Abstract
Motile phototrophic consortia are highly regular associations in which numerous cells of green sulfur bacteria surround a flagellated colorless β-proteobacterium in the center. To date, seven different morphological types of such consortia have been described. In addition, two immotile associations involving green sulfur bacteria are known. By employing a culture-independent approach, different types of phototrophic consortia were mechanically isolated by micromanipulation from 14 freshwater environments, and partial 16S rRNA gene sequences of the green sulfur bacterial epibionts were determined. In the majority of the lakes investigated, different types of phototrophic consortia were found to co-occur. In all cases, phototrophic consortia with the same morphology from the same habitat contained only a single epibiont phylotype. However, morphologically indistinguishable phototrophic consortia collected from different lakes contained different epibionts. Overall, 19 different types of epibionts were detected in the present study. Whereas the epibionts within one geographic region were very similar (Dice coefficient, 0.582), only two types of epibionts were found to occur on both the European and North American continents (Dice coefficient, 0.190). None of the epibiont 16S rRNA gene sequences have been detected so far in free-living green sulfur bacteria, suggesting that the interaction between epibionts and chemotrophic bacteria in the phototrophic consortia is an obligate interaction. Based on our phylogenetic analysis, the epibiont sequences are not monophyletic. Thus, the ability to form symbiotic associations either arose independently from different ancestors or was present in a common ancestor prior to the radiation of green sulfur bacteria and the transition to the free-living state in independent lineages. The present study thus demonstrates that there is great diversity and nonrandom geographical distribution of phototrophic consortia in the natural environment.
Motile phototrophic consortia are associations of bacterial cells in which a flagellated colorless β-proteobacterium is surrounded by numerous cells of green sulfur bacteria, the so-called epibionts (42, 44). The barrel-shaped, motile phototrophic consortia were first described in the early 20th century (7, 35). Since their discovery, seven different morphotypes, namely, “Chlorochromatium aggregatum,” “Chlorochromatium glebulum,” “Chlorochromatium magnum,” “Chlorochromatium lunatum,” “Pelochromatium roseum,” “Pelochromatium roseo-viride,” and “Pelochromatium selenoides,” have been described on the basis of morphology and the cellular arrangement of the epibionts (42).
The cellular arrangement in phototrophic consortia is always very regular, and the cell division of the partner bacteria proceeds in a highly coordinated fashion (43). Furthermore, rapid signal transfer between the epibionts and the central bacterium has been demonstrated (19). These observations indicate that the two bacterial partners are tightly and specifically associated. Theoretically, such a highly specific association could have emerged only once during evolution. Indeed, initial analyses of the phylogenetic affiliations of five types of phototrophic consortia from two lakes indicated that the epibionts form a single cluster within the green sulfur bacterial radiation, whereas most other free-living members of this phylum are more distantly related (20). However, the available data are not sufficient to elucidate whether a change from the symbiotic life style to the independent life style or vice versa has occurred multiple times during the radiation of the green sulfur bacteria.
Phototrophic consortia have been reported to occur in numerous freshwater lakes and ponds worldwide (8, 40, 43). Since consortia have been found to thrive exclusively in the chemocline of freshwater lakes at low concentrations of dissolved sulfide and low light intensities, they are assumed to occupy a narrow and well-defined ecological niche (43). Based on the postulate that bacteria are ubiquitous (2, 4), competitive exclusion of species with identical ecological niches would be expected to result in low overall diversity. In contrast, endemism of microorganisms would result in significantly greater global diversity (16, 17), since such diversity is maintained by geographic isolation (48). In the case of phototrophic consortia, it is not known whether the identical morphotypes observed in lakes on different continents harbor the same types of bacteria or whether the diversity surmounts the seven morphotypes that are currently recognized.
In the present study, the 16S rRNA gene sequences of phototrophic consortia were analyzed in order to (i) obtain more information on the actual diversity of the green sulfur bacterial epibionts, (ii) obtain a broader view of the geographical distribution of different types, and (iii) assess whether the switch from the symbiotic life style to the independent life style (or vice versa) occurred once or more frequently during the radiation of green sulfur bacteria. We used a culture-independent approach (20), in which different types of phototrophic consortia are mechanically isolated by micromanipulation and 16S rRNA gene sequences of epibionts are obtained after a highly sensitive group-specific amplification step, followed by separation by denaturing gradient gel electrophoresis (DGGE). So far, there is not an amplification method with comparable sensitivity for the 16S rRNA genes of β-proteobacteria. In addition, no single 16S rRNA gene sequence of a central bacterium is available at this time. Therefore, primers specific for central bacteria of phototrophic consortia have not been developed so far. Consequently, the present study was limited to the green sulfur bacterial epibionts.
MATERIALS AND METHODS
Study sites.
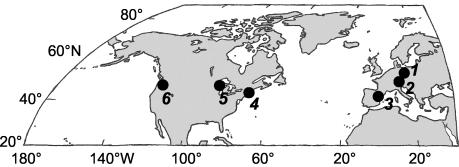
Fourteen lakes and ponds were selected based on previous studies and previously published data. The study sites are located in six different geographic regions (Fig. 1) and comprise three lakes in Germany, two lakes in Spain, one pond in Massachusetts, seven lakes in Michigan, and one lake in the state of Washington (Table 1). In each of the lakes, an anoxic, sulfide-containing hypolimnion builds up after the onset of summer stratification. Concomitantly, dense accumulations of anoxygenic phototrophic bacteria and phototrophic consortia develop in the chemocline (5, 14, 23, 24, 25, 27, 43, 50). The physicochemical conditions in the chemoclines during summer stratification were comparable in the lakes (Table 1). In Oyster Pond (Woods Hole, Mass.), the surface sediment usually becomes anoxic during the summer and is subsequently colonized by anoxygenic phototrophic bacteria. Although not detectable directly by microscopy in sediment samples, phototrophic consortia can be enriched from this sediment if the samples are incubated under appropriate conditions (Overmann, unpublished data) (Table 1). During the present study, the sampling locations were visited in the years from 1998 to 2001 between July and October (Table 1).
FIG. 1.
Geographic regions sampled during the present study. The numbers refer to Table 1, which lists the lakes studied and provides details on environmental parameters.
TABLE 1.
Selected physicochemical parameters and phototrophic consortia in the chemoclines of different lakes
| Lake or pond | Characteristic environmental parametersa
|
Reference(s) | Sampling date (day/mo/yr) | Depth (m) | Morphotype | Epibiont phylotypeb | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chemo- cline (m) | Temp (°C) | pH | Light intensity (μmol · m−2 · s−1) | Sulfide concn (μM) | ||||||
| Geographical region 1c | ||||||||||
| Dagow | 6.0 | 10 | 7.35 | 0.7 | 10 | 26 | 7/7/1998 | 6.4 | “C. aggregatum” | M |
| “C. magnum” CmD | E | |||||||||
| “P. roseum” PRDBD7 | K | |||||||||
| “P. latum” PRDBD8 | N | |||||||||
| Haussee | 6.0 | 13.7 | 7.5 | 2.4 | — f | H.-D. Babenzien, | 20/7/1999 | 6.5 | “C. aggregatum” CaH | C |
| personal | “P. roseum” PrH | G | ||||||||
| comunication | “P. selenoides” PSH | D | ||||||||
| Geographical region 2 | ||||||||||
| Schleinsee | 6 | 8-10 | 7.8 | 0.5-5 | 30 | 14 | 9/9/2001 | 7 | “P. roseum” 25 | K |
| Geographical region 3 | ||||||||||
| Sisó | 2.75 | 15 | 7.2 | — | 200 | Unpublished data | 23/8/1999 | 2.75 | “C. aggregatum” CaS | P |
| Coromina | 1.5 | 20 | 7.4 | 0.01 | 10 | 24 | 22/8/1999 | 2.5 | “C. aggregatum” CaC | P |
| Geographical region 4 | ||||||||||
| Oyster Pondd | NAe | 23 | 7 | 7 | 100 | Unpublished data | 30/6/2000 | 0.01 | “C. aggregatum” CaSp | L |
| Geographical region 5 | ||||||||||
| Wintergreen | 3.5 | 17 | 8.1 | 0.95 | 192 | 24, 25 | 4/9/2001 | 4.2 | “C. aggregatum” 7b-2 | M |
| “C. aggregatum” 8a-2 | N | |||||||||
| “C. magnum” 13-2 | H | |||||||||
| “P. latum” 29-2 | N | |||||||||
| Baker | 7 | 6 | 7.5 | 2.94 | 102 | 24, 25 | 4/9/2001 | 6 | “C. aggregatum” 11b | D |
| “C. magnum” 14-2 | H | |||||||||
| “P. roseum” 1a-2 | O | |||||||||
| “P. latum” 1b-2 | N | |||||||||
| Sheffer | — f | — | — | — | — | — | 4/9/2001 | 5.8 | “C. aggregatum” 9b-2 | D |
| “C. magnum” 9a | H | |||||||||
| “P. roseum” 18a | O | |||||||||
| “P. roseum 18c-2 | B | |||||||||
| “P. latum” 18b | N | |||||||||
| “C. vacuolata” | D | |||||||||
| Mud | 4 | 15 | 7 | 0.4 | 8 | 24, 25 | 4/9/2001 | 3.5 | “C. magnum” 52 | A |
| Leach | 3.5 | 21 | 7.2 | 1.52 | 87 | 24, 25 | 4/9/2001 | 7 | “C. aggregatum” 10b-2 | N |
| “C. magnum” 10a | H | |||||||||
| “P. roseum” 20 | O | |||||||||
| Round | 4.5 | 12 | 7.5 | 3.27 | 133 | 24, 25 | 4/9/2001 | 4.7 | “C. aggregatum” 4b-2 | N |
| “C. magnum” 14-2 | H | |||||||||
| “P. latum” 19-2 | N | |||||||||
| Cassidy | 8 | 5 | 7.4 | 0.05 | 62 | 24, 25 | 4/9/2001 | 6.7 | “C. aggregatum” 5-2 | D |
| “C. magnum” 10 | H | |||||||||
| “C. lunatum” 12-2 | D | |||||||||
| “P. roseum” 16a-2 | O | |||||||||
| “P. latum” 2b | N | |||||||||
| Geographical region 6 | ||||||||||
| Echo | — | — | — | — | — | — | 10/10/1998 | 10 | “C. aggregatum” CaEL | J |
| “C. glebulum” ELGSB5 | F | |||||||||
| “C. magnum” ELGSB2 | H | |||||||||
| “P. roseum” PrEL | B | |||||||||
Data from the literature. See the references cited for geographical coordinates of individual lakes.
See the phylogenetic tree in Fig. 5.
See Fig. 1.
Phototrophic consortia were not detectable in sediment samples, but they were enriched under the conditions provided.
NA, not applicable.
—, no information available.
Water sampling.
Water samples were obtained from the deepest parts of the lakes by employing a bilge pump connected to gas-tight isoversinic tubing. The inlet of the tubing consisted of two polyvinyl chloride cones that were 1 cm apart (34). This device allowed reproducible sampling of different water layers at 5-cm intervals. Sampling depths were chosen based on the vertical distribution of anoxygenic photosynthetic bacteria as determined by light and phase-contrast microscopy.
Samples from European lakes were placed in autoclaved 1-liter glass bottles and kept in the dark to avoid damage to the phototrophic bacteria, which occurs at the high light intensities at the lake surface (43). The bottles were sealed gas tight to prevent abiotic oxidation of sulfide, and then they were transported back to the laboratory at the in situ temperature and processed within 8 h after sampling. Samples obtained from North American lakes were transferred to 10-ml gas-tight screw-cap glass tubes. Anoxic conditions were maintained by addition of 200 μM neutralized (pH 7.3) sulfide solution (45). The glass tubes were shipped on artificial ice and by courier to the home laboratory, and they were processed within 48 h.
Isolation of intact phototrophic consortia and molecular fingerprinting of epibionts.
Phototrophic consortia were mechanically separated from the chemocline microbial community by using a micromanipulator connected to an inverted microscope (18, 19). At first, water samples containing phototrophic consortia were evenly spread on coverslips by squeezing 100 μl of a water sample between two acetone-cleaned coverslips (60 by 20 mm). Excess water was removed with a paper tissue, and the coverslips were separated and air dried aseptically. After drying, different morphotypes of phototrophic consortia could still be distinguished by bright-field and phase-contrast microscopy based on size and on the color, number, and shape of the epibionts.
Batches of 5 to 45 consortia with the same morphology were collected by micromanipulation and directly subjected to PCR amplification. 16S rRNA gene fragments that were 540 bp long were amplified by employing oligonucleotide primers GC 357f and GSB 840r and the PCR conditions described previously (41). Our method permitted highly sensitive and specific amplification of 16S rRNA gene fragments of the epibionts (20). A particular fragment was chosen because it included region V3, which is the largest of the highly variable regions in the 16S rRNA gene (11). In order to check for the presence of different epibionts in the same morphotype of phototrophic consortia, the resulting amplification products were separated by denaturing gradient gel electrophoresis (DGGE) according to melting behavior (39). The 6% (wt/vol) polyacrylamide DGGE gels contained a linear gradient of 35 to 70% denaturing agents. After the gels were stained with ethidium bromide, individual DNA bands were excised from each DGGE gel, recovered by electroelution, reamplified, and sequenced as described previously (41).
Phylogenetic analysis of 16S rRNA gene sequences of the epibionts.
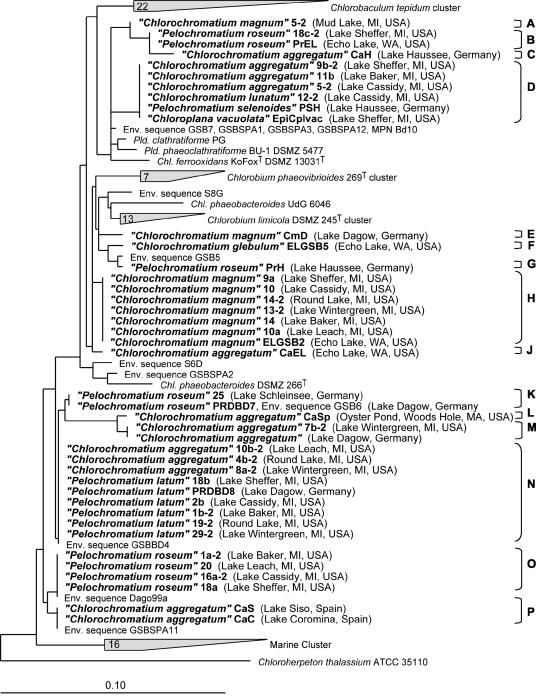
Phylogenetic analysis of 16S rRNA gene sequences of the epibionts was performed by using the ARB phylogeny package (37). The program Fast Aligner V1.03 was used for alignment of all 16S rRNA gene sequences of green sulfur bacteria available through the National Center for Biotechnology Information website (1). The sequence of Chloroherpeton thalassium ATCC 35110T was chosen as the outgroup, since it has been shown to branch at the root of the green sulfur bacteria and significantly deeper than all other known green sulfur bacteria (40). The alignment was manually corrected based on secondary structure information for Chlorobium vibrioforme ATCC 6030, and a phylogenetic tree was constructed from all sequences longer than 1,300 bp by using the maximum-likelihood program DNA_ML. After this, the partial 16S rRNA gene sequences of epibionts were inserted into the phylogenetic tree by using the Parsimony Interactive tool without changing the overall tree topology.
Small differences between partial 16S rRNA gene sequences can be caused by sequencing errors and hence can lead to overestimation of the sequence diversity of epibionts. In order to identify such potential sequencing errors, sequence pairs which differed at only one or two nucleotide positions were reassessed based on secondary structure information for the 16S rRNA molecule (20). We investigated whether nucleotide changes in double-stranded regions of the 16S rRNA molecule were compensated for by nucleotide changes in the opposite strand. Substitutions which resulted in the loss of Watson-Crick base pairing were regarded as sequencing errors and not included in the phylogenetic analysis. Based on our assessment, all sequences that differed at only one nucleotide position were treated as a single sequence type. Only two of the sequences (environmental sequence GSB5 and “P. roseum” PrH [see Fig. 5]) differed at two confirmed nucleotide positions. All other 16S rRNA gene sequences analyzed in the present study differed at three or more nucleotide positions. Consequently, the estimate of microbial diversity presented in this paper is a conservative estimate.
FIG. 5.
Phylogenetic tree of 16S rRNA gene sequences of green sulfur bacterial epibionts and their relatives. Distinct phylotypes are indicated by uppercase letters A through P. Bar = 0.1 fixed point mutation per nucleotide position. Pld., Pelodictyon; Chl., Chlorobium; Env., environmental.
Comparison of epibionts in different geographic regions.
The presence or absence of distinct types of epibionts was used to compare the communities of phototrophic consortia in lakes within the same geographic area or in lakes in different regions.
The binary similarity of communities in different lakes was computed by employing the coincidence index of Dice (D) (12): D = 2a/(2a + b + c), where a is the number of epibiont types present in the two environments compared, b is the number of types found exclusively in the first environment, and c is the number of epibiont types present in the second environment.
Chemotaxis assays.
To date, the chemotactic response of intact phototrophic consortia has been studied only for specimen from a single lake in eastern Germany (19, 28). The close proximity of a laboratory to Lake Sisó provided the opportunity to investigate the chemotactic behavior of the population of consortia in this second lake. Chemotaxis was tested by incubation of rectangular capillaries (length, 50 mm; inside dimensions, 0.1 by 1.0 mm; capacity, 5 μl; Vitro Dynamics, Rockaway, N.J.) filled with diluted test substances. Sterile 100 mM stock solutions of sulfide, thiosulfate, glycerol, acetate, pyruvate, lactate, propionate, citrate, succinate, 2-oxoglutarate, and glycine were prepared anoxically in Hungate tubes sealed with butyl rubber septa and flushed with N2. For preparation of organic acids, the corresponding sodium salts were used, and the pH of each stock solution was adjusted to 7.3 with NaOH. This pH value was chosen based on the pH values determined previously for the chemocline water of Lake Sisó (pH 7.2 to 7.4) (Table 1) (27, 43). All compounds were diluted to a final concentration of 500 μM with filter-sterilized (cellulose nitrate membrane filters; pore size, 0.2 μm; Sartorius, Göttingen, Germany), anoxic chemocline water. Controls were filled with sterile, anoxic chemocline water only. The capillaries were immediately sealed with plasticine (Idena, Berlin, Germany) at one end, and then the open end of each capillary was inserted into a sample containing consortia (19). Incubation lasted for 3 to 5 h.
After incubation, each capillary was recovered and immediately closed with plasticine. The phototrophic consortia were counted directly in each capillary by phase-contrast and dark-field microscopy at magnifications of ×100 and ×400.
RESULTS AND DISCUSSION
Individual populations of phototrophic consortia as detected by molecular fingerprinting.
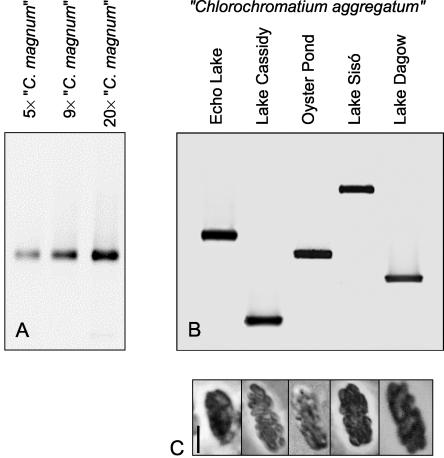
Theoretically, phototrophic consortia could form randomly from bacterial cells which encounter each other by chance. Under these conditions, morphologically identical consortia would be expected to harbor different types of green sulfur bacteria. To test this hypothesis, the epibionts associated with particular phototrophic consortia were analyzed by 16S rRNA gene sequencing. Different numbers of “C. magnum” were picked from Lake Cassidy, and the 16S rRNA gene fragments of the epibionts were amplified and subsequently separated by DGGE. Irrespective of the sample size, all fingerprints exhibited the same melting behavior (Fig. 2A). Sequencing confirmed that each band contained the same 16S rRNA gene sequence (phylotype). Similarly, identical 16S rRNA gene sequences were obtained for “P. roseum” isolated from Lake Dagow when batches of 10 and 45 individual consortia were used (data not shown). Each other type of consortia, if it was obtained from one lake, always yielded a single 16S rRNA gene fingerprint and nucleotide sequence (Fig. 2B and 3). It is unlikely that additional types of epibionts were present in the same consortia but were missed by our approach because of the following considerations. DGGE fingerprinting has been shown to detect the 16S rRNA gene sequences which represent more than 1% of all sequences present in a DNA sample (39). The mean number of epibionts per consortium was determined to be 19.9 ± 4.4 in “P. roseum” and 36 ± 4.4 in “C. magnum” (43). Based on its sensitivity, the DGGE fingerprinting method is therefore suitable for detecting even a single cell of a second phylotype per phototrophic consortium. Evidently, phototrophic consortia with the same morphology which share the same habitat contain only a single epibiont phylotype.
FIG. 2.
(A) Epibiont 16S rRNA gene fragments amplified from different numbers of “C. magnum” consortia obtained from Lake Cassidy and separated by DGGE. (B) Epibiont 16S rRNA gene fragments amplified from the same “C. aggregatum” morphotype found in four different lakes in North America and one lake in eastern Germany. Fragments were separated by DGGE. The gradient of denaturant in the gels shown in panels A and B was a gradient from 40% (top) to 60% (bottom). Negative images of ethidium bromide-stained gels are shown. (C) Phase-contrast photomicrographs of the different “C. aggregatum” consortia corresponding to the DGGE fingerprints from the five lakes in panel B. Bar, 5 μm.
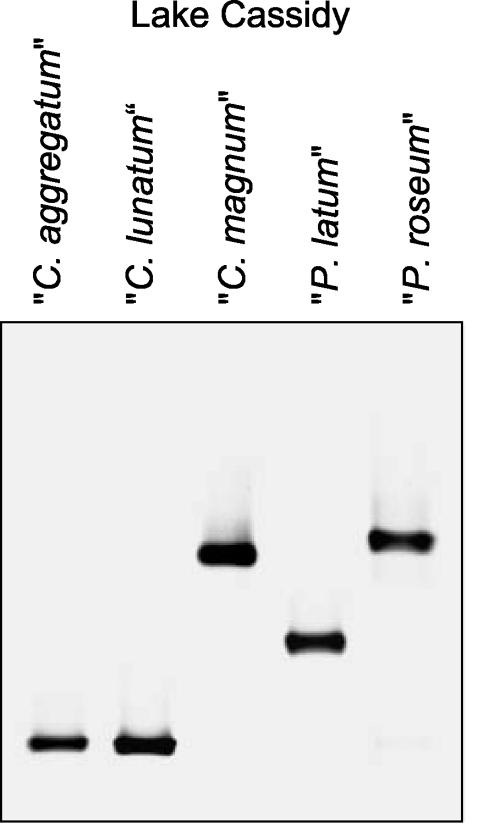
FIG. 3.
Diversity of epibionts of phototrophic consortia in Lake Cassidy. 16S rRNA gene fragments were amplified from all different morphotypes of phototrophic consortia present and were subsequently separated by DGGE. A negative image of an ethidium bromide-stained gel is shown.
Different results were obtained when morphologically similar phototrophic consortia were obtained from different lakes, however. Phylogenetic fingerprinting revealed that different green sulfur bacterial phylotypes were present in “C. aggregatum” from European and North American lakes (Fig. 2B), although the consortia were identical with respect to shape and the arrangement and color of the epibionts (Fig. 2C). Overall, seven different phylotypes were detected for “C. aggregatum,” three different 16S rRNA sequences were detected for “C. magnum,” and four phylotypes were detected for the brown “P. roseum” consortia (Table 1). We concluded that morphologically indistinguishable consortia which occur in geographically distant locations frequently harbor distinct epibionts.
Detection of a novel type of phototrophic consortia.
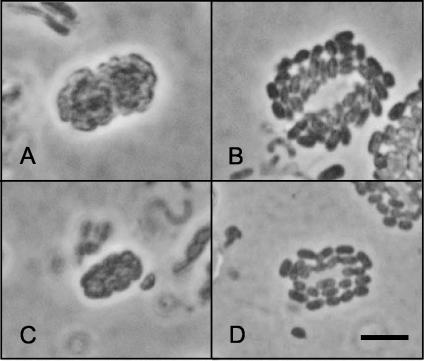
Water samples from five Michigan lakes and Lake Dagow (eastern Germany) contained a previously undescribed morphological type of phototrophic consortium. This type consisted of 44 to 58 cells of a brown epibiont which were associated with a single central colorless motile bacterium (Fig. 4A and B). Epibionts were arranged in several layers around the central rod, resulting in a more stumpy shape for the intact consortium (Fig. 4A) compared to the shape of “P. roseum” (Fig. 4C and D). The novel type of consortium always contained identical 16S rRNA gene sequences in Michigan as well as in German lakes (Table 1 and Fig. 5) (phylotype N), and the epibionts differed from all other brown epibionts. Based on these data, the name “Pelochromatium latum” is proposed for the newly discovered phototrophic consortium. Although we found only one phylotype for “P. latum,” it is feasible that similar consortia which harbor other epibiont phylotypes may be found in the future. As indicated by the quotation marks, however, “P. latum” has no standing in nomenclature and, like all previously published designations of phototrophic consortia, is only used to refer to a particular morphotype.
FIG. 4.
Comparison of two morphotypes, “P. latum” (A and B) and “P. roseum” (C and D). The images are phase-contrast photomicrographs of consortia in the intact state (A and C) and in the disintegrated state produced by squeezing consortia on agar-coated microscope slides (43) (B and D). Bar, 5 μm.
Diversity of epibionts of phototrophic consortia.
To date, seven different morphotypes have been described based on morphology and the cellular arrangement of their epibionts (20, 40, 42). In “C. aggregatum,” the colorless bacterium is surrounded by green rod-shaped bacteria, while brown epibionts are found in “P. roseum.” Consortia of the “C. glebulum” type are bent and contain gas-vacuolated green epibionts. “C. magnum” contains a larger number (∼36) of green epibionts than “C. aggregatum” and has a globular morphology. “P. roseo-viride” has an inner layer of brown cells and an outer layer consisting of green bacteria. Finally, “C. lunatum” and “P. selenoides” harbor epibionts which are half-moon shaped and are green and brown, respectively. In addition, two immotile associations (“Chloroplana vacuolata” and “Cylindrogloea bacterifera”) have been described, and these appear to occur more rarely.
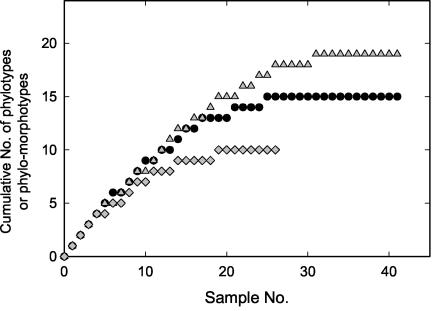
The results presented above demonstrate that the phylogenetic diversity of the epibionts in phototrophic consortia significantly surpasses the diversity deduced solely from morphology. Our systematic survey of phototrophic consortia from the 14 lakes yielded a total of 41 partial 16S rRNA gene sequences (Fig. 5 and Table 1). Among these, 15 distinct sequence types (phylotypes in Fig. 5 and Tables 1 and 2) were detected. A cumulative plot of the phylotypes versus the sample numbers reached saturation (Fig. 6). In the lakes studied, all types of phototrophic consortia co-occur in the same layer (24) and could therefore be analyzed from the same water sample. Therefore, our results indicate that the majority of epibiont phylotypes which were present in the lakes sampled were indeed recovered by our approach.
TABLE 2.
16S rRNA signature sequences and biogeographical distribution of the different epibiont types
| Phylo- type | Accession no. | 16S rRNA nucleotide signature(s)a
|
Morphotype | Phylo- morphotype | Presence in geographical regionsb
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 409 | 453-454 | 476 | 479 | 613 | 624 | 627 | 638 | 658 | 746 | 748 | 1 | 2 | 3 | 4 | 5 | 6 | ||||
| A | AJ578401 | “C. magnum” | A | X | ||||||||||||||||
| B | AJ578402 | “P. roseum” | B | X | X | |||||||||||||||
| C | AJ578404 | “C. aggregatum” | C | X | ||||||||||||||||
| D | AJ578405 | “P. selenoides” | D1 | X | ||||||||||||||||
| “C. lunatum” | D2 | X | ||||||||||||||||||
| “C. aggregatum” | D3 | X | ||||||||||||||||||
| “C. vacuolata” | D4 | X | ||||||||||||||||||
| E | AJ272094 | “C. magnum” | E | X | ||||||||||||||||
| F | AJ272092 | G | “C. glebulum” | F | X | |||||||||||||||
| G | AJ578410 | “P. roseum” | G | X | ||||||||||||||||
| H | AJ578411 | U | “C. magnum” | H | X | X | ||||||||||||||
| J | AJ272091 | “C. aggregatum” | J | X | ||||||||||||||||
| K | AJ578417 | “P. roseum” | K | X | X | |||||||||||||||
| L | AJ578418 | AC | A | G | Cc | A | U | “C. aggregatum” | L | X | ||||||||||
| M | AJ272090 | AC | A | G | C | A | G | U | “C. aggregatum” | M | X | X | ||||||||
| N | AJ578423 | “C. aggregatum” | N1 | X | ||||||||||||||||
| “P. latum” | N2 | X | X | |||||||||||||||||
| O | AJ578428 | C | A | “P. roseum” | O | X | ||||||||||||||
| P | AJ578432 | A | “C. aggregatum” | P | X | |||||||||||||||
E. coli numbering.
See Fig. 1. There were 8 phylomorphotypes in Europe and 13 phylomorphotypes in North America. We found 7, 1, 1, 1, 10, and 4 phylomorphotypes in regions 1, 2, 3, 4, 5, and 6, respectively. Two, one, two, one, seven, and one lakes were sampled in regions 1, 2, 3, 4, 5, and 6, respectively, and the numbers of phylomorphotypes per lake in these regions (as calculated from the data in Table 1) were 3.5, 1, 1, 1, 3.7, and 4, respectively.
Changes are complementary according to the secondary structure (20).
FIG. 6.
Comparison of the total diversity of phylotypes of epibionts (solid circles) and of phylotypes with distinct morphologies (phylomorphotypes) (shaded triangles) present in the data set. Also included is a cumulative plot for epibiont types found in Michigan lakes (shaded diamonds). Random numbers were generated and assigned to the strains (Sample No.), and the sequential detection of different phylotypes and phylomorphotypes was plotted.
Further analysis of our data demonstrated that the genomic diversity of the epibionts is even greater than the phylogenetic diversity since certain epibionts showed considerable differences in morphology and pigmentation. This was observed for some “C. aggregatum” consortia, “C. lunatum,” “P. selenoides,” and “C. vacuolata” (phylotype D in Table 2 and Fig. 5). Also, epibionts from another “C. aggregatum” consortium and from “P. latum” were phylogenetically identical (phylotype N in Table 2 and Fig. 5). Despite their identical 16S rRNA gene sequences, these epibiont cells clearly differ from each other. Thus, the epibionts of “C. aggregatum” are green and rod shaped, whereas those of “P. selenoides” are vibrioid and brown (phylotype D). The epibionts in “P. latum” are brown, whereas those in “C. aggregatum” are green (phylotype N). Like other members of the Chlorobiaceae, green epibionts of phototrophic consortia harbor bacteriochlorophyll c as a light-harvesting pigment, whereas brown strains and epibionts contain bacteriochlorophyll e (19, 26). It is well established that both sets of pigments are constitutively expressed and that the two sets are mutually exclusive (40). Consequently, the different shapes and pigmentations of epibionts with identical partial 16S rRNA gene sequences indicate that the epibionts with the same phylotype differ genetically. Therefore, 16S rRNA gene sequences alone are not sufficient for classification of the epibionts of phototrophic consortia and for assessment of their diversity. While sequences of the internal transcribed spacer regions of the rrn operon or of functional genes potentially provide higher resolution and may be used to distinguish between the different epibionts of phototrophic consortia in natural populations, the currently available amplification methods do not provide the necessary sensitivity and specificity. Therefore, only the combination of phylogenetic, morphological, and pigment data currently provide the means to describe the full diversity of green sulfur bacterial epibionts of phototrophic consortia.
In order to arrive at a more appropriate estimate of the total diversity of consortium epibionts, epibionts with identical phylotypes but different morphologies were considered separate types (D1to D4, N1, and N2 in Table 2). If each unique combination of a phylotype and a morphotype (phylomorphotype) was counted separately, a total of 19 different types could be recognized in our data set (Fig. 6). Again, the cumulative plot of the different phylomorphotypes reached saturation, indicating that further sampling in the six geographic regions at best would yield only a few, very rare additional types of epibionts. In the individual geographic regions, the highest number of morphologically different phototrophic consortia was encountered in Michigan lakes (Table 2). In this region, the 26 phototrophic consortia analyzed yielded 10 different types of epibionts. The repeated detection of identical epibiont phylomorphotypes (Fig. 6) indicates that essentially the entire diversity of epibionts had been recovered from this geographic area. In contrast, eight different types of epibionts were detected in 10 analyses of consortia in the European lakes. Consequently, the cumulative plot of epibiont types did not reach saturation (not shown in Fig. 6), and more types of epibionts are to be expected to occur in other European lakes.
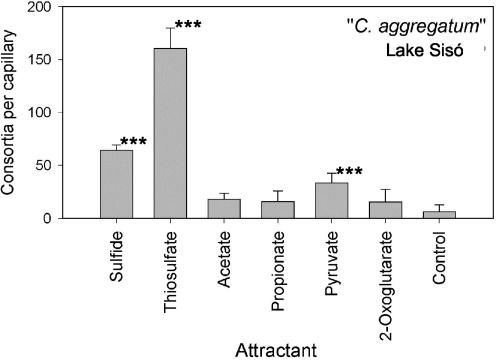
The presence of a single type of epibiont in each type of phototrophic consortium appears to be a general feature of all types of phototrophic consortia investigated (Fig. 2 and 3). Since an amplification method with comparable sensitivity does not exist for the 16S rRNA genes of the central β-proteobacteria, the present study was limited to the green sulfur bacterial epibionts. It remains to be determined whether the same colorless motile bacterium occurs in different phototrophic consortia or whether each of the different types of epibionts is associated with another central bacterium. However, the first evidence for physiological differences between the central bacteria of different consortia comes from our chemotaxis experiments with phototrophic consortia in Lake Sisó (Fig. 7). The chemotaxis towards organic carbon compounds is likely to be mediated by the central motile bacterium (28). “C. aggregatum” from Lake Sisó was attracted by pyruvate (Fig. 7). By comparison, the morphologically similar “C. aggregatum” from Lake Dagow was reported to respond to 2-oxoglutarate but not to pyruvate (19), and intact “P. roseum” consortia exhibited a pronounced chemotaxis towards sulfide and weakly responded to 2-oxoglutarate (28).
FIG. 7.
Chemotactic accumulation of “C. aggregatum” from Lake Sisó. The bars indicate the numbers of phototrophic consortia counted in capillaries after incubation for 3 to 5 h. Three or four parallel experiments were performed. The error bars indicate standard deviations of the counts. Values that differ from the control value at a high level of significance (P < 0.001) are indicated by three asterisks.
Implications for the evolution of epibionts.
Epibionts of all consortia investigated to date represent unique 16S rRNA sequence types and so far have not been found in a free-living state. Together with the rapid signal transfer demonstrated previously (19), our findings support the view that the interaction between the green sulfur bacteria and the chemotrophic motile bacteria is an obligate interaction. Epibionts of phototrophic consortia form several distinct phylogenetic clusters (Fig. 5). This suggests either that the ability to form symbiotic associations arose independently from different ancestors or, alternatively, that the common ancestor of the green sulfur bacteria was symbiotic. In the latter case, several descendant lineages would have developed the ability to sustain an independent life style. In any event, our phylogenetic analysis implies that the switch from the symbiotic state to the free-living state (or vice versa) occurred more than once during the radiation of the green sulfur bacteria.
Biogeography of phototrophic consortia.
For the most part it is assumed that physical or geographical barriers do not exist for prokaryotes (36), and it has been suggested that the high population densities of microorganisms drive rapid, large-scale dispersal across physical and geographical barriers (16, 17). Species like Escherichia coli, Salmonella enterica, or Haemophilus influenzae consist of a limited number of clones; each, however, has a worldwide distribution (38). For Neisseria meningitidis, particular genotypes have been detected on different continents (9). Evidently, dispersal of such human pathogens and commensals is rapid enough to permit widespread geographic distribution not only of bacteria with identical 16S rRNA gene sequences (phylotypes) but even of cells with the same genotype. For most other free-living or nonhuman pathogens and symbionts investigated, endemicity seems to be limited to the level of genotypes (strains or subspecies) rather than bacterial species (phylotypes). This is true for very different bacteria like the fluorescent pseudomonads, the gram-positive organism Renibacterium salmoninarum, or strains of the symbiotic cyanobacterium Nostoc sp. (10, 30, 32).
While studies of marine and freshwater microorganisms indicate that most of the species are ubiquitous (3, 13, 15, 16, 17, 21, 22, 46, 51), some soil bacteria appear to have a more limited distribution (10, 47). Limited migration between North America and Central America has been shown for Rhizobium leguminosarum biovar phaseoli (47). Cosmopolitan species could not be detected among psychrophilic sea ice bacteria from Arctic and Antarctic samples (49). Similarly, 16S rRNA gene sequencing of different populations of Achromatium oxaliferum suggested a high degree of endemism, since identical sequences were never recovered from geographically separated freshwater sediments (29). Hence, endemic species of bacteria may exist in some rare cases.
Our data permit a first assessment of the biogeography of phototrophic consortia. A pairwise comparison of the types of epibionts between the Michigan lakes (excluding Mud Lake, which contained only a single type of epibiont) yielded a mean value for the Dice coefficient of 0.582 ± 0.162 (n = 15). This high value shows that communities of phototrophic consortia in lakes in this geographic area are highly similar. In contrast, only two types of epibionts (phylomorphotypes M and N2) were found to occur on the two continents investigated (Fig. 5 and Table 2). The Dice coefficient for the pairwise comparison between North American and European epibionts was only 0.190. Thus, there is a nonrandom biogeographical pattern and a low level of similarity of epibionts between the two continents. Theoretical considerations indicate that a higher level of similarity between North American and European lakes is unlikely. A high level of similarity (i.e., a Dice coefficient of 0.58) between North American and European lakes would be possible only if at least 20 different types of epibionts occurred in Europe and at least 50% of these types were also present in North American lakes. In reality, much less (25%) of the epibiont types detected in Europe were also present in North America. While the possibility of greater diversity (≥20 epibiont types) cannot be excluded for European lakes (since the data set for this continent is incomplete [see above]), it appears highly unlikely that our random sampling procedure selectively excluded just those epibionts which are common to North American and European lakes.
Theoretically, the environmental conditions in North American lakes could differ significantly from those in European lakes and thereby select for different epibiont types. In the case of phototrophic consortia this is highly unlikely for two reasons. First, different epibionts were detected in lakes with very similar physicochemical conditions if they were located on different continents. For example, Lake Dagow, Schleinsee, and Mud Lake were very similar with respect to environmental conditions (Table 1). Whereas the phylotype K epibiont of Schleinsee was also found in Lake Dagow (both European lakes), the unique phylotype A found in Mud Lake (located in North America) was not found in the two other lakes. Second, in 9 of the 14 lakes investigated, multiple (i.e., up to six different) types of consortia occurred simultaneously in the same environment (Tables 1 and 2). The latter finding in particular supports the conclusion that environmental conditions are not the selective factor governing the geographic distribution of the epibionts of phototrophic consortia.
It has been shown that common soil bacteria, like Curtobacterium citreum, Bacillus megaterium, Arthrobacter globiformis, Microbacterium spp., Sphingomonas spp., Sinorhizobium spp., and Paracoccus spp., and fungal pathogens of plants are transported on dust particles and are capable of surviving long-range transport through the atmosphere (6, 31). Transport between continents occurs on a time scale of ∼6 days (33). In the case of epibionts of phototrophic consortia, the high similarity between populations in neighboring lakes and the pronounced intercontinental differences suggest that the dispersal of consortia is comparatively slow.
Conclusions.
During the present study, 16S rRNA gene sequences of epibionts of phototrophic consortia were found exclusively in the associated state. Although we cannot entirely rule out the possibility that epibionts also occur in the free-living state in other environments, our results strongly suggest that there is a close association and that there is specific adaptation of epibionts to the symbiotic state. Phototrophic consortia therefore do not form just by chance. Still, multiple changes from the symbiotic life style to the independent life style (or vice versa) must have occurred during the evolution of green sulfur bacteria, as indicated by the phylogenetic analysis.
Our survey of 14 different aquatic environments yielded 15 distinct phylotypes of epibionts and, when the differences in morphology and pigmentation were considered, a total of 19 phylomorphotypes. The first data on the chemotactic behavior of phototrophic consortia suggest that the central chemotrophic bacteria are also different in different types of consortia. The unexpected diversity of the epibionts of phototrophic consortia is distributed in a nonrandom fashion among different geographic regions. Phototrophic consortia therefore harbor a previously unrecognized diversity of green sulfur bacterial epibionts which appear to be only slowly dispersed over long geographic distances.
Acknowledgments
We thank Carlos Abella (University of Girona, Girona, Spain) for kindly providing samples from Michigan lakes and J. Staley (University of Seattle) for providing the water sample from Echo Lake. We are indebted to Ann-Katrin Manske for assistance with construction of the phylogenetic tree and to Kajetan Vogl for providing the epibiont sequence of “C. vacuolata.” J. Garcia-Gil kindly offered valuable support in the field and laboratory space at the University of Girona.
This study was supported by grants Ov 20/3-3 and Ov 20/10-1 from the Deutsche Forschungsgemeinschaft to J. Overmann.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baas-Becking, L. G. M. 1934. Geobiologie of Inleiding Tot de Milieukunde. W. P. van Stockum & Zoon N. V., The Hague, The Netherlands.
- 3.Beeder, J., R. K. Nilsen, J. T. Rosnes, T. Torsvik, and T. Lien. 1994. Archaeoglobus fulgidus isolated from hot North Sea oil field waters. Appl. Environ. Microbiol. 60:1227-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beijerinck, M. W. 1913. De infusies en de ontdekking der backteriën, Jaarboek van de Koninklijke Akademie v. Wetenschappen. Müller, Amsterdam, The Netherlands.
- 5.Borrego, C. M., J. Garcia-Gil, X. P. Cristina, X. Vila, and C. A. Abella. 1998. Occurrence of new bacteriochlorophyll d forms in natural populations of green photosynthetic sulfur bacteria. FEMS Microbiol. Ecol. 26:257-267. [Google Scholar]
- 6.Brown, J. K. M., and M. S. Hovmøller. 2002. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297:537-541. [DOI] [PubMed] [Google Scholar]
- 7.Buder, J. 1914. Chloronium mirabile. Ber. Dtsch. Bot. Ges. 31:80-97. [Google Scholar]
- 8.Caldwell, D. E., and J. M. Tiedje. 1975. A morphological study of anaerobic bacteria from the hypolimnia of two Michigan lakes. Can. J. Microbiol. 21:362-376. [DOI] [PubMed] [Google Scholar]
- 9.Caugant, D. A., L. F. Mocca, C. E. Frasch, L. O. Frøholm, W. E. Zollinger, and R. K. Selander. 1987. Genetic structure of Neisseria meningitidis populations in relation to serogroup, serotype, and outer membrane protein pattern. J. Bacteriol. 169:2781-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho, J.-C., and J. M. Tiedje. 2000. Biogeography and degree of endemicity of fluorescent Pseudomonas strains in soil. Appl. Environ. Microbiol. 66:5448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dams, E., L. Hendriks, Y. Van de Peer, J.-M. Neefs, G. Smits, I. Vandenbempt, and R. De Wachter. 1988. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 16:r87-r173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dice, L. R. 1945. Measures of the amount of ecologic association between species. Ecology 26:297-302. [Google Scholar]
- 13.Eguchi, M., M. Ostrowski, F. Fegatella, J. Bowman, D. Nichols, T. Nishino, and R. Cavicchioli. 2001. Sphingomonas alaskensis strain AF01, an abundant oligotrophic ultramicrobacterium from the North Pacific. Appl. Environ. Microbiol. 67:4945-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichler, B., and N. Pfennig. 1990. Seasonal development of anoxygenic phototrophic bacteria in a holomictic drumlin lake (Schleinsee, F.R.G.) Arch. Hydrobiol. 119:369-392. [Google Scholar]
- 15.Field, K. G., D. Gordon, T. Wright, M. Rappé, E. Urbach, K. Vergin, and S. J. Giovannonni. 1997. Diversity and depth-specific distribution of SAR11 cluster rRNA genes from marine planktonic bacteria. Appl. Environ. Microbiol. 63:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finlay, B. J. 2002. Global dispersal of free-living microbial eukaryote species. Science 296:1061-1063. [DOI] [PubMed] [Google Scholar]
- 17.Finlay, B. J., and K. J. Clarke. 1999. Ubiquitous dispersal of microbial species. Nature 400:828. [Google Scholar]
- 18.Fröhlich, J., and H. König. 1999. Rapid isolation of single microbial cells from mixed natural and laboratory populations with the aid of a micromanipulator. Syst. Appl. Microbiol. 22:249-257. [DOI] [PubMed] [Google Scholar]
- 19.Fröstl, J. M., and J. Overmann. 1998. Physiology and tactic response of the phototrophic consortium “Chlorochromatium aggregatum.” Arch. Microbiol. 169:129-135. [DOI] [PubMed] [Google Scholar]
- 20.Fröstl, J. M., and J. Overmann. 2000. Phylogenetic affiliation of the bacteria that constitute phototrophic consortia. Arch. Microbiol. 174:50-58. [DOI] [PubMed] [Google Scholar]
- 21.Fuhrman, J. A., K. McCallum, and A. A. Davis. 1993. Phylogenetic diversity of subsurface marine microbial communities from the Atlantic and Pacific oceans. Appl. Environ. Microbiol. 59:1294-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Pichel, F., L. Prufert-Bebout, and G. Muyzer. 1996. Phenotypic and phylogenetic analyses show Microcoleus chthonoplastes to be a cosmopolitan cyanobacterium. Appl. Environ. Microbiol. 62:3284-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasol, J. M., K. Jürgens, R. Massana, J. I. Calderón-Paz, and C. Pedrós-Alió. 1995. Mass development of Daphnia pulex in a sulfide-rich pond (Lake Cisó). Arch. Hydrobiol. 132:279-296. [Google Scholar]
- 24.Gich, F. 2001. Ecologia i caracterització pigmentària de bacteris fotosintètics amb nou tipus de bcl d. Ph.D. thesis. Institut d′Ecologia Aquatica, Universitat de Girona, Girona, Spain.
- 25.Gich, F., J. Garcia-Gil, and J. Overmann. 2001. Previously unknown and phylogenetically diverse members of the green nonsulfur bacteria are indigenous to freshwater lakes. Arch. Microbiol. 177:1-10. [DOI] [PubMed] [Google Scholar]
- 26.Glaeser, J., L. Baneras, H. Rütters, and J. Overmann. 2002. Novel bacteriochlorophyll e structures and species-specific variability of pigment composition in green sulfur bacteria. Arch. Microbiol. 177:475-485. [DOI] [PubMed] [Google Scholar]
- 27.Glaeser, J., and J. Overmann. 2003. Characterization and in situ carbon metabolism of phototrophic consortia. Appl. Environ. Microbiol. 69:3739-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glaeser, J., and J. Overmann. 2003. The significance of organic carbon compounds for in situ metabolism and chemotaxis of phototrophic consortia. Environ. Microbiol. 5:1053-1063. [DOI] [PubMed] [Google Scholar]
- 29.Gray, N. D., R. Howarth, A. Rowan, R. W. Pickup, J. G. Jones, and I. M. Head. 1999. Natural communities of Achromatium oxaliferum comprise genetically, morphologically, and ecologically distinct subpopulations. Appl. Environ. Microbiol. 65:5089-5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grayson, T. H., L. F. Cooper, F. A. Atienzar, M. R. Knowles, and M. L. Gilpin. 1999. Molecular differentiation of Renibacterium salmoninarum isolates from worldwide locations. Appl. Environ. Microbiol. 65:961-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffin, D. W., V. H. Garrison, J. R. Herman, and E. A. Shinn. 2001. African desert dust in the Caribbean atmosphere: microbiology and public health. Aerobiologia 17:203-213. [Google Scholar]
- 32.Guevara, R., J. J. Armesto, and M. Caru. 2002. Genetic diversity of Nostoc microsymbionts from Gunnera tinctoria revealed by PCR-STRR fingerprinting. Microb. Ecol. 44:127-136. [DOI] [PubMed] [Google Scholar]
- 33.Jaffe, D., T. Anderson, D. Covert, R. Kotchenruther, B. Trost, J. Danielson, W. Simpson, T. Berntsen, S. Karlsdottir, D. Blake, J. Harris, G. Carmichael, and I. Uno. 1999. Transport of Asian air pollution to North America. Geophys. Res. Lett. 26:711-714. [Google Scholar]
- 34.Jørgensen, B. B., J. G. Kuenen, and Y. Cohen. 1979. Microbial transformations of sulfur compounds in a stratified lake (Solar Lake, Sinai). Limnol. Oceanogr. 24:799-822. [Google Scholar]
- 35.Lauterborn, R. 1906. Zur Kenntnis der sapropelischen Flora. Allg. Bot. 2:196-197. [Google Scholar]
- 36.Lawrence, J. G. 2001. Catalyzing bacterial speciation: correlating lateral transfer with genetic headroom. Syst. Biol. 50:479-496. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig, W., O. Strunk, S. Klugbauer, N. Klugbauer, M. Weizenegger, J. Neumaier, M. Bachleitner, and K. H. Schleifer. 1998. Bacterial phylogeny based on comparative sequence analysis. Electrophoresis 19:554-568. [DOI] [PubMed] [Google Scholar]
- 38.Maynard Smith, J., N. H. Smith, M. O'Rourke, and B. G. Spratt. 1993. How clonal are bacteria? Proc. Natl. Acad. Sci. USA 90:4384-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muyzer, G., E. C. de Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Overmann, J. 2001. Green sulfur bacteria, p. 601-623. In G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams and Wilkins, Baltimore, Md. [Google Scholar]
- 41.Overmann, J., M. J. L. Coolen, and C. Tuschak. 1999. Specific detection of different phylogenetic groups of chemocline bacteria based on PCR and denaturing gradient gel electrophoresis of 16S rRNA gene fragments. Arch. Microbiol. 172:83-94. [DOI] [PubMed] [Google Scholar]
- 42.Overmann, J., and K. Schubert. 2002. Phototrophic consortia: model systems for symbiotic interrelations between prokaryotes. Arch. Microbiol. 177:201-208. [DOI] [PubMed] [Google Scholar]
- 43.Overmann, J., C. Tuschak, J. Fröstl, and H. Sass. 1998. The ecological niche of the consortium “Pelochromatium roseum.” Arch. Microbiol. 169:120-128. [DOI] [PubMed] [Google Scholar]
- 44.Pfennig, N. 1980. Syntrophic mixed cultures and symbiotic consortia with phototrophic bacteria: a review, p. 127-131. In G.Gottschalk, N. Pfennig, and D. Werner (ed.), Anaerobes and anaerobic infections. Gustav Fischer Verlag, Stuttgart, Germany.
- 45.Siefert, E., and N. Pfennig. 1984. Convenient method to prepare neutral sulfide solution for cultivation of phototrophic sulfur bacteria. Arch. Microbiol. 139:100-101. [Google Scholar]
- 46.Snyder, A. R., H. N. Williams, M. L. Baer, K. E. Walker, and O. C. Stine. 2002. 16S rDNA sequence analysis of environmental Bdellovibrio-and-like organisms (BALO) reveals extensive diversity. Int. J. Syst. Evol. Microbiol. 52:2089-2094. [DOI] [PubMed] [Google Scholar]
- 47.Souza, V., T. T. Nguyen, R. R. Hudson, D. Piñero, and R. E. Lenski. 1992. Hierarchical analysis of linkage disequilibrium in Rhizobium populations: evidence for sex? Proc. Natl. Acad. Sci. USA 89:8389-8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staley, J. T. 1999. Bacterial biodiversity: a time for place. ASM News 65:681-687. [Google Scholar]
- 49.Staley, J. T., and J. J. Gosink. 1999. Poles apart: biodiversity and biogeography of sea ice bacteria. Annu. Rev. Microbiol. 53:-215. [DOI] [PubMed] [Google Scholar]
- 50.Vila, X., C. A. Abella, J. B. Figueras, and J. P. Hurley. 1998. Vertical models of phototrophic bacterial distribution in the metalimnetic microbial communities of several freshwater North-American kettle lakes. FEMS Microbiol. Ecol. 25:287-299. [Google Scholar]
- 51.Zwart, G., W. D. Hiorns, B. A. Methé, M. P. van Agterveld, R. Huismans, S. C. Nold, J. P. Zehr, and H. J. Laanbroek. 1998. Nearly identical 16S rRNA sequences recovered from lakes in North America and Europe indicate the existence of clades of globally distributed freshwater bacteria. Syst. Appl. Microbiol. 21:546-556. [DOI] [PubMed] [Google Scholar]