Abstract
Importance
We provide novel evidence of specific clinical and neuroimaging features that may help for the in vivo prediction of underlying pathology in non-fluent/agrammatic primary progressive aphasia (nfvPPA) patients with progressive supranuclear palsy (PSP) or corticobasal degeneration (CBD) proved by autopsy.
Objective
To characterize the neurological, cognitive and neuroimaging features of patients with nvfPPA- in whom either PSP or CBD was eventually confirmed at autopsy- at initial presentation and at 1-year follow-up.
Design, Setting, and Participants
Prospective longitudinal clinical-pathological study was conducted in a tertiary research clinic that specialized in cognitive disorders. Patients (n=14) were evaluated between January 2002 and December 2014. Inclusion criteria were: a clinical diagnosis of nfvPPA; the availability of speech, language, and cognitive testing for at least one evaluation; magnetic resonance imaging within 6 months of initial evaluation, and a postmortem pathological diagnosis of PSP or CBD.
Main Outcomes and Measures
Clinical, cognitive, and neuroimaging longitudinal data were analyzed to characterize the whole nfvPPA-4R tau group and identify differences between nfvPPA-PSP and nfvPPA-CBD at presentation and longitudinally.
Results
Patient groups did not differ significantly in age, gender or handedness (nfvPPA-PSP: median [interquartile range] age 74 [67–76] years, 1/5 male: 1/4 left-handed; nfvPPA-CBD 65 [54–81] years, 3/9 male, 0/9 left-handed). Motor speech impairment and left frontal white matter atrophy were the most prominent common features. At presentation, dysarthria (Motor Speech Examination score median [interquartile range] nfvPPA-PSP: 4 [2–7], nfvPPA-CBD 0 [0–4]; p=0.02), depression (Geriatric Depression Scale score median [interquartile range] nfvPPA-PSP 19 [3–28], nfvPPA-CBD 4 [0–16]; p=0.04) and relatively selective white matter atrophy were typical of nfvPPA-PSP, while greater grey matter atrophy and a trend toward greater sentence comprehension deficits (sentence comprehension % correct median [interquartile range] nfvPPA-PSP 98 [80–100], nfvPPA-CBD 81 [65–98]; p=0.08) were found in nfvPPA-CBD. At follow-up after 1 year, we observed no significant differences in any speech or language measures. Furthermore, atrophy in PSP progressed within the subcortical/brainstem motor system generating greater oculomotor deficits (X2= 0.02) and swallowing difficulty (X2= 0.02); atrophy in CBD spread anteriorly in prefrontal regions consistent with their greater working memory impairment and development of behavioral symptoms.
Conclusion
In patients presenting with nfvPPA, presence of early severe dysarthria, relatively selective white matter atrophy at presentation and greater rate of change in the brainstem measured by longitudinal imaging may be useful for differentiating underlying PSP from CBD pathology during life.
Keywords: primary progressive aphasia, nonfluent/agrammatic variant, voxel based morphometry, corticobasal degeneration, progressive supranuclear palsy
INTRODUCTION
The non-fluent/agrammatic variant of primary progressive aphasia (nfvPPA) is a clinical syndrome strongly linked to underlying frontotemporal lobar degeneration (FTLD) pathology1,2. The majority of cases are caused by abnormal aggregation of the microtubule-associated protein tau (FTLD-tau) while most of the remaining cases are associated with the transactive response DNA binding protein of 43 kD (TDP) inclusions, usually type A (FTLD-TDP-A)2. The FTLD-tau cases are caused by either 4 repeat (4R) tauopathies - progressive supranuclear palsy (PSP) or corticobasal degeneration (CBD), or Pick’s disease, a 3 repeat tauopathy.
Motor speech (apraxia of speech [AOS] and dysarthria) and grammar impairment along with predominant left posterior frontal lobe and insular atrophy are well established features of clinically defined nfvPPA3–5. However, prospectively collected speech, language, and neuroimaging data in pathologically confirmed cohorts are scarce and, to our knowledge, no longitudinal neuroimaging study of pathologically confirmed nfvPPA has been conducted. Consequently, it is not known whether different types of FTLD-tau presenting as nfvPPA can be distinguished by early clinical and neuroimaging features or by their longitudinal trajectories. The small number of clinicopathological studies in nfvPPA1,6–11 show that 4R tauopathies, CBD and PSP, are the most common cause of nfvPPA making the identification of early clinical and neuroimaging biomarkers associated to these pathologies a matter of great interest. Despite significant clinical and pathological overlap, PSP and CBD are considered two distinct diseases presenting specific pathological lesions, biochemical features, and cellular and network vulnerabilities12,13. Also, recent evidence suggests that CBD and PSP may relate to distinct tau strains, which may require different therapies14. While it is possible that both diseases might respond to the same 4R-tau targeted therapy, the ability to differentiate these two syndromes at early stages when molecule-specific disease-modifying drugs are most likely to be effective may prove to be critical for successful treatment. Furthermore, the ability to prognosticate future clinical symptoms holds great value for patients and care-givers.
The purpose of this study was to characterize the early features and longitudinal trajectories of neurological, cognitive and neuroimaging impairment in patients with sporadic nfvPPA and autopsy confirmed PSP or CBD pathology.
METHODS
Subjects
Subjects were evaluated at the University of California San Francisco (UCSF) Memory and Aging Center (MAC) as part of a prospective, longitudinal research study between the years 2002–2014. Inclusion criteria: clinical diagnosis of nfvPPA according to current criteria5, availability of speech, language, and cognitive testing for at least one evaluation, magnetic resonance imaging (MRI) within 6 months of initial evaluation, and a postmortem pathological diagnosis of FTLD-4R-tau. This resulted in a cohort of 15 patients: 5 with pathologically confirmed PSP, 9 with CBD, and one with an unclassifiable 4R tauopathy. Tau immunohistochemistry demonstrated evidence of globose tangles and tufted astrocytes15 in all PSP and astrocytic plaques16 and thread-like inclusions in all CBD. Genetic screening for mutations in MAPT and Progranulin genes were negative in all subjects. Since our primary objective was to characterize and contrast features of nfvPPA-PSP and nfvPPA-CBD, the unclassifiable case of 4R tauopathy was excluded. Subjects were followed for 2.9 (± 1.6) years.
We recruited healthy controls from the San Francisco community. Controls were matched for age, gender, and scanner type and had a Clinical Dementia Rating Scale sum of boxes score of 0, a normal neurologic examination, and no cognitive complaints. All subjects underwent informed consent and the UCSF human research committee approved the study.
Clinical and cognitive data
All subjects received a standardized clinical evaluation17, the UCSF neuropsychological18, and speech and language19–21 batteries at initial visit and follow-up as described in previous reports. Speech production, motor speech and grammatical processing are of particular interest in nfvPPA and were considered in detail by reviewing videotaped evaluations22. A detailed description of the speech and language evaluation is included in the supplementary material (emethods-1).
Presence of clinical symptoms and neurological signs were compared between groups at presentation (PSP n=5; CBD n=9), at 1 year follow-up (PSP n=5; CBD n=6), and follow-up closest to time of death (PSP n=4; CBD n=5) using the Chi-squared test. The criteria used for the syndromic diagnosis of probable PSP and CBD are published previously23 and included in the supplemental material (emethods-2). We compared cognitive test scores between nfvPPA-PSP (n=5), nfvPPA-CBD (n=9), and controls (n=10) at initial evaluation and 1 year follow-up (PSP=4; CBD=6). Mann-Whitney U and Kruskal –Wallis tests were used for two and three group comparisons respectively. For analysis of longitudinal cognitive data, we performed a paired Wilcoxon test to compare performance at initial evaluation and follow-up within each group.
Neuroimaging
Acquisition
All patients and controls underwent whole-brain structural MRI using either a 1.5 T3, 3T24, or 4T25 scanner.
Subjects
Cross-sectional analysis: We compared nfvPPA-PSP (n=5) and nfvPPA-CBD (n=9) groups to each other and to healthy controls (n=80). Longitudinal analysis: Only subjects with two MRI scans performed on consecutive years and on the same scanner were included (5 nfvPPA-PSP, 5 nfvPPA-CBD, and 42 controls).
Voxel based morphometry (VBM) analysis
Image processing was performed using the unified segmentation procedure, DARTEL toolbox, and Pairwise Longitudinal Registration toolbox26 implemented in SPM12 according to standard procedures described elsewhere27,28. Whole brain analyses of differences in grey matter (GM) and white matter (WM) and differences in annual rate of volume change were investigated using an analysis of variance (ANOVA) test across groups, including age, gender, total intracranial volume (TIV), and scanner type as nuisance variables. For the figures, we depicted t-maps at a p<0.001 uncorrected threshold for better visualization of differences and similarities between groups. SPM Anatomy toolbox version 2.029 was used for reporting of GM coordinates (etables-1&2). Also see the supplementary material for a region-of-interest analysis (emethods-3; etable-3).
Neuropathology
Autopsies were performed at UCSF (n= 14), University of Pennsylvania (n=3), and Vancouver General Hospital (n=1). Pathological diagnosis was based on consensus guidelines for FTLD30 following standard procedures described previously17,31.
RESULTS
Demographic data (Table-1)
Table 1.
Demographic and cognitive data in nfvPPA-PSP, nfvPPA-CBD, and controls at initial visit.
| Demographic Data | All 4R tau | PSP (n=5) | CBD (n=9) | control (n=10) |
|---|---|---|---|---|
|
| ||||
| Gender (M/F) | 4/10 | 1/4 | 3/6 | 3/7 |
| Handedness (R/L) | 13/1 | 4/1 | 9/0 | 10/0 |
| Education, y | 17 (12–21) | 16.4 ± 3.9 | 18 (12–20) | 17 (14–20) |
| Age at symptom onset, y | 62.5 (51–79) | 15 (12–21) | 61 (51–79) | n/a |
| Age at initial evaluation, y | 66.5 (54–81) | 70 (62–72) | 65.3 ± 9.1 | 71.5 (57–78) |
| Survival, y | 7.23 (4.4–11.6) | 9.6 (6.4–11.6)c | 6.4 (4.4–10.3)c | n/a |
|
| ||||
| General Cognitive Data | ||||
|
| ||||
| MMSE | 27 (20–30)a | 28 (24–30)a | 27 (20–29)a | 30 (28–30) |
| CDR sum of boxes | 2 (0–4.5) | 1.5 (0–2.5) | 2 (1–4.5) | n/a |
| GDS total | 5 (0–28)a | 19 (3–28)ab | 4 (0–16) | 3.5 (0–13) |
| NPI total | 10.5 (1–50) | 16.5 (8–50) | 10.5 (1–38) | n/a |
| Digits Backward | 3 (2–6)a | 3 (2–6) | 3 (2–4)a | 5 (3–7) |
| Modified trails (lines per min) | 9.3 (0.5–32.3)a | 2 (0.5–32.3)a | 10.1 (4–26.3)a | 30 (14–40) |
| Calculation | 5 (2–5) | 5 (2–5) | 5 (3–5) | 5 (4–5) |
| Benson figure copy | 15 (13–17) | 15 (13–16) | 15 (13–17) | 16 (13–17) |
| Benson figure recall | 11.5 (3–17) | 10 (3–13) | 12 (9–17) | 14 (7–17) |
| CVLT-MS Total recall | 25 (16–34)a | 26 (16–28)a | 23.5 (17–34)a | 32 (26–35) |
| CVLT-MS 10min free recall | 6 (4–8)a | 7 (4–8)a | 6 (5–8)a | 8.5 (5–9) |
|
| ||||
| Language Cognitive test | ||||
|
| ||||
| AOS rating (MSE, 7) | 2 (1–4) | 1 (1–4) | 2 (1–4) | n/a |
| Dysarthria rating (MSE, 7) | 2 (0–7) | 4 (2–7)b | 0 (0–4)b | n/a |
| Speech fluency (WAB, 10) | 9 (4–10) | 9 (6–9) | 9 (4–10) | n/a |
| Information content (WAB, 10) | 10 (5–10) | 10 (5–10) | 10 (9–10) | n/a |
| Sequential commands (WAB, 80) | 73.5 (49–80)a | 80 (69–80)c | 68 (49–80)ac | 80 (76–80) |
| Grammar comprehension (%) | 81 (65–100)a | 98 (80–100)c | 81 (65–98)ac | 100 (92–100) |
| Repetition (WAB, 100) | 91.5 (52–100)a | 95 (52–100)a | 88 (64–100)a | 100 (96–100) |
| Word recognition (WAB, 60) | 60 (55–60) | 60 (59–60) | 60 (55–60) | 60 (60–60) |
| Boston Naming Test (BNT, 15) | 13.5 (11–15)a | 12 (11–15)a | 14 (11–15)a | 15 (14–15) |
| Phonemic fluency (D words) | 4.5 (0–13)a | 5 (2–13)a | 4 (0–6)a | 17 (14–24) |
| Semantic fluency (animals) | 9 (4–22)a | 9 (6–22)a | 9 (4–13)a | 26 (14–33) |
|
| ||||
| Spontaneous Speech sample analysis (Picnic scene) | ||||
|
| ||||
| Total narrative words | 66.5 (9–452)a | 69 (9–452) | 64 (14–131)a | 140 (89–238) |
| Words per minute | 55.5 (11–90.5)a | 65.9 (18.8–90.5)a | 54.8 (10.7–70.4)a | 154.7 (112–198) |
| Proportion of syntactic errors | 4 (0–35)a | 3.3 (0–11.11)a | 4.8 (0–35)a | 0 (0–1.02) |
| Proportion of words in sentences | 0.91 (0–1)a | 0.9 (0.6–1)a | 0.83 (0–1)a | 1 (0.88–1) |
| Proportion of distortions (per 100wrds) | 6.3 (0–33.3)a | 10.7 (1.5–33.3)a | 4 (0–31.25)a | 0 (0–1.33) |
p< 0.05 vs controls;
p< 0.05 PSP vs CBD;
Italicized= trend p≤0.10 PSP vs CBD. Kruskal-Wallis and post-hoc Mann-Whitney U tests performed. MMSE: minimental state examination. CDR: clinical dementia rating scale. GDS: geriatric depression scale. NPI: neuropsychiatric inventory. CVLT: California verbal learning test. AOS: apraxia of speech.
PSP and CBD did not differ significantly in age of symptom onset or age at initial evaluation. However, four out of five PSP and only 2 out of 9 CBD cases presented after the age of 65. PSP showed a trend (p = 0.058) towards longer survival following onset of first symptom.
General Cognitive and Language data
At initial evaluation (Table-1)
In nfvPPA-4R-tau, tests of general cognition (MMSE), memory, and executive function were significantly worse than controls. Speech and language measures showed impairment in measures of motor speech, verbal fluency, naming, and sentence comprehension.
nfvPPA-PSP was significantly more depressed than nfvPPA-CBD, and only nfvPPA-CBD was significantly worse than controls in a test of working memory (digits backward). All 14 patients showed AOS. Mixed hypokinetic and spastic dysarthria was present and rated as more severe than AOS in all of the nfvPPA-PSP cases. In CBD, dysarthria was present in only 4 out 9 cases. Dysarthria was significantly more severe in nfvPPA-PSP. Only nfvPPA-CBD was significantly worse than controls in both measures of sentence comprehension and showed a trend for lower scores compared to nfvPPA-PSP in these measures. No significant differences were found when directly comparing patient groups in the measures derived from the recorded speech sample. However, both groups scored significantly worse than controls in words per minute, distortions per hundred words, proportion of syntactical errors, and proportion of words in sentences. Only nfvPPA-CBD produced significantly fewer narrative words than controls.
At 1-year follow-up (Table-2)
Table 2.
Cognitive data at baseline and 1 year follow-up evaluation.
| All 4R tau (n=12)
|
PSP (n=5)
|
CBD (n=7)
|
||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
|
| ||||||
| MMSE | 27 (23–30) | 23 (15–29)1x | 28 (24–30) | 23 (18–29)y | 27 (23–29) | 23.5 (15–26)1x |
| Digits Backward | 3 (2–6) | 3 (0–6)1 | 3 (2–6) | 3 (0–6) | 3 (3–4) | 3 (0–3)1 |
| Benson figure copy | 15 (13–17) | 16 (9–17)3 | 15 (13–16) | 16 (9–17) | 15 (13–17) | 15.5 (14–17)3 |
| Benson figure recall | 11.5 (3–17) | 9 (5–16)3 | 10 (3–13) | 8 (5–13) | 12 (9–17) | 9.5 (7–16)3 |
|
| ||||||
| AOS (MSE, 7) | 2 (1–4) | 4 (1–7)3x | 1 (1–4) | 4.5 (1–7)1 | 2 (1–4) | 4 (3–7)2x |
| Dysarthria (MSE, 7) | 2 (0–7) | 5 (0–7)3y | 4 (2–7) | 5.5 (2–7)1 | 1 (0–4) | 2 (0–7)2 |
| Speech fluency (WAB, 10) | 9 (5–10) | 5 (1–9)2x | 9 (6–9) | 5.5 (1–9)1 | 9 (5–10) | 4.5 (2–9)1x |
| Information content (WAB, 10) | 10 (5–10) | 8 (4–10)2x | 10 (5–10) | 5.5 (4–10)1 | 10 (9–10) | 9 (5–9)1x |
| Sequential commands (WAB, 80) | 75.5 (61–80) | 78 (54–80)2 | 80 (69–80) | 78 (66–80)1 | 72 (61–80) | 77.5 (54–80)1 |
| Grammar comprehension (%) | 86.5 (74–100) | 73 (58–98)2x | 98 (80–100) | 80 (73–94)1y | 81 (74–98) | 71 (58–98)1y |
| Repetition (WAB, 100) | 88 (52–100) | 70.5 (12–98)2x | 95 (52–100) | 58 (23–98)1y | 88 (64–100) | 71 (12–98)1 |
| Word recognition (WAB, 60) | 60 (59–60) | 59 (58–60)2x | 60 (59–60) | 59 (58–60)1 | 60 (60–60) | 59 (58–60)1x |
| BNT (15) | 13.5 (11–15) | 12 (7–15)1x | 12 (11–15) | 12 (10–15) | 14 (11–15) | 11.5 (7–14)1 |
| Phonemic fluency | 5 (2–13) | 3.5 (1–13)2 | 5 (2–13) | 5 (1–13) | 5 (3–6) | 3 (2–9)2 |
| Semantic fluency | 10 (6–22) | 6.5 (1–20)2x | 9 (6–22) | 8 (1–20)x | 11 (6–13) | 5 (2–10)2x |
missing one case,
missing two cases,
missing three cases.
Longitudinal within group comparison:
Baseline vs Follow-up significant at p<0.05;
Baseline vs Follow-up trend at p<0.10;
Cross-sectional comparison between groups at time-point 2: Indented = p<0.05 vs Controls at follow-up. PSP and CBD did not differ significantly in any measure at time-point 2 when compared directly. Kruskal-Wallis and post-hoc Mann-Whitney U tests performed.
In nfvPPA-4R-tau, MMSE scores showed significant decline, while visuospatial and visual memory tests were still not significantly impaired compared to controls. Digits backward remained impaired but did not decline significantly. All speech and language measures declined significantly except phonemic fluency, sequential commands, and dysarthria (which only showed a trend towards significant decline).
At follow-up, cross-sectional comparisons did not show significant differences between patient groups in any cognitive measure. Accordingly, nfvPPA-CBD showed higher dysarthria scores and nfvPPA-PSP performed worse on grammar comprehension than before. However, longitudinal change in these measures was not significant. In nfvPPA-CBD longitudinal analysis showed significant decline in MMSE, AOS, speech fluency, and auditory word recognition (although patients continued to be relatively preserved in this single word comprehension task, as they missed only one out 60 items). nfvPPA-PSP showed significant decline in semantic fluency only. Both groups showed a trend towards significant decline in grammar comprehension.
Neurological symptoms and signs at initial and follow up evaluations (table-3)
Table 3. Neurological symptoms and signs at presentation, 1 year, >1 year follow-up evaluations and overall.
Number of cases that reported or presented each symptom or sign. Percentages in parenthesis.
| SYMPTOMS | Presentation | 1yr follow-up | >1yr follow-up | |||
|---|---|---|---|---|---|---|
| psp (n=5) | cbd (n=9) | psp (n=5) | cbd (n=6) | psp (n=4) | cbd (n=5) | |
|
| ||||||
| Swallowing complaints | 3 (60) | 1 (11) | 5 (100)a | 1 (17) | 4 (100) | 4 (80) |
| Reduced manual dexterity | 2 (40) | 2 (22) | 4 (80) | 2 (33) | 4 (100) | 3 (60) |
| Gait/Balance | 3 (60)a | 0 (0) | 3 (60) | 1 (17) | 4 (100) | 3 (60) |
| Falls | 2 (40)a | 0 (0) | 3 (60) | 0 (0) | 4 (100) | 2 (40) |
| Incontinence | 0 (0) | 0 (0) | 2 (40) | 0 (0) | 3 (80) | 1 (20) |
| Impulsive | 1 (20) | 4 (44) | 2 (40) | 5 (83) | 2 (40) | 5 (100) |
| Obsessive/Compulsive | 1 (20) | 2 (22) | 1 (20) | 2 (33) | 1 (20) | 3 (60) |
|
| ||||||
| SIGNS | ||||||
|
| ||||||
| Ocular movements* | 2 (40) | 1 (11) | 5 (100)a | 1 (17) | 4 (100) | 4 (80) |
| -Vertical movements worseˆ | 1 (20) | 0 (0) | 4 (80)a | 1 (17) | 4 (100) | 2 (40) |
| Buccofacial apraxia | 4 (80)a | 0 (0) | 5 (100) | 3 (50) | 4 (100) | 3 (60) |
| Asymmetric limb rigidity | 2 (40) | 2 (22) | 4 (80) | 2 (33) | 4 (100) | 3 (60) |
| Axial rigidity | 3 (60)a | 0 (0) | 3 (60) | 2 (33) | 4 (100) | 3 (60) |
| Limb Dystonia | 0 (0) | 2 (22) | 3 (60) | 1 (16) | 3 (75) | 3 (60) |
| Limb Apraxia | 3 (60) | 3 (33) | 3 (60) | 2 (33) | 4 (100) | 3 (60) |
| Postural instability | 1 (20) | 0 (0) | 2 (40) | 1 (17) | 4 (100) | 2 (40) |
| Cortical sensory/neglect | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (40) |
| Met probable PSP-S criteria | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 2 (50) | 0 (0) |
| Met probable CBD-S criteria | 0 (0) | 0 (0) | 3 (60) | 1 (16) | 4 (100) | 3 (60) |
Chi squared test performed
p< 0.05 PSP vs CBD.
Includes mild abnormalities such as decreased initiation, velocity, or amplitude of saccades.
Indicates vertical movements were more impaired than horizontal movements (only one PSP case presented clear vertical supranuclear gaze palsy at 1yr follow-up and thus met PSP-S criteria). PSP-S, CBD-S: PSP, CBD, syndrome (It was possible for one subject to meet both sets of diagnostic criteria).
At presentation, all cases reported difficulty with speech production as their initial and main complaint as well as the primary cause of impaired daily function. A significantly greater proportion of nfvPPA-PSP cases reported sensation of reduced balance and presence of at least 2 falls in the previous year. Also at presentation, a significantly greater proportion of nfvPPA-PSP cases showed buccofacial apraxia and mild axial rigidity in the neurological exam. At 1-year follow-up, more patients with nfvPPA-PSP complained of some swallowing difficulties and showed slower or lower amplitude of vertical than horizontal eye movements on neurologic exam. nfvPPA-CBD patients showed a trend for greater impulsive and obsessive-compulsive behaviors that were nevertheless present in both groups at follow-up.
Cross-sectional neuroimaging analysis at initial evaluation (figure-1)
Figure 1. Cross-sectional VBM at presentation of nfvPPA: 4R tau (n=14), PSP (n=5), and CBD (n=9).
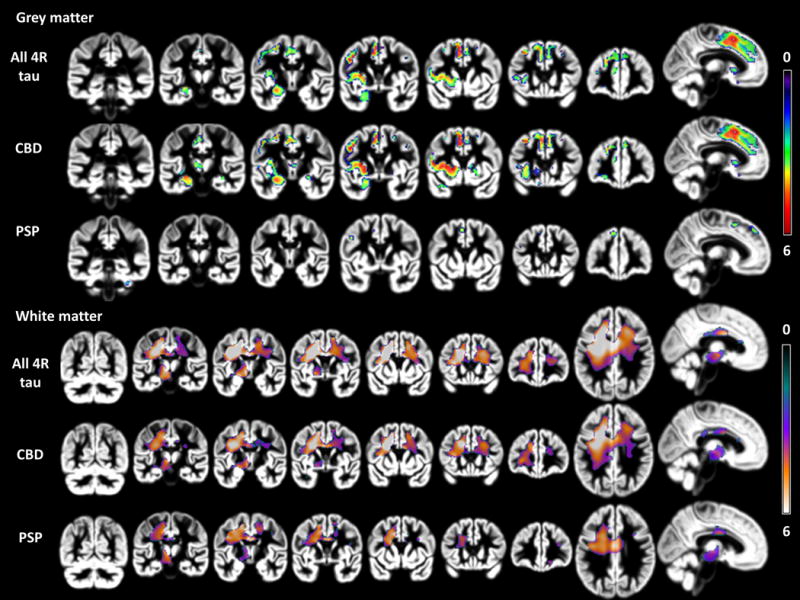
p<0.001 uncorrected for multiple comparisons; 3 group anova (PSP=5, CBD=9, controls= 80). 4 covariates (age, scanner, tiv, gender). Color bar indicates t-values (min: 0, max: 6). Images are in neurological view (left=left).
Grey Matter
nfvPPA-4R-tau showed atrophy primarily in a left posterior frontal insular- basal ganglia and superior medial frontal network. The most significant atrophy peaks were located in left precentral, middle and inferior frontal gyri, left medial supplemental motor area (SMA), left putamen, and left insula.
nfvPPA-CBD showed significant GM atrophy compared to controls in all regions mentioned above, while nfvPPA-PSP only showed small areas of significant GM atrophy in left SMA, precentral and middle frontal gyri, and right cerebellum. Direct group comparison showed greater GM atrophy in nfvPPA-CBD primarily in the left insula and putamen.
White Matter
nfvPPA-4R-tau showed extensive left frontal involvement predominantly affecting the WM between the striatum, premotor and prefrontal regions. Other smaller areas of significant atrophy were found in mid corpus callosum, underlying right premotor cortex, and in the midbrain-diencephalic junction.
Both pathological groups showed predominant WM atrophy beneath the left precentral gyrus and SMA and less significant atrophy in mid corpus callosum, right frontal, and left midbrain-diencephalic regions. As shown in figure 2, in nfvPPA-CBD atrophy extended considerably more anteriorly affecting WM underlying left frontal middle and inferior gyri. The relative proportion of GM to WM damage was strikingly different between patient groups, with PSP showing more WM than GM atrophy. Direct comparison of patient groups showed small regions of greater left prefrontal WM atrophy in nfvPPA-CBD.
Figure 2. Longitudinal VBM of nfvPPA: 4R tau (n=10), PSP (n=5), and CBD (n=5).
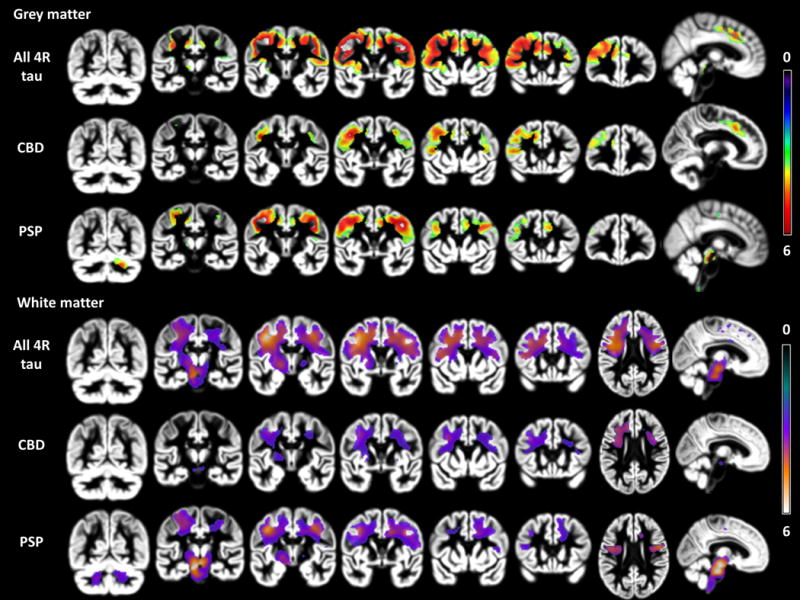
p<0.001 uncorrected for multiple comparisons; 3 group anova (PSP=5, CBD=5, controls= 42). 4 covariates (age, scanner, tiv, gender). Color bar indicates t-values (min: 0, max: 6). Images are in neurological view (left=left).
Longitudinal Neuroimaging analysis (figure-2)
Grey Matter
The area that showed greatest annual rate of change in nfvPPA-4R-tau included left precentral, middle frontal, and inferior frontal cortex. A homotopic area in the right hemisphere showed the second greatest rate of change followed by contiguous regions of bilateral SMA and middle cingulate cortex.
Both patient groups displayed significant longitudinal atrophy compared to controls in left precentral gyrus and SMA. nfvPPA- PSP showed more areas of significant GM longitudinal change including bilateral precentral, dorsal midbrain and right cerebellar regions. nfvPPA-CBD showed significant change in more anterior parts of left prefrontal cortex. Direct comparison did not reveal any significant differences.
White Matter
The area showing greatest rate of change in nfvPPA-4R-tau compared to controls was located underlying the left premotor region and extending anteriorly beneath prefrontal cortex and downwards through the corona radiata, posterior limb of the internal capsule, midbrain-diencephalic junction, left cerebral peduncle, and pons. Another less significant area of contraction was located in right frontal WM.
nfvPPA-CBD only showed significant longitudinal atrophy in one WM cluster underlying left precentral and middle frontal gyrus which extended farther anterior than in nfvPPA-PSP. In nfvPPA-PSP, the greatest rate of annual change included WM in the left half of the midbrain and pons and extended bilaterally into the cerebellar peduncles. Large areas of significant WM change were also visible underlying left and right precentral gyri. Direct comparison did not reveal any significant differences.
DISCUSSION
This study analyzed cross-sectional and longitudinal clinical, cognitive and neuroimaging data in a cohort of prospectively evaluated nfvPPA patients found to have CBD or PSP at autopsy. CBD was the most common pathological subtype in our cohort. Although the two groups showed major similarities, with AOS and left posterior frontal gray and white matter involvement being the most salient, common features, our results highlight specific characteristics that might help predict the nfvPPA presentation of PSP. In particular, the presence of severe dysarthria and greater WM than GM atrophy at presentation, and the appearance of midbrain anatomical and clinical signs at follow-up were typical of PSP. These findings are discussed in terms of previous literature on nfvPPA and on the anatomical structures involved.
It has been known for a decade that AOS and agrammatism are the most typical features of the nfvPPA clinical presentation3,4. In recent years, the term primary progressive apraxia of speech has been introduced when AOS is the main feature and no apparent agrammatism is detected32. In our experience, it is often difficult to judge whether grammar production is spared in patients with severe output difficulties. In our cohort, all patients were diagnosed by a speech pathologist as having AOS, while grammatical difficulties were variable and sometimes only detected in written language or at follow-up. Thirteen out of 14 of our patients could have been classified as having greater motor speech than grammatical deficits but nfvPPA-PSP had significantly more dysarthria and buccofacial apraxia at presentation. In contrast, nfvPPA-CBD was significantly worse than controls in sentence comprehension while nfvPPA-PSP was not. In the direct comparison between patient groups the difference in sentence comprehension was only a trend (p≤0.10). A recent clinicopathological study in nfvPPA suggested PSP is more likely when AOS dominates the syndrome whereas CBD is more likely when AOS and aphasia are equal6. In our cohort the presence of dysarthria, together with AOS, was responsible for greater motor speech deficits compared to grammar in patients with PSP. Dysarthria has previously been reported in pathologically confirmed PSP cases presenting as both PPA6 and Richardson’s syndrome33. The early predominance of motor speech deficits in both PSP and CBD supports a potential role as an outcome measure if one were to test a tau directed therapeutic in this population. However, more quantitative and reliable measures of AOS and dysarthria are needed for adequate assessment of change in these areas.
Consistent with their clinical presentation, CBD and PSP showed atrophy that overlapped in left SMA and precentral regions, important components in the motor speech production network21,34,35. These results are consistent with previous reports of cross-sectional neuroimaging in clinically3,36,37 and pathologically6,10,17,38 confirmed cases of nfvPPA. Our finding of early predominance of WM over GM atrophy in PSP is also in accordance with previous neuroimaging17,38 and quantitative pathology33 studies and may explain why dysarthria was more severe than AOS in PSP. In CBD, the atrophy extended further into left frontal GM and WM providing a substrate for their significantly impaired working memory and grammar comprehension compared to controls20,39. Early, severe WM damage has been proposed as typical of FTLD-tau pathology presenting as nfvPPA9. Our current results refine this association, suggesting that early predominant white versus gray matter atrophy should be considered as a possible neuroimaging biomarker of PSP pathology, but always in the context of a multi-domain approach considering clinical, molecular, genetic, and neuroimaging features.
Analyzing prospectively collected longitudinal data in pathologically confirmed nfvPPA was a unique opportunity of this study. Only PSP showed highly significant GM and WM longitudinal changes in the midbrain, particularly at the level of the cerebral and cerebellar peduncles presumably affecting the corticospinal tract, pontine crossing fibers, and other afferent and efferent cerebellar fibers. Accordingly, nfvPPA-PSP developed mild ocular and axial motor abnormalities. In contrast, nfvPPA-CBD showed greater longitudinal changes in prefrontal anterior, medial, and lateral GM and WM corresponding with their greater longitudinal decline in speech fluency and development of behavioral symptoms. Rohrer et al also found more prominent midbrain atrophy but less marked perisylvian atrophy in cases of nfvPPA that developed a typical PSP clinical syndrome compared to cases that did not, though this study did not include longitudinal imaging or pathological data36. Greater presence of behavioral symptoms in cases of CBD pathology was also reported in a recent study that compared cases of CBD versus PSP presenting as Richardson’s syndrome33. Similar to other longitudinal clinical-pathological reports40,41, CBD-syndrome was more common than Richardson’s syndrome at later visits. Our results might be relevant for prognosis in nfvPPA because significant initial dysarthria at presentation may indicate considerable subcortical disease and imminent swallowing and balance problems. This study also suggests that differential longitudinal neuroimaging changes in GM and WM may be a sensitive biomarker of disease-specific patterns of progression42.
Despite being the largest cohort of prospectively studied and pathologically confirmed nfvPPA that has been reported, this study is necessarily based on a relatively small sample which limits generalization of results and entails low-powered statistical analyses. To address this issue and help with interpretation of results, we included individual subject cognitive data in the supplementary material (etables-4&5). We also performed a region-of-interest (ROI) analysis to address the concern that nfvPPA-CBD’s larger sample size was driving the finding of more extensive atrophy in nfvPPA-CBD at presentation. The ROI analysis supports the VBM findings and is included in the supplementary material. Combining MRIs from three different scanners is also not ideal, however we controlled for this by matching controls and including it as a nuisance variable in the VBM analysis. Finally, DTI combined with tractography would have been the optimal technique to investigate WM damage in specific tracts. However, VBM was able to show important differences between groups that are consistent with a recent DTI tractography study in the same clinical population that included four (two PSP and two CBD) of the same subjects35.
In-vivo prediction of the pathology underlying the nfvPPA syndrome is an increasingly important endeavor as future molecule-specific treatments are developed. Our results indicate a promising role of combining early cross-sectional and longitudinal clinical and neuroimaging features in the in-vivo differentiation between nfvPPA-PSP and nfvPPA-CBD.
Supplementary Material
Acknowledgments
We thank the patients and their families for their dedication to the research, Ian Mackenzie for assistance with one of the autopsies, and John Kornak for assistance in the neuroimaging analysis.
STUDY FUNDING & ROLE OF FUNDERS:
This work was supported by the National Institutes of Health grants (NINDS R01 NS050915, P01 AG019724, P50 AG023501, K24 AG045333-01, R01 AG032306, U54NS092089, R01 AG038791); State of California (DHS 04-35516); Alzheimer’s Disease Research Center of California (09-11410 DHS/ADP/ARCC); Larry L. Hillblom Foundation; Koret Family Foundation; The Frontotemporal Lobar Degeneration Clinical Research Consortium; the McBean Family Foundation; and the Alfonso Martin Escudero Foundation. The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
AUTHOR DISCLOSURES
Dr. Santos, Dr. Mandelli, Dr. Binney, Dr. Ogar, Dr. Hubbard, Dr. Henry, Mr. Attygalle, Mr. Pakvasa, Ms. Rosenberg, Dr. Trojanowski, and Dr. Grinberg report no disclosures. Dr. Boxer is funded by grants R01 AG038791 and U54NS092089. He helped with the design and conceptualization of the study, analysis and interpretation of the data, drafting and revising the manuscript. Dr. Rosen is funded by grants K24 AG045333 and R01 AG032306. Dr. Seeley is funded by NIH grants P50 AG1657303, the John Douglas French Alzheimer’s Disease Foundation, Consortium for Frontotemporal Dementia Research, James S. McDonnell Foundation, Larry Hillblom Foundation, has received support for travel by the Alzheimer’s Association, and received payment for lectures by the Alzheimer’s Association, American Academy of Neurology, and Novartis Korea. Dr. Miller serves as board member on the John Douglas French Alzheimer’s Foundation and Larry L. Hillblom Foundation, serves as a consultant for TauRx, Ltd., Allon Therapeutics, Siemens, BMS, the Tau Consortium and the Consortium for Frontotemporal research, has received institutional support from Novartis, and is funded by NIH grants P50AG023501, P01AG019724, P50 AG1657303, and the state of CA. Dr. Gorno-Tempini is funded by NIH grant NINDS R01 NS050915.
Footnotes
AUTHOR CONTRIBUTIONS
Collection, management, analysis, or interpretation of the data: Dr. Santos, Dr. Mandelli, Dr. Binney, Dr. Ogar, Dr. Hubbard, Ms. Meese, Dr. Henry, Mr. Attygalle, Mr. Pakvasa, Ms. Rosenberg, Dr. Trojanowski, Dr. Grinberg, Dr. Boxer, Dr. Rosen, Dr. Seeley, Dr. Miller, Dr. Gorno-Tempini.
Preparation, review, or approval of the manuscript: Dr. Santos, Dr. Mandelli, Dr. Binney, Dr. Hubbard, Dr. Boxer, Dr. Rosen, Dr. Seeley, Dr. Miller, Dr. Gorno-Tempini.
Miguel A. Santos had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137(Pt 4):1176–1192. doi: 10.1093/brain/awu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88–97. doi: 10.1038/nrneurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman M. The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol. 2012;11(6):545–555. doi: 10.1016/S1474-4422(12)70099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(Pt 6):1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deramecourt V, Lebert F, Debachy B, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74(1):42–49. doi: 10.1212/WNL.0b013e3181c7198e. [DOI] [PubMed] [Google Scholar]
- 8.Grossman M, Xie SX, Libon DJ, et al. Longitudinal decline in autopsy-defined frontotemporal lobar degeneration. Neurology. 2008;70(22):2036–2045. doi: 10.1212/01.wnl.0000303816.25065.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caso F, Mandelli ML, Henry M, et al. In vivo signatures of nonfluent/agrammatic primary progressive aphasia caused by FTLD pathology. Neurology. 2014;82(3):239–247. doi: 10.1212/WNL.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohrer JD, Lashley T, Schott JM, et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain. 2011;134(Pt 9):2565–2581. doi: 10.1093/brain/awr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris JM, Gall C, Thompson JC, et al. Classification and pathology of primary progressive aphasia. Neurology. 2013;81(21):1832–1839. doi: 10.1212/01.wnl.0000436070.28137.7b. [DOI] [PubMed] [Google Scholar]
- 12.Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol. 1999;246(Suppl 2):Ii6–15. doi: 10.1007/BF03161076. [DOI] [PubMed] [Google Scholar]
- 13.Wakabayashi K, Takahashi H. Pathological heterogeneity in progressive supranuclear palsy and corticobasal degeneration. Neuropathology. 2004;24(1):79–86. doi: 10.1111/j.1440-1789.2003.00543.x. [DOI] [PubMed] [Google Scholar]
- 14.Sanders DW, Kaufman SK, DeVos SL, et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. 2014;82(6):1271–1288. doi: 10.1016/j.neuron.2014.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada T, McGeer PL, McGeer EG. Appearance of paired nucleated, Tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett. 1992;135(1):99–102. doi: 10.1016/0304-3940(92)90145-w. [DOI] [PubMed] [Google Scholar]
- 16.Feany MB, Dickson DW. Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol. 1995;146(6):1388–1396. [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SE, Rabinovici GD, Mayo MC, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol. 2011;70(2):327–340. doi: 10.1002/ana.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16(4):211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Gorno-Tempini ML, Murray RC, Rankin KP, Weiner MW, Miller BL. Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: a case report. Neurocase. 2004;10(6):426–436. doi: 10.1080/13554790490894011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson SM, Dronkers NF, Ogar JM, et al. Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. J Neurosci. 2010;30(50):16845–16854. doi: 10.1523/JNEUROSCI.2547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson SM, Henry ML, Besbris M, et al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133(Pt 7):2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord. 2007;21(4):S23–30. doi: 10.1097/WAD.0b013e31815d19fe. [DOI] [PubMed] [Google Scholar]
- 23.Boxer AL, Geschwind MD, Belfor N, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol. 2006;63(1):81–86. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- 24.Bettcher BM, Wilheim R, Rigby T, et al. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun. 2012;26(1):103–108. doi: 10.1016/j.bbi.2011.07.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Schuff N, Ching C, et al. Joint assessment of structural, perfusion, and diffusion MRI in Alzheimer’s disease and frontotemporal dementia. Int J Alzheimers Dis. 2011;2011:546871. doi: 10.4061/2011/546871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashburner J, Ridgway GR. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front Neurosci. 2012;6:197. doi: 10.3389/fnins.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 30.Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119(1):1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Annals of neurology. 2006;59(6):952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135(Pt 5):1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kouri N, Murray ME, Hassan A, et al. Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome. Brain. 2011;134(Pt 11):3264–3275. doi: 10.1093/brain/awr234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eickhoff SB, Heim S, Zilles K, Amunts K. A systems perspective on the effective connectivity of overt speech production. Philos Trans A Math Phys Eng Sci. 2009;367(1896):2399–2421. doi: 10.1098/rsta.2008.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandelli ML, Caverzasi E, Binney RJ, et al. Frontal white matter tracts sustaining speech production in primary progressive aphasia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(29):9754–9767. doi: 10.1523/JNEUROSCI.3464-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohrer JD, Paviour D, Bronstein AM, O’Sullivan SS, Lees A, Warren JD. Progressive supranuclear palsy syndrome presenting as progressive nonfluent aphasia: a neuropsychological and neuroimaging analysis. Mov Disord. 2010;25(2):179–188. doi: 10.1002/mds.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Josephs KA, Duffy JR, Strand EA, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013 doi: 10.1212/WNL.0b013e31829c5ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29(2):280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amici S, Brambati SM, Wilkins DP, et al. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. J Neurosci. 2007;27(23):6282–6290. doi: 10.1523/JNEUROSCI.1331-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128(Pt 9):1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- 41.Respondek G, Stamelou M, Kurz C, et al. The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord. 2014;29(14):1758–1766. doi: 10.1002/mds.26054. [DOI] [PubMed] [Google Scholar]
- 42.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


