Abstract
Background
The extent, rate, and source of endothelialization following coil embolization of saccular aneurysms remains poorly understood. We performed a whole tissue mount, dual immunohistochemical analysis of experimental aneurysms in order to characterize the state of endothelialization over time following platinum coil embolization.
Method and material
Elastase-induced rabbit aneurysms were created and treated with bare platinum coils. Samples were harvested at 4 and 8 weeks (n=6 for each). En face whole tissue mounts staining using antibodies for CD31 and α-smooth muscle actin to identify endothelial cells and smooth muscle cells, respectively. Sytox green stain was employed to demonstrate nuclear morphology for identification of inflammatory cells. Extent of endothelialization was measured in relation to the aneurysm neck-parent artery interface.
Results
At 4 weeks following coil embolization, very localized membranous tissue and neoendothelial cells were detected on the coil loops immediately adjacent to the parent artery-neck interface, but the remainder of the coil loops remained devoid of endothelial cells. At 8 weeks neo-endothelial cells were more confluent over the coils than at 4 weeks, and extended up to 900μm from the parent artery-neck interface. However, the surfaces of the coils farther than this region harbored no endothelial cells. Scattered inflammatory cells including neutrophils, and monocytes were observed on the coil surface at the neck center area, where the coil surface was bare grossly at the 4 and 8 weeks follow up.
Conclusion
Platinum coil embolization supports gradual but limited endothelialization, where endothelial cells migrate directly from the adjacent parent artery.
Keywords: Aneurysm, embolization, coils, endothelialization and healing
INTRODUCTION
The precise cellular response to platinum microcoil embolization of saccular aneurysms remains unclear. Rapid aneurysm thrombosis likely relates to coil-induced stasis, but subacute and chronic healing patterns have been poorly characterized to date[1–3]. The final common pathway of full endothelialization represents an ideal outcome to allow permanent closure of the treated aneurysm[4–6]. Human histologic samples have shown, even long after embolization, that varying degrees of endothelialization are present[5 7]. Animal studies have shown variation not only in degree of endothelialization, but also disparate conclusions regarding the exact source of endothelial cells over the neck[8–10]. Specifically, the relative importance of circulating versus local cells as precursors to endothelial cells over the treated aneurysm neck has yet to be determined; such determination would facilitate rational design of future devices. In the current study, we used whole tissue mounting staining for endothelial cells on coiled aneurysms to assess the extent and pattern of endothelial cells at the neck following the platinum embolization.
MATERIALS AND METHODS
In Vivo Experiments
The Institutional Animal Care and Use Committee approved all procedures prior to study initiation. Twelve elastase-induced saccular aneurysms were created in female New Zealand white rabbits (2.5–4 kg)[11]. Aneurysms were permitted to mature for at least 21 days after creation. Animals were anesthetized and a cut down was performed to gain access to the right common femoral artery. A microcatheter was inserted coaxially through the guiding catheter into the aortic arch. Heparin (100 U/kg) was administered intravenously. The size of the aneurysm cavity was assessed by direct comparison with radiopaque sizing devices during digital subtraction angiography (DSA). The volumetric occlusion rate was calculated as follows: Volume=π (dome)2 * (length)/6. Considering the aneurysm as a two-dimensional ellipsoid, we assumed the value of the aneurysm dome in this formula was equal to that of aneurysm width. Volumetric occlusion was calculated in real time, during aneurysm embolization, using the AngioCalc tool (http://www.angiocalc.com/index.aspx). Appropriate sized platinum coils (Heraeus Precious Metals GmbH and Co, Germany; diameter 0.0008 inches) were placed into the aneurysm, as in typical practice. Before and after each successive coil was placed, volumetric occlusion was calculated. Aneurysm cavities were packed as tightly as possible (>30% packing density) in all cases. Final DSAs were performed immediately following embolization.
Animals were given Buprenorphine SR, SQ once following coil embolization and were not medicated with anti-platelet agents. Four weeks (n=6) and 8weeks (n=6) after embolization, the coiled aneurysms were harvested for further study.
Tissue harvest and processing
After follow-up DSA was performed, the animals were then euthanized by using a lethal injection of pentobarbital. Median sternotomy and pericardiotomy were performed. Access to the left ventricle was obtained by direct puncture with a 20-gauge catheter. A small cut was made at the right atrial appendage. With pressure pump, heparinized saline (100U/ml) was continuously infused into the catheter, until the effluent from the right appendage was light pink. Then the animals were perfusion-fixed through the 20G catheter with 10% buffered formalin for 10 min followed by flushing with heparinized saline for 5 minutes. The coiled aneurysm was then harvested and immersed into tris-buffered saline (TBS). Under a dissection microscope (Leica MZ 125), the parent artery was cut longitudinally to expose the neck orifice for gross inspection to evaluate the tissue growth at the neck; the sample was then photographed using the MicroPublisher 5.0 RTV camera attached to the dissection microscope. After photography, the sample was fixed 10% formalin for 2 hours for further whole tissue mount staining. The adjacent left common carotid artery (LCCA) was harvested for positive and negative control.
Whole-mount en face immunostaining
The freshly harvested coiled aneurysms were fixed in 10% neutral buffered formalin for 2 hours at room temperature (RT); they were then washed with TBS for 15minutes with 4 changes. The aneurysms were then incubated with 5% normal donkey serum in TBST (Tris-Buffered Saline and Tween-20) for 1 hour at RT, followed by overnight incubation with primary antibodies against CD31 (1:30; Dako, Carpentaria, Calif), or smooth muscle actin (SMA) (1:20, Dako) at 4 °C. The aneurysms were then washed in TBS for 15 minutes with 3 changes; they were then incubated with a secondary antibody (Cy3 conjugated donkey anti-mouse IgG, 1:200; Jackson Immuno Research, West Groove, PA) for 2.5 hours at RT, they were then washed in TBS for 15 minutes with 4 changes. Sytox green (1:1000; Life Technologies, Grand Island, NY) served as a nuclear counterstain to identify inflammatory cells. Finally, samples were viewed and imaged with a fluorescence confocal microscope. A positive control was performed using the left CCA with the same antibodies; a negative control was performed with non-immune normal serum in place of the primary antibody
RESULTS
All 12 rabbits survived for the assigned time points and were submitted for histological evaluation. At 4 weeks, 2 (33%) of 6 aneurysms did not have coil loops over the neck orifice, with all the coil loops sitting deep within the aneurysm cavity, rendering dual immunostaining difficult. Four (67%) of 6 aneurysms had coil loops crossing over the neck orifice and touching the parent artery wall. In the peripheral portions of the neck limited, white, thin membranous tissue covered the coil loops; over the central areas of the neck the coil loops were grossly bare, without overlying tissue (Figure 1A),. The en face whole tissue mounting staining demonstrated very localized, single layers of CD31 positive endothelial cells corresponding to the thin, membranous areas noted above; those endothelial cells were present at the periphery only, were contiguous with the cells along the parent artery, and extended no more than 400μm from the parent artery-neck interface (Figure 1B–C). In the central areas, where the coil loops extended over the neck without touching the wall, neither CD31+ or SMA+ cells were detected; a few scattered inflammatory cells were present on the coil surface. Scattered or and diffused inflammatory cells including neutrophils and monocytes were seen on the coil surface or between the coil loops; either CD31 or SMA positive cells were not detected on or between the coil loops.
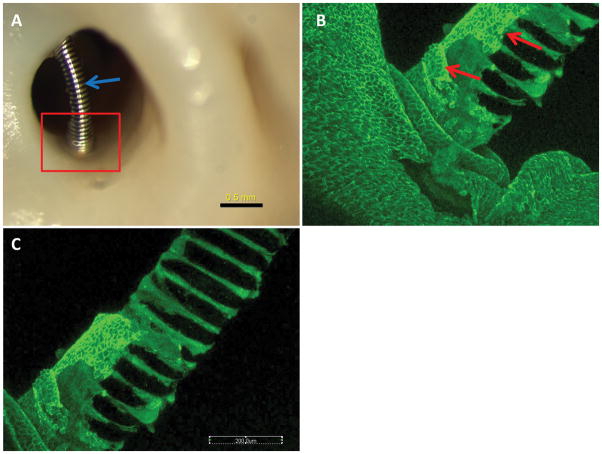
Figure 1. Aneurysm harvested at 4 weeks post embolization.
A, macrophotograh showing the localized membrane covering at the neck peripheral area (red rectangle), the coil segment which is far away from the wall (blue arrow) is bare, without tissue coverage. B–C, whole tissue mount staining (antibody for CD31), photography is taken from the rectangular area in A, showing the localized CD31 positive cells (red arrows) are covering the coil at the peripheral area, those cells continued up with the endothelial cells of the parent artery wall. The faraway portion of coil is bare, without CD31+ cells coverage.
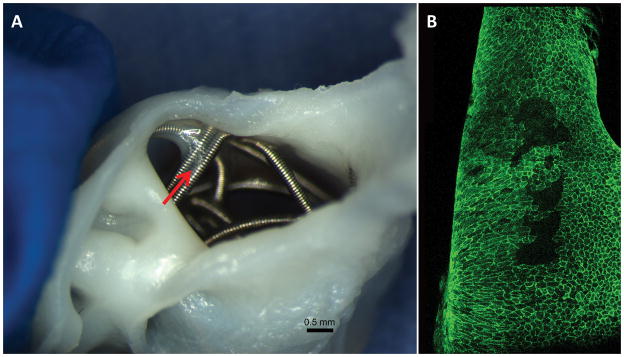
In the 8 week group, 1 aneurysm had coil loops deep within the cavity, which made confocal analysis impossible; this subject was excluded from further analysis. In the remaining 5 rabbits, 4 (80%) of 5 aneurysms had the coil loops cross over the neck orifice and touched the parent wall peripherally. Where the coil loop touched the parent artery wall, localized, thin membranous tissue was grossly visible (Figure 2A), where confluent CD31 positive endothelial cell covering was detected (Figure 2B). Compared with the aneurysms in the 4 week group, the endothelial cells in the 8 week group were more confluent, and extended along the coil loops toward the central area (Figure 3C–D), instead of localizing at the periphery area. Further, membranous tissue lined with CD31 positive cells was also present within the gaps between the coil loops at or and near the neck center area (Figure 3A–B). However, the maximum distance from the parent artery-neck interface to the leading edge of the endothelialization did not exceed 900μm microns in any subject. On the coil loop surfaces, where there was CD31 staining, a few sparse SMA positive cells were present, in which some were covered with CD31 positive cells (Figure 2E). Scattered inflammatory cells, primarily consisted of monocytes, were present on the coil surface where the coil loops were bare, without CD31 positive cell coverage.
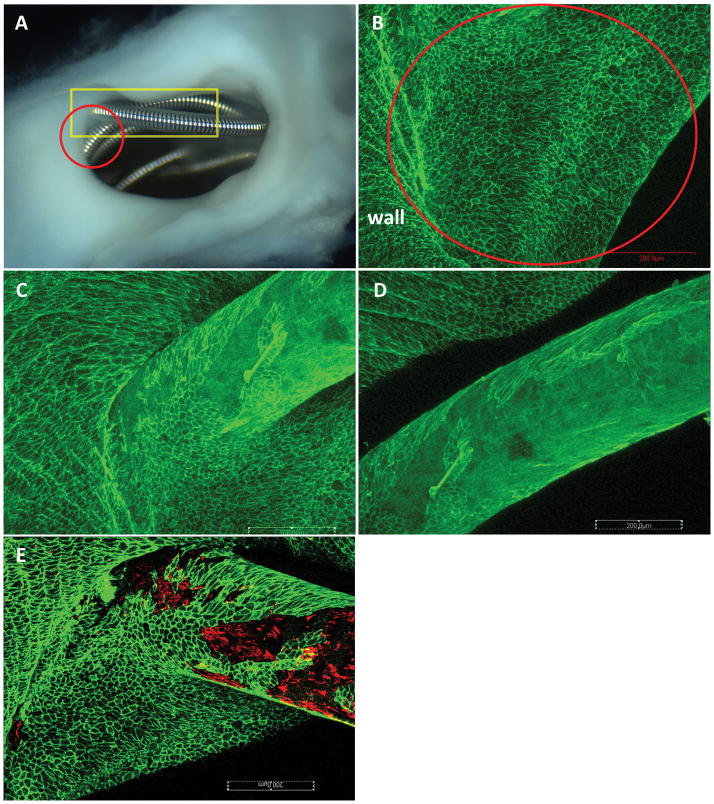
Figure 2. Aneurysm harvested at 8 weeks post embolization.
A, macro-photograph showing the coil loops at the neck orifice. Very thin membrane covering the coil loops at the peripheral area is observed (red circle). B, confocal microphotograph taken from the red circle area in A, showing the coil loops at the peripheral area in A within the red circle are completely covered with Cd31 positive cell ( large red circle area); those cells are confluent and continued up with the endothelial cells of the parent artery wall. C–D are taken from the rectangular area in A, showing the Cd31 positive cells covering on the coil surface not only limited to the periphery area,, extends to the center area. E, double staining for CD31 (green) and SMA (red), showing SMA positive cells are along with CD31 Positive cells on the coil surface at the peripheral area. (whole tissue mount staining: antibody for CD31 (A–D); double staining for CD31 and SMA (E), original magnificent 20X, water lens).
Figure 3. Aneurysm sample harvested at 8 weeks post embolization.
A, macro-photograph showing the membrane tissue between two coil segments at the center area (red arrow). B, confocal microphotograph showing the CD31 positive cells lining between the coil segments at the center area shown in A (red arrow) (whole tissue mount staining for antibody CD31, original magnification, 20x water lens).
DISCUSSION
In the current study, we demonstrated that the bare platinum coils can induce endothelial cell growth. However, at least up to 2 months following embolization, such growth is quite limited and is dependent on close contact between the coils and the junction of the neck and the parent artery. These findings suggest strongly that donor endothelial cells are resident along the parent artery rather than from circulating sources. These findings are important as they not only provide baseline, control data for future studies assessing modified coils, but also provide guidance to investigators focused on improving endothelialization over microcoils used to treat saccular aneurysms.
Endothelialization starts as early as 7 days following treatment in rabbit and swine aneurysm models following embolization[12 13]. In order to look at the progression of the endothelialization, chose 4 and 8 week time points for the en face endothelial staining. Both bone marrow-derived circulating stem cells and migration of endothelial cells from the adjacent vessel have been reported in the endothelialization of aneurysm neck following flow diverters treatment [12 14]. Studies showed that endothelial progenitor cells can promote the neck endothelium formation and help the aneurysm repair post coil embolization[15 16]. However, endothelial denudation has found to prevent recanalization after coil embolization in a canine vein pouch bifurcation model[17]. Pandey et al. developed an in vitro model for studying the mechanism of EC differentiation and proliferation on endovascular coils by seeding human umbilical vein ECs with coil segments and found EC attachment to the coils within hours of incubation[18]. They also observed expression of integrins, which promotes cell adherence to the extracellular matrix, in the coils seeded with ECs and reported cell cycle and cell signaling pathways were affected in ECs by platinum coils.
Hemodynamic factors may also influence the growth of endothelial cells at the aneurysm neck, depending on the aneurysm model, could influence the rate of endothelialization. The alteration of hemodynamics in the aneurysm following coil embolization is believed to promote adhesion of thrombocytes to the coil loops followed by fibrous organization and endothelialization[19–21]. In addition, shape of coil and configuration could also affect the endothelialization process. Reingers et al. compared endothelialization of aneurysm embolized with bare, bio-active and hydrogel-coated coils in the rabbit model and suggested a smooth and dense surface over the aneurysm neck is important for endothelial cells to bridge the aneurysm neck[8]. The lack of neointimal coverage at the aneurysm neck leads to the coil compaction and subsequently recanalization of the aneurysm[22]. The growth neointima derived from the adjacent wall observed in this is similar to the findings noted in flow diverter treated aneurysms[12]. Strategies that could promote endothelialization and neointimal growth at the neck would accelerate the permanent healing of the aneurysms.
There are several limitations in the current study. First, two month follow-up in the current study may not be long enough to monitor the complete growth pattern of EC following coil embolization. Second, although the results demonstrated in the current study suggested the neoendothelial cells over the coil loop at the neck were originally from the wall of the parent artery, the exact mechanism of neo-endothelial cells proliferation or migration following coil implantation remains unclear. Third, the assessment of endothelialization is only qualitative and the confocal microscopy is limited to the evaluation of the coils at the level of the neck.
CONLCUSION
Platinum coil can induce endothelial cells growth, which primarily started at the periphery area where the coil loop touched the parent wall. The neo-endothelial cells at aneurysm neck following endovascular device embolization likely originated from the parent artery wall.
Acknowledgments
We thank Covidien Inc. for generously providing the flow diverters for this study.
FUNDING STATEMENT
This work was supported by National Institutes of Health grant NS 076491.
Footnotes
COMPETING INTERESTS STATEMENT
None
CONTRIBUTORSHIP STATEMENT
YHD and IR contributed to the animal experiments and analysis of angiographic data.
YHD and IR contributed to the tissue staining, interpretation of data and drafting the manuscript.
DK and RK contributed to the conception and design and revising the article critically for important intellectual content.
DATA SHARING
All authors in this manuscript have read and approved submission of the manuscript. All authors have access to the raw data.
References
- 1.Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of Saccular Aneurysms Via Endovascular Approach .2. Preliminary Clinical-Experience. J Neurosurg. 1991;75(1):8–14. doi: 10.3171/jns.1991.75.1.0008. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 2.Guglielmi G, Vinuela F, Sepetka I, Macellari V. Electrothrombosis of Saccular Aneurysms Via Endovascular Approach .1. Electrochemical Basis, Technique, and Experimental Results. J Neurosurg. 1991;75(1):1–7. doi: 10.3171/jns.1991.75.1.0001. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 3.Workman MJ, Cloft HJ, Tong FC, et al. Thrombus formation at the neck of cerebral aneurysms during treatment with Guglielmi detachable coils. Am J Neuroradiol. 2002;23(9):1568–76. [PMC free article] [PubMed] [Google Scholar]
- 4.Bavinzski G, Richling B, Binder BR, et al. Histopathological findings in experimental aneurysms embolized with conventional and thrombogenic/antithrombolytic Guglielmi coils. Minim Invasive Neurosurg. 1999;42(4):167–74. doi: 10.1055/s-2008-1053392. [DOI] [PubMed] [Google Scholar]
- 5.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg. 1999;91(2):284–93. doi: 10.3171/jns.1999.91.2.0284. [DOI] [PubMed] [Google Scholar]
- 6.Sarrafzadeh A, Haux D, Kuchler I, Lanksch WR, Unterberg AW. Poor-grade aneurysmal subarachnoid hemorrhage: relationship of cerebral metabolism to outcome. J Neurosurg. 2004;100(3):400–6. doi: 10.3171/jns.2004.100.3.0400. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 7.Groden C, Hagel C, Delling G, Zeumer H. Histological findings in ruptured aneurysms treated with GDCs: six examples at varying times after treatment. AJNR Am J Neuroradiol. 2003;24(4):579–84. [PMC free article] [PubMed] [Google Scholar]
- 8.Reinges MH, Krings T, Drexler AY, et al. Bare, bio-active and hydrogel-coated coils for endovascular treatment of experimentally induced aneurysms. Long-term histological and scanning electron microscopy results. Interv Neuroradiol. 2010;16(2):139–50. doi: 10.1177/159101991001600205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai D, Ding YH, Danielson MA, et al. Histopathologic and Immunohistochemical Comparison of Human, Rabbit, and Swine Aneurysms Embolized with Platinum Coils. AJNR Am J Neuroradiol. 2005;26(10):2560–68. [PMC free article] [PubMed] [Google Scholar]
- 10.Dai D, Ding YH, Kadirvel R, et al. A longitudinal immunohistochemical study of the healing of experimental aneurysms after embolization with platinum coils. AJNR Am J Neuroradiol. 2006;27(4):736–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. American Roentgen Ray Society. AJR Am J Roentgenol. 2000;174(2):349–54. doi: 10.2214/ajr.174.2.1740349. [DOI] [PubMed] [Google Scholar]
- 12.Kadirvel R, Ding YH, Dai D, Rezek I, Lewis DA, Kallmes DF. Cellular mechanisms of aneurysm occlusion after treatment with a flow diverter. Radiology. 2014;270(2):394–9. doi: 10.1148/radiol.13130796. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitome-Mishima Y, Yamamoto M, Yatomi K, et al. Endothelial cell proliferation in Swine experimental aneurysm after coil embolization. PLoS One. 2014;9(2):e89047. doi: 10.1371/journal.pone.0089047. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li ZF, Fang XG, Yang PF, et al. Endothelial progenitor cells contribute to neointima formation in rabbit elastase-induced aneurysm after flow diverter treatment. CNS Neurosci Ther. 2013;19(5):352–7. doi: 10.1111/cns.12086. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aronson JP, Mitha AP, Hoh BL, et al. A novel tissue engineering approach using an endothelial progenitor cell-seeded biopolymer to treat intracranial saccular aneurysms Laboratory investigation. J Neurosurg. 2012;117(3):546–54. doi: 10.3171/2012.5.Jns091308. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 16.Wei HJ, Wang D, Chen JL, et al. Mobilization of circulating endothelial progenitor cells after endovascular therapy for ruptured cerebral aneurysms. Neurosci Lett. 2011;498(2):114–8. doi: 10.1016/j.neulet.2011.04.061. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 17.Raymond J, Guilbert F, Metcalfe A, Gevry G, Salazkin I, Robledo O. Role of the endothelial lining in recurrences after coil embolization: prevention of recanalization by endothelial denudation. Stroke. 2004;35(6):1471–5. doi: 10.1161/01.STR.0000126042.76153.f7. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 18.Pandey AS, San Antonio JD, Addya S, et al. Mechanisms of Endothelial Cell Attachment, Proliferation, and Differentiation on 4 Types of Platinum-Based Endovascular Coils. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.08.029. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrne JV, Hope JK, Hubbard N, Morris JH. The nature of thrombosis induced by platinum and tungsten coils in saccular aneurysms. AJNR Am J Neuroradiol. 1997;18(1):29–33. [PMC free article] [PubMed] [Google Scholar]
- 20.Ishihara S, Mawad ME, Ogata K, et al. Histopathologic findings in human cerebral aneurysms embolized with platinum coils: report of two cases and review of the literature. AJNR Am J Neuroradiol. 2002;23(6):970–4. [PMC free article] [PubMed] [Google Scholar]
- 21.Tong FC, Cloft HJ, Dion JE. Endovascular treatment of intracranial aneurysms with Guglielmi Detachable Coils: emphasis on new techniques. J Clin Neurosci. 2000;7(3):244–53. doi: 10.1054/jocn.1999.0211. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 22.Ozawa T, Tamatani S, Koike T, et al. Histological evaluation of endothelial reactions after endovascular coil embolization for intracranial aneurysm. Clinical and experimental studies and review of the literature. Interv Neuroradiol. 2003;9(Suppl 1):69–82. doi: 10.1177/15910199030090S109. IN.v9.Suppl 1.p69 [pii][published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]