Abstract
Background
Previous studies reported a correlation between the maximum standardised uptake value (SUVmax) obtained by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) and distant metastasis in nasopharyngeal carcinoma (NPC). However, an integrated model incorporating SUVmax and anatomic staging for stratifying metastasis risk has not been reported.
Results
The median SUVmax for primary tumour (SUV-T) and cervical lymph nodes (SUV-N) was 13.6 (range, 2.2 to 39.3) and 8.4 (range, 2.6 to 40.9), respectively. SUV-T (HR, 3.396; 95% CI, 1.451-7.947; P = 0.005), SUV-N (HR, 2.688; 95%CI, 1.250-5.781; P = 0.011) and N-classification (HR, 2.570; 95%CI, 1.422-4.579; P = 0.001) were identified as independent predictors for DMFS from multivariate analysis. Three valid risk groups were derived by RPA: low risk (N0-1 + SUV-T <10.45), medium risk (N0-1 + SUV-T >10.45) and high risk (N2-3). The three risk groups contained 100 (22.3%), 226 (50.3%), and 123 (27.4%) patients, respectively, with corresponding 3-year DMFS rates of 99.0%, 91.5%, and 77.5% (P <0.001). Moreover, multivariate analysis confirmed the RPA-based prognostic grouping as the only significant prognostic indicator for DMFS (HR, 3.090; 95%CI, 1.975-4.835; P <0.001).
Methods
Data from 449 patients with with histologically-confirmed, stage I-IVB NPC treated with radiotherapy or chemoradiotherapy were retrospectively analysed. A prognostic model for distant metastasis-free survival (DMFS) was derived by recursive partitioning analysis (RPA) combining independent predictors identified by multivariate analysis.
Conclusion
SUV-T, SUV-N and N-classification were identified as independent predictors for DMFS. An integrated RPA-based prognostic model for DMFS incorporating SUV-N and N-classification was proposed.
Keywords: nasopharyngeal neoplasms, metastasis, TNM staging, maximum standardized uptake value, recursive partitioning analysis
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is particularly prevalent in southern China, Southeast Asia, North Africa, the Middle East, and Alaska [1]. Radiotherapy is the primary treatment used for non-disseminated NPC [2, 3]. With advances in imaging and radiation therapies, local-regional control has exceeded 90% [4]. However, 20-30% of NPC patients eventually develop distant metastasis [5–8], which accounts for the majority of failures [7, 8]. Effort should therefore be made to stratify patients into different groups based on the risk of metastasis to tailor individualized treatments and improve outcomes.
N-classification in the TNM staging system is a measure of the extent of node involvement, and is currently the most reliable tool for assessing metastasis risk in NPC [9, 10]. However, there is remaining room for improvement in the correlation between the N classification and metastasis [11, 12], perhaps because N-classification is based solely on anatomic extent and lacks non-anatomic information such as tumour physiology.
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) imaging is used to probe glucose metabolism in tumour cells [13]. The maximal intensity of FDG uptake by the tumour (maximum standardized uptake value; SUVmax) is a valuable marker of tumour biological behaviour [13, 14] and a useful predictor of distant metastasis in NPC [15, 16]. However, an integrated model incorporating SUVmax and anatomic staging for stratifying metastasis risk has not been reported. Clinicians are therefore somewhat troubled as to how best to incorporate SUVmax into clinical decision-making. A valid approach for incorporating non-anatomic prognostic factors and anatomic staging into an integrated prognosis grouping was recently described [17], which significantly improved survival prediction compared with previous models. In the present study, we extended this approach by using recursive partitioning analysis (RPA) to develop an integrated prognostic model for metastasis that combines SUV parameters and N-classification.
RESULTS
Treatment failure and survival
The median follow-up time was 49.5 months (range, 3.37–67.9 months), and 385/402 (95.8%) of surviving patients were followed up for >3 years. A total of 84 patients experienced treatment failure, with 19/449 (4.2%), 21/449 (4.7%), and 53/449 (11.8%) developing local recurrence, regional recurrence, and distant metastases, respectively. 8/449 (1.8%) patients experienced both local-regional recurrence and distant metastases and 47/449 (10.5%) patients died- the causes of death were nasopharyngeal carcinoma (93.6%, 44/47), other diseases (2.1%, 1/47) and unknown causes (4.3%, 2/47). The 3-year distant metastasis-free survival, local relapse-free survival, regional relapse-free survival, disease-free survival and overall survival was 89.4%, 96.6%, 95.6%, 83.9% and 94.4%, respectively.
Prognosis of different N subcategories
In total, 75 (16.7%), 251 (55.9%), 76 (16.9%), and 47 (10.5%) patients were classified as N0-3, respectively. The 3-year DMFS decreased only very slightly with increasingly higher N category (96.0%, 93.2%, 81.1% and 71.7%, P <0.001). However, no significant differences were observed between N0 and N1 (P = 0.202) and N2 and N3 (P = 0.188).
Prognostic value of SUV-T and SUV-N in NPC
The SUVmax for primary tumours ranged from 2.2 to 39.3 (median, 13.6), and the optimal cut-off SUV-T value for distant metastasis was 10.45. This value was selected to classify patients into SUV-Thigh (≥10.45) and SUV-Tlow (<10.45) groups. Kaplan-Meier survival curves for the two groups (Figure 1A) showed that 3-year DMFS rates for the SUV-Thigh group (86.2% vs. 97.0%, P = 0.002) were significantly lower than the corresponding rates for the SUV-Tlow group.
Figure 1. Kaplan-Meier curves of DMFS for nasopharyngeal carcinoma groups.
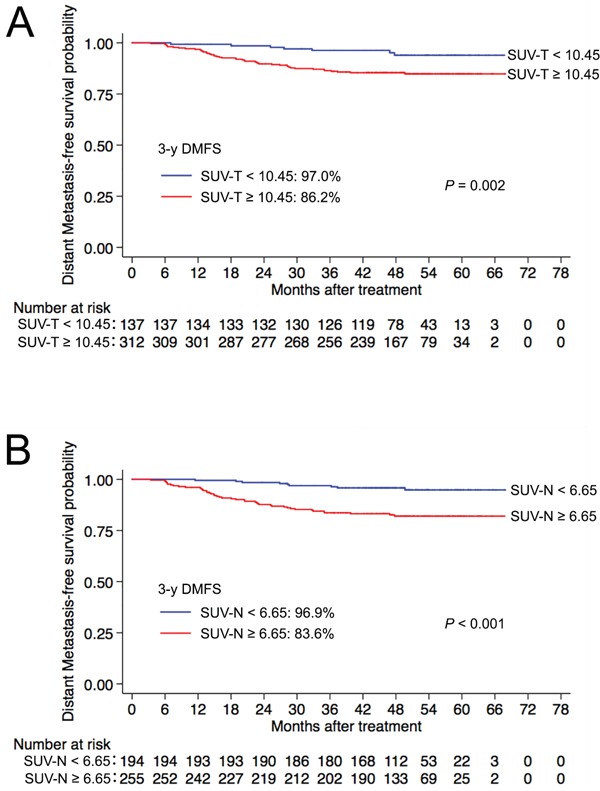
A. SUV-T and B. SUV-N. Abbreviations: SUV-T = SUVmax of the primary tumour; SUV-N = SUVmax of cervical lymph nodes; 3-y = 3-year; DMFS = distant metastasis-free survival; SUVmax = maximum standardized uptake value.
SUVmax for cervical lymph nodes ranged from 2.6 to 40.9 (median, 8.4), and the optimal cut-off SUV-N value for predicting distant metastasis was 6.65. This value was selected to classify patients into SUV-Nhigh (≥6.65) and SUV-Nlow (<6.65) groups. The 3-year DMFS rates for the SUV-N high group (83.6% vs. 96.9%, P <0.001) were significantly lower than the corresponding rates for the SUV-Nlow group (Figure 1B).
Multivariate analysis was performed to adjust for confounding factors. SUV-T (HR, 3.396; 95% CI, 1.451-7.947; P = 0.005) and SUV-N (HR, 2.688; 95% CI, 1.250-5.781; P = 0.011) were found to be independent prognostic factors for DMFS. Additionally, advanced N-classification (N2-3 vs. N0-1) was also associated with an increased risk of distant metastasis (HR, 2.570; 95%CI, 1.422-4.579; P = 0.001).
RPA-based prognostic model for DMFS
We then used RPA to develop an integrated prognostic model based on the independent prognostic factors identified from multivariate analysis (SUV-T, SUV-N and N-classification). Three valid risk groups were derived: low risk (N0-1 + SUV-T <10.45), medium risk (N0-1 + SUV-T >10.45) and high risk (N2-3). In total, 100 (22.3%), 226 (50.3%), and 123 (27.4%) patients belonged to low, medium and high risk groups, respectively, with corresponding 3-year DMFS rates of 99.0%, 91.5%, and 77.5% (P <0.001). Significant differences were observed between the three groups (Figure 2). Multivariate analysis that included host factors (sex, age), tumour factors (T-classification, N-classification), therapeutic intervention (chemotherapy) and RPA-based grouping confirmed the prognostic grouping as the only significant prognostic indicator for DMFS (HR, 3.090; 95% CI, 1.975-4.835; P <0.001; Table 1).
Figure 2.
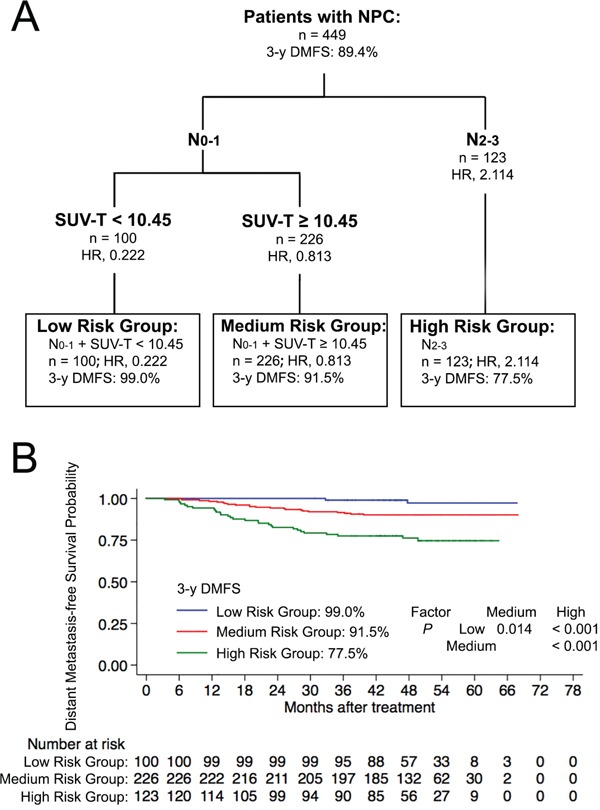
A. Prognostic model for DMFS using recursive partitioning analysis (RPA). B. Distant metastasis-free survival for derived prognostic groups. Abbreviations: 3-y = 3-year; DMFS = distant metastasis-free survival.
Table 1. Univariate and multivariate analysis of prognostic factors for DMFS in 449 patients with NPC.
| Variable | Univariate analysis | Multivariate analysis | |
|---|---|---|---|
| P-value | HR (95% CI) | P–value a | |
| Age | 0.067 | - | 0.196 |
| Gender | 0.667 | 0.994 | |
| Pathologyb (Keratinizing squamous cell carcinoma vs. Non-keratinizing carcinoma) |
0.484 | - | 0.968 |
| Tc (T1-2 vs. T3-4) | 0.033 | - | 0.155 |
| Nc (N0-1 vs. N2-3) | <0.001 | - | 0.576 |
| RPA group | <0.001 | 3.090(1.975-4.835) | <0.001 |
| Chemotherapy | 0.279 | - | 0.920 |
Abbreviations: NPC = nasopharyngeal carcinoma; HR = hazard ratio; CI = confidence interval; T = tumour; N = node; RPA = recursive partitioning analysis.
P-values were calculated using an adjusted Cox proportional hazards model.
Pathological type according to the 2005 World Health Organization classification of tumours.
According to the 7th UICC/AJCC staging system.
DISCUSSION
In this study, we firstly developed an integrated RPA-based prognostic model for DMFS that incorporated SUV-N and N-classification. Using multivariate analysis, the RPA-based prognostic grouping was the only significant indicator for DMFS.
The intensity of tumour FDG uptake is emerging as a valuable predictive factor of treatment outcome [18–20]. 18F-FDG uptake, measured by SUVmax, is correlated with the density and glucose metabolic rate of tumour cells. Tumours with a high pretreatment SUVmax are therefore likely to be dense and metabolically active, and are likely to have a poor prognosis [18]. Previous studies reported that the SUVmax of primary tumours or regional lymph nodes could predict distant failure in patients with NPC [15, 16], which is in accordance with our results.
Anatomic disease extent reflecting disease burden was the original basis of stage grouping of cancers in the TNM classification [9]. However, more and more non-anatomic prognostic factors are emerging [21, 22]. Even though the UICC and AJCC have recognized that prognostic classifications should extend beyond anatomic parameters alone, a method incorporating non-anatomic prognostic factors that meets the needs of practitioners and researchers has not been reported. Incorporating selected non-anatomic factors into the anatomic classification system while maintaining the consistency and sustainability of the TNM framework is perhaps the biggest challenge.
An RPA-based prognostic grouping incorporating anatomic staging, age, and smoking pack-years for human papilloma virus–related oropharyngeal carcinomas has been recently reported, and this has significantly improved survival prediction [17]. In the present study, we have extended this system by integrating an RPA-based prognostic algorithm with SUV-T and N-classification for predicting distant metastasis. The resultant model identified three distinct risk groups: low risk (N0-1 disease + SUV-T <10.45), medium risk (N0-1 disease + SUV-T >10.45), and high risk (N2-3 disease). This RPA-based prognostic model generated a more balanced distribution and offered superior hazard discrimination compared to N-classification alone, and was confirmed to be the only significant prognostic indicator for DMFS in multivariate analysis.
Despite the promising results, our study has some limitations. Firstly, the proposed model is derived from retrospective analysis of existing data from one institution, and a multi-institution study is needed to confirm our results. Secondly, pretreatment EBV DNA load has been demonstrated to be a valuable prognostic factor in NPC, but this data was only available for a few patients in our cohort and could not be incorporated in our model. Further studies are therefore needed to investigate whether adding EBV DNA data could further improve prediction of metastasis.
In conclusion, analysis of data from a large cohort of NPC patients allowed us to develop an integrated RPA-based prognostic model that incorporates SUV-N and N-classification. Our model performed better at predicting the likelihood of metastasis than previously reported models, and may prove useful for predicting distant metastasis and aiding treatment decisions in the clinic.
PATIENTS AND METHODS
Patients
This study was approved by the institutional review board, and the requirement to obtain informed consent was waived. From January 2010 to February 2012, 449 patients with stage I-IVB NPC treated at our institution received a positron emission tomography-computed tomography (PET-CT) examination before treatment followed by intensity-modulated radiotherapy (IMRT) with or without chemotherapy. All of the enrolled patients were of Chinese ethnicity. The median age was 46 years (range, 20- 77), with a male-to-female ratio of 3:1 (Table 2).
Table 2. Clinicopathological characteristics of 449 patients with NPC.
| Characteristics | No. of 449 patients |
|---|---|
| Sex | |
| Male | 338 (75.3%) |
| Female | 111 (24.7%) |
| Age (years) | |
| <50 | 302 (67.3%) |
| ≥50 | 147 (32.7%) |
| Histological typea | |
| Keratinizing squamous cell carcinoma | 4 (0.9%) |
| Non-keratinizing carcinoma | 445 (99.1%) |
| Chemotherapy | |
| Yes | 385 (85.7%) |
| No | 64 (14.3%) |
| T-categoryb | |
| T1 | 76 (16.9%) |
| T2 | 76 (16.9%) |
| T3 | 227 (50.6%) |
| T4 | 70 (15.6%) |
| N-categoryb | |
| N0 | 75 (16.7%) |
| N1 | 251 (55.9%) |
| N2 | 76 (16.9%) |
| N3 | 47 (10.5%) |
| Stageb | |
| I | 22 (4.9%) |
| II | 95 (21.2%) |
| III | 223 (49.7%) |
| IV | 109 (24.3%) |
Abbreviations: NPC = nasopharyngeal carcinoma; T = tumour; N = node.
Pathological type according to the 2005 World Health Organization classification of tumours.
According to the 7th edition of the UICC/AJCC staging system.
All patients underwent a pretreatment evaluation that included a complete patient history, physical examination, haematology and biochemistry profiles, MRI of the neck and nasopharynx, and PET-CT. All patients were staged according to the 7th edition of the International Union against Cancer/American Joint Committee on Cancer (UICC/AJCC) system [9].
PET/CT imaging
Serum glucose levels were measured in all NPC patients, all of whom fasted for at least 6 h before PET/CT scans, and individuals with a fasting plasma glucose >200 mg/dl were excluded. PET/CT imaging was performed with a combination PET/CT scanner (Discovery ST 16; GE Healthcare, Little Chalfont, UK) according to published guidelines [23]. Helical CT was performed from the head to the proximal thigh before PET acquisition, according to a standardized protocol and were 45-60 min after injection of 5.55 MBq/kg FDG. PET images were reconstructed from CT data for attenuation correction using an ordered-subset expectation maximization iterative reconstruction algorithm. SUVmax was determined for each region of interest using the whole-body attenuation corrected image and the following formula: SUVmax = tissue concentration of 18F-FDG / injected dose / body weight.
Treatment
The nasopharyngeal and neck tumour volumes of all patients were treated using radical radiotherapy based on IMRT for the entire treatment course. Institutional guidelines recommended radiotherapy only for stage I and concurrent chemoradiotherapy ± neoadjuvant/adjuvant chemotherapy for stage II-IVB. In total, 92.8% (308/332) of patients with stage III-IVB disease received concurrent chemoradiotherapy ± neoadjuvant/adjuvant chemotherapy. When possible, salvage treatments (intracavitary brachytherapy, surgery or chemotherapy) were provided and persistent disease or relapse was documented.
Follow-up
Patients were examined at least every three months during the first two years, and every six months during years 3-5 or until death. Evaluation during follow-up included a complete patient history, physical examination, haematology and biochemistry profiles, MRI of the neck and nasopharynx, chest radiography, abdominal sonography and a whole-body bone scan. Any residual disease found at the nasopharynx or cervical nodes within 6 months after completion of RT was regarded as local failure or regional failure, respectively. All distant metastases were diagnosed by clinical symptoms, physical examination, and imaging methods that included chest radiography, bone scan, MRI, CT, PET-CT, and abdominal sonography [24].
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA), and distant metastasis-free survival (DMFS) was the defined outcome and was calculated from the first day of treatment to the first distant metastasis. The area under the receiver-operating characteristic (ROC) curve was used to select the optimal cut-off point for SUV-T and SUV-N by maximizing the conditional Youden score, based on the method described by Hanley [25] and Zweig [26]. Survival rates were calculated using the Kaplan-Meier method and compared using the log-rank test [27]. Multivariate analysis based on the Cox proportional hazards model was used to calculate HRs and 95% confidence intervals (CIs), and to test the independent significance of different factors by backward elimination of insignificant variables [28] including host factors (sex, age), tumour factors (T-classification, N-classification), and therapeutic intervention (chemotherapy) as covariates.
Finally, we performed RPA for DMFS to derive prognostic groups that combined anatomic category with other survival predictors identified from multivariate analysis. The RPA algorithm is based on the optimized binary partition of predictors. The resultant subgroups were similar in terms of survival. All tests were two-sided, and P <0.05 was considered statistically significant.
Footnotes
CONFLICTS OF INTEREST
The authors declared no conflicts of interest.
GRANT SUPPORT
This work was supported by Grants from National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2014BAI09B10), Science and Technology Project of Guangzhou City, China (No. 14570006), the Planned Science and Technology Project of Guangdong Province (No. 2013B020400004), and Health & Medical Collaborative Innovation Project of Guangzhou City, China (201400000001).
REFERENCES
- 1.IARC. GLOBOCAN 2012:Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. 2012 Available at: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (Accessed: 27nd October 2015)
- 2.Chan AT. Nasopharyngeal carcinoma. Annals of oncology. 2010;21:vii308–312. doi: 10.1093/annonc/mdq277. [DOI] [PubMed] [Google Scholar]
- 3.Lee AW, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Seminars in radiation oncology. 2012;22:233–244. doi: 10.1016/j.semradonc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Tao CJ, Liu X, Tang LL, Mao YP, Chen L, Li WF, Yu XL, Liu LZ, Zhang R, Lin AH, Ma J, Sun Y. Prognostic scoring system for locoregional control among the patients with nasopharyngeal carcinoma treated by intensity-modulated radiotherapy. Chinese journal of cancer. 2013;32:494–501. doi: 10.5732/cjc.013.10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee N, Xia P, Quivey JM, Sultanem K, Poon I, Akazawa C, Akazawa P, Weinberg V, Fu KK. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. International journal of radiation oncology, biology, physics. 2002;53:12–22. doi: 10.1016/s0360-3016(02)02724-4. [DOI] [PubMed] [Google Scholar]
- 6.Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, Sun Y, Lin AH, Liu MZ, Ma J. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? International journal of radiation oncology, biology, physics. 2011;80:661–668. doi: 10.1016/j.ijrobp.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT, Zee BC, Law SC, Teo PM, Tung SY, Kwong DL, Lau WH. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. International journal of radiation oncology, biology, physics. 2005;61:1107–1116. doi: 10.1016/j.ijrobp.2004.07.702. [DOI] [PubMed] [Google Scholar]
- 8.Tham IW, Hee SW, Yeo RM, Salleh PB, Lee J, Tan TW, Fong KW, Chua ET, Wee JT. Treatment of nasopharyngeal carcinoma using intensity-modulated radiotherapy-the national cancer centre singapore experience. International journal of radiation oncology, biology, physics. 2009;75:1481–1486. doi: 10.1016/j.ijrobp.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Mao YP, Xie FY, Liu LZ, Sun Y, Tian L, Tang LL, Lin AH, Li L, Ma J. The seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma is prognostically useful for patients treated with intensity-modulated radiotherapy from an endemic area in China. Radiotherapy and oncology. 2012;104:331–337. doi: 10.1016/j.radonc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Yue D, Xu YF, Zhang F, Lin L, Mao YP, Li WF, Chen L, Sun Y, Liu LZ, Lin AH, Li L, Ma J. Is replacement of the supraclavicular fossa with the lower level classification based on magnetic resonance imaging beneficial in nasopharyngeal carcinoma? Radiotherapy and oncology. 2014;113:108–114. doi: 10.1016/j.radonc.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 12.Pan J, Xu Y, Qiu S, Zong J, Guo Q, Zhang Y, Lin S, Lu JJ. A Comparison Between the Chinese 2008 and the 7th Edition AJCC Staging Systems for Nasopharyngeal Carcinoma. American journal of clinical oncology. 2013 doi: 10.1097/COC.0b013e31828f5c96. [DOI] [PubMed] [Google Scholar]
- 13.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001;285:914–924. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- 14.Allal AS, Dulguerov P, Allaoua M, Haenggeli CA, El-Ghazi el A, Lehmann W, Slosman DO. Standardized uptake value of 2-[(18)F] fluoro-2-deoxy-D-glucose in predicting outcome in head and neck carcinomas treated by radiotherapy with or without chemotherapy. Journal of clinical oncology. 2002;20:1398–1404. doi: 10.1200/JCO.2002.20.5.1398. [DOI] [PubMed] [Google Scholar]
- 15.Hung TM, Wang HM, Kang CJ, Huang SF, Liao CT, Chan SC, Ng SH, Chen IH, Lin CY, Fan KH, Chang JT. Pretreatment (18)F-FDG PET standardized uptake value of primary tumor and neck lymph nodes as a predictor of distant metastasis for patients with nasopharyngeal carcinoma. Oral oncology. 2013;49:169–174. doi: 10.1016/j.oraloncology.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Chan SC, Chang JT, Wang HM, Lin CY, Ng SH, Fan KH, Chin SC, Liao CT, Yen TC. Prediction for distant failure in patients with stage M0 nasopharyngeal carcinoma: the role of standardized uptake value. Oral oncology. 2009;45:52–58. doi: 10.1016/j.oraloncology.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Huang SH, Xu W, Waldron J, Siu L, Shen X, Tong L, Ringash J, Bayley A, Kim J, Hope A, Cho J, Giuliani M, Hansen A, Irish J, Gilbert R, Gullane P, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. Journal of clinical oncology. 2015;33:836–845. doi: 10.1200/JCO.2014.58.6412. [DOI] [PubMed] [Google Scholar]
- 18.Xie P, Yue JB, Fu Z, Feng R, Yu JM. Prognostic value of 18F-FDG PET/CT before and after radiotherapy for locally advanced nasopharyngeal carcinoma. Annals of oncology. 2010;21:1078–1082. doi: 10.1093/annonc/mdp430. [DOI] [PubMed] [Google Scholar]
- 19.Lee SW, Nam SY, Im KC, Kim JS, Choi EK, Ahn SD, Park SH, Kim SY, Lee BJ, Kim JH. Prediction of prognosis using standardized uptake value of 2-[(18)F] fluoro-2-deoxy-d-glucose positron emission tomography for nasopharyngeal carcinomas. Radiotherapy and oncology. 2008;87:211–216. doi: 10.1016/j.radonc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Chan WK, Kwong DL, Yeung DW, Huang B, Khong PL. Prognostic impact of standardized uptake value of F-18 FDG PET/CT in nasopharyngeal carcinoma. Clinical nuclear medicine. 2011;36:1007–1011. doi: 10.1097/RLU.0b013e31821a29a4. [DOI] [PubMed] [Google Scholar]
- 21.Zhou GQ, Tang LL, Mao YP, Chen L, Li WF, Sun Y, Liu LZ, Li L, Lin AH, Ma J. Baseline serum lactate dehydrogenase levels for patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. International journal of radiation oncology, biology, physics. 2012;82:e359–365. doi: 10.1016/j.ijrobp.2011.06.1967. [DOI] [PubMed] [Google Scholar]
- 22.Chen YP, Zhao BC, Chen C, Shen LJ, Gao J, Mai ZY, Chen MK, Chen G, Yan F, Liu S, Xia YF. Pretreatment platelet count improves the prognostic performance of the TNM staging system and aids in planning therapeutic regimens for nasopharyngeal carcinoma: a single-institutional study of 2,626 patients. Chinese journal of cancer. 2015;34:137–146. doi: 10.1186/s40880-015-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, Townsend DW, Berland LL, Parker JA, Hubner K, Stabin MG, Zubal G, Kachelriess M, Cronin V, Holbrook S. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. Journal of nuclear medicine. 2006;47:885–895. [PubMed] [Google Scholar]
- 24.Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, Li WX, Chen YY, Xie FY, Liang SB, Chen Y, Xu TT, Li B, Long GX, Wang SY, Zheng BM, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. The Lancet Oncology. 2012;13:163–171. doi: 10.1016/S1470-2045(11)70320-5. [DOI] [PubMed] [Google Scholar]
- 25.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 26.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clinical chemistry. 1993;39:561–577. [PubMed] [Google Scholar]
- 27.Dinse GE, Lagakos SW. Nonparametric estimation of lifetime and disease onset distributions from incomplete observations. Biometrics. 1982;38:921–932. [PubMed] [Google Scholar]
- 28.Cox DR. Regression models and life- tables. J R Stat Soc Ser B (Methodological) 1972;34:187–220. [Google Scholar]


