Abstract
Background and Purpose
Mechanisms of both healing and complications, including spontaneous aneurysm rupture, remain unclear following flow diverter treatment. The aim of the current study was to compare the gene expression of various key molecules involved in the healing of aneurysms between aneurysms treated with microcoils and flow diverters.
Methods
Saccular aneurysms were created in rabbits. Aneurysms were either treated with coils (n=6) or with flow diverters (n=6). Aneurysms were harvested at four weeks following treatment and used for gene expression and zymography experiments. Genes with a fold change of 1.2 or greater were considered up regulated, whereas those with a fold change of 0.8 or less were considered down regulated.
Results
All coil embolized aneurysms were completely occluded at follow up. Two aneurysms were occluded and the remaining four samples were incompletely occluded in the flow diverter treated group. The following genes were expressed at lower levels in the flow diverter group as compared to the coiled aneurysm group: proteinases (matrix metalloproteinases -2 and -9), cellular markers (endothelial nitric oxide synthase and smooth muscle actin), and structural proteins (collagens and fibronectin). Genes related to inflammation (tumor necrosis factor-α and monocyte chemoattractant protein -1) were up regulated in flow diverter treated aneurysms compared to coil embolized aneurysms. Notably, the enzymatic activity of active MMP-9 was high in aneurysms treated with flow diverters.
Conclusion
Our findings may provide improved understanding of rupture risk and healing following aneurysm treatment and inform development of therapies aimed at lowering rupture risk and accelerating healing.
Keywords: Aneurysm, flow diversion, coil, embolization, gene expression, rabbit model
INTRODUCTION
Even after nearly 2 decades of clinical use, long-term occlusion rates with endovascular microcoils in large and broad-necked aneurysms remain poor. The introduction of flow diverters in the treatment of aneurysms alleviates the shortcomings of microcoils and has shown remarkable rate of complete occlusion of aneurysms, which are difficult to treat using other endovascular techniques.
Both of these approaches primarily elicit thrombus formation in the aneurysm cavity and then promote neointima formation across the neck to seal the aneurysm cavity from the circulation[1–4]. Coils act within the aneurysm cavity while flow diverters primarily work at the parent artery[5 6]. Studies aiming at exploring the mechanism of aneurysm healing following endovascular treatments are mostly focused at the tissue and cellular levels and a handful of preclinical experiments studied the molecular mechanisms[3 7–11]. However, the exact biological mechanism of action of these endovascular techniques remains poorly understood. Furthermore, rare but devastating reports of spontaneous aneurysm rupture following aneurysm therapy have diminished enthusiasm for flow diversion devices[12 13]. The mechanisms underlying propensity for aneurysm rupture following flow diversion treatment remain enigmatic.
The goal of the current study was to directly compare the gene expression of key molecules potentially involved in the healing of aneurysms as well as the activity of proteolytic enzymes between aneurysms treated with microcoils and flow diverters.
MATERIALS AND METHODS
Aneurysm creation, treatment and follow up
The Institutional Animal Care and Use Committee approved all procedures before initiation of the study. Elastase-induced saccular aneurysms were created in 12 New Zealand White rabbits (body weight, 3 to 4 kg). Detailed procedures for aneurysm creation have been described in depth elsewhere[14]. Aneurysms were permitted to mature for at least 3 weeks after creation. Rabbits were assigned to either coil or flow diverter treatment groups. Aneurysms were either embolized with platinum coils or treated with flow diverters (Pipeline Embolic Device™, Covidien Inc, CA) placed in the parent artery as previously described[15 16]. Digital subtraction angiography (DSA) of the aortic arch was performed immediately before or after treatment. At 4 weeks following treatment, a follow-up DSA of the aortic arch was performed. The animals were then euthanized by using a lethal injection of pentobarbital. The aneurysms and the parent artery were harvested, longitudinally cut into two halves and the samples were immediately snap-frozen in liquid nitrogen and kept frozen at −70°C until use. One half of the sample was used from gene expression and the other half was used for zymography analysis.
Angiographic Evaluation
Images from DSA obtained immediately after device implantation and just before sacrifice were evaluated. Follow-up DSA images were assessed by using a trichotomous scale (incomplete occlusion, near-complete occlusion, or complete occlusion)
Quantitative Real-time Polymerase Chain Reaction (qRT-PCR) Analysis
The mRNA expression of selected genes that have been associated with vascular remodeling of aneurysms was assessed by qRT-PCR. These selected genes are categorized into cell adhesion (vascular cell adhesion molecule-1 (VCAM-1) and selectin E), structural molecules (type VIII collagen, type I collagen and fibronectin), growth factors and cytokines (vascular endothelial growth factor 1 (VEGF-1), tumor necrosis factor- alpha (TNF- α), osteopontin and monocyte chemoattractant protein-1 (MCP-1)), proteinases and their inhibitors ((matrix metalloproteiase-2 and -9 (MMP-2 and -9), tissue inhibitor of MMPs-1 and -2 (TIMP-1 and -2) and cathepsin L), and cellular and oxidative stress markers (hypoxia-inducible factor 1 (HIF-1), heme oxygenase 1, endothelial nitric oxide synthase (eNOS), von Willebrand factor (vWF) and smooth muscle actin (SMA)). Briefly, total RNA was extracted from aneurysm tissue with RNeasy kit (Qiagen). First-strand complementary DNAs were synthesized from 500 ng of total RNA by using a synthesis system (SuperScript III First-Strand Synthesis System; Invitrogen, Carlsbad, Calif). Then RT-PCR was performed with a cycler (iCycler; Bio-Rad, Hercules, Calif) using SYBR® Green PCR kit (Invitrogen). The specific primers were designed from corresponding sequences obtained from GenBank (the genetic sequence database of the National Institutes of Health, National Center for Biotechnology Information) by using a Web tool (Primer 3; http://frodo.wi.mit.edu/primer3/)[17].
Gelatin zymography for MMPs
Soluble proteins were extracted from frozen samples[9]. Protein samples were separated by 10% zymogram gel (Bio-Rad). Gels were washed with renaturation buffer (Bio-rad) for 1 h, and then incubated for 48 h at 37°C in development buffer (Bio-rad). Then gels were stained with 0.5% Coomassie blue R-250. The gelatinolytic bands, representing the activities of MMPs, were scanned and analyzed densitometrically using Licor software.
Statistical analysis
Genes with a fold change of 1.2 or greater were considered up regulated, whereas those with a fold change of 0.8 or less were considered down regulated[10].
RESULTS
Angiographic results
Angiographic data are summarized in Table 1, and representative angiographic images are presented in Figure 1. There were no statistically significant differences in mean neck, width and height between coil and flow diverter treated groups. Of the six aneurysms treated with flow diverter devices, at 4 weeks post-treatment two aneurysms showed complete occlusion, two aneurysms showed near complete occlusion, and remaining two aneurysms were patent, whereas all six aneurysms treated with platinum coils showed stable occlusion at 4 weeks post-treatment. Representative angiographic images are presented in Figure 1.
Table 1.
Aneurysm geometry
| Flow diverter | Coil | |
|---|---|---|
| Neck width, mm | 3.7±0.9 | 3.3±1.6 |
| Width, mm | 4.5±1.4 | 3.7±0.6 |
| Height, mm | 9.4±2.5 | 8.0±1.3 |
Figure 1. Representative angiograms of treated aneurysms.
A. DSA image showing aneurysm cavity prior to coil treatment
B. Follow-up DSA image showing obliteration of aneurysm
C. DSA image showing aneurysm cavity prior to flow diverter placement
D. Follow-up DSA image showing complete occlusion of aneurysm
Gene and protein expression results
Cell adhesion and structural components
None of the adhesion molecules (VCAM and selectin E) showed differential expression between flow diverter and coil treated aneurysms. Among the structural molecules studied, both collagen I (0.18 fold) and fibronectin (0.32 fold) showed remarkably decreased expression in the flow diverter treated aneurysms compared coiled aneurysms, whereas collagen VIII (0.82 fold) exhibited no significant change between flow diverter and coil groups (Table 2).
Table 2.
Relative expression of genes in flow diverter treated aneurysms compared to coiled aneurysms
| Gene name | Median (range) | Expression changes |
|---|---|---|
| Adhesion molecules | ||
| VCAM | 0.94 (0.11–661.7) | No change |
| Selectin E | 0.91 (0.19–1.54) | No change |
| Structural molecules | ||
| Collagen VIII | 0.82 (0.07–180.39) | No change |
| Collagen I | 0.18 (0.06–1.38) | ↓ |
| Fibronectin | 0.32 (0.08–0.36) | ↓ |
| Vascular remodeling | ||
| MMP-2 | 0.43 (0.09–2.42) | ↓ |
| MMP-9 | 0.36 (0.02–94.03) | ↓ |
| Cathepsin L | 0.25 (0.07–1.1) | ↓ |
| TIMP-1 | 2.4 (0.11–1.13) | ↑ |
| TIMP-2 | 0.18 (0.02–2.51) | ↓ |
| Growth factors/cytokines/inflammatory molecules | ||
| Osteopontin | 2.4 (0.11–133.9) | ↑ |
| Monocyte chemoattractant protein | 1.29 (0.37–5.35) | ↑ |
| TNF- α | 1.37 (0.63–27.19) | ↑ |
| VEGF | 0.37 (0.28–1.13) | ↓ |
| Oxidative stress | ||
| HIF | 0.42 (0.06–251.6) | ↓ |
| HO-1 | 0.69 (0.15–1.84) | ↓ |
| Cellular markers | ||
| vWF | 0.35 (0.12–0.14) | ↓ |
| eNOS | 0.08 (0.02–0.14) | ↓ |
| SMA | 0.69 (0.34–0.72) | ↓ |
Growth factors, cytokines and inflammatory molecules
The expression profiles of osteopontin (2.4 fold) and pro-inflammatory genes TNF- α (1.3 fold) and MCP-1 (1.4 fold) were increased in aneurysms treated flow diverters compared to aneurysms treated with platinum coils. VEGF expression (0.37 fold) was significantly less in flow diverter treated samples.
Cellular and stress markers
Both the hypoxia marker, HIF-1 (0.42 fold) and oxidative stress marker, hemeoxygenase-1 (0.7 fold) were down regulated in flow diverted treated aneurysms compared to coiled aneurysms. Significantly decreased expression of both endothelial cell makers, vWF (0.35 fold) and eNOS (0.08) and smooth muscle cell marker, SMA (0.69 fold) was observed in the aneurysms of flow diverted treated animals.
Proteases and their inhibitors
Compared to flow diverter treated aneurysms, the expression of MMP-2, MMP-9 and cathepsin L were up regulated over two fold in coil embolized aneurysms. TIMP-1 expression (2.4 fold) was elevated and TIMP-2 expression (0.2 fold) was diminished in flow diverted treated group compared to coiled group.
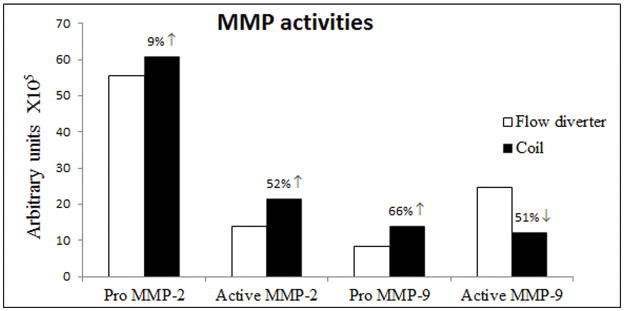
MMP activities
MMP zymography was performed to assess the activities of both inactive precursor and active functional forms of MMPs. Pro MMP-2 expression was not different between both coil and flow diverted treated samples whereas active MMP-2 expression was 52% higher in coiled aneurysms. Pro MMP-9 expression was diminished (66%) while active MMP-9 expression was increased two-fold in flow diverter treated aneurysms compared to coiled control aneurysm tissues (Figure 2A and 2B).
Figure 2.
Figure 2A. MMP-2 and MMP-9 enzymatic activities of aneurysm samples at 4 weeks following flow diverter and coil embolization on gelatin zymography.
Figure 2B. Densitometry analysis of activities of pro and active MMPs-2 and -9.
DISCUSSION
In this study, we compared the gene expression pattern between aneurysms treated with diverters and aneurysms embolized with coils to probe the mechanism of aneurysm healing following two different interventional approaches. The major finding of this study is that active MMP-9 involves in the remodeling of flow diverter treated aneurysms as evidenced by increase in the enzymatic activity of active MMP-9, despite a decrease in the level of mRNA. Our findings also demonstrate that the over expression of pro-inflammatory markers and under expression of structural genes and cellular markers in flow diverters treated aneurysms. There were no observed trends in the expression of VCAM-1 and selection E to suggest a role for adhesion molecules to account for flow diverter related remodeling.
The role of MMPs in the pathophysiology of cerebral aneurysms is well characterized and they have been also reported in the recanalization and recurrence of canine aneurysms embolized with gelatin sponges[8]. Similarly, high expression of MMP-9 has been associated with the healing of aneurysms following stent placement in the parent artery of a swine aneurysm model[7]. Diminished expression of pro-MMP-2 and pro-MMP-9 seen in the zymogram is correlated with the decreased gene expression of the respective genes in the flow diverter treated group. Interestingly, profound elevation of active MMP-9 in the flow diverter treated aneurysms indicates a major role of MMP-9 in the remodeling of aneurysm wall, which may be a possible explanation for the spontaneous rupture of aneurysms treated with flow diverters in patients[12 13]. Moreover, the activity of MMP-9 is important in cell migration of neointimal cells and the occlusion of aneurysms following flow diverters is thought to be derived from neointimal cell growth originating from the vessel wall[3]. The observed increase in the activity of active MMP-9 in the flow diverter treated group could also be related to the ongoing vascular remodeling in the aneurysm cavity. The activity is more important in predicting the function of an enzyme that the level of mRNA[18 19]. Our previous study comparing the gene expression pattern between aneurysms with high aspect (height to neck width) revealed high expression of hypoxia induced TIMP in high aspect ratio aneurysms[20]. Similarly, in the present study, an increase in the expression of TIMP-1 was observed in the flow diverter treated aneurysms. This could be related to active extracellular matrix turn over and vascular remodeling, which may lead to aneurysm rupture[21].
TNF- α is a pro-inflammatory cytokine with strong necrotic activity and is secreted by variety of cells including macrophages, endothelial cells and smooth muscle cells. The chemokine, MCP-1 recruits monocytes and neutrophils to the site of injury and promotes chemotaxis and inflammation. The deposition of inflammatory cells was noted in the struts of the flow diverters at the neck of the aneurysms. High levels of detected MCP-1 and TNF- α could be related to the inflammatory events in the aneurysm following flow diverter placement[3]. Chow et al. showed inflammatory infiltrates, mural thinning, and necrosis within the aneurysm of a delayed spontaneous rupture of a posterior inferior cerebellar artery aneurysm following treatment with flow diversion[12]. Increase in the level of TNF- α detected this study may be associated with the inflammation and necrotic cell death of spontaneous rupture of flow diverter treated aneurysms. TNF has also been shown to increase MMP-9 activity[22 23]. Moreover, high levels of both TNF- α and MMPs were reported in the ruptured human cerebral aneurysms[24] and inhibition of TNF- α has shown decreased incidence of aneurysm rupture in a mouse model[25]. The observed elevated levels of HIF and VEGF in coiled aneurysm could be related to the neo-vascularization of thrombus in the aneurysm[26 27], since the geometry of untreated rabbit aneurysms remains stable for up to 5 years [28].
Our study has several limitations. Neither flow diverter nor coils treated groups were compared with untreated aneurysms or normal control arteries. We assessed the gene expression of completely occluded, coiled aneurysms against incompletely occluded, flow diverter treated aneurysms. In addition, we did compare the differences between complete occlusion, near-complete occlusion and patent groups. Even though the rabbit aneurysm model employed in this study is similar to human aneurysms histologically[29], morphologically[30], biologically[31], and hemodynamically[32], further studies on human samples will be necessary to validate our findings. We only have one time point following embolization and the gene expression may change overtime.
CONCLUSION
In the rabbit saccular aneurysm model, active MMP-9 activity and genes involved in the production of proteins that function under inflammation were up regulated whereas genes coding for cellular markers and structural proteins were down regulated. These findings may be helpful to further understanding of the molecular pathways involved in the healing of aneurysms treated with either flow diverter devices or platinum coils.
Acknowledgments
We thank Covidien Inc. for generously providing the flow diverters for this study.
FUNDING STATEMENT
This work was supported by National Institutes of Health grant NS 076491.
Footnotes
COMPETING INTERESTS STATEMENT
None
CONTRIBUTORSHIP STATEMENT
Cole Puffer: Gene expression experiments, statistical analysis and manuscript drafting.
Daying Dai and Yong-Hong Ding: Animal experiments, including aneurysm creation, embolization and follow up, and tissue harvest.
Juan Cebral, David Kallmes and Ramanathan Kadirvel: Study design, data analysis and manuscript editing.
DATA SHARING
All of the authors in this manuscript have read and approved submission of the manuscript. All authors have access to the raw data.
References
- 1.Ribourtout E, Raymond J. Gene therapy and endovascular treatment of intracranial aneurysms. Stroke. 2004;35(3):786–93. doi: 10.1161/01.STR.0000117577.94345.CC. [DOI] [PubMed] [Google Scholar]
- 2.Mitome-Mishima Y, Yamamoto M, Yatomi K, et al. Endothelial cell proliferation in Swine experimental aneurysm after coil embolization. PLoS One. 2014;9(2):e89047. doi: 10.1371/journal.pone.0089047. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadirvel R, Ding YH, Dai D, Rezek I, Lewis DA, Kallmes DF. Cellular Mechanisms of Aneurysm Occlusion after Treatment with a Flow Diverter. Radiology. 2013 doi: 10.1148/radiol.13130796. radiol.13130796 [pii] published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li ZF, Fang XG, Yang PF, et al. Endothelial progenitor cells contribute to neointima formation in rabbit elastase-induced aneurysm after flow diverter treatment. CNS Neurosci Ther. 2013;19(5):352–7. doi: 10.1111/cns.12086. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991;75(1):8–14. doi: 10.3171/jns.1991.75.1.0008. [DOI] [PubMed] [Google Scholar]
- 6.D’Urso PI, Lanzino G, Cloft HJ, Kallmes DF. Flow diversion for intracranial aneurysms: a review. Stroke. 2011;42(8):2363–8. doi: 10.1161/STROKEAHA.111.620328. STROKEAHA.111.620328 [pii][published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 7.Bouzeghrane F, Darsaut T, Salazkin I, Ogoudikpe C, Gevry G, Raymond J. Matrix metalloproteinase-9 may play a role in recanalization and recurrence after therapeutic embolization of aneurysms or arteries. J Vasc Interv Radiol. 2007;18(10):1271–9. doi: 10.1016/j.jvir.2007.06.034. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 8.Darsaut T, Salazkin I, Ogoudikpe C, Gevry G, Bouzeghrane F, Raymond J. Effects of stenting the parent artery on aneurysm filling and gene expression of various potential factors involved in healing of experimental aneurysms. Interv Neuroradiol. 2006;12(4):289–302. doi: 10.1177/159101990601200401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadirvel R, Ding YH, Dai D, Lewis DA, Cloft HJ, Kallmes DF. Molecular indices of apoptosis activation in elastase-induced aneurysms after embolization with platinum coils. Stroke. 2007;38(10):2787–94. doi: 10.1161/STROKEAHA.107.486738. [DOI] [PubMed] [Google Scholar]
- 10.Kadirvel R, Ding YH, Dai D, Lewis DA, Kallmes DF. Differential gene expression in well-healed and poorly healed experimental aneurysms after coil treatment. Radiology. 2010;257(2):418–26. doi: 10.1148/radiol.10100362. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadirvel R, Ding YH, Dai D, Lewis DA, Kallmes DF. Proteomic analysis of aneurysm healing mechanism after coil embolization: comparison of dense packing with loose packing. AJNR Am J Neuroradiol. 2012;33(6):1177–81. doi: 10.3174/ajnr.A2940. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow M, McDougall C, O’Kelly C, Ashforth R, Johnson E, Fiorella D. Delayed spontaneous rupture of a posterior inferior cerebellar artery aneurysm following treatment with flow diversion: a clinicopathologic study. AJNR Am J Neuroradiol. 2012;33(4):E46–51. doi: 10.3174/ajnr.A2532. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulcsar Z, Houdart E, Bonafe A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol. 32(1):20–5. doi: 10.3174/ajnr.A2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. American Roentgen Ray Society. AJR Am J Roentgenol. 2000;174(2):349–54. doi: 10.2214/ajr.174.2.1740349. [DOI] [PubMed] [Google Scholar]
- 15.Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007;38(8):2346–52. doi: 10.1161/STROKEAHA.106.479576. [DOI] [PubMed] [Google Scholar]
- 16.Kallmes DF, Helm GA, Hudson SB, et al. Histologic evaluation of platinum coil embolization in an aneurysm model in rabbits. Radiology. 1999;213(1):217–22. doi: 10.1148/radiology.213.1.r99oc16217. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 17.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 18.Laurent JM, Vogel C, Kwon T, et al. Protein abundances are more conserved than mRNA abundances across diverse taxa. Proteomics. 2010;10(23):4209–12. doi: 10.1002/pmic.201000327. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svensson C, Welsh N, Krawetz SA, Welsh M. Exhibition of specific alterations in activities and mRNA levels of rat islet glycolytic and mitochondrial enzymes in three different in vitro model systems for attenuated insulin release. Diabetes. 1991;40(6):771–6. doi: 10.2337/diab.40.6.771. [DOI] [PubMed] [Google Scholar]
- 20.Kadirvel R, Ding YH, Dai D, Lewis DA, Kallmes DF. Differential expression of genes in elastase-induced saccular aneurysms with high and low aspect ratios. Neurosurgery. 2010;66(3):578–84. doi: 10.1227/01.NEU.0000365769.78334.8C. discussion 84. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin D, Sheng J, Yang X, Gao B. Matrix metalloproteinases and tissue inhibitors of metalloproteinases expression in human cerebral ruptured and unruptured aneurysm. Surg Neurol. 2007;68(Suppl 2):S11–6. doi: 10.1016/j.surneu.2007.02.060. discussion S16. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 22.Han SJ, Hawkins SM, Begum K, et al. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat Med. 2012;18(7):1102-+. doi: 10.1038/Nm.2826. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai CL, Chen WC, Hsieh HL, Chi PL, Hsiao LD, Yang CM. TNF-alpha induces matrix metalloproteinase-9-dependent soluble intercellular adhesion molecule-1 release via TRAF2-mediated MAPKs and NF-kappaB activation in osteoblast-like MC3T3-E1 cells. J Biomed Sci. 2014;21:12. doi: 10.1186/1423-0127-21-12. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayaraman T, Paget A, Shin YS, et al. TNF-alpha-mediated inflammation in cerebral aneurysms: a potential link to growth and rupture. Vasc Health Risk Manag. 2008;4(4):805–17. doi: 10.2147/vhrm.s2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starke RM, Chalouhi N, Jabbour PM, et al. Critical role of TNF-alpha in cerebral aneurysm formation and progression to rupture. J Neuroinflammation. 2014;11:77. doi: 10.1186/1742-2094-11-77. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim CS, Kiriakidis S, Sandison A, Paleolog EM, Davies AH. Hypoxia-inducible factor pathway and diseases of the vascular wall. J Vasc Surg. 2013;58(1):219–30. doi: 10.1016/j.jvs.2013.02.240. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 27.Abrahams JM, Forman MS, Grady MS, Diamond SL. Delivery of human vascular endothelial growth factor with platinum coils enhances wall thickening and coil impregnation in a rat aneurysm model. AJNR Am J Neuroradiol. 2001;22(7):1410–7. [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Y, Dai D, Kadirvel R, Lewis DA, Kallmes DF. Five-year follow-up in elastase-induced aneurysms in rabbits. AJNR Am J Neuroradiol. 2010;31(7):1236–9. doi: 10.3174/ajnr.A2056. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai D, Ding YH, Danielson MA, et al. Histopathologic and Immunohistochemical Comparison of Human, Rabbit, and Swine Aneurysms Embolized with Platinum Coils. AJNR Am J Neuroradiol. 2005;26(10):2560–68. [PMC free article] [PubMed] [Google Scholar]
- 30.Short JG, Fujiwara NH, Marx WF, Helm GA, Cloft HJ, Kallmes DF. Elastase-induced saccular aneurysms in rabbits: comparison of geometric features with those of human aneurysms. AJNR Am J Neuroradiol. 2001;22(10):1833–7. [PMC free article] [PubMed] [Google Scholar]
- 31.Mangrum WI, Farassati F, Kadirvel R, et al. mRNA expression in rabbit experimental aneurysms: a study using gene chip microarrays. AJNR Am J Neuroradiol. 2007;28(5):864–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng Z, Kallmes DF, Durka MJ, et al. Hemodynamics and anatomy of elastase-induced rabbit aneurysm models: similarity to human cerebral aneurysms? AJNR Am J Neuroradiol. 2011;32(3):595–601. doi: 10.3174/ajnr.A2324. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]