Abstract
Background. It is important to prepare response in advance to increase the efficiency of its execution. The process of response preparation is usually studied using the precueing paradigm. In this paradigm subjects have to employ the preceding information about further imperative stimulus to perform proper response preparation, which shortens the reaction time of subsequent response execution. Previous studies detected the impairment of response preparation in schizophrenia only with the help of electroencephalographic parameters, but not with the assessing of reaction time. Therefore, in this study we attempted to find a behavioural parameter that could detect impairment in response preparation of schizophrenia patients. It was recently found that appropriate response preparation not only shortens the reaction time but also increases its stability, which is measured with the intra-individual reaction time variability. It was also revealed that response stability could better find cognitive dysfunction in some studies of schizophrenia disorder than classical behavioural parameters. Hence, the main goal of this study was to verify if intra-individual reaction time variability could detect the impairment of response preparation in schizophrenia patients.
Materials and methods. In order to achieve the main purpose, we carried out a study with 14 schizophrenia patients and 14 control group subjects. We used precueing paradigm in our research, in which participants had to employ information about stimulus probability for the proper response preparation.
Results. Our main result showed that despite the responses of schizophrenia patients were faster to the high-probability stimulus than to the low-probability one (F (1, 13) = 30.9, p < 0.001), intra-individual reaction time variability did not differ in this group between the responses to more and less probable stimuli (F (1, 13) = 0.64, p = 0.44).
Conclusions. Results of the study suggest that people with schizophrenia were able to use precueing probabilistic information only to shorten their reaction time, but not to increase response stability. Therefore, it was found that intra-individual reaction time variability parameter could detect response preparation impairment in schizophrenia, and could be used in clinical purposes.
Keywords: response preparation, response stability, intra-individual reaction time variability, schizophrenia
Abstract
ATSAKO PARUOŠIMAS IR ASMENINIS REAKCIJOS LAIKO KINTAMUMAS ŠIZOFRENIJOS ATVEJU
Santrauka
Įžanga. Norint padidinti atsako atlikimo efektyvumą, svarbu paruošti jį iš anksto. Dažniausiai atsako paruošimo tyrimuose taikoma įspėjančio stimulo paradigma. Šioje paradigmoje tiriamieji turi pasinaudoti išankstine informacija apie artėjantį stimulą, kad galėtų tinkamai paruošti atsaką, o tai sutrumpintų atsako atlikimo laiką. Ankstesnieji tyrimai aptiko atsako paruošimo sutrikimą šizofrenijos atveju tik taikant elektroencefalografinius parametrus. Tačiau matuojant dažniausiai taikomą elgseninį parametrą – reakcijos laiką, rasti šio sutrikimo nepavyko. Tyrimo metu buvo bandoma nustatyti tokį elgseninį rodiklį, kuris galėtų aptikti atsako paruošimo sutrikimą sergantiems šizofrenija pacientams. Neseniai nustatyta, kad tinkamas atsako paruošimas ne tik sutrumpina reakcijos laiką, bet ir padidina atsako stabilumą, kuris vertinamas asmeninio reakcijos laiko kintamumo matavimu. Taip pat išsiaiškinta, kad atsako stabilumo parametras gali geriau nustatyti kai kurių kognityvinių funkcijų sutrikimus šizofrenijos atveju negu klasikiniai elgseniniai parametrai. Pagrindinis šio tyrimo tikslas – patikrinti, ar asmeninis reakcijos laiko kintamumas gali parodyti atsako paruošimo sutrikimą sergantiesiems šizofrenija.
Metodika. Ištyrėme 14 sergančiųjų šizofrenija pacientų ir 14 kontrolinės grupės sveikų tiriamųjų. Tyrime taikyta įspėjančio stimulo paradigma, kai dalyviai tinkamam atsako paruošimui turėjo pasinaudoti informacija apie artėjančių stimulų tikimybę.
Rezultatai. Pagrindiniai tyrimo rezultatai parodė, kad nežiūrint į tai, jog sergantys šizofrenija pacientai greičiau reagavo į didesnės tikimybės stimulą (F (1, 13) = 30,9, p < 0,001), jų asmeninio reakcijos laiko kintamumas reaguojant į abu stimulus buvo vienodas (F (1, 13) = 0,64, p = 0,44).
Išvados. Tyrimas patvirtino, kad sergantieji šizofrenija galėjo pasinaudoti išankstine tikimybine informacija, tačiau tai tik sutrumpino reakcijos laiką, bet nepadidino atsakų stabilumo. Nustatyta, kad asmeninis reakcijos laiko kintamumo parametras gali aptikti atsako paruošimo sutrikimą šizofrenijos atveju.
Raktažodžiai: atsako paruošimas, atsako stabilumas, asmeninis reakcijos laiko kintamumas, šizofrenija
INTRODUCTION
It is well known that in order to increase efficiency of response execution it is important to prepare it in advance (1). The process of response preparation is usually studied using the precueing paradigm (e. g. 2–5, 1). This paradigm relies on two types of stimuli: precue and imperative one. The precue stimulus provides advance information about the subsequent imperative stimulus, and the former requires a certain response. It was found that the reaction time (RT) decreases with the increasing amount of information provided by the precue. This particular reduction of response time (a precueing effect) is related to the response preparation processes occurring in the foreperiod interval – a timescale between precue and imperative stimuli (for review, see 1).
The precue stimulus can provide different types of precedent information regarding the imperative signal. For instance, it may define its location or time of occurrence (4, 5). However, employing of probabilistic information about the upcoming imperative stimulus has a particular advantage in such studies. Prediction of future situation is not always exact in everyday life. Rather, our brain makes probabilistic predictions of different future events and prepares adequate responses. Therefore, using information about upcoming stimulus probability in the precueing paradigm makes the experiment closer to real life conditions (6–8).
It was found that the higher the probability of imperative stimulus is, the faster the subjects respond to it (e. g. 9–13). For example, in Miller’s (1998) experiment the probability of one imperative stimulus was 75%, while the probability of another one was accordingly 25%. The RT result showed that subjects were more prepared to react to the higher probability imperative stimulus. This was confirmed by occurrence of the Lateralized Readiness Potential (LRP) during the foreperiod showing that response time reduction to more probable stimulus is related to the process of response preparation. LRP is defined as the difference between potentials at contra- and ipsilateral central electrodes processing before the movement of a certain hand. This parameter serves as an index of lateralized motor preparation and it appears in case participants prepare movement of one hand more than another (e. g. 14).
Previous studies showed that different brain processes linked to future events such as foresight (15), anticipation (16–18) and planning (19–22) are impaired in schizophrenia patients. However, it was revealed that people with schizophrenia are able to employ different precue information (including a probabilistic one) to produce faster reaction time. This evidence suggested that response preparation is not impaired in the case of this disorder (4, 23–25). Nevertheless, electroencephalographic studies detected that response preparation is reduced in people with schizophrenia when assessed with the LRP parameter, although not all researches found the significant reduction (26–28, 25).
While the attempts to discover the impairment of response preparation in the case of schizophrenia disorder with the help of RT measuring failed, we tried in our study to find the behavioural parameter, which could detect this impairment. The previous electroencephalographic study suggested that response preparation not only speeds up the reaction, but it also increases its stability (13). Response stability is measured with intra-individual reaction time variability (IIV). This latter parameter is based on the reaction time standard deviation and it provides useful predictive information about cognitive functioning (29–31). Numerous studies of IIV have been published during the last decades, particularly in the field of psychiatric and neurological disorders. For instance, response stability reductions were found in the case of frontal lobe lesions (32, 33), schizophrenia (34–36, 30, 31), dementia (37, 38), attention deficit hyperactivity disorder (ADHD) (39, 40) and Parkinson’s disease (41). In addition, exploring of IIV is also used in gerontology studies (37).
The previous studies found that employing of IIV parameter has some advantage as compared with classic response measurements, because it can detect more subtle differences between cognitive functioning in healthy persons and in potential patients (42–44, 33, 31). Bearing in mind all previous information, we provided a hypothesis that assessing of response stability could detect the impairment of response preparation in schizophrenia patients. Therefore, the main purpose of our study was to analyze if people with schizophrenia are able to use probabilistic information in response preparation to increase not only the speed of reaction but also its stability. The result of such analysis would not only help to investigate the subtle characteristic of response preparation in the case of schizophrenia disorder but also can be employed in clinical purposes.
MATERIALS AND METHODS
Participants
Fourteen schizophrenia inpatients (5 males and 9 females) were recruited from the Republican Vilnius Psychiatric Hospital for this study. Diagnosis of schizophrenia was made by clinicians, according to the International Classification of Diseases criteria (ICD-10; 45). The mean age of patients was 36.5 years (SD = 14.2). The control group comprised 14 healthy volunteers (7 males and 7 females), the mean age was 41.4 years (SD = 13.4). All individuals of both groups were right-handed and had normal or corrected-to-normal vision. This study was approved by the local Medical Ethic Committee.
Procedure
The study was carried out in the Electrophysiology Research Department of Republican Vilnius Psychiatric Hospital in a specially equipped laboratory room that eliminates extraneous visual and audible disturbances. Participants were seated at a comfortable distance in front of a computer monitor. They had to use the right hand in response to the stimuli. Before the beginning of the study, the participants were instructed to place the middle finger on the right button and the index finger on the left button of a response device.
The method of our study was based on Miller’s (1998) research. The experimental task was developed and performed using the E-Prime 2.0 software (Psychology Software Tools, Inc.). At the beginning of each trial, a precue stimulus showing a picture of an outstretched left hand (mirroring the participant right hand) holding fingers in a prepared position appeared in the center of the screen. After a foreperiod of 1 s one of two possible imperative stimuli (‘0’ symbol or ‘X’ letter) followed the precue signal. The stimuli were shown one at a time in a light colour on the black background. Participants were asked to respond with the middle finger to the ‘0’ stimulus and with the index finger to the ‘X’ one.
The probability of the ‘0’ imperative stimulus was 75%, while the ‘X’ stimulus appeared with 25% probability. The participants were not informed about the probabilities of stimuli during the study. They had from 150 ms until 2000 ms to perform the response and were instructed to react as fast and as accurately as possible.
After the response was performed, short feedback signaling if the response was correct or wrong appeared in the center of the screen. The study consisted of six series of 32 trials occurring in a random order. The first series was considered as a practice and was not counted in the final analysis.
RESULTS
Data analysis
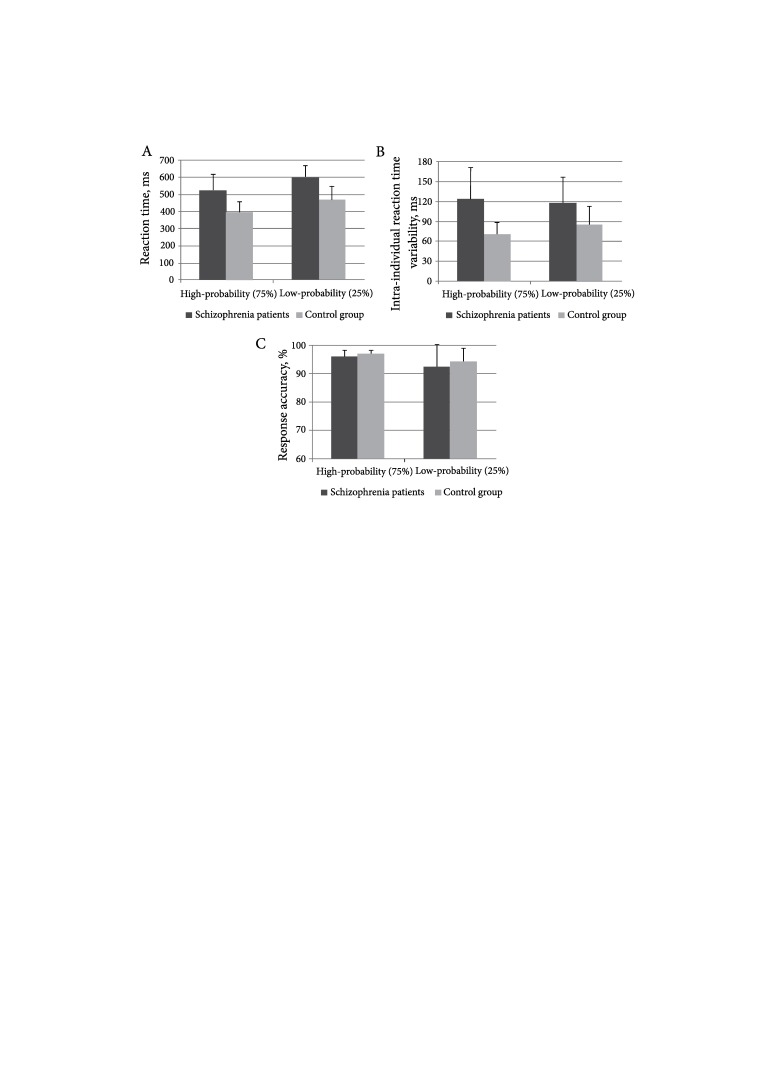
We eliminated all trials with wrong key responses. All remaining trials, which had reaction times 2.5 SD slower or faster than the mean for each participant, were considered to be outliers and were also eliminated from the result analysis. 2.54% of trials were eliminated in the schizophrenia group and 2.45% of trials were eliminated in healthy subjects. Reaction times, intra-individual reaction time variability and response accuracy were counted for all remaining trials considered as accurate. Following Elvevåg et al. (2000), who had also investigated response performance to different target probabilities in schizophrenia, we provided empirical log odds transformation of response accuracy data. The results with the statistical values are presented in the Figure and the Table. The results of response accuracy in the Figure and the Table represent the untransformed data.
Table.
Comparison of reaction time, intra-individual reaction time variability and response accuracy to high- and low-probability stimuli
| High-probability stimulus (75%) | Low-probability stimulus (75%) | Statistic | ||
|---|---|---|---|---|
| F (1, 13) | p | |||
| Schizophrenia patients | ||||
| Reaction time, ms (SD) | 519 (101) | 589 (82) | 30.9 | <0.001 |
| Intra-individual reaction time variability, ms (SD) | 124 (47) | 118 (39) | 0.64 | 0.44 |
| Response accuracy, % (SD) | 95.6 (2.6) | 92.3 (7.8) | 3.3 | 0.09 |
| Control group | ||||
| Reaction time, ms (SD) | 394 (64) | 467(83) | 52.4 | <0.001 |
| Intra-individual reaction time variability, ms (SD) | 70 (19) | 85 (28) | 15.8 | <0.01 |
| Response accuracy, % (SD) | 96.7 (1.5) | 94.1 (4.8) | 7.5 | <0.05 |
Response execution
At first, we investigated the ability to execute responses in schizophrenia patients when compared with healthy subjects. In order to achieve this purpose, we investigated three different response parameters in both groups. The RT, IIV and response accuracy were analyzed separately for the responses to the high- and low-probability stimuli (Figure). The one-way ANOVA analysis revealed that schizophrenia patients had significantly slower reaction time than the control group, responding to the high-probability stimulus (F (1, 26) = 15.4, p = 0.001) and to the low-probability one (F (1, 26) = 15.3, p = 0.001) (Figure, A). It has also been found that the clinical group had reliably higher IIV than healthy subjects when they responded to both the more probable stimulus (F (1, 26) = 16.1, p < 0.001) and the less probable one (F (1, 26) = 6.7, p < 0.05) (Figure, B). Nevertheless, the analysis of response accuracy did not reveal any difference between schizophrenia patients and the control group in the cases of both types of responses: to the high-probability stimulus (F (1, 26) = 2, p = 0.17) and the low-probability one (F (1, 26) = 0.1, p = 0.76) (Figure, C).
Figure.
Comparison of reaction time, intra-individual reaction time variability and response accuracy between schizophrenia patients and control group subjects
Response preparation
Secondly, we investigated the main goal of our study – exploring behavioural parameters of response preparation in people with schizophrenia. In order to achieve this purpose, we compared the reaction time, IIV and response accuracy between the responses to high- and low-probability stimuli separately in both groups (Table). The presence of a statistically significant difference between the latter parameters was an indicator of the ability of using the probabilistic precueing information to the appropriate response preparation. According to the one-way repeated measure ANOVA analysis, both groups were reliably faster responding to the more probable stimuli than to the less probable ones. Nevertheless, IIV was significantly lower in responses to the high-probability stimuli than to the low-probability ones only in the control group subjects. The response accuracy was higher in reactions to the high-probability stimuli. However, the difference was significant only in the subjects of the control group.
DISCUSSION
This study was carried out to examine subtle behavioural properties of response preparation in the case of schizophrenia disorder. Despite a relatively small number of participants that could essentially affect the results, the obtained statistical p values have a high significance level. The former evidence suggests that the results of the study were reliable enough. The results of this study would not only expand our understanding about the process of response preparation but also can be used in clinical purposes. At first, we have analyzed behavioural parameters of response execution which revealed that schizophrenia patients were slower than healthy subjects responding to both high- and low probability stimuli. Such data coincides with the results of previous studies (24, 25). The previous studies also found that response stability of schizophrenia patients is lower than the response stability of healthy subjects (34–36, 30, 31). The results of our study have supplemented this evidence with finding that this data is also valid in case when stimuli have different probabilities. In this case, schizophrenia patients are less stable than healthy subjects responding to both high- and low-probability stimuli.
Investigating the response preparation process, we have revealed that people with schizophrenia are able to prepare response enough to react faster to high-probability stimuli that to low-probability ones. Similar findings were obtained in previous researches, in which schizophrenia patients also had to employ preceding probabilistic information (24, 25). However, the most important analysis of our study was assessing response preparation with the IIV parameter. It was found that responses to high-probability stimuli are more stable than to low-probability ones only in the group of healthy subjects. This result coincides with the previous finding (13). Nevertheless, we did not detect any difference between the stability of responses to more and less probable stimuli in the schizophrenia group. This finding showed that response preparation in schizophrenia patients was not intact enough to provide a more stable response to the more probable stimulus. Therefore, our study revealed that the impairment of response preparation in schizophrenia could be detected only by assessing the IIV parameter. In general, this evidence coincides with the findings of previous studies, which revealed that this parameter could detect the impairment in the nervous system disorder in cases when classical behavioural test results could not find it (33, 31).
Previous electroencephalographic studies detected impairment of response preparation in people with schizophrenia only with LRP measuring. Hence, additionally to the electroencephalographic parameter we have found a behavioural one, which was also able to assess reduction of response preparation in the case of schizophrenia disorder. Behavioural parameters have some advantages as compared with electroencephalographic ones in the clinical practice. For instance, they do not need any special EEG equipment, are faster and easier to obtain. Finding of a new behavioural parameter specific to schizophrenia disorder can help in diagnostic purposes and in assessing of the efficiency of the medical treatment.
The mechanism of subtle impairment of response preparation in schizophrenia can be related to the pathology of frontal cortex neurons in these patients. Previous studies found the impairment in response preparation after the damage of the frontal cortex (46–48). However, it is well known that the activity of neurons in the frontal cortex is also disturbed in the case of schizophrenia (e. g. 49–52). The former evidence determines the similarities of cognitive impairment in frontal lobe lesion and schizophrenia patients (e. g. 53–56). Nevertheless, in the case of schizophrenia, disturbance of the frontal cortex neurons is not as severe as after frontal lobe damage. This evidence could be a reason why impairment of response preparation cannot be detected in schizophrenia patients with a help of reaction time measuring as it takes place in the case of frontal cortex damage. We suggest that impairment of response preparation in the case of schizophrenia is more subtle than after lesion of the frontal cortex, and it can be detected only with assessing of response stability.
CONCLUSIONS
Our study revealed that schizophrenia patients were able to use precueing probabilistic information in response preparation only speeding up the reaction time but not increasing its stability. Therefore, it was revealed that in the case of schizophrenia disorder intra-individual reaction time variability could detect impairment of response preparation (contrary to the parameter of reaction time). This finding showed the new advantages of employing response stability measurements in scientific and clinical studies.
Denisas Dankinas, Sigita Mėlynytė, Aldona Šiurkutė, Kastytis Dapšys
References
- Leuthold H, Sommer W, Ulrich R.. Preparing for action: inferences from CNV and LRP. J Psychophysiol. 2004; 18(2): 77–88. [Google Scholar]
- Leonard JA.. Advance information in sensorimotor skills. Q J Exp Psychol. 1953; 5: 41–9. [Google Scholar]
- Rosenbaum DA.. Human movement initiation: Specification of arm, direction and extent. J Exp Psychol Gen. 1980; 109: 444–74. [DOI] [PubMed] [Google Scholar]
- Carnahan H, Chua R, Elliott D, Velamoor VR,Carnahan CJ.. Effects of schizophrenia and prefrontal leukotomy on movement preparation and generation. J Clin Exp Neuropsychol. 1994; 16(2): 253–60. [DOI] [PubMed] [Google Scholar]
- Leuthold H, Sommer W, Ulrich R.. Partial advance information and response preparation: inferences from the lateralized readiness potential. J Exp Psychol Gen. 1996; 125(3): 307–23. [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, Von Cramon DY.. Predicting events of varying probability: uncertainty investigated by fMRI. Neuroimage. 2003; 19(2): 271–80. [DOI] [PubMed] [Google Scholar]
- Feigenberg IM.. Motor reaction time and probabilistic prognosis. Hum Physiol. 2008; 34(5): 581–91. [PubMed] [Google Scholar]
- Bruhn P.. Emergence of spontaneous anticipatory hand movements in a probabilistic environment. Adv Cogn Psychol. 2013; 9(2): 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBerge D, Legrand R, Hobbie RK.. Functional identification of perceptual and response biases in choice reaction time. J Exp Psychol. 1969; 79: 295–9. [DOI] [PubMed] [Google Scholar]
- Blackman AR.. Influence of stimulus and response probability on decision and movement latency in a discrete choice reaction task. J Exp Psychol. 1972; 92(1): 128–33. [DOI] [PubMed] [Google Scholar]
- Heuer H.. Choice between finger movements of different and identical form: The effect of relative signal frequency. Psychol Res. 1982; 44(4): 323–42. [Google Scholar]
- Miller J.. Effects of stimulus-response probability on choice reaction time: Evidence from the lateralized readiness potential. J Exp Psychol Gen Human. 1998; 24(5): 1521–34. [Google Scholar]
- Dankinas D, Parciauskaite V, Dapsys K.. Intra-individual reaction time variability and response preparation: an EEG study. Acta Neurobiol Exp. 2015; 75: 462–8. [PubMed] [Google Scholar]
- Coles MGH.. Modern mind-brain reading: psychophysiology, physiology, and cognition. Psychophysiology. 1989; 26: 251–69. [DOI] [PubMed] [Google Scholar]
- Eack S, Keshavan M.. Foresight in schizophrenia: a potentially unique and relevant factor to functional disability. Psychiat Serv. 2008: 59: 256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Miller RM, Duncan CC, Hoffman RE, Mathalon DH.. When it’s time for a change: failures to track context in schizophrenia. Int J Psychophysiol. 2010; 78: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn JK, Horan WP, Kring AM, Simons RF, Green MF.. Impaired anticipatory event-related potentials in schizophrenia. Int J Psychophysiol. 2010; 77: 141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH.. Anticipating the future: automatic prediction failures in schizophrenia. Int J Psychophysiol. 2012; 83: 232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Rushe T, Woodruffe PWR, Murray RM.. Problem solving in schizophrenia: a specific deficit in planning ability. Schizophr Res. 1995; 14: 235–46. [DOI] [PubMed] [Google Scholar]
- Jogems-Kosterman BJM, Zitman FG, Van Hoof JJM, Hulstijn W.. Psychomotor slowing and planning deficits in schizophrenia, Schizophr Res. 2001; 48: 317–33. [DOI] [PubMed] [Google Scholar]
- Holt DV, Rodewald K, Rentrop M, Funke J, Weisbrod M, Kaiser S.. The Plan-a-Day approach to measuring planning ability in patients with schizophrenia. J Int Neuropsych Soc. 2011; 17: 327–35. [DOI] [PubMed] [Google Scholar]
- Holt DV, Wolf J, Funke J, Weisbrod M, Kaiser S.. Planning impairments in schizophrenia: Specificity task independence and functional relevance. Schizophr Res. 2013; 149: 174–9. [DOI] [PubMed] [Google Scholar]
- Fuller R, Jahanshahi M.. Impairment of willed actions and use of advance information for movement preparation in schizophrenia. J Neurol Neurosurg Psychiatry. 1999; 66: 502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvevåg B, Weinberger DR, Suter JC, Goldberg TE.. Continuous performance test and schizophrenia: a test of stimulus response compatibility working memory response readiness or none of the above? Am J Psychiat. 2000; 157: 772–80. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Kappenman ES, Fuller RL, Robinson B, Summerfelt A, Gold JM.. Impaired response selection in schizophrenia: Evidence from the P3 wave and the lateralized readiness potential. Psychophysiology. 2009; 46: 776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayanidis F, Nicholson R, Schall U, Meem L, Fulham R, Michie PT.. Switching between univalent task-sets in schizophrenia: ERP evidence of an anticipatory task-set reconfiguration deficit. Clin Neurophysiol. 2006; 117: 2172–90. [DOI] [PubMed] [Google Scholar]
- Kieffaber PD, O’Donnell BF, Shekhar A, Hetrick WP.. Event related brain potential evidence for preserved attentional set switching in schizophrenia. Schizophr Res. 2007; 93: 355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM.. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. J Abnorm Psychol. 2002; 111: 22–41. [PubMed] [Google Scholar]
- MacDonald SW, Nyberg L, Bäckman L.. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006; 29(8): 474–80. [DOI] [PubMed] [Google Scholar]
- Roalf DR, Ruparel K, Verma R, Elliott MA, Gur RE, Gur RC.. White matter organization and neurocognitive performance variability in schizophrenia. Schizophr Res. 2013, 143(1): 172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YS, Kim SN, Shin NY, Jung WH, Hur JW, Byun MS, et al.. Increased intra-individual variability of cognitive processing in subjects at risk mental state and schizophrenia patients. PloS one. 2013; 8(11): e78354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, Alexander MP.. Staying on the job: the frontal lobes control individual performance variability. Brain. 2003; 126(11): 2363–80. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S.. Effects of focal frontal lesions on response inhibition. Cereb Cortex. 2007; 17(4): 826–38. [DOI] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, Weinberger DR.. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry. 2004; 161(3): 490–500. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Roth A, Rentrop M, Friederich HC, Bender S, Weisbrod M.. Intra-individual reaction time variability in schizophrenia, depression and borderline personality disorder. Brain Cogn. 2008; 66(1): 73–82. [DOI] [PubMed] [Google Scholar]
- Cole VT, Weinberger DR, Dickinson D.. Intra-individual variability across neuropsychological tasks in schizophrenia: a comparison of patients, their siblings, and healthy controls. Schizophr Res. 2011; 129(1): 91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, Macdonald SWS, Dixon RA.. Variability in reaction time performance of younger and older adults. J Gerontol B Psychol Sci Soc Sci. 2002; 57(2): 101–15. [DOI] [PubMed] [Google Scholar]
- Tales A, Leonards U, Bompas A, Snowden RJ, Philips M, Porter G, et al.. Intra-individual reaction time variability in amnestic mild cognitive impairment: a precursor to dementia? J Alzheimers Dis. 2012; 32(2): 457–66. [DOI] [PubMed] [Google Scholar]
- Henríquez-Henríquez MP, Billeke P, Henríquez H, Zamorano FJ, Rothhammer F, Aboitiz F.. Intra-individual response variability assessed by ex-Gaussian analysis may be a new endophenotype for attention-deficit/hyperactivity disorder. Front Psychiatry. 2015; 197(5): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belle J, van Raalten T, Bos DJ, Zandbelt BB, Oranje B, Durston S.. Capturing the dynamics of response variability in the brain in ADHD. Neuroimage Clin. 2015; 7: 132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camicioli RM, Wieler M, de Frias CM, Martin WRW.. Early untreated Parkinson’s disease patients show reaction time variability. Neurosci Lett. 2008; 441: 77–80. [DOI] [PubMed] [Google Scholar]
- Collins LF, Long CJ.. Visual reaction time and its relationship to neuropsychological test performance. Arch Clin Neuropsychol. 1996; 11(7): 613–23. [PubMed] [Google Scholar]
- Hultsch DF, Macdonald SWS, Hunter MA, Levy- Bencheton J, Strauss E.. Intraindividual variability in cognitive performance in older adults: Comparison of adults with mild dementia, adults with arthritis, and healthy adults. Neuropsychology. 2000; 14(4): 588–98. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M.. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006; 60(10): 1088–97. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. England: Gaskell Royal College of psychiatrists; 1992. [Google Scholar]
- Verfaellie M, Heilman KM.. Response preparation and response inhibition after lesions of the medial frontal lobe. Arch Neurol. 1987: 44(12); 1265–71. [DOI] [PubMed] [Google Scholar]
- Turken U, Swick D. Response selection in the human anterior cingulate cortex. Nat Neurosci. 1999; 2(10): 920–4. [DOI] [PubMed] [Google Scholar]
- Triviño M, Correa Á, Arnedo M, Lupiáñez J.. Temporal orienting deficit after prefrontal damage. Brain. 2010; 133(4): 1173–85. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF.. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia: I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986; 43(2): 114–24. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Illowsky BP.. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia: III. A new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry. 1988; 45(7); 609–15. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, et al.. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000; 10(11): 1078–92. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW.. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012; 35(1): 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, Morris DL.. Schizophrenic syndromes and frontal lobe performance. Br J Psychiatry. 1991; 158(3): 340–5. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW.. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res. 1999; 37(3): 251–70. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD.. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001; 158(7): 1105–13. [DOI] [PubMed] [Google Scholar]
- Christensen BK, Patrick RE, Stuss DT, Gillingham S, Zipursky RB.. Verbal episodic memory impairment in schizophrenia: a comparison with frontal lobe lesion patients. Clin Neuropsychol. 2013; 27(4); 647–66. [DOI] [PubMed] [Google Scholar]