Abstract
Continuous flow ventricular assist devices (cfVADs) while effective in advanced heart failure, remain plagued by thrombosis related to abnormal flows and elevated shear stress. To limit cfVAD thrombosis, patients utilize complex anti-thrombotic regimens built upon a foundation of aspirin (ASA). While much data exists on ASA as a modulator of biochemically-mediated platelet activation, limited data exists as to the efficacy of ASA as a means of limiting shear-mediated platelet activation, particularly under elevated shear stress common within cfVADs. We investigated the ability of ASA (20, 25 and 125 μM) to limit shear-mediated platelet activation under conditions of: 1) constant shear stress (30 dyne/cm2 and 70 dyne/cm2); 2) dynamic shear stress, and 3) initial high shear exposure (70 dyne/cm2) followed by low shear exposure – i.e. a platelet sensitization protocol, utilizing a hemodynamic shearing device providing uniform shear stress in vitro. The efficacy of ASA to limit platelet activation mediated via passage through a clinical cfVAD system (DeBakey Micromed) in vitro was also studied. ASA reduced platelet activation only under conditions of low shear stress (38% reduction compared to control, n = 10, p < 0.004), with minimal protection at higher shear stress and under dynamic conditions (n = 10, p > 0.5) with no limitation of platelet sensitization. ASA had limited ability (25.6% reduction in platelet activation rate) to modulate shear-mediated platelet activation induced via cfVAD passage. These findings, while performed under “deconstructed” non-clinical conditions by utilizing purified platelets alone in vitro, provide a potential contributory mechanistic explanation for the persistent thrombosis rates experienced clinically in cfVAD patients despite ASA therapy. An opportunity exists to develop enhanced pharmacologic strategies to limit shear-mediated platelet activation at elevated shear levels associated with mechanical circulatory support devices.
Keywords: aspirin, thrombosis, platelets, shear, ventricular assist devices, mechanical circulatory support
Introduction
Mechanical circulatory support (MCS) devices have emerged as vital life-saving therapeutic systems for failing patients with advanced and end-stage heart failure (1). Despite their efficacy, MCS systems remain limited by post-implantation thrombotic complications (2). Continuous flow Ventricular Assist Devices (cfVADs), in particular, are plagued by intra-device thrombus buildup, reduced pump output with recurrent heart failure, and thromboembolic events- e.g. stroke, pump stop, and potential death (3). The high incidence of thromboembolic events in cfVADs is largely due to non-physiological flows within these devices, where platelets, the principal cellular clotting elements in blood, are exposed to extremely elevated shear stresses. In an attempt to limit MCS thrombosis, patients are burdened with complex, life-long anti-thrombotic pharmacologic regimens (4). The foundation of all regimens employed today is Aspirin (5).
Aspirin, i.e. acetylsalicylic acid (ASA), has been utilized for over sixty years as a clinical antithrombotic agent (6), with initial use described by Craven (7). ASA has been shown to inhibit platelet function by permanently acetylating cyclooxygenase (COX), both COX-1 and COX-2 isoforms, responsible for prostaglandin and thromboxane synthesis (8, 9). Over the years, ASA has been increasingly employed as a vital agent in limiting intra-arterial thrombosis related to atherosclerotic coronary disease, acute coronary syndromes, vulnerable plaque, carotid artery disease and intra-arterial stent use (8, 10–13). While all of these conditions impart shear stress to platelets, the intensity of stress, the time of exposure to stress, and the number of platelets exposed is significantly less than the stress accumulation (total dose of shear stress and exposure time) experienced by platelets exposed to cfVADs. Furthermore, the increase in stress accumulation is generally linked with an increase in platelet activation (14, 15).
In the present study we hypothesized that ASA, while an effective antiplatelet agent targeting a biochemical, i.e. thromboxane A2 pathway of platelet activation, will have limited efficacy as an agent modulating shear-mediated platelet activation, particularly at significantly elevated or “hyper-shear” levels common in VADs. As such, utilizing gel-filtered platelets, we first examined the efficacy of ASA to modulate shear-mediated platelet activation in vitro – examining exposure to both constant and dynamic shear stress, extracted from platelet flight trajectories obtained from a clinical VAD (16, 17). Secondly, we examined the ability of ASA to modulate shear-mediated “sensitization,” i.e. continued platelet activation over time following initial rapid shear exposure – a phenomenon described previously by our group (18). Finally, we examined the efficacy of ASA to limit VAD-mediated platelet activation in vitro, utilizing platelets obtained from normal human volunteers following in vivo aspirin ingestion and exposure.
Materials and Methods
Platelet preparation
Whole blood (30 ml) was drawn via venipuncture into 3 ml acid-citrate dextrose (ACD-A) from consenting healthy adult volunteers of both sexes who had not taken aspirin or ibuprofen for two weeks, in accordance with a University of Arizona IRB-approved protocol. Whole blood was centrifuged at 500g for 15 min to obtain platelet-rich plasma (PRP), which was filtered through a column of Sepharose 2B beads (Sigma-Aldrich, St. Louis, MO, USA) to collect gel-filtered platelets (GFP) (18, 19). GFP were diluted to a count of 20,000/μl in HEPES-modified Tyrode’s buffer, with 3 mM CaCl2 added 10 min prior to experiments (18, 20).
Exposure of gel-filtered platelets to Aspirin
Platelets were treated with ASA dissolved in sodium bicarbonate solution (324 mg ASA, 965 mg citric acid, and 1744 mg sodium hydrogen carbonate in 50 ml double-distilled H2O), diluted to 20, 25 or 125 μM final concentration, and incubated at 37°C for ten min. prior to shear exposure. For each ASA-treated platelet sample, a paired control experiment with platelets exposed to solvent vehicle alone (control) was performed on the same day. To verify the consistent reactivity of ASA, GFP were pre-incubated with ASA (125 μM) and then treated with Arachidonic Acid (AA, 25 μM). Samples for platelet activation measurements were taken at 0, 10 and 30 min.
Exposure of Aspirin-treated platelets to constant and dynamic shear stress
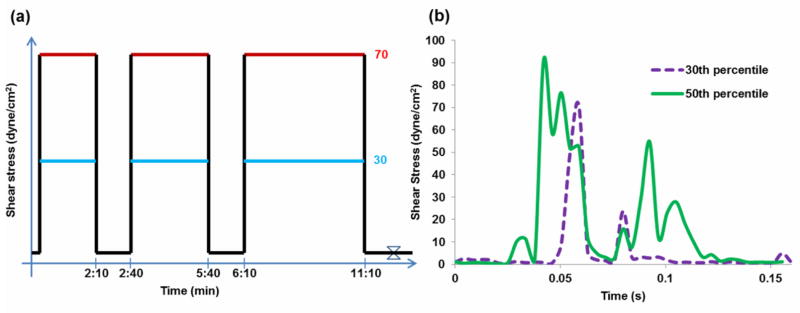
Platelets were exposed to shear stress in the Hemodynamic Shearing Device (HSD), a computer-controlled cone-plate-Couette viscometer that replicates dynamic shear conditions found in blood recirculating devices (14, 15, 21). GFP were pre-treated with ASA (25 or 125 μM) ten min prior to shear exposure, which included either constant or dynamic conditions. In the constant shear stress experiments, platelets were exposed to 30 dyne/cm2 or 70 dyne/cm2 for a total exposure time of 10 min, with samples taken at 0, 2, 5 and 10 min (Figure 1a). At each time point, the HSD was slowed down to 1 dyne/cm2 for 30 s for sampling. For the dynamic experiments, the waveforms utilized were extracted from the probability density function (PDF) of the shear stress conditions found in the DeBakey VAD (16). This function describes the stress accumulation, or product of shear stress and exposure time, experienced by the platelets and is calculated along thousands of simulated platelet trajectories (16). The PDF represents the device thrombogenicity “footprint” and highlights potential thrombotic “hotspot” trajectories. For the purpose of the study, we exposed platelets to waveforms corresponding to the 30th and 50th percentiles of the PDF (Figure 1b). The magnitude of the waveforms was scaled by a factor of 52.5 due to the limitation of the HSD (maximum shear stress of 108 dyne/cm2 at 1 cP). Shear stress exposure was repeated at 110 passages per min for 10 min. Platelets were sampled at 0, 2, 5 and 10 min. Platelet activation was measured as detailed below.
Figure 1. Constant and dynamic shear stress waveforms.
Gel-filtered platelets (GFP) were exposed to a) variable magnitudes of constant shear stress for variable durations and b) dynamic shear stress waveforms representing a single exposure in the DeBakey VAD.
Shear-induced sensitization of in vitro aspirin-treated platelets
GFP, prepared as above, were pre-treated with 20 μM ASA 10 min prior to shear experiments. Control GFP were prepared with the addition of the solvent vehicle alone 10 min prior to exposure. Both forms of GFP were then exposed to shear stress in the HSD. Platelets were sheared at 70 dyne/cm2 for 40 s, followed by a subsequent low shear period of 1 dyne/cm2, for a total experimental duration of 15 min. Exposure to 1 dyne/cm2 for 15 min served as the negative shear control. Samples for activation measurements were taken at time 0, 40 s, 3 min, and every 3 min thereafter.
In vitro flow loop study with platelets pre-treated with ASA in vivo
Healthy adult volunteers were utilized for this study after providing informed consent in accordance with a Stony Brook University IRB-approved protocol (22). Whole blood, 30 ml, was obtained from all subjects as a baseline. Subjects then ingested 1000 mg buffered ASA (two 500 mg Ascriptin tablets, Novartis, East Hanover, NJ) and returned 2 h later for a second 30 ml blood donation. GFP were isolated from both samples as described above and diluted to a count of 15,000/μl in HEPES-modified Tyrode’s buffer with 5 mM Ca++ added 10 min prior to experiments. The platelet mixture, 120 ml, was then exposed to shear stress via circulation through an in vitro flow loop for 30 min at 37°C. The circuit included a DeBakey VAD (MicroMed Cardiovascular Inc., Houston, TX, USA), with the inlet and outlets connected via a short 0.5″ I.D. Tygon R3603 loop tubing and a 0.25″ I.D. Tygon R3603 flow resistor tubing of 47″ length. The VAD was operated at 4L/min and 9500 rpm, corresponding to a pressure rise of ~70 – 80 mmHg across the pump (16). Platelet samples were then taken via a silicone sampling port downstream of the VAD at 0, 10, 20, and 30 min for measurement of platelet activation.
Platelet activation measurement
Platelet activation state (PAS) was measured using a modified prothrombinase based assay (23) which is routine in our laboratory and correlates strongly with P-selectin expression, as quantified with flow cytometry (24) as well as with phosphatidylserine expression (FITC-Annexin V) (25). Briefly, this technique employs acetylated prothrombin to determine the rate of thrombin generation. The use of acetylated prothrombin blocks the feedback action of generated thrombin on platelets, ensuring linear kinetics during the assay and quantitative measurement of PAS (23). PAS values were normalized against fully activated platelets obtained by sonication (10 W for 10 s, Branson Sonifier 150 with microprobe, Branson, MO) (18, 19, 22). PAS values are therefore expressed as a fraction of the maximal thrombin-generating capacity, with a maximum value of 1.0.
Statistical Analysis
Prior to statistical analyses, the Shapiro-Wilk normality test was run for all data populations. For the constant and dynamic shear stress experiments, we calculated the differences in PAS between 0 and 10 min, ΔPAS, which was compared between ASA-treated platelets and control. The Platelet Activation Rate (PAR) for each experiment was obtained from the slopes of lines fit to normalized PAS values. For the in vivo ASA treatment, differences in PAR were obtained by subtracting the PAR calculated for ASA-treated platelet experiments from PAR obtained for paired control experiments. The percentage change in the PAR was calculated by dividing this difference by the control PAR and multiplying by 100. Parametric or non-parametric (Kruskal-Wallis) one-way ANOVA was performed, depending on the normality of the data, with Tukey’s post hoc test used to compare the PAR for the different groups tested. Paired samples Student’s t-tests were used to compare the ASA-treated and control PAR values for each set of VAD experiments, while differences between the PAR for control and ASA-treated experiment were compared to a value of 0, which represents the condition where PAR for ASA-treated and untreated platelets are identical. Significance was achieved for p < 0.05. Results are presented as the mean ± standard error of the mean (S.E.M.), unless otherwise stated.
Results
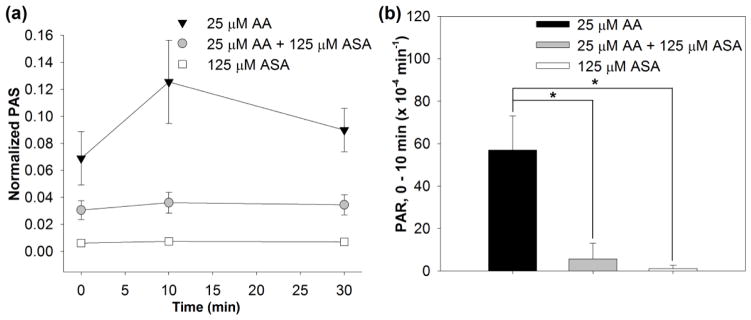
As a first step we examined the ability of the PAS assay to detect platelet activation via arachidonic acid (AA) and inhibition of activation via addition of ASA – i.e. biochemical activation and inhibition of activation. We selected this as our starting point as the PAS assay has largely been utilized for quantifying shear-mediated activation (14, 18, 25). AA-treated platelets revealed a clear increase in PAS between 0 and 10 min (Figure 2). In contrast, ASA-pretreated platelets demonstrated inhibition of activation. Both mean PAS (0.12 vs 0.04, p < 0.01) and PAR between 0 and 10 min (Table 1) demonstrated the protective effect and maintenance of the inhibitory effect of ASA after 10 min of incubation with AA. These results served to establish as a baseline the efficacy of the PAS assay for examining the effect of ASA in the outlined shear studies of this investigation.
Figure 2. The effect of ASA on platelets exposed to arachidonic acid (AA).
a) Platelet activation in time after treatment with AA alone (black), ASA and then AA (gray) or ASA alone (white). b) Platelet activation rate (PAR) after 10 min in the same conditions as a) (n = 4, *p < 0.01).
Table 1. Platelet Activity Rate (PAR) after static exposure to aspirin (ASA) and arachidonic acid (AA).
Mean PAR values were obtained from the slope of lines fit to PAS values for the first 10 min of platelet exposure to ASA and AA.
| Experimental conditions | PAR, 0–10 min (×10−4min−1) | p vs. 25 μM AA |
|---|---|---|
| 25 μM AA | 57 ± 16 | - |
| 25 μM AA + 125 μM ASA | 5.5 ± 7.4 | 0.001 |
| 125 μM ASA | 1.1 ± 1.6 | 0.004 |
Effect of Aspirin in Modulating Shear-Mediated Platelet Activation In Vitro
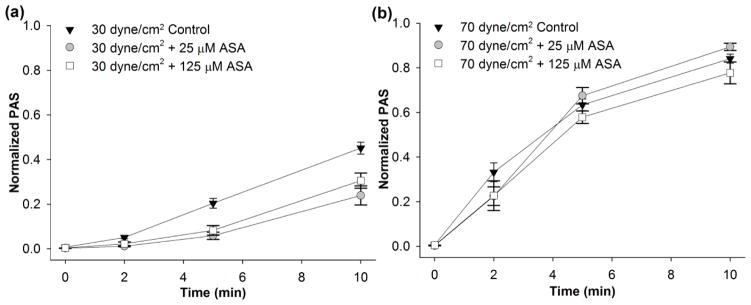
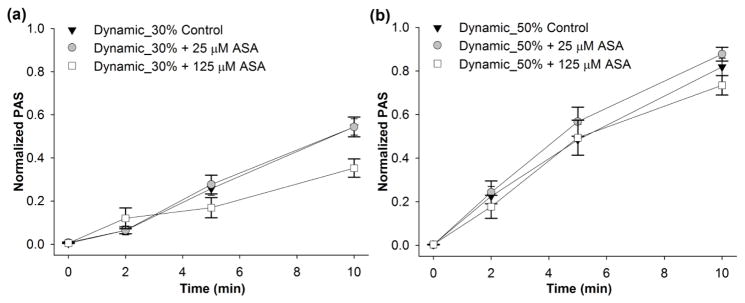
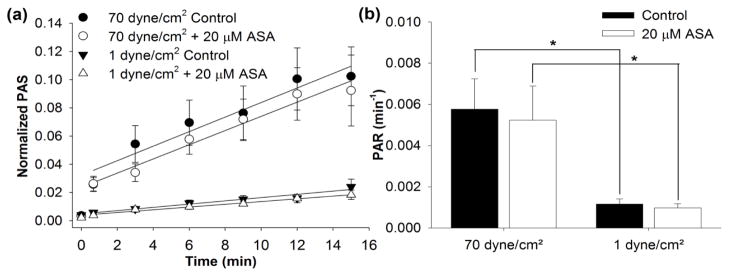
We then investigated the effect of constant shear stress on platelets pre-treated with 25 and 125 μM ASA. These ASA concentrations correspond to clinical use dosages of 81 mg/day or 325 mg/day, respectively (4). For constant shear conditions, a significant reduction in the ΔPAS after 10 min (Table 2) was observed for 30 dyne/cm2 exposure for both ASA concentrations when compared to control (Figure 3a, n = 10, p < 0.004). This corresponds to a 31.8% and 45.4% reduction in ΔPAS after 25 and 125 μM ASA pre-treatment, respectively. However, no significant reduction was observed for the 70 dyne/cm2 condition (Table 2, Figure 3b, n = 10, p > 0.1). Similarly, platelets treated with 125 μM ASA and exposed to the 30th percentile DeBakey VAD waveform showed a 35.2% reduction in ΔPAS compared to untreated platelets (Table 2, Figure 4a, n = 10, p < 0.05), However, 25 μM ASA-treated platelets showed no significant reduction compared to the control at 30th percentile shear stress waveform exposure (Figure 4a, n = 10, p > 0.5). Furthermore, ASA treatment had no effect on platelets exposed to the 50th percentile shear stress waveform (Table 2, Figure 4b, n = 10, p > 0.5). We also observed that platelets exposed to either the 30 dyne/cm2 constant shear stress condition or the 30th percentile dynamic VAD waveform showed a convex PAS behavior with respect to exposure time, whereas concave behavior was observed for the 70 dyne/cm2 constant shear condition and 50th percentile dynamic VAD waveform condition.
Table 2. Mean normalized ΔPAS for control and ASA-treated platelets subjected to different shear stress profiles.
Mean ΔPAS values were calculated from the difference in PAS between the 0 and 10 min samples obtained from the HSD.
| Experimental conditions | Normalized ΔPAS, 0 – 10 min | p vs. control |
|---|---|---|
| a) 30 dyne/cm2 | ||
| control | 0.44 ± 0.03 | - |
| 25 μM ASA | 0.30 ± 0.03 | < 0.001 |
| 125 μM ASA | 0.24 ± 0.04 | 0.004 |
| b) 70 dyne/cm2 | ||
| control | 0.83 ± 0.02 | - |
| 25 μM ASA | 0.89 ± 0.02 | 0.12 |
| 125 μM ASA | 0.77 ± 0.05 | 0.43 |
| c) Dynamic_30% | ||
| control | 0.54 ± 0.04 | - |
| 25 μM ASA | 0.54 ± 0.05 | 0.94 |
| 125 μM ASA | 0.35 ± 0.04 | 0.002 |
| d) Dynamic_50% | ||
| control | 0.82 ± 0.04 | - |
| 25 μM ASA | 0.87 ± 0.03 | 0.29 |
| 125 μM ASA | 0.73 ± 0.04 | 0.15 |
Figure 3. The effect of ASA on constant shear-mediated platelet activation.
The effect of ASA (25 or 125 μM) was investigated after exposure to a) 30 dyne/cm2 and b) 70 dyne/cm2 (n = 10).
Figure 4. The effect of ASA on dynamic shear-mediated platelet activation.
The effect of ASA (25 or 125 μM) was investigated after exposure to the dynamic waveforms extracted from simulation in the DeBakey VAD corresponding to the a) 30th (Dynamic_30%) and b) 50th percentile (Dynamic_50%) of the PDF function (n=10).
Effect of Aspirin in Modulating Shear-Induced Platelet Sensitization
We evaluated whether in vitro treatment with ASA modulated shear-induced platelet sensitization, or the priming of platelets for further activation subsequent to high shear stress exposure.(18) Platelets exposed to 70 dyne/cm2 for 40 s, followed by 1 dyne/cm2 showed a significant increase in the subsequent PAR during this follow-on exposure when compared to platelets only exposed to 1 dyne/cm2 for a similar time period (Figure 5, n = 10, p < 0.01). However, treatment with 20 μM ASA yielded a non-significant PAR reduction of 9.4% and 15.7% after 70 dyne/cm2 and 1 dyne/cm2 pre-exposure, respectively (Figure 5b, n = 10, p > 0.5). This indicates that 20 μM ASA does not significantly attenuate shear-induced platelet sensitization after exposure to constant pathological shear stress.
Figure 5. Platelet sensitization after in vitro ASA treatment.
a) For both 20 μM ASA-treated and control platelets, PAS was measured after initial 40 s exposure to 70 dyne/cm2, and during subsequent exposure 1 dyne/cm2. b) Sensitization PAR rates for ASA-treated and control platelets were calculated from PAS values subsequent to 70 dyne/cm2 and 1 dyne/cm2 pre-exposure (n = 10, *p < 0.01).
Effect of Aspirin in Modulating VAD-mediated (Elevated Shear) in Vitro
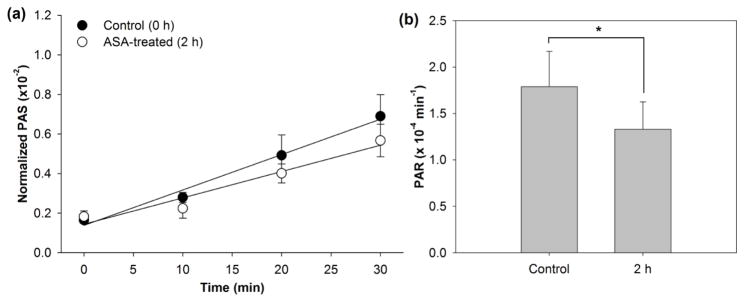
In vivo ASA treated-platelets (1000 mg ASA loading dose), sampled 2h after ASA ingestion, showed only a 0.46 ± 0.14 (× 10−4) min−1, or 25.6%, reduction in PAR following exposure to shear imparted by a clinical VAD when compared with paired control experiments (Figure 6, n = 7, p < 0.001). This reduction in platelet activation was observed to be 3-fold less than that achieved in prior work by our group through design optimization of the original DeBakey VAD (16).
Figure 6. Platelet activation in DeBakey VAD 2h post-in vivo ASA treatment.
a) PAS was measured for 1000 mg ASA-treated platelets and untreated platelets recirculated for 30 min through the VAD, with b) PAR determined from the slope of lines fit to PAS values (n = 7, p < 0.001).
Discussion
The overall goal of the present study was to examine the ability of ASA - a traditional anti-thrombotic and anti-platelet agent, operating via biochemical pathway inhibition, to limit shear-mediated platelet activation. In the present study, we utilized a “deconstructionist strategy,” – i.e. utilizing pure, gel-filtered platelets, to examine the efficacy of ASA to limit shear-mediated platelet activation under conditions devoid of other potentially pro-thrombotic blood constituents. Further, we utilized the HSD – affording exact control of the shear exposure history – to examine the effects of both constant and dynamic shear. As such, we demonstrate that ASA has a dichotomous effect with regard to its ability to limit shear-mediated platelet activation. At the low shear stress levels tested, i.e. 30 dynes/cm2 constant shear stress or 30th percentile of the PDF (stress accumulation of 1.25 dyne-s/cm2 per 545 ms passage (dynamic shear stress)), ASA demonstrated a clear ability to limit shear-mediated activation. In contrast at elevated shear levels tested, i.e. 70 dynes/cm2 constant shear stress or 50th percentile of the PDF (stress accumulation of 2.94 dyne-s/cm2 per 545 ms passage (dynamic shear stress)), ASA was unable to limit shear-mediated platelet activation. Furthermore, ASA did not significantly reduce platelet sensitization – i.e. subsequent activation following an initial high shear exposure. Finally, ASA had limited ability to prevent shear-mediated platelet activation for platelets subjected to elevated shear via cyclic passage through a cfVAD operating under normal clinical conditions (speed, flow rate) in vitro.
Ability of Aspirin to Modulate Shear-Mediated Platelet Activation
There are few studies in the literature which have examined the ability of ASA to limit shear mediated-platelet activation (11, 22, 26–28). Konstantopoulos et al. utilized a simple cone and plate viscometer system to demonstrate that the platelet response to shear stress was insensitive to ASA, rather being limited by agents that elevated platelet cyclic AMP levels (29). In their study, shear stress profiles used were those of constant shear with a maximum shear stress level tested of 140 dynes/cm2. The present study extends these observations along two lines. Herein we have utilized dynamic shear stress exposure, in addition to constant shear stress, and demonstrated the limited efficacy of ASA in this more aggressive setting. Furthermore in the present study, we utilize actual hyper-shear levels as experienced clinically via utilization of a clinical VAD – the DeBakey VAD, with peak shear stress > 800 dynes/cm2 (16). In this emulation of a clinical scenario, ASA offers no significant protective effect as to limitation of platelet activation.
ASA was also examined as to its ability to prevent shear-induced platelet aggregation mediated by ADP and vWF multimers (11). Here, the authors showed that shear-induced aggregation is mediated by either large plasma type vWF multimers, endogenous released platelet vWF forms, or unusually large vWF multimers derived from endothelial cells. Furthermore, they showed that aggregation requires ADP and is not inhibited significantly by ASA.
Due to its poor protective action on shear-mediated platelet aggregation, ASA has been used as control to prove the effectiveness of other more recent antiplatelet agents. In a study presented by Tsuchikane et al., 211 patients with 273 lesions who underwent successful percutaneous transluminal coronary angioplasty (PTCA) were randomly assigned to the cilostazol (200 mg/d) group or ASA (250 mg/d) control group (28). Cilostazol appeared to significantly reduce restenosis and target lesion revascularization rates after successful PTCA compared to ASA. More recently, an in vitro study conducted by Sheriff et al. compared the efficacy of ASA on platelets subjected to shear stress, proving that in vitro treatment with antiplatelet drugs such as ASA is as effective as in vivo metabolized ASA in testing the effect of reducing shear-induced platelet activation (22).
The advance afforded by the present study is the utilization of the HSD for shear stress exposure. In the HSD, shear exposure histories may be very finely regulated (15, 21, 30), with a dynamic response time of 4 ms and with defined control of shear stress magnitude (up to 108 dyne/cm2 at 1 cP viscosity). Furthermore, the HSD uniformly exposes a small sample volume (2.5 ml) to shear stress waveforms extracted from computational fluid dynamics (CFD) simulations of cardiovascular devices, including mechanical heart valves (14, 31) and cfVADs. In addition, the HSD set-up allows real-time sampling to examine the evolution of PAS over time.
Two strategies of shear stress exposure were chosen for this study – constant (continuous) and dynamic shear stress. The logic of this approach was to first examine the inhibitory efficacy of ASA under a simple, constant stress and then to follow with examination of the effect of dynamic varying conditions, i.e. those experienced by platelets with actual flight trajectories derived from passage through a VAD. Under constant low level shear, i.e. that within the normal physiological range experienced in the arterial vasculature (8 – 60 dynes/cm2) (32), ASA demonstrates some inhibition. Our results are consistent with studies that observed non-significant reduction in ASA-treated platelet aggregation after 30 s exposure to 30 and 60 dyne/cm2 in a cone-plate viscometer (11), and significant reduction in thrombus volume after 5 min exposure of canine blood to 56 dyne/cm2 (1600 s−1) in a parallel-plate flow chamber (33). However, when shear stress exposure was elevated to levels experienced with cardiovascular pathologies, such as arterial stenosis, or at elevated shear regions within a cfVAD, ASA was unable to prevent shear-mediated platelet activation. This reflects prior observations with aggregation in cone-and-plate viscometers at 108 and 120 dyne/cm2 (11, 34), collagen-induced thrombus formation above 80 dyne/cm2 in a perfusion chamber (35), and collagen- or vWF-induced platelet adhesion at 50 dyne/cm2 (36). Moreover, as with constant shear stress exposure, when platelets were exposed to pathologic dynamic shear stress levels, with net stress accumulation of 3235 dyne-s/cm2 (10 min at 110 passages/min for 50th percentile waveform), ASA afforded no protection for shear-mediated activation. Inhibition of activation was only observed after pre-treatment with 125 μM for platelets exposed to a net stress accumulation of 1378 dyne-s/cm2 (10 min at 110 passages/min for 30th percentile waveform).
Inability of Aspirin to Prevent Platelet Sensitization
We previously observed that platelets briefly exposed to elevated magnitudes of constant or dynamic shear stress, such as those found in cardiovascular devices or pathologies, continue to activate during subsequent low shear stress exposure (15, 18). This behavior, termed as shear-induced platelet sensitization, has been observed in purified platelets independent of coagulation factors typically present in plasma (18). In addition, sensitized platelets may activate quiescent platelets that have not been exposed to pathological conditions (18). In the present study, we observed that pre-treatment with 20 μM ASA yielded non-significant attenuation of this sensitization effect (Figure 5). Both ADP (released during the minimal lysis during the high shear stress phase) and microparticles generation were previously found to play a role in shear-induced platelet sensitization (18). While the effect of COX-1 pathway modulation on platelet sensitization is not yet understood, it has been shown that shear-induced platelet aggregation is significantly dependent on the presence of ADP, whereas ASA, and therefore COX-1 inhibition, has a minimal effect (11). Therefore, it appears that ASA pre-treatment is unlikely to inhibit the shear-induced sensitization response. This may partially explain why approximately 26% of VAD patients show aspirin resistance or persistent platelet activation despite antithrombotic therapy (37).
Efficacy of in vivo ASA in Limiting in vitro cfVAD Shear-mediated Platelet Activation
Our results overall demonstrate that subject-ingested in vivo ASA has limited ability to prevent and reduce shear-mediated platelet activation in our in vitro cfVAD loop model. In the present study we observed that treatment with maximum strength ASA (1000 mg, sampled 2h after ingestion) reduced the platelet activation rate by 25.6% after shear exposure with a DeBakey VAD. However, an earlier study by our group showed that 20 h after ASA ingestion, this reduction was all but absent (22). These results highlight the necessity of continuous monitoring and administration of ASA therapy to control the level of platelet activation under shear stress exposure. Unfortunately, this continuous administration of drugs could lead to other complications, such as bleeding and hemorrhage (4, 5). This finding has clinical relevance as present mechanical circulatory support systems, i.e. cfVADs, rely primarily on ASA as the main antiplatelet agent of current clinical anti-thrombotic regimens, with warfarin providing anticoagulant effects (4, 5). While our studies are strictly in vitro, utilizing gel-filtered platelets alone rather than whole blood, and must be viewed within that context, they do suggest to the clinical community that relying on ASA alone as the clinical “insurance” to prevent shear-mediated platelet activation may be very limited. Further, these results highlight the impact of shear for situations such as a cfVAD with high degrees of platelet exposure, in contradistinction to an arterial stenosis. In the case of a VAD, the entire blood volume, with contained platelets, circulates through regions of high shear within a short exposure time. In contrast, for a high-grade isolated arterial stenosis, as is seen in coronary artery disease, only a small fraction of platelets within the blood volume are actually exposed to elevated shear.
Limitations of the Present Study
In the present study we utilized isolated gel-filtered platelets in order to examine the direct effect of fluid shear stress on platelet activation, and ASA potential to modulate this process. By using purified platelets instead of whole blood (WB), or platelet-rich plasma we neglect the contribution of red blood cells (RBCs), white cells and plasma proteins to the platelet activation process. In particular, RBCs have been shown to increase platelet diffusivity as a function of 1) shear rate and hematocrit through platelet margination and 2) shear rate through localized mixing and cell-cell collisions (38–42). In our experimental protocols, we assumed that platelet margination played a limited role during our tests (absence of RBCs) and that the platelet concentration used (20000 per μl) minimized platelet collisions and cross-talk. RBCs can also contribute to thrombosis and hemostasis by releasing substances that act as platelet agonists. In fact, when hemolysis occurs, RBCs mainly release hemoglobin, ADP and LDH in the blood plasma. These elements have variable and contributory effects on thrombosis and platelet activation (43).
Whole blood also includes plasma proteins that contribute to platelet activation. Some of the prothrombinase complex factors (factor Xa, Ca2+), which are normally present in blood plasma in their inactive form, play a key role in the platelet activation process by enhancing the conversion of prothrombin to thrombin. In our study, due to the absence of these factors in GFP, this mechanism of activation was not considered. Nonetheless, the absence of the positive feedback on platelet activation provided by prothrombinase complex factors was necessary for the correct performance of the PAS assay, and these factors were reintroduced during the assay. It is worth clarifying that the main goal of our work was not to study platelet behavior through replication of the biological environment present in vivo. Rather, our goal was to assess the effect of ASA on platelet activation by measuring its ability to protect platelets under a range of shear stress conditions. To this end, GFP was considered the best platelet preparation for the detection of activation using the PAS assay.
Future perspectives
In recent years, a combinational approach consisting of coupling low dose ASA with other antiplatelet agents has been introduced for the clinical management of VAD-patients (4). Several research groups have begun evaluating the effect of ASA in combination with other drugs (8, 12, 13, 44–49). Nonetheless, the effectiveness of such combined therapeutic approaches after exposure to defined dynamic shear stress profiles has been poorly studied and defined. The results described herein provides a base upon which future studies examining the effects of combinational therapies may be examined. Further, future studies are planned to contrast the efficacy of ASA in limiting shear-mediated platelet activation in whole blood with that observed herein with GFP. Finally, our findings suggest that mechanotransduction of shear leading to platelet activation operates via mechanisms that are independent or insensitive to prostaglandin/thromboxane inhibition (50).
Conclusions
The present study utilizing purified gel-filtered platelets demonstrates that ASA, a routine, basal component of clinical antithrombotic therapy for prevention of platelet activation in patients with a variety of cardiovascular pathologies, has limited efficacy at best in modulating shear-mediated platelet activation under flow conditions and shear levels existent in mechanical circulatory support devices such as cfVADs. Our study goes beyond what has been termed “aspirin resistance” (50) to demonstrate, in circumstances of supra-physiologic shear stress exposure and accumulation, a domain of “limited or lack” of ASA effect. ASA demonstrated only moderate efficacy under conditions of low shear and with no significant efficacy under high shear. Similarly, ASA was unable to limit platelet sensitization – i.e. subsequent activation following an initial high shear exposure. Finally, ASA had limited ability to prevent shear-mediated platelet activation for platelets subjected to elevated shear via cyclic passage through a cfVAD. While our study is performed under in vitro conditions, it provides potential mechanistic insight for the persistent thrombosis rates experienced clinically in cfVAD patients despite ASA therapy. Utilizing ASA with cfVADS remains the present clinical standard-of-care. However, our results reveal the need for future studies aimed at consideration of combinational drug strategies or new agents or means for limiting shear-mediated platelet activation under elevated “hyper-shear” conditions as experienced in patients with cfVADs with persistent thrombotic risk.
Table 3.
Summary.
What is known on this topic
|
What this paper adds
|
Highlights.
ASA has limited efficacy in modulating high shear-mediated platelet activation (SMPA)
ASA is unable to limit shear-mediated platelet sensitization
ASA has limited efficacy in modulating SMPA for shear associated with cfVADs
Need exists for strategies to modulate SMPA with “hypershear” (600–1000 dynes/cm2)
Acknowledgments
Funding Source
This work was supported by the National Institute of Biomedical Imaging and Bioengineering Quantum Grant Award No. 5U01EB012487-00 and by Fondazione Cariplo grant no. 2241-2011
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peura JL, Colvin-Adams M, Francis GS, et al. Recommendations for the use of mechanical circulatory support: device strategies and patient selection: a scientific statement from the American Heart Association. Circulation. 2012;126:2648–67. doi: 10.1161/CIR.0b013e3182769a54. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Kormos RL, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2014;33:12–22. doi: 10.1016/j.healun.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29:S1–39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Ensor CR, Paciullo CA, Cahoon WD, Jr, et al. Pharmacotherapy for mechanical circulatory support: a comprehensive review. Ann Pharmacother. 2011;45:60–77. doi: 10.1345/aph.1P459. [DOI] [PubMed] [Google Scholar]
- 5.Von Ruden SA, Murray MA, Grice JL, et al. The pharmacotherapy implications of ventricular assist device in the patient with end-stage heart failure. J Pharm Pract. 2012;25:232–49. doi: 10.1177/0897190011431635. [DOI] [PubMed] [Google Scholar]
- 6.Miner J, Hoffhines A. The discovery of aspirin’s antithrombotic effects. Tex Heart Inst J. 2007;34:179–86. [PMC free article] [PubMed] [Google Scholar]
- 7.Craven LL. Coronary thrombosis can be prevented. J Insur Med. 1950;5:47–8. [PubMed] [Google Scholar]
- 8.Cattaneo M. Aspirin and clopidogrel: efficacy, safety, and the issue of drug resistance. Arterioscler Thromb Vasc Biol. 2004;24:1980–7. doi: 10.1161/01.ATV.0000145980.39477.a9. [DOI] [PubMed] [Google Scholar]
- 9.Patrono C, Rocca B. Aspirin: promise and resistance in the new millennium. Arterioscler Thromb Vasc Biol. 2008;28:s25–32. doi: 10.1161/ATVBAHA.107.160481. [DOI] [PubMed] [Google Scholar]
- 10.Burch JW, Stanford N, Majerus PW. Inhibition of platelet prostaglandin synthetase by oral aspirin. J Clin Invest. 1978;61:314–9. doi: 10.1172/JCI108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moake JL, Turner NA, Stathopoulos NA, et al. Shear-induced platelet aggregation can be mediated by vWF released from platelets, as well as by exogenous large or unusually large vWF multimers, requires adenosine diphosphate, and is resistant to aspirin. Blood. 1988;71:1366–74. [PubMed] [Google Scholar]
- 12.Taylor ML, Misso NL, Stewart GA, et al. The effects of varying doses of aspirin on human platelet activation induced by PAF, collagen and arachidonic acid. Br J Clin Pharmacol. 1992;33:25–31. doi: 10.1111/j.1365-2125.1992.tb03996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooke GE, Liu-Stratton Y, Ferketich AK, et al. Effect of platelet antigen polymorphism on platelet inhibition by aspirin, clopidogrel, or their combination. J Am Coll Cardiol. 2006;47:541–6. doi: 10.1016/j.jacc.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Xenos M, Girdhar G, Alemu Y, et al. Device Thrombogenicity Emulator (DTE) - Design optimization methodology for cardiovascular devices: A study in two bileaflet MHV designs. J Biomech. 2010;43:2400–9. doi: 10.1016/j.jbiomech.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheriff J, Soares JS, Xenos M, et al. Evaluation of shear-induced platelet activation models under constant and dynamic shear stress loading conditions relevant to devices. Ann Biomed Eng. 2013;41:1279–96. doi: 10.1007/s10439-013-0758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girdhar G, Xenos M, Alemu Y, et al. Device thrombogenicity emulation: a novel method for optimizing mechanical circulatory support device thromboresistance. PLoS One. 2012;7:e32463. doi: 10.1371/journal.pone.0032463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu WC, Girdhar G, Xenos M, et al. Thromboresistance comparison of the HeartMate II ventricular assist device with the device thrombogenicity emulation- optimized Heart Assist 5 VAD. J Biomech Eng. 2014;136:021014. doi: 10.1115/1.4026254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheriff J, Bluestein D, Girdhar G, et al. High-shear stress sensitizes platelets to subsequent low-shear conditions. Ann Biomed Eng. 2010;38:1442–50. doi: 10.1007/s10439-010-9936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz-Heik K, Ramachandran J, Bluestein D, et al. The extent of platelet activation under shear depends on platelet count: differential expression of anionic phospholipid and factor Va. Pathophysiol Haemost Thromb. 2005;34:255–62. doi: 10.1159/000093104. [DOI] [PubMed] [Google Scholar]
- 20.Neuenschwander P, Jesty J. A comparison of phospholipid and platelets in the activation of human factor VIII by thrombin and factor Xa, and in the activation of factor X. Blood. 1988;72:1761–70. [PubMed] [Google Scholar]
- 21.Nobili M, Sheriff J, Morbiducci U, et al. Platelet activation due to hemodynamic shear stresses: damage accumulation model and comparison to in vitro measurements. ASAIO J. 2008;54:64–72. doi: 10.1097/MAT.0b013e31815d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheriff J, Girdhar G, Chiu WC, et al. Comparative efficacy of in vitro and in vivo metabolized aspirin in the DeBakey ventricular assist device. J Thromb Thrombolysis. 2014;37:499–506. doi: 10.1007/s11239-013-0997-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jesty J, Bluestein D. Acetylated prothrombin as a substrate in the measurement of the procoagulant activity of platelets: elimination of the feedback activation of platelets by thrombin. Anal Biochem. 1999;272:64–70. doi: 10.1006/abio.1999.4148. [DOI] [PubMed] [Google Scholar]
- 24.Claiborne TE, Girdhar G, Gallocher-Lowe S, et al. Thrombogenic potential of Innovia polymer valves versus Carpentier-Edwards Perimount Magna Aortic Bioprosthetic Valves. ASAIO J. 2011;57:26–31. doi: 10.1097/MAT.0b013e3181fcbd86. [DOI] [PubMed] [Google Scholar]
- 25.Jesty J, Yin W, Perrotta P, et al. Platelet activation in a circulating flow loop: combined effects of shear stress and exposure time. Platelets. 2003;14:143–9. doi: 10.1080/0953710031000092839. [DOI] [PubMed] [Google Scholar]
- 26.Minami N, Suzuki Y, Yamamoto M, et al. Inhibition of shear stress-induced platelet aggregation by cilostazol, a specific inhibitor of cGMP-inhibited phosphodiesterase, in vitro and ex vivo. Life Sci. 1997;61:PL 383–9. doi: 10.1016/s0024-3205(97)00986-7. [DOI] [PubMed] [Google Scholar]
- 27.Tomizawa A, Ohno K, Jakubowski JA, et al. Comparison of antiplatelet effects of prasugrel and ticagrelor in cynomolgus monkeys by an ELISA-based VASP phosphorylation assay and platelet aggregation. Thromb Haemost. 2013;110:769–76. doi: 10.1160/TH13-03-0260. [DOI] [PubMed] [Google Scholar]
- 28.Tsuchikane E, Fukuhara A, Kobayashi T, et al. Impact of cilostazol on restenosis after percutaneous coronary balloon angioplasty. Circulation. 1999;100:21–6. doi: 10.1161/01.cir.100.1.21. [DOI] [PubMed] [Google Scholar]
- 29.Konstantopoulos K, Wu KK, Udden MM, et al. Flow cytometric studies of platelet responses to shear stress in whole blood. Biorheology. 1995;32:73–93. doi: 10.3233/bir-1995-32106. [DOI] [PubMed] [Google Scholar]
- 30.Girdhar G, Bluestein D. Biological effects of dynamic shear stress in cardiovascular pathologies and devices. Expert Rev Med Devices. 2008;5:167–81. doi: 10.1586/17434440.5.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alemu Y, Girdhar G, Xenos M, et al. Design Optimization of a Mechanical Heart Valve for Reducing Valve Thrombogenicity-A Case Study with ATS Valve. ASAIO J. 2010;56:389–96. doi: 10.1097/MAT.0b013e3181e65bf9. [DOI] [PubMed] [Google Scholar]
- 32.Hathcock JJ. Flow effects on coagulation and thrombosis. Arterioscler Thromb Vasc Biol. 2006;26:1729–37. doi: 10.1161/01.ATV.0000229658.76797.30. [DOI] [PubMed] [Google Scholar]
- 33.Roux SP, Sakariassen KS, Turitto VT, et al. Effect of aspirin and epinephrine on experimentally induced thrombogenesis in dogs. A parallelism between in vivo and ex vivo thrombosis models. Arterioscler Thromb. 1991;11:1182–91. doi: 10.1161/01.atv.11.5.1182. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T, Uchiyama S, Yamazaki M, et al. Effects of dipyridamole and aspirin on shear-induced platelet aggregation in whole blood and platelet-rich plasma. Cerebrovasc Dis. 2002;14:234–8. doi: 10.1159/000065669. [DOI] [PubMed] [Google Scholar]
- 35.Barstad RM, Orvim U, Hamers MJ, et al. Reduced effect of aspirin on thrombus formation at high shear and disturbed laminar blood flow. Thromb Haemost. 1996;75:827–32. [PubMed] [Google Scholar]
- 36.Turner NA, Moake JL, Kamat SG, et al. Comparative real-time effects on platelet adhesion and aggregation under flowing conditions of in vivo aspirin, heparin, and monoclonal antibody fragment against glycoprotein IIb-IIIa. Circulation. 1995;91:1354–62. doi: 10.1161/01.cir.91.5.1354. [DOI] [PubMed] [Google Scholar]
- 37.Houel R, Mazoyer E, Boval B, et al. Platelet activation and aggregation profile in prolonged external ventricular support. J Thorac Cardiovasc Surg. 2004;128:197–202. doi: 10.1016/j.jtcvs.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 38.Aarts PA, Heethaar RM, Sixma JJ. Red blood cell deformability influences platelets--vessel wall interaction in flowing blood. Blood. 1984;64:1228–33. [PubMed] [Google Scholar]
- 39.Goldsmith HL, Turitto VT. Rheological aspects of thrombosis and haemostasis: basic principles and applications. ICTH-Report--Subcommittee on Rheology of the International Committee on Thrombosis and Haemostasis. Thromb Haemost. 1986;55:415–35. [PubMed] [Google Scholar]
- 40.Jordan A, David T, Homer-Vanniasinkam S, et al. The effects of margination and red cell augmented platelet diffusivity on platelet adhesion in complex flow. Biorheology. 2004;41:641–53. [PubMed] [Google Scholar]
- 41.Zhao R, Kameneva MV, Antaki JF. Investigation of platelet margination phenomena at elevated shear stress. Biorheology. 2007;44:161–77. [PubMed] [Google Scholar]
- 42.Du VX, Huskens D, Maas C, et al. New insights into the role of erythrocytes in thrombus formation. Semin Thromb Hemost. 2014;40:72–80. doi: 10.1055/s-0033-1363470. [DOI] [PubMed] [Google Scholar]
- 43.Helms CC, Marvel M, Zhao W, et al. Mechanisms of hemolysis-associated platelet activation. J Thromb Haemost. 2013;11:2148–54. doi: 10.1111/jth.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gresele P, Momi S, Falcinelli E. Anti-platelet therapy: phosphodiesterase inhibitors. Br J Clin Pharmacol. 2011;72:634–46. doi: 10.1111/j.1365-2125.2011.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- 46.Lenz TL, Hilleman DE. Aggrenox: a fixed-dose combination of aspirin and dipyridamole. Ann Pharmacother. 2000;34:1283–90. doi: 10.1345/aph.10079. [DOI] [PubMed] [Google Scholar]
- 47.Verro P, Gorelick PB, Nguyen D. Aspirin plus dipyridamole versus aspirin for prevention of vascular events after stroke or TIA: a meta-analysis. Stroke. 2008;39:1358–63. doi: 10.1161/STROKEAHA.107.496281. [DOI] [PubMed] [Google Scholar]
- 48.Adams RJ, Albers G, Alberts MJ, et al. Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2008;39:1647–52. doi: 10.1161/STROKEAHA.107.189063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ratnatunga CP, Edmondson SF, Rees GM, et al. High-dose aspirin inhibits shear-induced platelet reaction involving thrombin generation. Circulation. 1992;85:1077–82. doi: 10.1161/01.cir.85.3.1077. [DOI] [PubMed] [Google Scholar]
- 50.Gasparyan AY, Watson T, Lip GY. The role of aspirin in cardiovascular prevention: implications of aspirin resistance. J Am Coll Cardiol. 2008 May 13;51(19):1829–43. doi: 10.1016/j.jacc.2007. [DOI] [PubMed] [Google Scholar]