Abstract
There is much interest in the use of seed-applied bacteria for biocontrol and biofertilization, and several commercial products are available. However, many attempts to use this strategy fail because the seed-applied bacteria do not colonize the rhizosphere. Mechanisms of rhizosphere colonization may involve active bacterial movement or passive transport by percolating water or plant roots. Transport by other soil biota is likely to occur, but this area has not been well studied. We hypothesized that interactions with soil nematodes may enhance colonization. To test this hypothesis, a series of microcosm experiments was carried out using two contrasting soils maintained under well-defined physical conditions where transport by mass water flow could not occur. Seed-applied Pseudomonas fluorescens SBW25 was capable of rhizosphere colonization at matric potentials of −10 and −40 kPa in soil without nematodes, but colonization levels were substantially increased by the presence of nematodes. Our results suggest that nematodes can have an important role in rhizosphere colonization by bacteria in soil.
The presence of certain bacteria in the rhizosphere and rhizoplane is associated with several widely reported benefits to plants. These benefits include growth promotion by enhancing nutrient uptake by roots and antagonistic effects against plant pathogens (4, 38, 45). Consequently, rhizosphere bacteria have been developed as biological fertilizers and biological control agents (4, 33, 46). In many instances such biofertilizers and biocontrol agents are applied as seed dressings (15, 45). To be effective, the seed-coated bacteria must colonize the rhizosphere, and the varying success of many field applications may be explained by differences in the extent of rhizosphere colonization (36, 45, 46). Thus, improving our understanding of rhizosphere colonization may help to develop more effective biological fertilizers and biocontrol agents.
Two mechanisms control rhizosphere colonization by seed-applied bacteria: active bacterial motility and passive movement (8). Comparisons between wild-type motile rhizobacteria and nonmotile mutants have shown that bacterial motility (and presumably chemotaxis to root exudates) is of great benefit in rhizosphere colonization (6, 9, 23, 29, 32). However, root extension rates can greatly exceed rates of bacterial movement in soil, suggesting that passive movement is likely to be an important additional mechanism under field conditions. Passive movement may be caused by percolating water, movement on the root surface, or interactions with other biota (8). Percolating water (e.g., rainfall, irrigation) can be a major transporting agent of bacteria from seeds to roots, as water frequently flows preferentially down root channels (11, 34, 44). While some studies have demonstrated transport by extending roots, with bacteria actively multiplying when mucigel or other root exudates are used (5, 19, 26, 31, 43), other studies have not demonstrated this phenomenon (10, 14, 30, 33, 47). This implies that physical or biological factors can limit transport by this mechanism. The least well-studied mechanism for colonization by seed-applied bacteria is that through their interactions with other soil biota, and most evidence that this occurs is anecdotal (24). In soils, nematodes are by far the most abundant animals, with numbers typically ranging from 1 to 100 per g of soil (35, 37). In addition, whereas other abundant soil faunal groups (e.g., mites, collembola) tend to be confined to the soil surface and litter layers, nematodes are found throughout the root profile. Thus, we hypothesized that nematodes can enhance rhizosphere colonization in soil. This can occur as a result of the combined effect of transport of bacteria by nematodes, which has already been demonstrated in sand and on agar plates, (13, 16, 35) and possibly by nutritional effects associated with nitrogen excretion and mineralization by nematodes (1, 22, 39).
We tested our hypothesis by using colonization of the wheat rhizosphere by Pseudomonas fluorescens SBW25 as a model system. This is a well-studied bacterium capable of colonizing roots and shoots of several plant species (3, 16, 17, 42). Because abiotic factors, such as soil type, temperature, and soil matric potential, are known to strongly influence all soil-based biological processes and particularly root colonization (24), we used two contrasting soils and devised a robust regimen for maintaining these soils under defined physical conditions. We used an experimental system designed to ensure that there was minimal mass water flow. Because the size of water-filled pores in the soil is inversely related to matric potential (the negative value of the soil water suction), which exerts a major influence on bacterial motility (24) and nematode movement (48), we did all experiments at two matric potentials, −10 and −40 kPa. These were chosen to represent soil at field capacity and also a drier soil, but one that would still allow active growth and movement of plant roots, bacteria, and nematodes.
MATERIALS AND METHODS
Test organisms.
Winter wheat cv. Savannah (Advanta Seeds, Forfar, United Kingdom) was used as the test plant for colonization by P. fluorescens SBW25 (CEH, Oxford, United Kingdom). The bacterium was chromosomally marked with kanamycin resistance (Kmr) genes (3, 18) as a selective marker. Nematode species used were the bacterial feeders Acrobeloides thornei DWF1109, Acrobeloides maximum, Cruznema sp. strain Rosario (all from Paul De Ley, University of California—Riverside), and Caenorhabditis elegans N2 (Christina Lagido, University of Aberdeen, United Kingdom).
Preparation of soil and matric potentials.
Top soils from Craibstone (a Countesswells series sandy loam, with 73.9% sand, 20% silt, 6.1% clay, and 5% organic matter, pH 6.2) and Cruden Bay (Tipperty series clay loam, with 43.4% sand, 32.3% silt, 24.3% clay, and 3% organic matter, pH 6.4) were collected from arable sites in Aberdeenshire, Scotland. Soils were air dried and sieved (pore size, 2.5 mm in diameter) prior to storage. The relationship between gravimetric water content (GWC) and matric potential was determined by using 600-g lots of dry soil packed to a dry bulk density of 1 g cm−3. Various amounts of water were added to soils, and microtensiometers (Delta-T Devices, Cambridge, United Kingdom) and a Richard's psychrometer were used to measure matric potentials in the range of 0 to −80 kPa and >−80 kPa, respectively, according to the manufacturer's instructions. GWC of the soils was calculated from their weight before and after oven drying at 105°C for 24 h.
Removal of natural nematode populations from soils was achieved by pasteurization. Soils were wetted to a GWC of 20%, left to equilibrate for 72 h, and then pasteurized at 60°C for 72 h.
Surface sterilization, bacterial preparation, coating, and germination of wheat seeds.
Wheat seeds (∼60 to 70 seeds) were treated by using an oxytetracycline/silver nitrate method (41). For seed coating, P. fluorescens SBW25 (Kmr) was grown at 25°C with shaking at 200 rpm for 20 h in 10 ml of Luria-Bertani broth. A bacterial pellet was formed by centrifugation from 6 ml of culture, washed in 1 ml of one-quarter-strength Ringer's solution (Oxoid), and then recentrifuged. The resulting bacterial pellet was suspended in sterile 1% (wt/vol) high-viscosity carboxy methyl cellulose in one-quarter-strength Ringer's solution (16) and was applied to 4 g of surface-sterilized wheat by using a spatula. Coated seeds were air dried for 2 h and then germinated on moist filter paper in the dark at 25°C for 24 h. Effectiveness of the coating was determined by soaking several air-dried seeds in one-quarter-strength Ringer's solution for 2 h, preparing a 10-fold dilution series from which 10-μl aliquots were plated onto Luria-Bertani agar.
Construction and preparation of microcosms.
Microcosms, 25 by 25 by 1 cm, were constructed from sheets of 2-mm-thick Perspex, with removable front panels to allow watering and harvesting of plants. Soils of a known GWC were added to microcosms in order to give a dry bulk density of 1.2 g cm−3 in every experiment. The desired matric potential was then establish by adding the required weight of water to achieve the correct GWC, based on the equation w = (GWCR − GWCi)(1 + GWCi), where w was the amount of water to add, GWCR was the required GWC, and GWCi was the GWC of the soil added to the microcosm. Weights of the microcosms (without front plate and cladding strips), the added soil, added water, and their combined weights were recorded. Established microcosms were left for 24 h to equilibrate prior to planting a single germinated wheat seed into each microcosm at a depth of 1 cm directly below the top spacer gap. Filled microcosms were placed in aluminum boxes (15 to a box) and were transferred to high-light growth cabinets running 12-h day-night cycles set at 15 and 17°C, respectively. Minitensiometers (SWT5; Delta-T Devices) and thermistors were added to microcosms and were logged on a DL2e datalogger (Delta-T Devices) every 10 min. Data were downloaded and analyzed on days 4, 7, and 11, immediately prior to watering, and again on day 14. After removing the front plate, a weight of water sufficient to maintain the required matric potentials was added as a fine mist by using an aspirator. Temperatures in the microcosms and the air temperature in the growth cabinet were monitored with thin-wire thermistors and a DataHog (Skye Instruments, Powys, United Kingdom).
Influence of nematodes on rhizosphere colonization.
All four nematode species were cultivated by using a modification of the foam chip method (7). Foam chips coated with 30 ml of nematode growth broth (12) with 500 μl of canola oil were used to fill 250-ml conical flasks and then were autoclaved. Nematodes from established monoxenic petri dish cultures with Escherichia coli HB101 were used to inoculate flasks that were incubated for approximately 3 weeks at 15°C prior to harvest. Nematodes were harvested by using methods previously described (7). The nematode cultures of different species were mixed in approximately equal numbers prior to being added to pasteurized soils to give a mixed nematode community. The experiment had a three-way factorial design: two soils types (sandy loam and clay loam) maintained at two matric potentials (−10 and −40 kPa) with two nematode treatments (no nematodes or mixed nematode community added at 8 nematodes g−1 of soil). Five microcosms were prepared per treatment, and the entire experiment was repeated (10 replicates in total). Microcosms were prepared and incubated as described above for 2 weeks.
Root systems were recovered from microcosms by using a dissecting probe, and the degree of rhizosphere colonization was estimated. Roots and attached soil were divided into primary roots by cutting just below the seed. Each primary root was measured and cut into thirds according to length, and each section of each root was placed into one-quarter-strength Ringer's solution. Root sections and adhering soil were then sonicated for 5 min. The resulting bacterial suspensions were vortexed and serially diluted in one-quarter-strength Ringer's solution, and replicate 10-μl aliquots of each dilution were grown on selective agar plates. Colonies were counted after 28 h of incubation at 25°C, and the CFU per centimeter of the root system was estimated for each section.
Numbers of surviving nematodes were estimated by randomly selecting 10-g samples of soil and placing them in Baermann funnels (25) for 24 h. Numbers of extracted nematodes were counted with a dissecting microscope.
Statistical analysis.
All data were log10 transformed to achieve normality and homogeneity of variance and then were subjected to analysis of variance (ANOVA). When ANOVA showed significant treatment effects or interactions, individual means were compared by using the least-significant-difference test.
RESULTS
Establishment and maintenance of soil physical conditions.
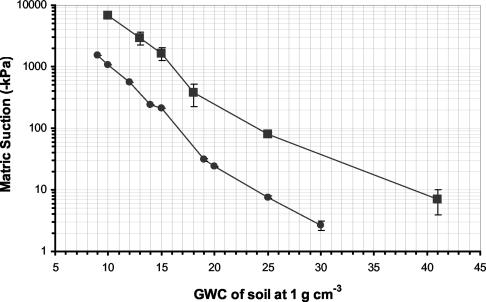
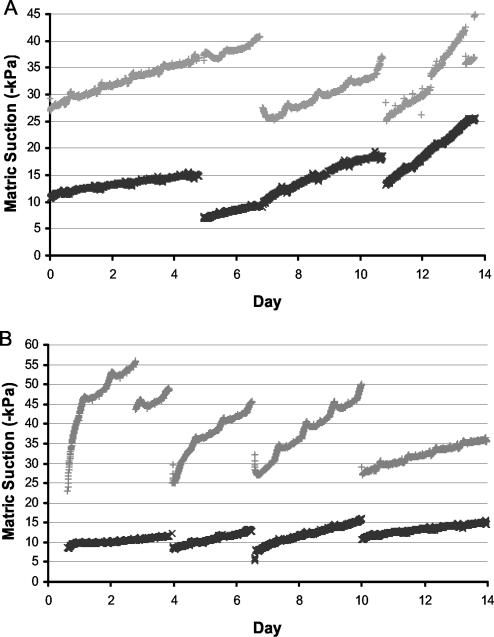
Establishing and maintaining soil matric potential was fundamental to this study. The matric potentials of the two soils after wetting to a range of GWC (Fig. 1) were used to establish microcosms at −10 and −40 kPa by adjusting the GWC of the sandy loam soil to 26 and 19% and the clay loam to 42 and 30%, respectively. After establishment the matric potentials of the microcosms were logged. Microcosms were rewetted roughly every 3 days, which enabled the matric potentials of the two soils to be maintained around −10 and −40 kPa (Fig. 2) in all experiments.
FIG. 1.
Soil water uptake characteristics of Craibstone (•) and Cruden Bay (▪) soils. Error bars represent the standard deviation of the mean (n = 6).
FIG. 2.
Mean matric potential in Cruden Bay (A) and Craibstone (B) microcosms, established at matric potentials of −10 (+) and −40 kPa (×).
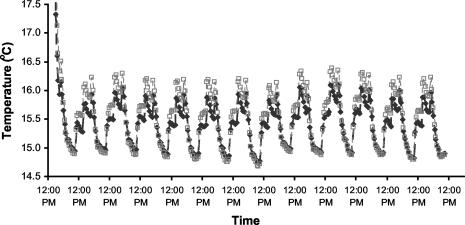
Setting the day temperature in the growth cabinets 2°C lower than the night temperature to offset the effect of radiative heating of the microcosms reduced the magnitude of diurnal temperature fluctuations in the microcosms to less than ±1°C (Fig. 3.). Although some thermally driven water movement may have occurred in the microcosms, because diurnal fluctuations in matric potential were small to negligible (Fig. 2) we assume that water movement was negligible and did not have a significant effect on rhizosphere colonization in our experiments.
FIG. 3.
Typical temperature readings taken at depths of 8 cm (♦) and 16.5 cm (□) from the soil surface in the microcosms.
Influence of nematodes on root colonization.
The mean log10 numbers (± standard deviations) of recoverable bacteria applied per seed for the two replicate experiments were 5.87 ± 5.32 and 5.43 ± 5.23. Pasteurization of soil was successful at removing the indigenous nematode communities, because no nematodes were recovered by Baermann funnel extractions from either of the untreated soils. Mean nematode numbers recovered from treated soils at the start of the experiment was 69 ± 19.1 nematodes per 10 g of dry weight. At the end of the 14-day experiment ANOVA showed no differences in nematode recovery between the two soil types, but there was a significant difference between soils held at different matric potentials with nematode survival being better in the drier soil (F1,4 = 15.97, P = 0.016; mean number of nematodes per 10 g of soil was 31.1 at −10 kPa and 65.1 at −40 kPa; standard error of difference was 8.47). Soil type did not interact significantly with matric potential in terms of numbers of recovered nematodes.
ANOVA of bacterial colonization data of the entire data set revealed that there was a highly significant difference in bacterial colonization between the two soil types (F1,48 = 25.69, P < 0.001), with mean log10 CFU cm of root−1 being 6.81 (clay loam) and 6.37 (sandy loam); standard error of difference was 0.13; 118 degrees of freedom. As would be expected, soil type interacted with matric potential but not with nematode treatment or root section, and the three-way interaction was not significant. Thus, for ease of presentation, further analysis was done on each of the two soil types separately.
Clay loam soil.
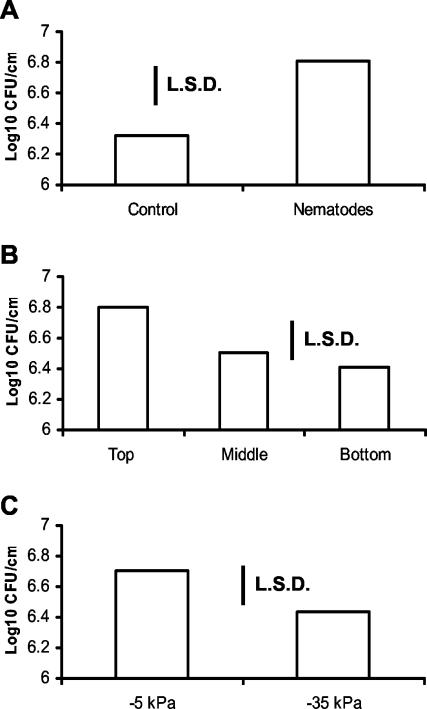
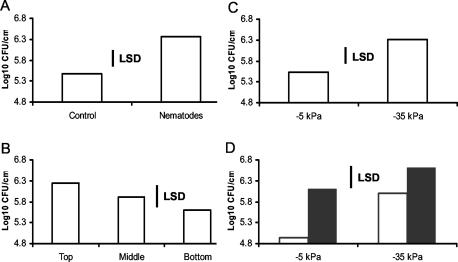
ANOVA revealed highly significant differences in colonization (log10 CFU cm of root−1) caused by the presence of nematodes (F1,48 = 14.14, P < 0.001) and significant differences between soil matric potentials (F1,48 = 4.08, P = 0.049) and root sections (F2,48 = 3.34, P < 0.044).
There were significantly more bacteria on roots in microcosms containing nematodes than in control microcosms (Fig. 4A). In addition, bacterial colonization was significantly higher in the uppermost section of the root compared to that of lower sections (Fig. 4B), and there was a greater degree of colonization in the wetter soils (−10 kPa) than in the drier soil (−40 kPa) (Fig. 4C). None of these factors interacted significantly.
FIG. 4.
Mean log10 CFU of P. fluorescens SBW25 per centimeter of wheat root grown in microcosms of clay loam soil in relation to the presence or absence of nematode community (A), position on roots that had been divided into equal thirds from the spermosphere (top) to the root tip (bottom) (B), and matric potential of soil (C). LSD, least significant difference. P = 0.05, 48 degrees of freedom.
Sandy loam soil.
Results for sandy loam soil were generally similar to those for the clay loam soil. There were highly significant differences in colonization (log10 CFU per centimeter of root) caused by the presence of nematodes (F1,48 = 51.47, P < 0.001), with the difference being more pronounced than that in the clay loam. Again there were significant differences between soil matric potentials (F1,48 = 39.91, P < 0.001) and between root sections (F2,48 = 9.22, P < 0.001). Significantly more bacteria were present on roots of nematode-treated plants than in controls (Fig. 5A), and bacterial colonization was significantly greater in the upper root sections than in lower sections (Fig. 5B). Unlike the clay loam soil, colonization was greater in the drier soils (−40 kPa) than in wetter soils (−10 kPa) (Fig. 5C). In this soil, unlike the clay loam, the factors of matric potential and nematode treatment interacted significantly (F2,48 = 4.59, P = 0.037). In this case, the effect of nematodes was more pronounced in soils with matric potential of −10 kPa than in soils at −40 kPa (Fig. 5D).
FIG. 5.
Mean log10 CFU of P. fluorescens SBW25 per centimeter of wheat root grown in microcosms of sandy loam soil in relation to presence or absence of nematode community (A), position on roots that had been divided into equal thirds from the spermosphere (top) to the root tip (bottom) (B), and matric potential of soil (C). (D) The interaction between matric potential and presence (▪) or absence (□) of nematodes. LSD, least significant difference. P = 0.05, 48 degrees of freedom.
DISCUSSION
In the absence of percolating water, the mechanisms of rhizosphere colonization are not well understood. Using microcosms and antibiotic-marked bacteria as a tool for exploring the mechanisms of rhizosphere colonization, we tested our hypothesis that nematodes may enhance colonization. The ability to control the soil matric potential, and in particular to eliminate mass water flow, was crucial to the success of these experiments. Our protocols for achieving these goals were successful: fluctuations in matric potential were small, and our watering regimen using a fine mist on the open microcosms ensured no net directional mass flow of water. The system has much potential for studying the ecology and behavior of a wide range of microorganisms and soil mesofauna under specific physical conditions, and it may be used for fundamental or applied studies. It is particularly suitable for studies using genetically marked organisms. The soils used represent the sandy and clayey ends of possible soil textures with very different particle size distributions. Thus, we believe that the system could be used with soils of all textural classes.
P. fluorescens SBW25 is a well-known colonizer of both plant roots and plant shoots (3, 16, 17, 42); thus, it is not surprising that this bacterium was able to colonize the entire length of the rhizosphere in the absence of percolating water and transport by other organisms. Presumably the motile SBW25 is capable of utilizing plant exudates as a carbon source and colonizes by a combination of active growth and motility. The general trends shown in our data are consistent with previous observations. In all experiments there was a decrease in bacterial numbers further down the root, as has been previously recorded with seed-applied bacteria (2). Numbers of colonizing bacteria tended to be higher in the clay loam soil than in the sandy loam. This is in agreement with studies using model soils in which viability of bacteria was positively correlated with the percentage of clay and was negatively correlated with the percentage of sand (24). There were no clear trends in our work between soil matric potential and colonization.
In spite of the good colonizing capability of P. fluorescens on its own, the presence of nematodes led to substantial increases in root colonization in all microcosms, irrespective of soil type or matric potential. Until this study, all experiments investigating nematode transport of bacteria were done either on agar, sands, or model soils (13, 27, 28). This is the first study demonstrating enhanced colonization of rhizobacteria in soils and clearly demonstrates the strong increase in colonization these animals may cause. It is not possible from our work to determine the precise mechanisms by which nematodes promote rhizosphere colonization. Transport by nematodes is a likely mechanism, because nematodes have been shown to be capable of transporting bacteria on their cuticles; also, bacteria-eating nematodes, such as those used in this study, can transport bacteria by passage through the intestine (35). Another possible explanation is that nitrogen and/or carbon mineralization and nitrogen excretion by nematodes could also have promoted growth of bacteria, as nematodes have been implicated in these processes (1, 22, 39). However, these nutritional effects are unlikely to account for the almost 10-fold increase in bacterial numbers seen in our experiments. It is well documented that grazing of bacterial populations can increase their activity, but any benefit in increasing bacterial growth has to be balanced against nematode consumption of bacteria. Bacteria-eating nematodes consume large numbers of bacteria, on the order of 6.61 × 105 to 15.22 × 105 cells per μg of nematodes per day (21). Thus, while the presence of bacteria-eating nematodes can increase plant biomass (20, 40) and bacterial activity (20), their presence tends to decrease total bacterial numbers and biomass (1, 20). Ultimately, the relative contribution of movement and nutritional effects is likely to depend on soil type, soil physical conditions, and soil nutrient status.
In the moister soils (−10 kPa) there was significant nematode mortality, with an approximate 50% reduction in nematode numbers during the course of the experiment. It could be argued that in this treatment enhanced bacterial colonization could result from mineralization of nematode carbon promoting bacterial growth. We think this is unlikely, because the nematodes were distributed evenly throughout the microcosms so that nematode death in the rhizosphere could only account for very small amounts of carbon. Furthermore, the nematode effect was similar in the −40 kPa soils, where there was no significant nematode mortality. The fact that nematodes survived much better in the drier soils supports the idea that nematode movement, feeding, and reproduction in soil is facilitated by thin rather than thick water films (48).
While this study looked merely at the effects of nematodes on promoting downward movement and colonization of seed-applied bacteria to colonize the rhizosphere, it is quite likely that the ubiquitous nature of nematodes in soils means that they interact with many soil bacteria and promote many bacterial processes. This area of soil ecology clearly warrants further research.
Acknowledgments
We thank the BBSRC (BIRE Initiative) for funding this work.
Mark Bailey and Tracey Timms-Wilson (Centre for Ecology and Hydrology), Paul DeLey (University of California—Riverside), and Chris Thornton (University of Exeter, Exeter, United Kingdom) are acknowledged for providing strains and technical advice.
REFERENCES
- 1.Anderson, R. V., W. D. Gould, L. E. Woods, C. Cambardella, R. E. Ingham, and D. C. Coleman. 1983. Organic and inorganic nitrogenous losses by microbivorous nematodes in soil. Oikos 40:75-80. [Google Scholar]
- 2.Bahme, J. B., and M. N. Schroth. 1987. Spatial-temporal colonization patterns of a rhizobacterium on underground organs of potato. Phytopathology 77:1093-1100. [Google Scholar]
- 3.Bailey, M. J., A. K. Lilley, I. P. Thompson, P. B. Rainey, and R. J. Ellis. 1995. Site directed chromosomal marking of a fluorescent pseudomonad isolate from the phytosphere of sugar beet; stability and potential for marker gene transfer. Mol. Ecol. 4:755-763. [DOI] [PubMed] [Google Scholar]
- 4.Bashan, Y. 1998. Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol. Adv. 16:729-770. [Google Scholar]
- 5.Bashan, Y., and H. Levanoy. 1989. Wheat root tips for passive vertical transfer of Azospirillum brasilense. J. Gen. Microbiol. 135:2899-2909. [Google Scholar]
- 6.Bashan, Y., and G. Holguin. 1995. Inter-root movement of Azospirillum brasilense and subsequent root colonization of crop and weed seedlings growing in soil. Microbial Ecol. 29:269-281. [DOI] [PubMed] [Google Scholar]
- 7.Bedding, R. A. 1981. Low cost in vitro mass production of Neoaplectana and Heterorhabditis species (Nematoda) for field control of insect pests. Nematologica 27:109-114. [Google Scholar]
- 8.Benizri, E., E. Baudoin, and A. Guckert. 2001. Root colonization by inoculated plant growth-promoting rhizobacteria. Biocontrol Sci. Technol. 11:557-574. [Google Scholar]
- 9.Boelens, J., M. Vande Woestyne, and W. Verstraete. 1994. Ecological importance of motility for the plant growth promoting rhizopseudomonas strain ANP15. Soil Biol. Biochem. 26:269-277. [Google Scholar]
- 10.Bowen, G. D., and A. D. Rovira. 1992. The rhizosphere: the hidden half of the hidden half, p. 641-669. In Y. Waisel, A. Eshel, and U. Kafkafi (ed.), Plant roots: the hidden half. Marcel Decker, New York, N.Y.
- 11.Bowers, J. H., and J. L. Parke. 1993. Colonization of pea (Pisum sativum) taproots by Pseudomonas fluorescens: effects of soil temperature and bacterial mobility. Soil Biol. Biochem. 25:1693-1701. [Google Scholar]
- 12.Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cayrol, J. C., C. Couderc, and I. Evrard. 1977. Studies of the relationships between free-living soil nematodes and nodule bacteria of leguminous plants. Rev. Zool. Agric. Pathol. Veg. 76:77-89. [Google Scholar]
- 14.Catlow, H. Y., A. R. Glenn, and M. J. Dilworth. 1990. The use of transposon-induced non-motile mutants in assessing the significance of motility of Thizobium leguminosarum biovar trifolii for movement in soils. Soil Biol. Biochem. 22:331-336. [Google Scholar]
- 15.Ciccillo, F., A. Fiore, A. Bevivino, C. Dalmastri, S. Tabacchioni, and L. Chiarini. 2002. Effects of two different application methods of Burkholderia ambifaria MCI 7 on plant growth and rhizosphere bacterial diversity. Environ. Microbiol. 4:238-245. [DOI] [PubMed] [Google Scholar]
- 16.De Leij, F. A. A. M., E. J. Sutton, J. M. Whipps, J. S. Fenlon, and J. M. Lynch. 1995. Impact of field release of genetically modified Pseudomonas fluorescens on indigenous microbial populations of wheat. Appl. Environ. Microbiol. 61:3443-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Leij, F. A. A. M., C. E. Thomas, M. J. Bailey, J. M. Whipps, and J. M. Lynch. 1998. Effect of insertion site and metabolic load on the environmental fitness of genetically modified Pseudomonas fluorescens isolate. Appl. Environ. Microbiol. 64:2634-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dijkstra, A. F., G. H. N. Scholten, and J. A. Van Ven. 1987. Colonization of wheat seedlings (Triticum aestivum) by Pseudomonas fluorescens and Bacillus subtilis. Biol. Fertil. Soils 4:41-46. [Google Scholar]
- 20.Djigal, D., A. Rauman, T. A. Diop, J. L. Chotte, and C. Villenave. 2004. Influence of bacterial feeding nematodes (Cepholobidae) on soil microbial communities during maize growth. Soil Biol. Biochem. 36:323-331. [Google Scholar]
- 21.Ferris, H., R. C. Venette, and S. S. Lau. 1997. Population energetics of bacterial feeding nematodes: carbon and nitrogen budgets. Soil Biol. Biochem. 29:1183-1194. [Google Scholar]
- 22.Ferris, H., R. C. Venette, H. R. van der Meulen, and S. S. Lau. 1998. Nitrogen mineralization by bacterial-feeding nematodes: verification and measurement. Plant Soil 203:159-171. [Google Scholar]
- 23.Gamliel, A., and J. Katan. 1992. Chemotaxis of fluorescent pseudomonads towards seed exudates and germinated seeds in solarized soil. Phytopathology 80:328-332. [Google Scholar]
- 24.Gammack, S. M., E. Paterson, J. S. Kemp, M. S. Cresser, and K. Killham. 1992. Factors affecting the movement of microorganisms in soils, p. 263-305. In G. Stotzky and J. M. Bollag (ed.), Soil biochemistry, vol. 7. Marcel Dekker, New York, N.Y. [Google Scholar]
- 25.Hooper, D. J. 1986. Extraction of free-living stages from soil, p. 5-31. In J. F. Southey (ed.), Laboratory methods for work with plant and soil nematodes. HMSO, London, United Kingdom.
- 26.Howie, W. J., R. J. Cook, and D. M. Weller. 1987. Effects of soil matric potential and cell motility on wheat root colonisation by fluorescent pseudomonads suppressive to take-all. Phytopathology 77:286-292. [Google Scholar]
- 27.Jatala, P., H. J. Jenson, and S. A. Russel. 1974. Pristiounchus iheritieri as a carrier of Rhizobium japonicum. J. Nematol. 6:130-131. [PMC free article] [PubMed] [Google Scholar]
- 28.Knox, O. G. G., K. Killham, C. E. Mullins, and M. J. Wilson. 2003. Nematode enhanced microbial colonization of the wheat rhizosphere. FEMS Microbiol. Lett. 225:227-233. [DOI] [PubMed] [Google Scholar]
- 29.Lugtenberg, B., and L. Dekkers. 1999. What makes Pseudomonas bacteria rhizosphere competent? Environ. Microbiol. 1:9-13. [DOI] [PubMed] [Google Scholar]
- 30.Madsen, E., and M. Alexander. 1982. Transport of Rhizobium and Pseudomonas through soil. J. Soil Sci. Soc. Am. 42:557-560. [Google Scholar]
- 31.Mawdsely, J. L., and R. G. Burns. 1994. Root colonization by a Flavobacterium species and the influence of percolating water. Soil Biol. Biochem. 26:861-870. [Google Scholar]
- 32.Parke, J. L. 1991. Root colonisation by indigenous and introduced microorganisms, p. 33-42. In D. L. Glaser and P. B. Cregan (ed.), The rhizosphere and plant growth. Kluwer, Dordrecht, The Netherlands.
- 33.Parke, J. L., R. Moen, A. D. Rovira, and G. D. Bowen. 1986. Soil water flow affects the rhizosphere distribution of a seed borne biological control agent, Pseudomonas fluorescens. Soil Biol. Biochem. 18:583-588. [Google Scholar]
- 34.Patterson, E., J. S. Kemp, S. M. Gammack, E. A. Fitzpatrick, M. S. Cresser, C. E. Mullins, and K. Killham. 1993. Leaching of genetically modified Pseudomonas fluorescens through intact soil microcosms: influence of soil type. Biol. Fertil. Soils 15:308-314. [Google Scholar]
- 35.Poinar, G. O., and E. L. Hansen. 1986. Associations between nematodes and bacteria. Helminthol. Abst. Ser. B 55:61-81. [Google Scholar]
- 36.Rattray, E. A. S., J. A. Tyrrel, J. I. Prosser, L. A. Glover, and K. Killham. 1993. Effect of soil bulk density and temperature on wheat rhizosphere colonisation by lux-marked Pseudomonas fluorescens. Eur. J. Soil Biol. 29:73-82. [Google Scholar]
- 37.Ritz, K., and D. L. Trudgill. 1999. Utility of nematode community analysis as an integrated measure of the functional state of soils: perspectives and challenges. Plant Soil 212:1-11. [Google Scholar]
- 38.Ryder, M. H., and A. D. Rovira. 1993. Biological control of take-all of glasshouse-grown wheat using strains of Pseudomonas corrugata isolated from wheat field soil. Soil Biol. Biochem. 25:311-320. [Google Scholar]
- 39.Savin, M. C., J. H. Görres, D. A. Neher, and J. A. Amador. 2001. Uncoupling of carbon and nitrogen mineralization: role of microbivorous nematodes. Soil Biol. Biochem. 33:1463-1472. [Google Scholar]
- 40.Setala, H., P. Kulmala, J. Mikola, and A. M. Markkola. 1999. Influence of ectomycorrhiza on the structure of detrital food webs in pine rhizosphere. Oikos 87:113-122. [Google Scholar]
- 41.Speakman, J. B., and W. Kruger. 1983. A comparison of methods to surface sterilise wheat seeds. Trans. Br. Mycol. Soc. 80:374-376. [Google Scholar]
- 42.Thompson, I. P., A. K. Lilley, R. J. Ellis, P. A. Bramwell, and M. J. Bailey. 1995. Survival, colonization and dispersal of a genetically modified Pseudomonas fluorescens (SBW25) in the phytosphere of field grown sugar beet. Bio/Technology 13:1493-1497. [Google Scholar]
- 43.Trevors, J. T., J. D. Van Elsas, L. S. Van Overbeek, and M. E. Starodub. 1990. Transport of genetically engineered Pseudomonas fluorescens strain through a soil microcosm. Appl. Environ. Microbiol. 56:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Elsas, J. D., J. T. Trevors, and L. S. Van Overbeek. 1991. Influence of soil properties on the vertical movement of genetically marked Pseudomonas fluorescens through large soil microcosms. Biol. Fertil. Soils 25:416-420. [Google Scholar]
- 45.Weller, D. M. 1988. Biological control of soil-borne pathogens in the rhizosphere with bacteria. Annu. Rev. Phytopathol. 26:379-407. [Google Scholar]
- 46.Weller, D. M., and L. S. Thomashow. 1994. Current challenges in introducing beneficial microorganisms into the rhizosphere, p. 1-18. In F. O'Gara, D. N. Dowling, and B. Boesten (ed.), Molecular ecology of rhizosphere micro-organisms. VCH, Weinheim, Germany.
- 47.Worrall, V., and R. J. Roughley. 1991. Vertical movement of Rhizobium leguminosarum var trifolii in soil as influenced by soil water potential and water flow. Soil Biol. Biochem. 23:485-486. [Google Scholar]
- 48.Yeates, G. W., J. L. Dando, and T. G. Shepherd. 2002. Pressure plate studies to determine how moisture affects access of bacterial feeding nematodes to food in soil. Eur. J. Soil Sci. 53:355-365. [Google Scholar]