Abstract
Classical anesthetics of the γ-aminobutyric acid type A receptor (GABAA)-enhancing class (e.g., pentobarbital, chloral hydrate, muscimol, and ethanol) produce analgesia and unconsciousness (sedation). Dissociative anesthetics that antagonize the N-methyl-D-aspartate (NMDA) receptor (e.g., ketamine, MK-801, dextromethorphan, and phencyclidine) produce analgesia but do not induce complete loss of consciousness. To understand the mechanisms underlying loss of consciousness and analgesia induced by general anesthetics, we examined the patterns of expression of c-Fos protein in the brain and correlated these with physiological effects of systemically administering GABAergic agents and ketamine at dosages used clinically for anesthesia in rats. We found that GABAergic agents produced predominantly delta activity in the electroencephalogram (EEG) and sedation. In contrast, anesthetic doses of ketamine induced sedation, followed by active arousal behaviors, and produced a faster EEG in the theta range. Consistent with its behavioral effects, ketamine induced Fos expression in cholinergic, monoaminergic, and orexinergic arousal systems and completely suppressed Fos immunoreactivity in the sleep-promoting ventrolateral preoptic nucleus (VLPO). In contrast, GABAergic agents suppressed Fos in the same arousal-promoting systems but increased the number of Fos-immunoreactive neurons in the VLPO compared with waking control animals. All anesthetics tested induced Fos in the spinally projecting noradrenergic A5–7 groups. 6-hydroxydopamine lesions of the A5–7 groups or ibotenic acid lesions of the ventrolateral periaqueductal gray matter (vlPAG) attenuated antinociceptive responses to noxious thermal stimulation (tail-flick test) by both types of anesthetics. We hypothesize that neural substrates of sleep-wake behavior are engaged by low-dose sedative anesthetics and that the mesopontine descending noradrenergic cell groups contribute to the analgesic effects of both NMDA receptor antagonists and GABAA receptor-enhancing anesthetics.
Indexing terms: sedation, antinociception, supraspinal analgesia, tail-flick
Anesthetics that modulate the γ-aminobutyric acid type A receptor (GABAA; by direct gating of the Cl channel or by potentiating the effects GABA; hereafter referred to as GABAA agents) induce both analgesia and loss of consciousness (sedation) and are associated with a slow-wave electroencephalogram (EEG) pattern and depressed CNS function (for review see Sloan, 1998). In contrast, anesthetics that act by antagonizing N-methyl-D-aspartate (NMDA) receptors (e.g., ketamine, MK-801, phencyclidine, dextromethorphan, and nitrous oxide) produce analgesia but also preserve some aspects of consciousness (dissociative anesthesia). These NMDA receptor antagonist anesthetics are associated, especially at subanesthetic doses, with a fast, theta-range EEG (5–7 Hz) and maintenance of sympathetic tone (Yamamura et al., 1981; Carruba et al., 1987; Marquis et al., 1989; Sagratella et al., 1992; Mattia and Moreton, 1996). There is an increase in the EEG delta power (<4 Hz) during subsequent episodes of sleep after 3 hours of “arousal” produced by ketamine (Campbell and Feinberg, 1996), which is similar to sleep behavior after sleep deprivation. Subanesthetic doses of ketamine produce behaviors that resemble schizophrenia in humans (for reviews see Moghaddam, 1994; Abi-Saab et al., 1998).
Similarities and differences between the mechanisms of NMDA receptor antagonists and GABAA agents in modulating analgesia, anesthesia, and sleep-wake behavior raise questions regarding their mutual sites of action. With respect to areas involved in sleep and wakefulness, animal studies have shown that ketamine and other NMDA antagonists, including phencyclidine, dextromethorphan, and MK-801, increase Fos immunoreactivity and 2-[14C]deoxyglucose uptake (an indicator of cellular metabolic activity) in the limbic system, cerebral cortex, and thalamus; Duncan et al., 1999, 2000; Miyamoto et al., 2000; Jahng et al., 2001). Conversely muscimol, a GABAA direct agonist, has been found to reduce Fos expression in the cerebral cortex and histaminergic tuberomammillary nucleus (TMN) while increasing it in the sleep-promoting ventrolateral preoptic nucleus (VLPO; Nelson et al., 2002). However, a more general overview of the effects of different classes of anesthetics on Fos expression throughout the brain has not appeared.
Research on analgesic mechanisms has revealed that the brain maintains strong descending modulation of nociceptive neurons in the dorsal horn of the spinal cord (for review see Millan, 2002). The first supraspinal site identified as participating in pain modulation was the ventrolateral periaqueductal gray matter (vlPAG; Yeung et al., 1977). Subsequently, the rostral ventromedial medulla (RVM) was found to modulate pain transmission (Fields et al., 1983; Mitchell et al., 1998). The analgesic functions of the vlPAG and the RVM are partially mediated by descending serotoninergic and noradrenergic systems (Brodie and Proudfit, 1984; Barbaro et al., 1985; Jensen and Yaksh, 1986). Three pontine noradrenergic cell groups (A5–7) that receive input from the vlPAG (Bajic et al., 2001) and project to the spinal dorsal horn (Clark and Proudfit, 1992) have been reported to modulate endogenous antinociception (Martin et al., 1999) and to mediate antinociceptive effects of morphine (Sawynok and Reid, 1987), nitrous oxide (Sawamura et al., 2000), and isoflurane (Kingery et al., 2001), via α2-adrenoceptors on dorsal horn neurons. There is some evidence that supraspinal descending control may also be involved in analgesia produced by GABAB receptor agonists, such as baclofen (for review see Sawynok, 1987); however, it is not clear whether GABAA agents activate the same descending nor-adrenergic systems. Because spinal cord transection or systemic α2-adrenoceptor antagonists can attenuate the analgesic effects of ketamine (Tomemori et al., 1981; Crisp et al., 1991), the pontine noradrenergic descending projection is also thought to mediate the antinociceptive effects of ketamine.
We therefore hypothesized that a key mechanism of action for both classes of anesthetics may be to engage endogenous brain systems that control sedation and analgesia. To explore those possibilities, we examined Fos immunoreactivity throughout the brain, with particular attention to the sleep-wake regulatory system and to the descending A5–7 noradrenergic neurons following administration of anesthetics. We also determined whether lesions of A5–7 noradrenergic cell groups with 6-hydroxydopamine and the ventrolateral PAG using the excitotoxin ibotenic acid alter antinociceptive responses to anesthetics.
MATERIALS AND METHODS
Animals
Pathogen-free adult male Sprague Dawley rats (275–300 g; Harlan) were individually housed under controlled conditions (12 hours light starting at 07:00 AM, 200 lux) in an isolated ventilated chamber maintained at 20–22°C and had free access to food and water. All protocols were approved by the Institutional Animal Care and Use Committees of Beth Israel Deaconess Medical Center and Harvard Medical School.
EEG/EMG and sleep recording
The surgery and EEG/EMG data collection and analyses have been described in detail previously (Lu et al., 2000, 2001, 2002). Briefly, animals were anesthetized with chloral hydrate (7% in saline, 350 mg/kg), and four stainless-steel EEG screw electrodes were implanted into the skull, in the frontal (two) and parietal (two) bones, and two flexible EMG wire electrodes were placed in the nuchal muscles. The electrodes were fixed into sockets stabilized to the skull with dental cement. The sockets were connected via flexible recording cables and a commutator to a Grass polygraph (West Warwick, RI) and a Macintosh computer. The amplified EEG/EMG signals were digitized by the ICELUS program (M. Opp, University of Michigan, Ann Arbor). Animals were allowed to recover from surgery for at least 1 week, including 2 days of adaptation to the EEG/EMG cables before recording. Wakefulness, NREM sleep, and REM sleep were scored as previously described in 12-second epochs (Lu et al., 2000, 2001, 2002). Percentages of sleep and wake states per hour were calculated based on scored results of EEG/EMG. The ICELUS program performs a fast Fourier analysis on the EEG to provide a continuous plot of the power in the delta (0.5–4 Hz) and theta (4–7 Hz) ranges. In addition, it provides analysis of the delta and theta power in the EEG over 1-hour blocks during the recording period. Although it is not possible to compare EEG power measurement between animals (because EEG amplitude is affected by lead placement and other technical factors), we did compare the EEG power in the delta and theta bands in the same animals in the pre- and posttreatment states.
Administration of anesthetics
We examined the effects of administration of four GABAA-class anesthetics and one NMDA antagonist anesthetic on behavior, EEG, tail-flick latency, and cFos expression throughout the brain. One week after surgery for sleep recording, rats received intraperitoneal (i.p.) injections of ethanol (1.0 g/kg, n = 5) or pentobarbital (40 mg/kg, n = 4), chlorate hydrate (350 mg/kg, n = 5), or muscimol (5 mg/kg, n = 5) at 19:00 (i.e., onset of the dark period, because these caused sedation, which is most easily measurable when the animals would otherwise be awake) or ketamine (300 mg/kg, n = 2; 150 mg/kg, n = 3; 60 mg/kg, n = 3; 30 mg/kg, n = 3) at 10:00 AM (i.e., early in the light period, because it activated the EEG, and this was most easily measurable during the time of day when the animals would ordinarily be asleep). For spontaneous waking and sleeping controls, we injected 1.0 ml saline (i.p.) at either 19:00 (n = 4) and 10:00 (n = 4), respectively. All rats were perfused 2 hours after drug or saline injection. During this 2 hours, EEG/EMG was continuously recorded. We also delivered the gas anesthetic isoflurane to four rats in an airtight chamber from 19:00 to 21:00. The construction of the chamber did not allow us to measure EEG/EMG during the delivery of isoflurane, but we did measure the righting reflex and EEG for 10 minutes directly after the isoflurance exposure (Fig. 1).
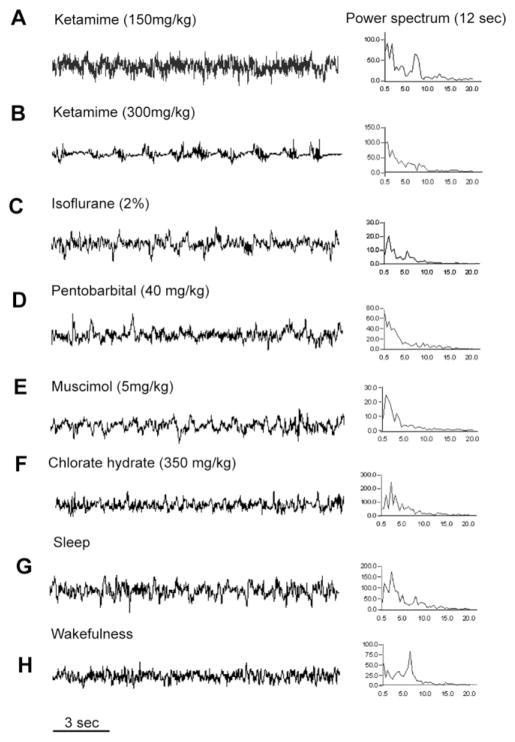
Fig. 1.
Effects on the electroencephalogram (EEG) induced by ketamine (A,B) and GABAA anesthetics (C–F), compared with normal NREM sleep (G) and wakefulness (H). For each state, a 12-second epoch of typical EEG is shown on the left, and the power spectrum for that same epoch on the right. Note that doses of ketamine up to 150 mg/kg (twice that used typically for rat anesthesia; A) increased both delta power (0.5–4.0 Hz, associated with sleep) and theta power (4–7 Hz, associated with arousal and REM sleep) in the EEG, whereas only much higher doses (300 mg/kg) predominantly increased delta power (B). All GABAA agents induced an EEG pattern dominated by slow, delta waves (C–F). The x-axis in the power spectrum represents the frequency band that was sampled, and the y-axis represents the power in that frequency band, in arbitrary units.
Lesions of A5, A6, and A7 noradrenergic cell groups
Rats were anesthetized with chloral hydrate (350 mg/kg) and secured in a stereotaxic frame, and the skull was exposed. After a small hole was drilled into the skull and the dura was incised, a fine glass micropipette (20 μm tip diameter) was stereotaxically positioned with its tip in the fourth ventricle (coordinates 7.65 mm anteroposterior, 0 mm mediolateral, and 4.8 mm dorsoventral with respect to bregma; Paxinos and Watson, 1998), and 8 μl of 6% 6-hydroxydopamine (n = 8) or 8 μl saline (n = 6) was injected by air pressure into the fourth ventricle. These rats did not require wake-sleep recording, so we did not implant EEG/EMG electrodes in this group. Animals were allowed to recover for at least 7 days prior to testing.
Lesions of the vlPAG
While deeply anesthetized with chloral hydrate (350 mg/kg), rats received bilateral sterotaxic injection of 30 nl 10% ibotenic acid at coordinates: −7.65 mm anterioposterior, ±0.5 mm mediolateral, 5.2 mm dorsoventral with respect to bregma; Paxinos and Watson, 1998). These animals did not receive EEG/EMG implants. Animals were allowed to recover for at least 7 days prior to testing.
Tail-flick latency
After 1 week of recovery from sham and lesion surgeries, rats were tested for their basal responses to a thermal noxious stimulus by using the tail-flick test. Rats were loosely restrained with a towel, and three-fouths of the tail was immersed in a 53°C water bath. The latency of the tail-flick response was recorded by a stopwatch, with a cut-off time at 15 seconds to prevent tissue injury. In both lesioned and sham-injection control groups, we measured the latency of tail-flick at baseline; then anesthetics were administrated, and tail flick was measured again 15–30 minutes later.
Histology
Two hours after receiving a test dose of anesthetics, animals received an overdose of chloral hydrate (500 mg/kg) and were immediately perfused transcardially with 50 ml saline, followed by 500 ml 10% formalin. Brains were removed, postfixed for 4 hours in 10% formalin, and then equilibrated in 20% sucrose in phosphate-buffered saline (PBS; pH 7.4).
The brains were sectioned at 40 μm on a freezing microtome in four series, and cFos expression was examined throughout the brain, with particular attention to areas of the brain involved in wake-sleep regulation and antinociception. One series was immunostained for Fos and counterstained with thionin. Sections from three adjacent series were selected for double immunostaining of Fos + choline acetyltransferase (ChAT; basal forebrain and pons), Fos + tyrosine hydroxylase (TH; midbrain and pons), Fos + orexin (lateral hypothalamus), or Fos + serotonin (midbrain and pons). Sections were washed in PBS and incubated in primary antiserum: rabbit polycolonal antibody against c-Fos (1:150,000; AB5; Oncogene Sciences). rabbit polycolonal antibody against orexin A (1:50,000; T-4074; Peninsula Laboratories, Belmont, CA), polycolonal goat antibody against ChAT (1:2,000; AB144; Chemicon, Temecula, CA), polyclonal goat antibody against 5-HT (1:10,000; AB125; Chemicon), or mouse monocolonal antibody against TH (1:30,000; lot No. 22941; DiaSorin, Stillwater, MN).
Sections were then washed in PBS and incubated in biotinylated secondary antiserum (against appropriate species IgG; 1:1,000; Vector, Burlingame, CA) in PBS for 1 hour, washed in PBS, and incubated in ABC reagents for 1 hour. Sections were then washed again and incubated in a solution of 0.06% 3,3-diaminobenzidine tetrahydrochloride (DABp; Sigma) and 0.02% H2O2. The sections were stained brown with DAB (ChAT, TH, 5-HT, or orexin) or black (Fos) by DAB plus 0.05% cobalt chloride and 0.01% nickel ammonium sulfate. Because Fos stains only the nuclei of labeled neurons, it is compatible even with rabbit antisera that stain only the cytoplasmic compartment.
Antibody characterization
The Fos antibody was raised against a synthetic peptide including residues 4–17 from human c-Fos. This antibody stained a single band of 55 kD m.w. on Western blots from rat brain (manufacturer’s technical information). Staining with this antibody was seen in the ventrolateral preoptic nucleus only during sleep and in the tuberomammillary nucleus and lateral hypothalamic orexin neurons during wakefulness (Estabrooke et al., 2001; Gaus et al., 2002).
The antibody against orexin A was raised against the orexin peptide Pyr-Pro-Leu-Pro-Asp-Cys-Cys-Arg-Gln-Lys-Thr-Cys-Ser-Cys-Arg-Leu-Tyr-Glu-Leu-Leu-His-Gly-Ala-Gly-Asn-His-Ala-Ala-Gly-Ile-Leu-Thr-Leu-NH2. On immunoblots from rat brain, it recognizes a pair of bands representing the orexin peptide at about 3.5 kD m.w. (manufacturer’s technical information). The pattern of cellular morphology and distribution that is stained is limited to the lateral hypothalamus and is identical to previous reports (Peyron et al., 1998; Chou et al., 2003).
The antibody against ChAT was raised against human placental enzyme, and on Western blot of rat brain it stains a single band of approximately 62 kD m.w. (manufacturer’s technical information). In the basal forebrain and the mesopontine tegmentum, it stains a pattern of cellular morphology and distribution identical to previous reports (Chou et al., 2002).
The antibody against 5-HT was raised against 5-hyroxytryptamine conjugated to polylysine by glutaraldehyde. It stains a pattern of cellular morphology and distribution identical to previous reports for the serotoninergic system in the mesopontine tegmentum of rat (Lu et al., 2006). For immunohistochemical controls for the secondary antibody, Fos, ChAT, TH, 5-HT and orexin A primary antibodies were omitted, and the tissue showed no immunoreactivity above background.
Cell counting
Cell counts were obtained in the cingulate cortex, components of the sleep (ventrolateral preoptic nucleus) and waking (basal forebrain cholinergic neurons, lateral hypothalamic orexin neurons, locus coeruleus) systems, antinociceptive (ventrolateral periaqueductal gray matter, A5 and A7 catecholamine) cell groups, and several additional cell groups (ventral lateral septum, lateral habenular nucleus, and Edinger-Westphal nucleus) that showed conspicuous changes in c-Fos expression after administering anesthetics. For each cell group, neurons with immunoreactive (c-Fos+) nuclei were counted on both sides of the brain in three adjacent 1:4 sections through the center of the cell group. All counts are expressed as the mean number of neurons per section on one side of the brain. For cell groups that were clearly defined on Nissl staining, such as the Edinger-Westphal nucleus, the lateral habenular nucleus, and the tuberomammillary nucleus, the region to be counted was defined by outlining the cell group in the Nissl-stained sections. For cell groups with less well-defined borders, counting boxes were constructed (see Lu et al., 2000), and counts were made on both sides of the brain. For the VLPO cluster, a box of 0.4 mm × 0.3 mm was used, with its ventromedial corner at the edge of the optic chiasm; for the lateral septal nucleus, a box of 0.3 mm × 0.3 mm was placed with its lateral margin at the wall of the lateral ventricle and its ventral border just above the bed nucleus of the stria terminalis. For the ventrolateral PAG, a box of 0.3 mm × 0.3 mm was placed with its medial edge aligned with the lateral edge of the cerebral aqueduct and its ventral border even with ventral extent of the aqueduct. For the rostral ventromedial medulla, a box of the same size was placed with its medial edge on the midline of the brainstem and its ventral border along the dorsal edge of the pyramidal tract. Other cell groups, such as the basal forebrain cholinergic neurons, lateral hypothalamic orexin neurons, and locus coeruleus, and A5 and A7 cell groups were defined immunocytochemically (for choline acetyltransferase, orexin, and tyrosine hydroxylase, respectively). For all labeled cell groups, cell profiles with a clearly visualized nucleus were counted in three adjacent 1:4 sections through the center of the cell group. In each case, nuclear diameters were measured and counts were corrected by using Abercrombie’s formula (N = n · [T/(T + D)]), where n = cell count, T = section thickness, and D = nucleus diameter). Stereological methods were not applicable, because the antibodies do not penetrate the entire thickness of the sections. Counts are presented as mean ± SEM for one side of one section. Brightness and contrast of images were optimized for visualization purposes, and images were created in Adobe Photoshop and Illustrator.
Statistical analyses
ANOVA with post hoc Student’s paired t-test was used to determine significant differences (P < 0.05) in the Fos-ir neurons in the control (sleep and waking controls) and posttreatment (two-way ANOVA) groups and in latency of tail-flick between lesioned and control groups at baseline and in response to anesthetics (one-way ANOVA).
RESULTS
Effects of anesthetics on EEG and sleep-wake behavior
We examined the EEG and its power spectrum after administering the different anesthetics, to confirm that the responses were similar to those previously reported (Yamamura et al., 1981; Carruba et al., 1987; Marquis et al., 1989; Russell and Graybeal, 1992; Sagratella et al., 1992; Mattia and Moreton, 1996; Plourde et al., 1997; Sloan, 1998). GABAergic agents were tested at 19:00, because they produced sedation, which was most easily distinguished from normal behavior during the normal waking phase.
Consistent with previous reports (Sloan, 1998), anesthetic doses of GABAA-class drugs such as ethanol (1.0 g/kg), pentobarbital (40 mg/kg), chlorate hydrate (350 mg/kg), or muscimol (5 mg/mg) injected intraperitoneally at 19:00 induced sedation and a slow-wave EEG in the delta (1–4 Hz) range for the next 2 hours (Fig. 1). These doses of drugs also completely suppressed REM sleep. Because isoflurance was delivered as a gas in a closed chamber, we were not able to determine the composition of slow-wave vs. REM states while animals were under anesthesia. However, recordings immediately after taking animals out of the chamber disclosed predominantly high-voltage delta activity (Fig. 1C)
Also, as previously reported, ketamine at doses at or below the range used for clinical anesthesia in rats (75–100 mg/kg) produced mainly a fast EEG with a prominent theta component. This was associated with excited behavior and wake-like Fos expression, so ketamine was given at 10:00, when animals are usually sleeping, so that it would be easier to distinguish the behavioral and histological differences from control animals. Subanesthetic doses of ketamine (30 and 60 mg/kg, i.p.) injected at 10:00 produced arousal behaviors (moving constantly with head weaving and ataxia) with a prominent peak in the theta range in the EEG power spectrum. Doses of ketamine in the high anesthetic range (150 mg/kg) initially resulted in immobility (sedation) for approximately 40–60 minutes, followed by arousal behaviors. During the period of behavioral sedation, peaks were seen both in the delta range (similar to sleeping animals) and in the theta range (similar to awake animals; Fig. 1). Only at a dosage well above the usual clinical range (300 mg/kg) did ketamine produce a prolonged anesthetic level of sedation with predominantly delta EEG for the entire 2 hours (Fig. 1).
Effects of anesthetics on Fos expression in the brain
To compare Fos expression caused by sedation or arousal induced by anesthetics to that induced by natural sleep and wake behavior, we perfused two groups of rats at 21:00 (n = 4, waking controls, at the same time as animals that received GABAergic agents) or 12:00 (n = 4, sleeping controls, at the same time as animals that received ketamine).
In general, both subanesthetic (30 and 60 mg/kg) and anesthetic doses (150 mg/kg) of ketamine produced patterns of Fos expression in the CNS that were similar to wakefulness. In contrast, GABAergic anesthetics induced overall Fos expression in the CNS similar to that during sleep. However, there was a common pattern of increased Fos expression in the ventrolateral septal nucleus, the lateral habenular nucleus, and the Edinger-Westphal nucleus as well as the pontine noradrenergic cell groups (A5, A6, A7 groups) seen after both types of anesthetic administration that wass not seen in the sleeping or waking controls.
Cerebral cortex
During wakefulness, large numbers of Fos-immunoreactive neurons were seen throughout the cerebral cortex, particularly in layers 2–3 and 5. In sleeping animals, only a few scattered cells were seen. Ketamine at doses up to 150 mg/kg induced Fos expression by large numbers of neurons throughout the entire cerebral cortex, but this was most prominent in the cingulate, somatosensory, auditory, visual, and motor areas. Waking controls also showed high levels of Fos expression throughout the cerebral cortex. At 300 mg/kg ketamine, Fos expression in most of the cerebral cortex was significantly reduced to a level similar to that in sleeping control animals, although Fos expression in the cingulate cortex remained at waking levels. Sleeping controls and rats receiving GABAergic agents showed relatively few cortical Fos-immunoreactive (-ir) neurons, and these were evenly dispersed throughout the cortex. Among the animals that received GABAergic drugs, those that were treated with pentobarbital and isoflurane had the fewest Fos-ir neurons in the cerebral cortex.
Thalamus
In sleeping rats, Fos-ir neurons were seen mainly in the paraventricular thalamic nucleus. In awake rats, additional Fos-ir neurons were found in the midline thalamic nuclei. Similarly, after ketamine administration at all doses (up to 300 mg/kg), more Fos-ir neurons were seen in the midline thalamus than were seen during natural sleep or following treatment with GABAergic agents. Other thalamic nuclei contained few Fos-ir neurons under any conditions.
Cholinergic (ChAT+) cell groups
Fos expression in basal forebrain cholinergic neurons was seen during spontaneous wakefulness, but not sleep, and was similarly induced by ketamine at doses up to 150 mg/kg. Although ketamine caused strong Fos expression in the cholinergic neurons in both the horizontal and the vertical limbs of the nucleus of the diagonal band and the substantia innominata, during spontaneous waking Fos immunoreactivity was seen only in the cholinergic neurons in the substantia innominata (Fig. 2, Table 1). In the pons, Fos-ir neurons were not seen in the cholinergic cells of the laterodorsal tegmental (LDT) or the pedunculopontine tegmental (PPT) nuclei (Fig. 3) under any conditions, including waking and after ketamine administration.
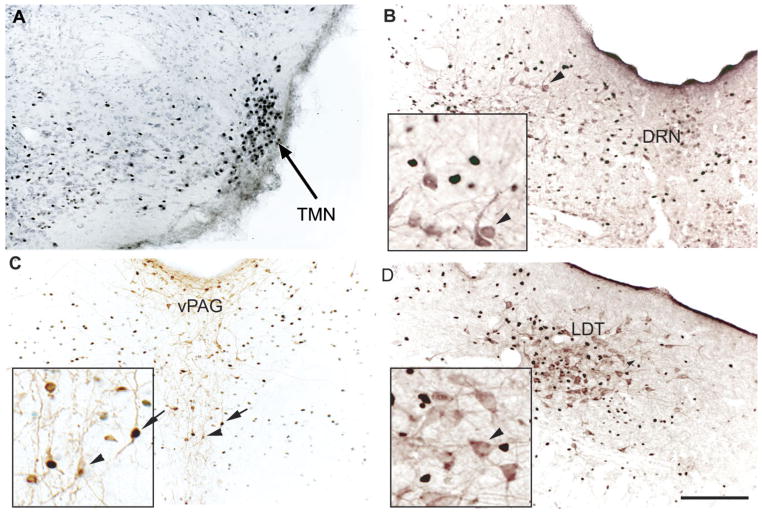
Fig. 2.
Ketamine-induced Fos immunoreactivity (black) in the cholinergic cells labeled by choline acetyltransferase immunoreactivity (purple) in the basal forebrain (A,B) and the orexinergic cells in the medial and lateral perifornical (PeriF) fields of the lateral hypothalamus (C,D). Arrows point to double-labeled cells; arrowheads point to single labeling (without Fos expression). Insets show these same cells at higher magnification. Scale bar = 200 μm.
TABLE 1.
Fos Expression (Mean/Section/Side) in Sleep-Wake and Supraspinal Analgesic Control Systems Following Administration of Anesthetics1
| Cg | VLPO | BF | Orx | TMN | vPAG | LC | A5 | A7 | vLS | LHb | EW | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ketamine | 72.3 ± 7.6** | 0** | 65.0 ± 5.2## | 68 ± 3.8**# | 59.4 ± 5.0# | 25.2 ± 1.7 | 79.8 ± 5.6**## | 7.2 ± 1.0**# | 8.5 ± 2.8*# | 23.5 ± 2.7 | 35.3 ± 4.1**## | 44.8 ± 3.3**## |
| Muscimol | 36.2 ± 3.7** | 8.3 ± 1.0*## | 0## | 0## | 0## | 0## | 77.5 ± 6.9**## | 6.9 ± 1.5*# | 6.8 ± 1.1*# | 50.6 ± 3.9*# | 70.5 ± 3.3**## | 48.6 ± 3.6**## |
| Ethanol | 31.4 ± 4.1** | 7.8 ± 0.9*## | 0## | 0## | 0## | 0## | 70.2 ± 6.1**## | 5.6 ± 1.8*# | 7.0 ± 1.3*# | 61.2 ± 4.3**## | 22.0 ± 1.7**## | 56.0 ± 3.9**## |
| Pentobarbital | 4.6 ± 2.6## | 11.2 ± 1.8*## | 0## | 0## | 0## | 0## | 23.7 ± 0.8**## | 0 | 0 | 32.7 ± 2.5*# | 67.2 ± 5.5**## | 22.0 ± 2.5*# |
| Chloral hydrate | 16.7 ± 4.3## | 12.0 ± 2.1*## | 0## | 0## | 0## | 0## | 63.2 ± 3.9**## | 5.5 ± 1.6*# | 6.5 ± 1.9*# | 36.0 ± 4.1*# | 8.7 ± 3.2*# | 23.2 ± 3.8*# |
| Isoflurane | 7.4 ± 2.5## | 10.6 ± 2.5*## | 0## | 0## | 0## | 0## | 26.0 ± 1.9**## | 0 | 0 | 0 | 0 | 21.2 ± 3.7*# |
| Sleeping control | 6.2 ± 2.9 | 32.2 ± 2.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18.0 ± 1.5 | 0 | 11.0 ± 2.1 |
| Waking control | 63.4 ± 5.1 | 0 | 17.4 ± 0.9 | 43.7 ± 4.3 | 40.0 ± 3.5 | 23.7 ± 1.9 | 0 | 0 | 0 | 21.4 ± 2.1 | 0 | 13.5 ± 1.9 |
Cg, cingulate cortex; VLPO, ventral preoptic nucleus; BF, basal forebrain cholinergic cells; Orx, perifornical orexin cells; TMN, tuberomammillary nucleus; vlPAG, ventrolateral periaquductal gray dopamine cells; LC, locus coeruleus; A5 and A7, noradrenergic cell groups; LHb, lateral habenular nucleus; LS, ventral lateral septum; EW, Edinger-Westphal nucleus.
P < 0.05 (vs. sleep control).
P < 0.01 (vs. sleep control).
P < 0.05 (vs. waking control).
P < 0.01 (vs. waking control).
Fig. 3.
Ketamine induced Fos immunorectivity in the tuberomam-millary nucleus (TMN; A; section counterstained with light Nissl stain), the dorsal raphe nucleus (DRN) nonserotoninergic cells (B; purple immunohistochemical staining for serotonin), the ventral periaqueductal gray matter (vPAG) dopaminergic cells (C; brown immunohistochemical staining for tyroxine hydroxylase), and the laterodorsal tegmental (LDT) noncholinergic cells (D; purple immunohistochemical staining for choline acetyltransferase). Arrows indicate double labeling; arrowheads indicate single labeling (without Fos expression). Scale bar = 200 μm.
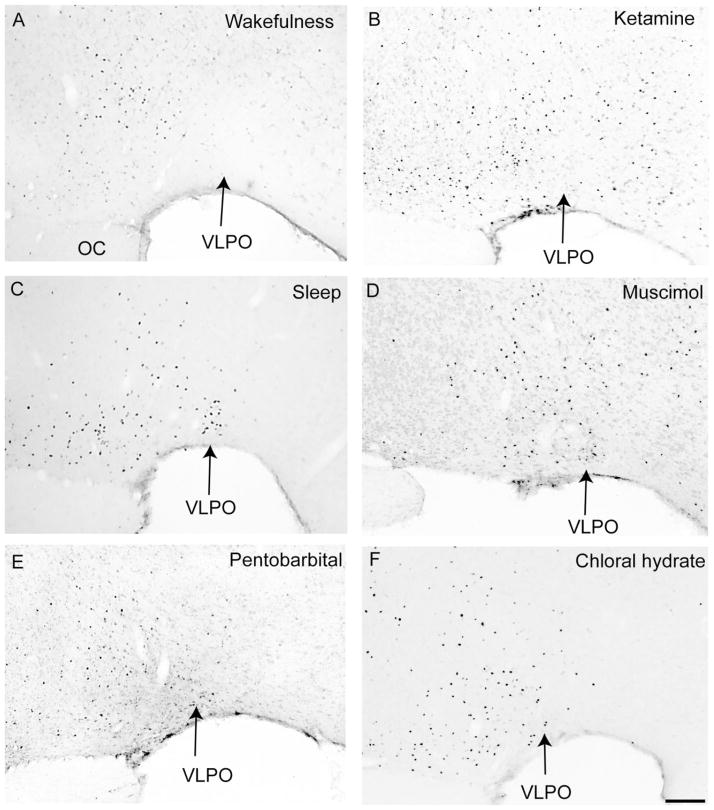
VLPO and median preoptic nucleus
Fos expression in the VLPO was nearly absent in waking control and ketamine-treated rats (Fig. 4, Table 1). Sleeping control animals and those treated with GABAergic drugs demonstrated a similar pattern of Fos-ir neurons in the VLPO complex (Fig. 4), although GABAergic agents produced about one-third the number of Fos-ir neurons in the VLPO cluster as were seen during natural sleep (Fig. 4, Table 1). Fos-ir neurons were seen in the median preoptic nucleus (MnPO) in sleeping control animals; however, this Fos pattern was absent in all other groups.
Fig. 4.
Effects of systemic anesthetics on Fos expression in the ventrolateral preoptic nucleus (VLPO). Wakefulness (A) and ketamine (B) completely suppress Fos expression in the VLPO, whereas spontaneous sleep (C), muscimol (D), pentobarbital (E), and chlorate hydrate (F) all are associated with Fos expression in the VLPO. OC, optic chiasm. Scale bar = 200 μm.
Tuberomammillary (TMN) and orexinergic cell groups
Many Fos-ir neurons were seen in the ventrolateral cluster of the histaminergic TMN both in waking animals and after ketamine at doses up to 150 mg/kg. Fos staining was also seen in orexin-ir cells in awake animals (approximately 45% of perifornical orexin-ir neurons) and after these doses of ketamine (approximately 60% of perifornical orexin-ir neurons; Fig. 2, Table 1). Fos expression was absent in orexin-ir neurons in sleeping controls or following treatment with GABAergic agents or 300 mg/kg ketamine.
Midbrain dopaminergic neurons
During wakefulness, Fos immunoreactivity was seen in about 40% of TH-ir cells in the ventral PAG (Fig. 3). Ketamine administration at doses up to 150 mg/kg produced a similar amount of Fos activation. Fewer than 2% of TH-ir cells were Fos-ir in the ventral tegmental area (VTA; A10) and in the substantia nigra (SN; A9) in ketamine-treated rats or in waking control animals. Fos staining was not seen in any of the midbrain dopaminergic cell groups following natural sleep, or with injections of ketamine at 300 mg/kg, or after any of the GABAergic agents, with the exception that ethanol induced Fos expression in the VTA.
Mesopontine raphe serotoninergic neurons
Although many more Fos-ir cells were seen in the dorsal and median raphe nuclei in the anesthetic-treated groups than in waking or sleeping controls, Fos was rarely coexpressed with serotonin immunoreactivity (Fig. 3) in any of the groups of animals examined.
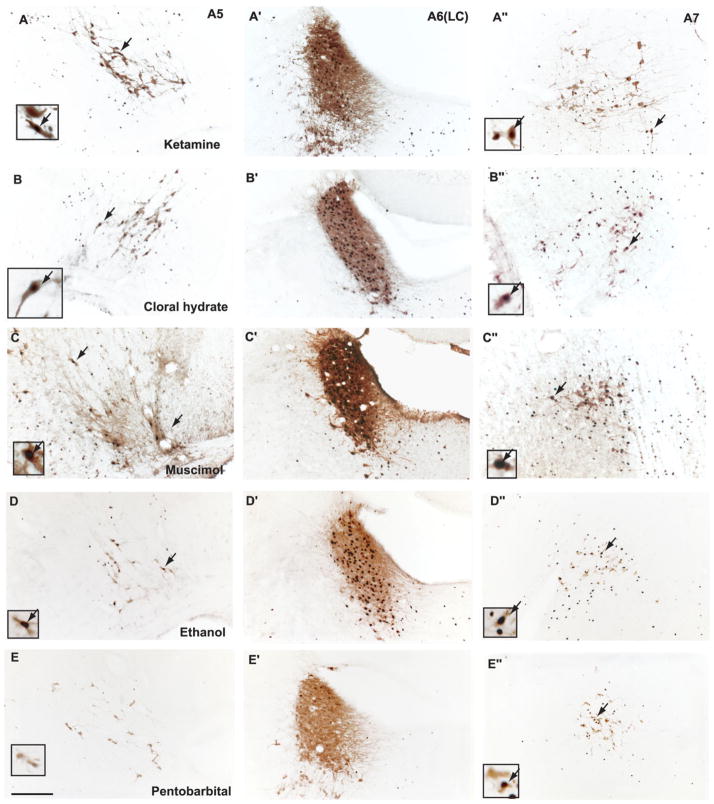
Pontine noradrenergic neurons
Neurons in the A5–7 groups rarely showed Fos immunoreactivity during either naturally occurring sleep or wakefulness. By contrast, all anesthetic agents (including 300 mg/kg of ketamine) induced Fos expression in the TH-ir noradrenergic A5, A6, and A7 groups (Fig. 5, Table 1). Among agents tested, ketamine and muscimol produced the greatest Fos expression, pentobarbital and isoflurane the least (Table 1).
Fig. 5.
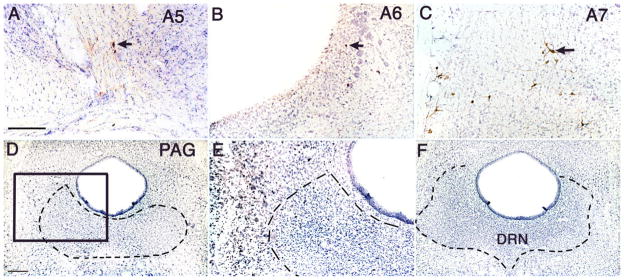
Fos induction in the pontine noradrenergic A5, A6, and A7 groups following administration of ketamine (A,A′,A″), chloral hydrate (B,B′,B″), muscimol (C,C′,C″), ethanol (D,D′,D″), or pentobarbital (E,E′,E″). Arrows indicate selected doubled-labeled neuron [tyrosine hydroxylase (TH) and Fos], which are shown at higher magnification in the insets. Note that all groups of anesthetics caused Fos expression in A5–7 noradrenergic neurons, although this was least prominent with pentobarbital [Fos was seen in the LC (A6) but rarely see in A5 and A7 groups]. Scale bar = 200 μm.
vlPAG and RVM
We examined Fos expression in these areas that are thought to modulate nociception. There were very few Fos-ir cells in the RVM (<5 Fos-ir cells/section/side) or in the vlPAG (<10 Fos-ir cells/section/side) either in waking or in sleeping states or following injections of the anesthetics.
Fos expression in other areas
Ethanol and chloral hydrate induced strong Fos immunoreactivity in the magnocellular part of the paraventricular nucleus of the hypothalamus and in the supraoptic nucleus, which may be due to a disturbance of osmolar homeostasis caused by drug-induced diuresis. However, few Fos-ir neurons were seen with any treatment in the parvicellular parts of the paraventricular hypothalamic nucleus, the central nucleus of the amygdala, or the bed nucleus of the stria terminalis, all of which typically show Fos expression under stressful conditions (Day et al., 2004; Ma and Morilak, 2004). After administration of each of the anesthetics with the exception of isoflurane, substantial Fos expression was also seen in the ventrolateral septum, the lateral habenular nucleus, and the Edinger-Westphal nucleus. Isoflurane induced Fos expression only in the Edinger-Westphal nucleus (Table 1).
Effects of lesions of the A5, A6, and A7 groups or the vlPAG on tail-flick latency
All of the anesthetics induced Fos expression in the A5–7 cell groups, which have been implicated in descending modulation of nociception. To test the role of activation of the A5–7 groups in producing analgesia, we made selective lesions of the A5–7 cell groups by injection of 6-hydroxydopamine into the fourth ventricle, as we have previously reported (Lu et al., 2006). Our previous studies had established that these lesions have little if any effect on wake-sleep states, so we examined thermal nociceptive responses after administration of anesthetics. In addition, we were interested in the sources of activation of the A5–7 groups during anesthesia. Given that the anesthetics enhance GABAA receptor-mediated inhibition or inhibit NMDA receptors, we hypothesized that activation of the A5–7 cell groups most likely was due to disinhibition (i.e., decreased firing of an inhibitory input). Previous studies indicate that the vlPAG innervates the A5–7 cell groups (Bajic et al., 2001), and we hypothesized that anesthetics may inhibit GABAergic cells in the vlPAG, thus releasing descending noradrenergic inhibition of nociception. We therefore also placed ibotenic acid lesions targeting the vlPAG and examined thermal nociceptive responses during light anesthesia. In these experiments, the doses of anesthetics were titrated so that the latencies of the tail flick in control animals (unlesioned) were no more than 15 seconds, so that changes in latency were measurable.
Intraventricular injection of 6-hydroxydopamine killed >90% of the noradrenergic cells in the A5, A6 and A7 groups (Fig. 6), without affecting other catecholaminergic cell groups in the midbrain (A8–10 groups), medulla (A1 and A2 groups), or hypothalamus (A11–14 groups). Ibotenic acid injected into the vlPAG produced extensive cell loss in this area but also partially damaged the dorsal raphe nucleus (Fig. 6).
Fig. 6.
Sections showing selective cell loss in TH-labeled noradrenergic A5–7 groups (A–C; cf Fig. 5) caused by fourth ventricular injection of 6-hydroxydopamine; and complete cell loss in the ventrolateral PAG (but also including the DRN) after ibotenic acid injections at that site (rostral level, D,E; caudal level, F). Arrows indicate the few surviving TH-immunoreactive cells in A–C. The boxed area in D is magnified in E, demonstrating only glial nuclei in the region of the lesion. Scale bar = 200 μm in A (applies to A–C); 200 μm in D (applies to D,F); 400 μm for E.
At baseline, animals with the vlPAG lesions showed tail-flick latencies slightly shorter than, although not statistically significantly different from, those of controls. Animals with A5–7 lesions had significantly shorter latencies than controls (Table 2). Ketamine (60 mg/kg), chloral hydrate (300 mg/kg), and ethanol (1.0 mg/kg) substantially prolonged the tail-flick latencies in the control group (Table 2). However, in animals with vlPAG or A5–7 lesions, after those same anesthetics, the latencies were similar to those of the unlesioned animals without anesthetics.
TABLE 2.
Effects of Anesthetics on Tail-Flick Latency (Sec) in Rats With Lesions of the A5–7 Groups or vlPAG
| Baseline | Ketamine (60 mg/kg) | Chloral hydrate (300 mg/kg) | Ethanol (1.0 g/kg) | |
|---|---|---|---|---|
| Control (n = 6) | 5.6 ± 0.3 | >15 | 13.8 ± 0.3 | 10.4 ± 0.6 |
| PAG lesion (n = 6) | 5.1 ± 0.3 | 6.2 ± 0.2** | 6.1 ± 0.2** | 7.0 ± 0.4** |
| A5–7 lesion (n = 8) | 3.7 ± 0.1* | 5.6 ± 0.3** | 6.0 ± 0.3** | 5.0 ± 0.6** |
P < 0.05 comparing PAG lesion or A5–7 lesion and control state.
P < 0.01 comparing PAG lesion or A5–7 lesion and control state.
DISCUSSION
Our results show that a wide range of GABAA agonist or potentiating agents, including pentobarbital, chlorate hydrate, muscimol, isoflurane, and ethanol, induce sedation that is characterized by increased delta power in the EEG as well as suppression of Fos expression in the arousal systems (with the exception of the LC) and a modest increase in Fos expression in the VLPO. In contrast, doses of the NMDA receptor antagonist ketamine at or just below those used in clinical rat anesthesia elicit arousal behaviors and increased EEG theta activity with increased Fos expression in the central arousal system, including the basal forebrain cholinergic, the TMN histaminergic, the hypothalamic orexinergic, the ventral PAG dopaminergic, and the LC noradrenergic neurons, with total suppression of Fos expression in the VLPO. Only at doses of ketamine 50–100% greater than those used in clinical rat anesthesia (150 mg/kg) is behavioral sedation and EEG delta activity produced, but only transiently, and there is still excess EEG theta activity and a pattern of increased Fos expression in the arousal systems and cerebral cortex 2 hours after administration. Only at 300 mg/kg of ketamine did we see a predominantly delta-frequency EEG and suppression of Fos expression in the cerebral cortex and arousal system at 2 hours after dosing, and even then there was excess Fos expression in the cingulate cortex and the intralaminar thalamus. Thus, at typical anesthetic dosages recommended for ketamine use in rats (75–100 mg/kg), it would appear that the predominant effect would be to cause arousal, not anesthesia. Perhaps for this reason, ketamine is often given in combination with other drugs such as xylazine, dexmeditomidine, and acepromazine, all of which as α2-adrenergic agonists are sedating but also are not anesthetic agents. Although these combinations are frequently used as rodent “anesthetics,” their effects on cortical Fos expression has not, to our knowledge, been tested. Given our finding that these doses of ketamine primarily induce cortical activation, not depression, this issue certainly deserves investigation.
In addition, all agents tested induced Fos in the A5, A6, and A7 noradrenergic neurons, and chemical ablation of the A5–7 groups significantly decreased tail-flick latency and diminished the analgesic effects of both classes of anesthetics on tail-flick latency. Lesions of the vlPAG, which provides GABAergic input to the A5–7 cell groups (Bajic et al., 2000, 2001), also attenuated anesthetic-induced analgesia.
We hypothesize that the GABAA-potentiating anesthetic agents, particularly at relatively low dosages, act at least in part via endogenous sleep-wake regulatory systems to produce their characteristic EEG and behavioral responses and at least in part through the A5–7 cell groups and the vlPAG to cause analgesia. In contrast, ketamine at commonly used dosages (under 100 mg/kg) appears to cause activation of endogenous arousal systems, although it activates the same endogenous antinociceptive system as the GABAergic agents.
Technical considerations
Although we classified GABAA-agonist or -potentiating agents as a single group, in fact this is a very diverse class of drugs that act on different subunits of the GABAA receptor (Rudolph and Möhler, 2004). The GABAA receptors are composed of heterogeneous combinations of five subunits drawn from at least 19 different possibilities, and different GABAA drugs bind preferentially to specific combinations of these subunits. In addition, GABAA receptors can be located presynaptically or postsynaptically or even extrajunctionally, so the actions of the GABAergic agents at specific sites can be quite difficult to predict. On the other hand, we found that the changes in c-Fos expression and putative mechanisms of analgesia were similar across this group.
Although only one NMDA receptor antagonist was tested, previous studies have found that low doses of other NMDA receptor antagonists, including phencyclidine, MK801, and nitrous oxide, also produce a fast EEG and are associated with increased Fos expression and glucose uptake in the cerebral cortex, thalamus, and hippocampus (Dragunow and Faull, 1990; Sharp, 1997; Duncan et al., 1999; Miyamoto et al., 2000). At doses of ketamine, phencyclidine, and MK801 used for rat anesthesia, there is an increase in EEG delta power, which may represent an inhibitory action on the CNS, but, even at these doses of ketamine, the endogenous arousal systems remained activated, and the cerebral cortex showed extensive Fos expression, similar to wakefulness.
We found it necessary to test the GABAergic drugs during the normal wake cycle (7–9 PM), because the effect of the drug was difficult to distinguish if the animals were already asleep. Conversely, we had to test ketamine during the normal sleep cycle (10 AM to noon), because it caused arousal and activation of brain systems that ordinarily express Fos during wakefulness. This approach produces a potential circadian confound in our data (i.e., would the two classes of drugs have similar effects if tested at the same time of day?). In our preliminary experiments, we did in fact give the ketamine at night (during the wake phase). This caused intoxicated (ataxia, headweaving) wakefulness, EEG changes, and a pattern of Fos expression that were essentially identical to those reported here during the day. However, when compared with nighttime awake controls, the effects of ketamine on wake time, EEG, and Fos expression in the cerebral cortex and arousal systems were not as salient. We therefore chose instead to compare each group of animals with controls who were killed at the same time of day, and to use the time of day for each group that brought out most clearly the features of its physiological and histological effects.
Anesthetic interactions with endogenous sleep-wake control systems
The patterns of Fos expression in the sleep-wake regulatory system induced by NMDA receptor-antagonist and by GABAA-potentiating anesthetics were consistent with the sleep-wake states and EEG that were observed. Ketamine at doses up to 60 mg/kg produced arousal behaviors and theta range EEG activity and induction of Fos immunoreactivity in the arousal system, particularly in the histaminergic TMN, the orexinergic hypothalamic, the dopaminergic ventral PAG, the noradrenergic LC, and the cholinergic basal forebrain neurons. Although ketamine did not induce Fos in serotoninergic cells in the DRN or in cholinergic cells in the LDT/PPT, it is possible that these cells were activated but unable to produce Fos, insofar as they also do not show Fos expression during natural wakefulness. The 150 mg/kg dose of ketamine caused brief sedation, but the animals soon awakened and showed hyperarousal, so that by 2 hours after drug administration the Fos expression in the cerberal cortex, the arousal system, and the VLPO was similar to lower dosages that caused only arousal. At the 300 mg/kg dose of ketamine, which caused 2 hours of sedation, Fos expression was suppressed in the cerebral cortex, the arousal system, the sleep-promoting VLPO cells, and the sleep-active median preoptic nucleus (Sherin et al., 1996; Gong et al., 2000; Lu et al., 2002; Suntsova et al., 2002). The “arousal effect” (intoxicated waking) of ketamine in the dosage range used for clinical rat anesthesia is consistent with a strong rebound of subsequent sleep with high delta power (Campbell and Feinberg, 1996), as if ketamine at typical “anesthesic” dosages represents prolonged wakefulness to the brain, rather than sleep. As with ketamine, nitrous oxide significantly induces Fos expression in cerebral cortex and thalamus (Kaiyala et., 2003).
Systemic administration of GABAergic agents produced a slow-wave EEG pattern and sedation, a pattern consisten with many GABAergic agents that have been studied previously (Corner et al., 1984; Lancel and Faulhaber, 1996; Lancel et al., 1997). In agreement with these behavioral changes, GABAergic agents suppressed Fos expression in arousal systems and induced Fos in the sleep-active VLPO (though to a lesser extent than that seen during spontaneous sleep). We had previously found that nearly all neurons in the VLPO that express Fos during sleep also express galanin mRNA (see Gaus et al., 2002). However, we did not examine whether the Fos-ir neurons in the VLPO during anesthesia contained galanin, raising the question of whether the Fos-ir neurons in the VLPO during anesthesia may represent a population different from that of VLPO neurons that are activated during sleep. We think this is unlikely, in that nearly all neurons in the VLPO cluster, where we examined Fos expression during anesthesia, contain galanin mRNA. However, the direct test of this hypothesis must await further studies.
Although our study cannot determine whether the sleep effects are due to direct action of GABAergic agents on the VLPO, Mendelson and colleagues (Mendelson, 1996; Tung et al., 2001) have reported that microinjection of pentobarbital, propofol, or ethanol into the medial preoptic area produced a slow EEG and sedation, suggesting that these GABA agonists may activate the VLPO and/or MnPO. Nelson and colleagues (2002, 2003) demonstrated that systemic administration of sedative doses of dexmedetomidine (an α2-adrenoceptor agonist), pentobarbital, or muscimol all induced Fos in the VLPO. Taken together with the current data, these observations suggest that activation of the VLPO is a common property of both natural sleep and many forms of drug-induced sedation. We hypothesize that GABAA-mediated activation of the VLPO results from direct presynaptic inhibition of inhibitory inputs to the VLPO, although GABAA drugs may also produce VLPO activation by inhibiting its targets in the arousal system. This hypothesis will require further study by examining the effects of VLPO lesions on anesthetic responses.
The role of the LC in causing arousal has been controversial (for review see Aston-Jones, 2005; Lu et al., 2006). Although LC neurons are activated by many arousing stimuli, lesions of the LC do not alter amounts of baseline wakefulness or sleep, nor do they alter the sedating effects of α2-adrenergic agonists such as clonidine (Nassif et al., 1983; Nassif-Caudarella et al., 1986). Furthermore, the noradrenergic input to the VLPO, which inhibits it via α2 receptors (Gallopin et al., 2000), largely originates from the ventrolateral medulla (A1 neurons), not from the LC (Chou et al., 2002). Thus the surprising activation of the LC by all classes of anesthetics may reflect a role not in arousal but rather in descending antinociception.
Other components of the ascending arousal system, by contrast, show suppression of Fos expression during anesthetic-induced sedation. Inhibition of the TMN may be particularly important in producing sedation, insofar as injection of GABAA agonists into the TMN region produces a state of sedation (Lin et al., 1989) and injection of a GABAA antagonist, gabazine, into the TMN region blocks EEG synchronization and sedation induced by subsequent administration of systemic GABAergic agents such as muscimol or pentobarbital (Nelson et al., 2002).
A major difference between anesthetic sedation and sleep is that sleep is a regulated state and is reversible with stimulation. In addition, sleep is composed of NREM sleep and REM sleep, and there are regular, physiologically mediated transitions between the two states during natural sleep. GABAergic agents, such as barbiturates, ethanol, or chloral hydrate, are used at low dosages to promote sleep. These drugs, but not ketamine, at low doses induce Fos expression in the VLPO, suggesting that the activation of the VLPO may be a component of their mechanism of action in sedation. On the other hand, even low-dose GABAA drugs suppress REM sleep, indicating that the sleep-like state induced by enhanced GABAergic transmission is not equivalent to natural sleep. At higher dosages, animals can no longer be aroused, which may be due to direct GABA-mediated inhibition of the neocortex. Similarly, ketamine at higher doses (300 mg/kg) may also directly inhibit neocortical activity significantly. Thus at higher doses of anesthetics, the deeply sedated (unarousable) state is likely to be secondary to direct inhibition of the neocortex and other components of sensory and motor systems, rather than effects on the endogenous sleep-wake regulatory system. However, the same drugs at lower dosages, within the clinical therapeutic range, may work at least in part by engaging endogenous brain systems for regulating sleep and wakefulness.
Analgesic mechanism of general anesthetics
Similarly, although high doses of general anesthetics as well as narcotics inhibit neurons widely in the CNS, including direct inhibition of dorsal horn neurons in the spinal cord, at lower dosages supraspinal antinociceptive descending projections may make a substantial contribution to analgesia. Sawamura et al. (2000) showed that nitrous oxide (an NMDA antagonist) failed to induce antinociceptive effects after chemical lesions of the A5, A6, and A7 groups in rats or in α2B-adrenoceptor knockout mice or after lesions in the PAG (Zuniga et al., 1987). Several lines of evidence suggest that ketamine also activates the supraspinal antinociceptive descending pathway. Tomemori et al. (1981) showed that the analgesic effects of ketamine in the tail-flick test disappear following C1 cervical transection in rats, and ketamine analgesia is absent following lesions of the spinal dorsolateral funiculus (Crisp et al., 1991), which contains the descending noradrenergic axons. Kawamata et al. (2000) demonstrated that intrathecal yohimbine (an α2-adrenoreceptor antagonist) attenuates the analgesic effects of ketamine in the paw withdrawal test. A recent study showed that systemic administration of clonidine (an α2-adrenoreceptor agonist) produced analgesia but did not induce Fos in the A6 and A7 groups, and thus presumably caused direct α2-mediated antinociception at the dorsal horn (Fukuda et al., 2006). Our findings provide a direct test demonstrating that analgesic effects of ketamine depend on the nor-adrenergic A5–7 cell groups.
Although it is believed that GABAergic (GABAA and GABAB receptor) agonists activate supraspinal mechanisms to induce analgesia (for review see Sawynok, 1987), the supraspinal sites have not previously been identified. Kingery and colleagues (2002) showed that systemic administration of yohimbine (an α2-antagonist) inhibited the antinociceptive effect of isoflurane, indicating that spinal α2 adrenoceptors mediate an isoflurane-evoked descending spinal antinociceptive effect. Here, we show that isoflurane and other GABAergic agents induce Fos in A5–7 neurons and that discrete lesions of these cell groups block the analgesic effects of ethanol and chloral hydrate, strongly suggesting that GABAergic agents exert their antinociceptive effects at least in part by acting on the same supraspinal pathways as NMDA receptor antagonists.
Because GABAA agonists are inhibitory and NMDA receptor antagonists prevent excitation, we hypothesized that these agents would activate A5–7 neurons by disinhibition. A likely source for inhibition of A5–7 groups is the vlPAG. There are relatively few sources of GABAergic innervation of the LC, including the VLPO, the lateral hypothalamic cells containing melanin-concentrating hormone, and the vlPAG (Verret et al., 2003). Among these, the vlPAG has consistently been implicated in supraspinal control of analgesia. Focal injections of many analgesic agents, including opiates (Yeung et al., 1977), nociceptin (Bytner et al., 2001), cannabinoids (Lichtman et al., 1996), GABAA receptor agonists (Levy and Proudfit, 1979; Foong and Satoh, 1984), and NMDA receptor antagonists (Vaccarino et al., 1997), into the ventrolateral PAG produce antinociception. The ventrolateral PAG contains mu- and delta-opioid and GABAA receptors (Williams and Beitz, 1990; Kalyuzhny and Wessendorf, 1998; Commons et al., 2001; Wang and Wessendorf, 2002), and mu receptors and NMDA receptors are expressed by the same neurons (Commons et al., 1999). In vitro, opiates and canabinoids have been shown to activate mu receptors on the presynaptic GABAergic terminals in the vlPAG (Vaughan et al., 1997a,b). Moreover, Budai et al. (1998) showed that the PAG-mediated antinociceptive effects on the sacral dorsal horn involve activation of α2-adrenergic receptors. Finally, lesions of the vlPAG attenuate antinociceptive effects of nitrous oxide (Zuniga et al., 1987). In our experiments, chronic lesions of the vlPAG did not affect the tail flick latency. However, it is likely that the noradrenergic dis-inhibition experienced with acute loss of vlPAG action habituates with time, as the nociceptive system resets itself. On the other hand, after vlPAG chronic lesions, our animals lost most of their analgesic response to both GABAA drugs and ketamine. These results suggest that both GABAA drugs and NMDA receptor antagonist anesthetics target the ventrolateral PAG, disinhibiting the A5–7 groups and inducing analgesia, at least at dosages commonly used for sedation and light anesthesia (where the animals are still sufficiently rousable to produce a tail-flick response). Residual analgesia seen at these dosages after vlPAG or A5–7 lesions could represent other potential antinociceptive pathways that may be activated, e.g., brainstem or spinal projections from the Edinger-Westphal nucleus, which projects to the dorsal horn and was also activated by all classes of anesthetics. On the other hand, the mechanisms of analgesia, like those of sedation, are also probably dose dependent. Thus, at higher dosages, it is likely that the anesthetics act at both spinal and supraspinal levels.
The dose at which the mechanism of GABAA anesthetics switches from activation of sleep and analgesic mechanisms to direct global inhibition of the targets of the arousal system (cortex and thalamus) and nociceptive system (dorsal horn and its forebrain targets) may depend on the properties of individual anesthetics. However, our data suggest that, at low dosages, the activation of endogenous sleep and analgesic systems contributes substantially to the sedative and antinociceptive effects of GABAA anesthetics.
CONCLUSION
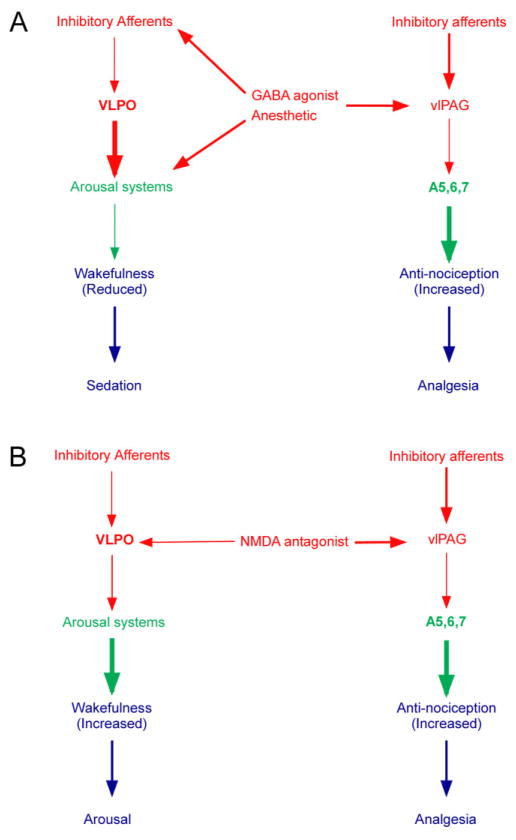
Our data show that a wide range of general anesthetics at the lower end of their effective dosage ranges engage endogenous brain wake-sleep and analgesic pathways and that, particularly at relatively low dosages, these mechanisms may play a key role in producing the anesthetic state. Specifically, we propose that GABAergic anesthetics appear to cause sedation by activating the VLPO and inhibiting the TMN, and analgesia is caused by inhibition of the vlPAG and disinhibition of the A5–7 noradrenergic groups, which produces antinociception (Fig. 7). NMDA receptor antagonists may cause analgesia by activating the same descending antinociceptive pathways, but their effects on arousal systems are profoundly different.
Fig. 7.
Summary diagrams of proposed anesthesia models for GABAA agonist anesthetics (A) and NMDA antagonist ketamine (B). Red arrows show putative inhibitory pathways; green arrows show putative excitatory pathways. The width of the red and green arrows illustrates the change in firing rate caused by the drugs. Thus a GABAA agonist will reduce the activity of inhibitory afferents to the VLPO, increasing its firing rate, which in turn uses GABAA receptors to inhibit the TMN (which is also directly inhibited by the drug); this reduces wakefulness. The GABAA agonist drug at the same time inhibits the GABAergic vlPAG neurons, which have reduced firing, thus disinhibiting the A5–7 cell groups, which fire faster and have an antinociceptive effect at the level of the spinal dorsal horn. Ketamine at dosages usually used for rat anesthesia directly blocks activation of the VLPO, thus reducing its activity and releasing the TMN to increase wakefulness. However, ketamine blocks activity in the vlPAG, thus disinhibiting the A5–7 noradrenergic neurons to produce an antinociceptive effect at the level of the spinal dorsal horn.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: NS 33987; Grant number: NS051609; Grant number: HL60292; Grant sponsor: Medical Research Council; Grant number: G9817980; Grant sponsor: Chelsea and Westminster Health Care NHS Trust; Grant number: 010ANA007; Grant number: 010ANA008.
We thank Quan Hue Ha and Man Xu for excellent technical help and Drs. David Hopkins and Marshall Devor for insightful comments on earlier versions of the manuscript.
LITERATURE CITED
- Abi-Saab WM, D’Souza DC, Moghaddam B, Krystal JH. The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry Suppl. 1998;2:104–109. doi: 10.1055/s-2007-979354. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G. Brain structures and receptors involved in alertness. Sleep Med Suppl. 2005;1:S3–7. doi: 10.1016/s1389-9457(05)80002-4. [DOI] [PubMed] [Google Scholar]
- Bajic D, Proudfit HK, Van Bockstaele EJ. Periaqueductal gray neurons monosynaptically innervate extranuclear noradrenergic dendrites in the rat pericoerulear region. J Comp Neurol. 2000;427:649–662. doi: 10.1002/1096-9861(20001127)427:4<649::aid-cne11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Bajic D, Van Bockstaele EJ, Proudfit HK. Ultrastructural analysis of ventrolateral periaqueductal gray projections to the A7 catecholamine cell group. Neuroscience. 2001;104:181–197. doi: 10.1016/s0306-4522(01)00052-5. [DOI] [PubMed] [Google Scholar]
- Barbaro NM, Hammond DL, Fields HL. Effects of intrathecally administered methysergide and yohimbine on microstimulation-produced antinociception in the rat. Brain Res. 1985;343:223–229. doi: 10.1016/0006-8993(85)90738-3. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Proudfit HK. Hypoalgesia induced by the local injection of carbachol into the nucleus raphe magnus. Brain Res. 1984;291:337–342. doi: 10.1016/0006-8993(84)91266-6. [DOI] [PubMed] [Google Scholar]
- Budai D, Harasawa I, Fields HL. Midbrain periaqueductal gray (PAG) inhibits nociceptive inputs to sacral dorsal horn nociceptive neurons through alpha2-adrenergic receptors. J Neurophysiol. 1998;80:2244–2254. doi: 10.1152/jn.1998.80.5.2244. [DOI] [PubMed] [Google Scholar]
- Bytner B, Huang YH, Yu LC, Lundeberg T, Nylander I, Rosen A. Nociceptin/orphanin FQ into the rat periaqueductal gray decreases the withdrawal latency to heat and loading, an effect reversed by (Nphe(1))nociceptin(1–13)NH(2) Brain Res. 2001;922:118–124. doi: 10.1016/s0006-8993(01)03161-4. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. NREM delta stimulation following MK-801 is a response of sleep systems. J Neurophysiol. 1996;76:3714–3720. doi: 10.1152/jn.1996.76.6.3714. [DOI] [PubMed] [Google Scholar]
- Carruba MO, Bondiolotti G, Picotti GB, Catteruccia N, Da Prada M. Effects of diethyl ether, halothane, ketamine and urethane on sympathetic activity in the rat. Eur J Pharmacol. 1987;134:15–24. doi: 10.1016/0014-2999(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22:977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. Projections of neurons in the ventromedial medulla to pontine catecholamine cell groups involved in the modulation of nociception. Brain Res. 1991;540:105–115. doi: 10.1016/0006-8993(91)90496-i. [DOI] [PubMed] [Google Scholar]
- Commons KG, Van Bockstaele EJ, Pfaff DW. Frequent colocalization of mu opioid and NMDA-type glutamate receptors at postsynaptic sites in periaqueductal gray neurons. J Comp Neurol. 1999;408:549–559. [PubMed] [Google Scholar]
- Commons KG, Beck SG, Rudoy C, Van Bockstaele EJ. Anatomical evidence for presynaptic modulation by the delta opioid receptor in the ventrolateral periaqueductal gray of the rat. J Comp Neurol. 2001;430:200–208. [PubMed] [Google Scholar]
- Corner M, Scholte R, Korf J, Mirmiran M. Electrocortical activity patterns during chloral hydrate induced sleep in developing rats. Brain Res Bull. 1984;12:77–81. doi: 10.1016/0361-9230(84)90218-1. [DOI] [PubMed] [Google Scholar]
- Crisp T, Perrotti JM, Smith DL, Stafinsky JL, Smith DJ. The local monoaminergic dependency of spinal ketamine. Eur J Pharmacol. 1991;194:167–172. doi: 10.1016/0014-2999(91)90101-u. [DOI] [PubMed] [Google Scholar]
- Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull RL. MK-801 induces c-fos protein in thalamic and neocortical neurons of rat brain. Neurosci Lett. 1990;111:39–45. doi: 10.1016/0304-3940(90)90341-6. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Miyamoto S, Leipzig JN, Lieberman JA. Comparison of brain metabolic activity patterns induced by ketamine, MK-801 and amphetamine in rats: support for NMDA receptor involvement in responses to subanesthetic dose of ketamine. Brain Res. 1999;843:171–183. doi: 10.1016/s0006-8993(99)01776-x. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Miyamoto S, Leipzig JN, Lieberman JA. Comparison of the effects of clozapine, risperidone, and olanzapine on ketamine-induced alterations in regional brain metabolism. J Pharmacol Exp Ther. 2000;293:8–14. [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Vanegas H, Hentall ID, Zorman G. Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature. 1983;306:684–686. doi: 10.1038/306684a0. [DOI] [PubMed] [Google Scholar]
- Foong FW, Satoh M. The periaqueductal gray is the site of the antinociceptive action of carbamazepine as related to bradykinin-induced trigeminal pain. Br J Pharmacol. 1984;83:493–497. doi: 10.1111/j.1476-5381.1984.tb16512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Furukawa H, Hisano S, Toyooka H. Systemic clonidine activates neurons of the dorsal horn, but not the locus coeruleus (A6) or the A7 area, after a formalin test: the importance of the dorsal horn in the antinociceptive effects of clonidine. J Anesth. 2006;20:279–283. doi: 10.1007/s00540-006-0426-5. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, Audinat E, Mühlethaler M, Serafin M. Identification of sleep-promoting neurons in vitro. Nature. 2000;40:992–995. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115:285–294. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Gong H, Szymusiak R, King J, Steininger T, McGinty D. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. Am J Physiol Regul Integr Comp Phys. 2000;279:R2079–R2088. doi: 10.1152/ajpregu.2000.279.6.R2079. [DOI] [PubMed] [Google Scholar]
- Jahng JW, Zhang TY, Lee S, Kim DG. Effects of dextromethorphan on nocturnal behavior and brain c-Fos expression in adolescent rats. Eur J Pharmacol. 2001;431:47–52. doi: 10.1016/s0014-2999(01)01409-1. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Examination of spinal monoamine receptors through which brainstem opiate-sensitive systems act in the rat. Brain Res. 1986;363:114–27. doi: 10.1016/0006-8993(86)90663-3. [DOI] [PubMed] [Google Scholar]
- Kaiyala KJ, Thiele TE, Watson CH, Ramsay DS. Nitrous oxide-induced c-Fos expression in the rat brain. Brain Res. 2003;967:73–80. doi: 10.1016/s0006-8993(02)04219-1. [DOI] [PubMed] [Google Scholar]
- Kalyuzhny AE, Wessendorf MW. Relationship of mu- and delta-opioid receptors to GABAergic neurons in the central nervous system, including antinociceptive brainstem circuits. J Comp Neurol. 1998;392:528–547. [PubMed] [Google Scholar]
- Kawamata T, Omote K, Sonoda H, Kawamata M, Namiki A. Analgesic mechanisms of ketamine in the presence and absence of peripheral inflammation. Anesthesiology. 2000;93:520–528. doi: 10.1097/00000542-200008000-00032. [DOI] [PubMed] [Google Scholar]
- Kingery WS, Sawamura S, Agashe GS, Davies MF, Clark JD, Zimmer A. Enkephalin release and opioid receptor activation does not mediate the antinociceptive or sedative/hypnotic effects of nitrous oxide. Eur J Pharmacol. 2001;427:27–35. doi: 10.1016/s0014-2999(01)01193-1. [DOI] [PubMed] [Google Scholar]
- Kingery WS, Agashe GS, Guo TZ, Sawamura S, Davies MF, Clark JD, Kobilka BK, Maze M. Isoflurane and nociception: spinal alpha2A adrenoceptors mediate antinociception while supraspinal alpha1 adrenoceptors mediate pronociception. Anesthesiology. 2002;96:367–374. doi: 10.1097/00000542-200202000-00023. [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J. The GABAA agonist THIP (gaboxadol) increases non-REM sleep and enhances delta activity in the rat. Neuroreport. 1996;7:2241–2245. doi: 10.1097/00001756-199609020-00036. [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J, Schiffelholz T, Mathias S, Deisz RA. Muscimol and midazolam do not potentiate each other’s effects on sleep EEG in the rat. J Neurophysiol. 1997;77:1624–1629. doi: 10.1152/jn.1997.77.3.1624. [DOI] [PubMed] [Google Scholar]
- Levy RA, Proudfit HK. Analgesia produced by microinjection of baclofen and morphine at brain stem sites. Eur J Pharmacol. 1979;57:43–55. doi: 10.1016/0014-2999(79)90102-x. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- Lin JS, Sakai K, Vanni-Mercier G, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–240. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Lesions of ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhang YH, Chou T, Gaus SG, Shiromani P, Saper CB. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci. 2001;21:4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Bjorkum AA, Xu M, Gaus SG, Shiromani P, Saper CB. Selective activation of the extended ventrolateral preoptic nucleus during REM sleep. J Nueorsci. 2002;22:4568–4576. doi: 10.1523/JNEUROSCI.22-11-04568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Jhou TC, Saper CB. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci. 2006;26:193–202. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Induction of FOS expression by acute immobilization stress is reduced in locus coeruleus and medial amygdala of Wistar-Kyoto rats compared with Sprague-Dawley rats. Neuroscience. 2004;124:963–972. doi: 10.1016/j.neuroscience.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Marquis KL, Gussio R, Webb MG, Moreton JE. Cortical EEG changes during the self-administration of phencyclinoids. Neuropharmacology. 1989;28:1193–1198. doi: 10.1016/0028-3908(89)90210-4. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Gupta NK, Loo CM, Rohde DS, Basbaum AI. Differential effects of neurotoxic destruction of descending noradrenergic pathways on acute and persistent nociceptive processing. Pain. 1999;80:57–65. doi: 10.1016/s0304-3959(98)00194-8. [DOI] [PubMed] [Google Scholar]
- Mattia A, Moreton JE. Electroencephalographic (EEG), EEG power spectra, and behavioral correlates in rats given phencyclidine. Neuropharmacology. 1986;25:763–769. doi: 10.1016/0028-3908(86)90093-6. [DOI] [PubMed] [Google Scholar]
- Mendelson WB. Sleep induction by microinjection of pentobarbital into the medial preoptic area in rats. Life Sci. 1996;59:1821–1828. doi: 10.1016/s0024-3205(96)00400-6. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Lowe D, Fields HL. The contribution of the rostral ventromedial medulla to the antinociceptive effects of systemic morphine in restrained and unrestrained rats. Neuroscience. 1998;87:123–133. doi: 10.1016/s0306-4522(98)00119-5. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Leipzig JN, Lieberman JA, Duncan GE. Effects of ketamine, MK-801, and amphetamine on regional brain 2-deoxyglucose uptake in freely moving mice. Neuropsychopharmacology. 2000;22:400–412. doi: 10.1016/S0893-133X(99)00127-X. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Recent basic findings in support of excitatory amino acid hypotheses of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:859–870. doi: 10.1016/0278-5846(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Nassif S, Kempf E, Cardo B, Velley L. Neurochemical lesion of the locus coeruleus of the rat does not suppress the sedative effect of clonidine. Eur J Pharmacol. 1983;91:69–76. doi: 10.1016/0014-2999(83)90363-1. [DOI] [PubMed] [Google Scholar]
- Nassif-Caudarella S, Kempf E, Velley L. Clonidine-induced sedation is not modified by single or combined neurochemical lesions of the locus coeruleus, the median and dorsal raphe nuclei. Pharmacol Biochem Behav. 1986;25:1211–1216. doi: 10.1016/0091-3057(86)90114-0. [DOI] [PubMed] [Google Scholar]
- Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABAA receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Sagratella S, Pezzola A, Popoli P, Scotti de Carolis AS. Different capability of N-methyl-D-aspartate antagonists to elicit EEG and behavioural phencyclidine-like effects in rats. Psychopharmacology. 1992;109:277–282. doi: 10.1007/BF02245874. [DOI] [PubMed] [Google Scholar]
- Sawamura S, Kingery WS, Davies MF, Agashe GS, Clark JD, Kobilka BK, Hashimoto T, Maze M. Antinociceptive action of nitrous oxide is mediated by stimulation of noradrenergic neurons in the brainstem and activation of α2B adrenoceptors. J Neurosci. 2000;20:9242–9251. doi: 10.1523/JNEUROSCI.20-24-09242.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawynok J. GABAergic mechanisms of analgesia: an update. Pharmacol Biochem Behav. 1987;26:463–474. doi: 10.1016/0091-3057(87)90148-1. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Reid A. Effect of 6-hydroxydopamine-induced lesions to ascending and descending noradrenergic pathways on morphine analgesia. Brain Res. 1987;419:156–165. doi: 10.1016/0006-8993(87)90579-8. [DOI] [PubMed] [Google Scholar]
- Sharp JW. Phencyclidine (PCP) acts at sigma sites to induce c-fos gene expression. Brain Res. 1997;758:51–58. doi: 10.1016/s0006-8993(97)00025-5. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Sloan TB. Anesthetic effects on electrophysiologic recordings. J Clin Neurophysiol. 1998;15:217–226. doi: 10.1097/00004691-199805000-00005. [DOI] [PubMed] [Google Scholar]
- Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol. 2002;543:665–677. doi: 10.1113/jphysiol.2002.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomemori N, Komatsu T, Shingu K, Urabe N, Seo N, Mori K. Activation of the supraspinal pain inhibition system by ketamine hydrochloride. Acta Anaesthesiol Scand. 1981;25:355–359. doi: 10.1111/j.1399-6576.1981.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Vaccarino AL, Clemmons HR, Mader GJ, Jr, Magnusson JE. A role of periaqueductal grey NMDA receptors in mediating formalin-induced pain in the rat. Neurosci Lett. 1997;236:117–119. doi: 10.1016/s0304-3940(97)00770-2. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Christie MJ. Actions of the ORL1 receptor ligand nociceptin on membrane properties of rat periaqueductal gray neurons in vitro. J Neurosci. 1997a;17:996–1003. doi: 10.1523/JNEUROSCI.17-03-00996.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997b;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- Verret L, Goutagny R, Fort P, Cagnon L, Salvert D, Léger L, Boissard R, Salin P, Peyron C, Luppi PH. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wessendorf MW. Mu- and delta-opioid receptor mRNAs are expressed in periaqueductal gray neurons projecting to the rostral ventromedial medulla. Neuroscience. 2002;109:619–634. doi: 10.1016/s0306-4522(01)00328-1. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Craig AD. Association of spinal lamina I projections with brainstem catecholamine neurons in the monkey. Exp Brain Res. 1996;110:151–162. doi: 10.1007/BF00228547. [DOI] [PubMed] [Google Scholar]
- Williams FG, Beitz AJ. Ultrastructural morphometric analysis of GABA-immunoreactive terminals in the ventrocaudal periaqueductal grey: analysis of the relationship of GABA terminals and the GABAA receptor to periaqueductal grey-raphe magnus projection neurons. J Neurocytol. 1990;19:686–696. doi: 10.1007/BF01188037. [DOI] [PubMed] [Google Scholar]
- Yamamura T, Fukuda M, Takeya H, Goto Y, Furukawa K. Fast oscillatory EEG activity induced by analgesic concentrations of nitrous oxide in man. Anesth Analg. 1981;60:283–288. [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Projections of substance P-immunoreactive neurons located in the ventromedial medulla to the A7 noradrenergic nucleus of the rat demonstrated using retrograde tracing combined with immunocytochemistry. Brain Res. 1990;532:329–332. doi: 10.1016/0006-8993(90)91777-e. [DOI] [PubMed] [Google Scholar]
- Yeung JC, Yaksh TL, Rudy TA. Concurrent mapping of brain sites for sensitivity to the direct application of morphine and focal electrical stimulation in the production of antinociception in the rat. Pain. 1977;4:23–40. doi: 10.1016/0304-3959(77)90084-7. [DOI] [PubMed] [Google Scholar]
- Zuniga J, Joseph S, Knigge K. Nitrous oxide analgesia: partial antagonism by naloxone and total reversal after periaqueductal gray lesions in the rat. Eur J Pharmacol. 1987;142:51–60. doi: 10.1016/0014-2999(87)90653-4. [DOI] [PubMed] [Google Scholar]