four of five lakes tested showed a significant increase in VLP in response to the addition of mitomycin C (Table 4). The sample that did not show a significant increase in VLP was from Lake Skärlen, the lake with one of the lowest concentrations of DOC sampled in this study. We did not find any correlation between DOC concentration and induction (P > 0.05; number of samples, 5).
TABLE 4.
Estimation of percentages of lysogenic bacteria in the total bacterial community in five lakesa
| Lake | DOC concn (mg of C/liter) | VLP (control) (107/ml) | Mitomycin C (% of the control) | Lysogeny (%) |
|---|---|---|---|---|
| Skärlen | 3.3 | 1.65 ± 0.12 | 103.5 NS | NA |
| Feresjön | 5.6 | 1.57 ± 0.08 | 129.3** | 31.8 |
| Homehultsjön | 8.0 | 3.38 ± 0.29 | 109.7* | 13.5 |
| Stråken | 10.8 | 2.10 ± 0.09 | 127.4** | 23.1 |
| Skärshultsjön | 14.6 | 2.62 ± 0.49 | 126.8* | 18.1 |
VLP values are given as the means ± standard deviations. NA, not applicable; NS, not significant; *, 0.05 < P < 0.1; **, P < 0.05.
Effect of carbon and nutrient amendments on the dynamics of bacteria and viruses.
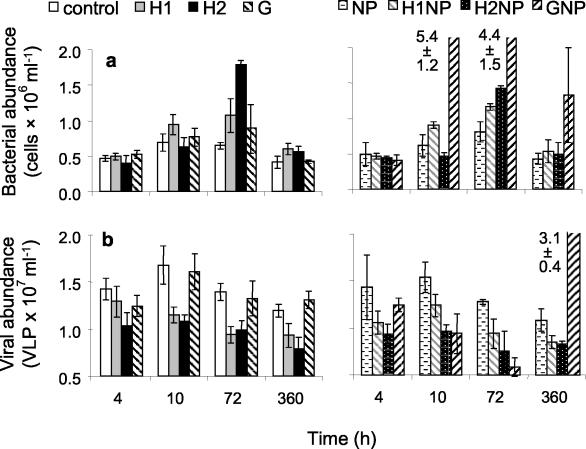
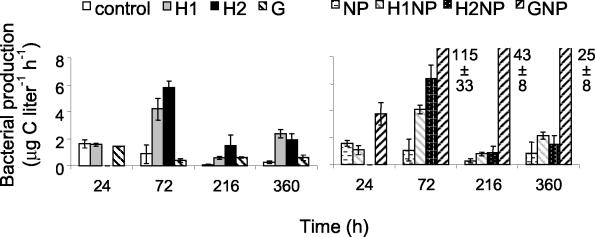
Bacterial abundance and production increased when humic matter and glucose in combination with nutrients were added to Lake Skärlen, a clear water lake (Fig. 1 and 2). Repeated measurements of ANOVA revealed that additions of inorganic nutrients in combination with a carbon source and additions of fulvic acids alone stimulated bacterial growth as measured by abundance and production (Table 5). Additions of 11 mg of C liter−1 of humic matter (i.e., treatments H2 and H2NP) had an initial negative effect on bacterial growth during the first 48 h. Thereafter, levels of bacterial abundance and production were significantly higher in all treatments with the fulvic acid addition relative to the control (P < 0.05, as determined by MANOVA). The addition of nitrogen and phosphorus (NP treatment) or glucose alone did not significantly affect bacterial abundance and production (Table 5).
FIG. 1.
Changes in bacterial (a) and viral (b) abundances in the laboratory experiment over time. Treatments are no amendments (control), fulvic acid addition (H1 and H2; final concentrations of 5 and 11 mg of C liter−1, respectively), glucose addition (G; final concentration of 11 mg of C liter−1), nitrogen and phosphorus addition (NP; final concentrations of 10 and 1 μM, respectively), fulvic acid at two concentrations and nutrient addition (H1NP and H2NP), and glucose and nutrient additions (GNP). Results are given as the means ± standard deviations (number of samples, 3).
FIG. 2.
Changes in bacterial production over time. Treatments are the same as described in the legend of Fig. 1. Results are given as the means ± standard deviations (number of samples, 3). G, glucose.
TABLE 5.
Results of repeated-measures ANOVA and subsequent Dunnett post hoc testsa
| Treatment | Virus abundance | Bacterial production | VBR |
|---|---|---|---|
| NP | NS | NS | NS |
| H1 | − | + | − |
| H2 | − | NSb | − |
| G | NS | NS | NS |
| H1NP | − | + | − |
| H2NP | − | NSb | − |
| GNP | NSb | + | −c |
Significant negative (−) and positive (+) effects in the variables analyzed relative to the control are indicated. NS, not significantly different.
Interaction between time and treatment was found. Therefore, a MANOVA test was performed and indicated a significant initial inhibition followed by significant stimulation.
Interaction between time and treatment was found. Therefore, a MANOVA test was performed and indicated a significant initial inhibition followed by no significant difference by the end of the experiment.
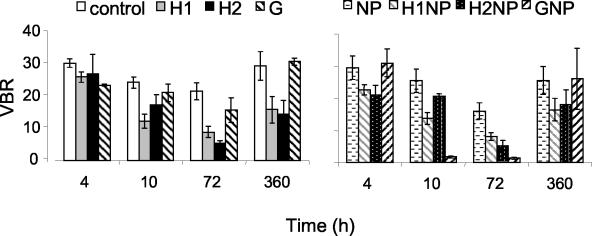
Viral abundance ranged from 42 to 257% of the control VLP concentrations. Overall, the virus counts were significantly lower in the treatments with the fulvic acid additions than in the control (mean, 67.4% ± 6.5% of the control). Virus counts did not differ significantly among the control and treatments with glucose or inorganic nutrients. VBRs during the entire laboratory experiment ranged from 1 to 31 for all treatments (Fig. 3). Significantly lower values were found for the treatments with the fulvic acid additions than for the control. A lower VBR was also found in the GNP treatment; however, no differences between the GNP treatment and the control were found after 360 h (P > 0.05, as determined by MANOVA). Statistically significant lysogeny was detected on only one occasion during the laboratory experiment (13% lysogeny in the H1NP treatment after 72 h of incubation), while a small nonsignificant trend of increase was found in the other fulvic acid treatments relative to the control (data not shown).
FIG. 3.
Changes in VBRs over time. Treatments are the same as described in the legend of Fig. 1. Results are given as the means ± standard deviations (number of samples, 3). G, glucose.
DISCUSSION
No relationship was found between virus counts and the variables analyzed in the lake survey. Nevertheless, a negative relationship was found between VBRs and DOC concentration, suggesting that the mortality of bacteria caused by viruses is lower in humic lakes than in clear water lakes. Burst size, infection rates, and factors that can influence the loss and production of viruses and bacteria can strongly affect VBRs (40). Vrede et al. (35) found no differences in burst size and the frequency of infected cells between a humic and a clear water lake in Sweden. Therefore, the data suggest that infection and/or replication rates of viruses are negatively influenced by the presence of humic substances as viruses may be adsorbed to humic matter and/or replication may be inhibited (18). HMW DOM (>30 kDa) has been implicated as a significant factor of virus decay in coastal waters (23). Humic substances are complex mixtures containing aliphatic and aromatic carboxyl and hydroxyl functional groups that have important binding properties (26) and might bind viruses in freshwaters. The effects of humic substances on infectivity have been studied by using different approaches. For instance, irreversible inactivation of human immunodeficiency viruses was observed after treatment with synthetic humic substances (a compound designated HS-1500) in cell culture medium (28). Indeed, medicines produced from coal-derived humic and fulvic acids have been used recently with promising results for the treatment of human immunodeficiency virus-infected patients (34). Lu et al. (18) found that humic-like substances negatively affect the in vitro replication of influenza virus. It is worth noting that the concentration of humic substances used in these clinical studies is comparable to natural concentrations found in humic lakes (i.e., up to 50 mg of C liter−1).
Results from the laboratory experiment supported the findings of the lake survey and showed that humic substances had a negative effect on virus counts and VBRs. Interestingly, the addition of glucose alone did not significantly affect viral abundance relative to the controls, suggesting that the type and not quantity of carbon was responsible for the observed changes in the abundance of viruses. DOC-normalized absorptivity in the fulvic acid treatments is far higher than in the treatment with glucose additions only, which is indicative of the presence of HMW DOM in the fulvic acid treatments (Table 1). Therefore, HMW DOM is probably the main reason for the lower viral abundance in the treatments with fulvic acid additions. Indeed, we also found a significant negative correlation between DOC-normalized absorptivity and VBRs in the lake survey (Table 3). One could argue that the lower number of viruses found in the treatments with the fulvic acid additions might be a result of interference in the microscope counts due to the presence of humus. Our methodological tests showed a decrease of 15% in virus counts after the addition of fulvic acids in fixed samples, which was not significantly different statistically from counts of the controls. We have no reason to believe that the difference between virus counts in fixed samples with and without fulvic acids was due to the quality of the slides or their brightness, since we found no significant differences in the speeds of particles fading between these treatments. The decrease of approximately 34% in viral abundance due to the presence of fulvic acids relative to the control is far higher than the 15% decrease in viral abundance due to a possible interference of humic substances during the microscope counts.
Our laboratory investigation demonstrated a positive response of bacteria to additions of humic substances, whereas viral abundance and the VBR decreased significantly relative to the controls. The relationship between the VBR and bacterial abundance is complex, and both positive (11, 38) and negative (14, 20, 33) relationships have been found. An increase in bacterial abundance without a corresponding increase in virus abundance is often explained by the growth of phage-resistant bacterial communities (24, 41) or by virus adsorption to bacteria (33). We cannot discount the possibility that the decrease in the VBR after the fulvic acid addition is because of such responses. The adsorption without successful infection may be one of the mechanisms for viral inhibition due to the presence of fulvic acids, in addition to the possible direct binding of viruses to fulvic acids, which is also proposed in this study. This mechanism is supported by the study of Lu et al. (18). These authors added humic acids under in vitro conditions after the viral adsorption phase and found an inhibition in the viral protein synthesis. According to Lu et al. (18), the addition of humic acids inhibited the endonuclease activity of viral RNA polymerase of the influenza virus, which has an important role in the viral synthesis that occurs after virus enters the cells. Since we have worked with a mixed community of bacteria and viruses, different mechanisms might be implicated. We also found a remarkable decrease in the VBR and viral abundance in combination with an increase in bacterial abundance and production after the GNP treatment. The dynamics of virus and bacterial abundance in the GNP treatments may be explained by virus adsorption, followed by a successful infection, since at the end of the experiment (i.e., after 360 h), the number of viruses in this treatment increased considerably and the VBR was not any longer significantly different from that of the control. This suggests that a lytic infection was occurring in the GNP treatment between 10 and 360 h. In the fulvic acid treatments, both viral abundance and the VBR remained significantly lower after 360 h, suggesting that if viruses were adsorbed by bacteria, their replication was significantly delayed or inactivated.
In aquatic ecosystems, lysogeny may be one strategy for viruses to survive harsh conditions (40). The production and survival of lytic viruses are only possible if the rates of destruction and inactivation are lower than rates of production (39). Therefore, we have hypothesized that the presence of HMW DOM in humic lakes would benefit the establishment of lysogenic species in comparison to a clear water lake. Although no relationship was found between lysogeny and DOC concentration during the lake survey, lysogeny was not detected in the lake with the lowest DOC-normalized absorptivity (i.e., 1.2 liters m−1 mg of DOC−1), while the other surveyed lakes (i.e., DOC-normalized absorptivity between 3.0 and 3.9 liters m−1 mg of DOC−1) showed significant levels of lysogeny. The incidence of lysogeny found in our study, although conservative, is high compared to findings of similar studies in marine and coastal systems (37, 39), suggesting that lysogeny might be an important mechanism in humic lakes. If we had used a burst size of 9, as found in two Swedish lakes by Vrede et al. (35), the incidence of lysogeny would range between 45 and 106% in the humic lakes. However, we did not find any significant increase in lysogeny after fulvic acid additions in the laboratory, except on one occasion, and we found a slightly nonsignificant increase on two more occasions. One possible explanation is that the negative effects of the fulvic acid additions on the infection and replication rates of viruses would be similar whether the virus is temperate or virulent. Moreover, while amendments had an effect on the bacterial and viral abundances, the duration of the experiment (i.e., 360 h) may have been insufficient to change the proportion of lysogens in the sample, given that they were not present at the beginning of the experiment (Table 4, Lake Skärlen).
In conclusion, our data indicate that the abundance of virus is negatively influenced by humic substances. The lower viral abundance in the treatments with fulvic acids in comparison to the control may be due to one or a combination of different factors such as binding to HMW substances, destruction, lower infectivity (23, 28), or lower replication rates (18) of viruses in an HMW DOM environment. The nature of the interactions between viruses and humic substances may be complex and demand further investigation. In addition, our results suggest that lysogeny might be an important ecological strategy in humic lakes. Results from this study suggest that the way viruses control bacterial assemblages in humic lakes may be significantly different from that of clear water systems.
Acknowledgments
This work was supported by the European Commission (Marie Curie Individual Fellowship, contract HPMF-CT-2002-02116) to A.M.A. and by the Swedish Research Council (VR) to W.G. (contract 621-2002-3828).
Edward Adams and two anonymous referees gave valuable comments and suggestions on an early version of the manuscript.
REFERENCES
- 1.Bergh, Ø., K. Y. Børsheim, G. Bratbak, and M. Heldal. 1989. High abundance of viruses found in aquatic environments. Nature 340:467-468. [DOI] [PubMed] [Google Scholar]
- 2.Bettarel, Y., T. Sime-Ngando, C. Amblard, and H. Laveran. 2000. A comparison of methods for counting viruses in aquatic systems. Appl. Environ. Microbiol. 66:2283-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bratbak, G., M. Heldal, S. Norland, and T. F. Thingstad. 1990. Viruses as partners in spring bloom microbial trophodynamics. Appl. Environ. Microbiol. 56:1400-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, F., J. R. Lu, B. J. Binder, Y. C. Liu, and R. E. Hodson. 1992. Application of digital image analysis and flow cytometry to enumerate marine viruses stained with SYBR Gold. Appl. Environ. Microbiol. 67:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cochlan, W. P., J. Wikner, G. F. Steward, D. C. Smith, and F. Azam. 1993. Spatial distribution of viruses, bacteria, and chlorophyll a in neritic, oceanic and estuarine environments. Mar. Ecol. Prog. Ser. 92:77-87. [Google Scholar]
- 6.Corinaldesi, C., E. Crevatin, P. Del Negro, M. Marini, A. Russo, S. Fonda-Umani, and R. Danovaro. 2003. Large-scale spatial distribution of viroplankton in the Adriatic Sea: testing the trophic state control hypothesis. Appl. Environ. Microbiol. 69:2664-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuhrman, J. A. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399:541-548. [DOI] [PubMed] [Google Scholar]
- 8.Fuhrman, J. A., and R. T. Noble. 1995. Viruses and protists cause similar bacterial mortality in coastal seawater. Limnol. Oceanogr. 40:1236-1242. [Google Scholar]
- 9.Fuhrman, J. A., and F. Azam. 1980. Bacterioplankton secondary production estimates for coastal waters of British Columbia, Antarctica, and California. Appl. Environ. Microbiol. 39:1085-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granéli, W., M. Lindell, and L. Tranvik. 1996. Photo-oxidative production of dissolved inorganic carbon in lakes of different humic content. Limnol. Oceanogr. 41:698-706. [Google Scholar]
- 11.Hara, S., I. Koike, K. Terauchi, H. Kamiya, and E. Tanoue. 1996. Abundance of viruses in deep oceanic water. Mar. Ecol. Prog. Ser. 145:269-277. [Google Scholar]
- 12.Hennes, K. P., and C. A. Suttle. 1995. Direct counts of viruses in natural waters and laboratory cultures by epifluorescence microscopy. Limnol. Oceanogr. 40:1050-1055. [Google Scholar]
- 13.Jespersen, A. M., and K. Christoffersen. 1987. Measurements of chlorophyll-a from phytoplankton using ethanol as extraction solvent. Arch. Hydrobiol. 109:445-454. [Google Scholar]
- 14.Jiang, S. C., and J. H. Paul. 1994. Seasonal and diel abundance of viruses and occurrence of lysogeny/bacteriocinogeny in the marine environment. Mar. Ecol. Prog. Ser. 104:163-172. [Google Scholar]
- 15.Karlsson, J., M. Jansson, and A. Jonsson. 2002. Similar relationships between pelagic primary and bacterial production in clearwater and humic lakes. Ecology 83:2902-2910. [Google Scholar]
- 16.Laybourn-Parry, J., M. Walton, J. Young, A. Shrine, and R. I. Jones. 1994. The protozooplankton and bacterioplankton of a large oligotrophic lake-Loch Ness, Scotland. J. Plankton Res. 16:1655-1670. [Google Scholar]
- 17.Laybourn-Parry, J., J. S. Hofer, and R. Sommaruga. 2001. Viruses in the plankton of freshwater and saline Antarctic lakes. Freshw. Biol. 46:1279-1287. [Google Scholar]
- 18.Lu, F. J., S. N. Tseng, M. L. Li, and S. R. Shih. 2002. In vitro anti-influenza virus activity of synthetic humate analogues derived from protocatechuic acid. Arch. Virol. 147:273-284. [DOI] [PubMed] [Google Scholar]
- 19.Maranger, R., D. F. Bird, and S. K. Juniper. 1994. Viral and bacterial dynamics in Arctic sea ice during the spring algal bloom near Resolute, N. W. T. Canada. Mar. Ecol. Prog. Ser. 111:121-127. [Google Scholar]
- 20.Maranger, R., and D. F. Bird. 1995. Viral abundance in aquatic systems: a comparison between marine and fresh waters. Mar. Ecol. Prog. Ser. 121:217-226. [Google Scholar]
- 21.Noble, R. T., and J. A. Fuhrman. 1998. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat. Microb. Ecol. 14:113-118. [Google Scholar]
- 22.Noble, R. T., and J. A. Fuhrman. 2000. Rapid virus production and removal as measured with fluorescently labeled viruses as tracers. Appl. Environ. Microbiol. 66:3790-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noble, R. T., and J. A. Fuhrman. 1997. Virus decay and its causes in coastal waters. Appl. Environ. Microbiol. 63:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noble, R. T., M. Middelboe, and J. A. Fuhrman. 1999. Effects of viral enrichment on the mortality and growth of heterotrophic bacterioplankton. Aquat. Microb. Ecol. 18:1-13. [Google Scholar]
- 25.Paul, J. H., and S. C. Jiang. 2001. Lysogeny and transduction, p. 105-125. In J. H. Paul (ed.), Marine microbiology. Methods in microbiology, vol. 30. Academic Press, London, United Kingdom. [Google Scholar]
- 26.Perdue, E. M. 1998. Chemical composition, structure, and metal binding properties, p. 41-61. In D. O. Hessen and L. Tranvik (ed.), Aquatic humic substances: ecology and biogeochemistry. Ecological studies, vol. 133. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 27.Proctor, L. M., and J. A. Fuhrman. 1990. Viral mortality of marine bacteria and cyanobacteria. Nature 343:60-62. [Google Scholar]
- 28.Schneider, J., R. Weis, C. Männer, B. Kary, A. Werner, B. J. Seubert, and U. N. Riede. 1996. Inhibition of HIV-1 in cell culture by synthetic humate analogues derived from hydroquinone: mechanism of inhibition. Virology 218:389-395. [DOI] [PubMed] [Google Scholar]
- 29.Smith, D. C., and F. Azam. 1992. A simple economical method for measuring bacterial protein synthesis rate in seawater using 3H-leucine. Mar. Microb. Food Webs 6:107-114. [Google Scholar]
- 30.Steward, G. F., D. C. Smith, and F. Azam. 1996. Abundance and production of bacteria and viruses in the Bering and Chukchi Seas. Mar. Ecol. Prog. Ser. 131:287-300. [Google Scholar]
- 31.Tranvik, L. 1989. Bacterioplankton growth, grazing mortality and quantitative relationship to primary production in a humic and a clearwater lake. J. Plankton. Res. 11:985-1000. [Google Scholar]
- 32.Tranvik, L. 1998. Degradation of dissolved organic matter in humic waters by bacteria, p. 259-284. In D. O. Hessen and L. Tranvik (ed.), Aquatic humic substances: ecology and biogeochemistry. Ecological studies, vol. 133. Springer-Verlag, Heidelberg, Germany. [Google Scholar]
- 33.Tuomi, P., K. M. Fagerbakke, G. Bratbak, and M. Heldal. 1995. Nutritional enrichment of a microbial community: the effects on activity, elemental composition, community structure and virus production. FEMS Microbiol. Ecol. 16:123-134. [Google Scholar]
- 34.Van Rensburg, C. E. J., J. Dekker, R. Weis, T. L. Smith, E. J. Van Rensburg, and J. Schneider. 2002. Investigation of the anti-HIV properties of oxihumate. Chemotherapy 48:138-143. [DOI] [PubMed] [Google Scholar]
- 35.Vrede, K., U. Stensdotter, and E. S. Lindström. 2003. Viral and bacterioplankton dynamics in two lakes with different humic contents. Microb. Ecol. 46:406-415. [DOI] [PubMed] [Google Scholar]
- 36.Weinbauer, M. G., U. Christaki, J. Nedoma, and K. Šimek. 2003. Comparing the effects of resource enrichment and grazing on viral production in a meso-eutrophic reservoir. Aquat. Microb. Ecol. 31:137-144. [Google Scholar]
- 37.Weinbauer, M. G., and C. A. Suttle. 1999. Lysogeny and prophage induction in coastal and offshore bacterial communities. Aquat. Microb. Ecol. 18:217-225. [Google Scholar]
- 38.Weinbauer, M. G., D. Fuks, S. Puskaric, and P. Peduzzi. 1995. Diel, seasonal, and depth-related variability of viruses and dissolved DNA in the northern Adriatic Sea. Appl. Environ. Microbiol. 59:4074-4082. [DOI] [PubMed] [Google Scholar]
- 39.Weinbauer, M. G., and C. A. Suttle. 1996. Potential significance of lysogeny to bacteriophage production and bacterial mortality in coastal waters of the Gulf of Mexico. Appl. Environ. Microbiol. 62:4374-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wommack, K. E., and R. R. Colwell. 2000. Viroplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 64:69-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yager, P. L., T. L. Connelly, B. Mortazavi, K. E. Wommack, N. Bano, J. E. Bauer, S. Opsahl, and J. T. Hollibaugh. 2001. Dynamic bacterial and viral response to an algal bloom at subzero temperatures. Limnol. Oceanogr. 46:790-801. [Google Scholar]