Abstract
Integrins αvβ3 and αvβ5 regulate angiogenesis and invasiveness in cancer, potentially by modulating activation of the transforming growth factor (TGF)-β pathway. The randomized phase III CENTRIC and phase II CORE trials explored the integrin inhibitor cilengitide in patients with newly diagnosed glioblastoma with versus without O6-methylguanine DNA methyltransferase (MGMT) promoter methylation. These trials failed to meet their primary endpoints.
Immunohistochemistry was used to assess the levels of the target integrins of cilengitide, αvβ3 and αvβ5 integrins, of αvβ8 and of their putative target, phosphorylation of SMAD2, in tumor tissues from CENTRIC (n=274) and CORE (n=224).
αvβ3 and αvβ5 expression correlated well in tumor and endothelial cells, but showed little association with αvβ8 or pSMAD2 levels. In CENTRIC, there was no interaction between the biomarkers and treatment for prediction of outcome. In CORE, higher αvβ3 levels in tumor cells were associated with improved progression-free survival by central review and with improved overall survival in patients treated with cilengitide.
Integrins αvβ3, αvβ5 and αvβ8 are differentially expressed in glioblastoma. Integrin levels do not correlate with the activation level of the canonical TGF-β pathway. αvβ3 integrin expression may predict benefit from integrin inhibition in patients with glioblastoma lacking MGMT promoter methylation.
Keywords: glioblastoma, integrin, pSmad, TGF-β, biomarker
INTRODUCTION
Integrins are a family of 24 heterodimeric cell surface receptors that participate in signal transduction during many cellular processes. They are also involved in cellular communication with the extracellular matrix, e.g. during adhesion, motility, migration, invasion and angiogenesis. Their abundant expression in tumor-associated endothelial cells [1, 2] and presumed biological roles led to integrins αvβ3 and αvβ5 being identified and validated as therapeutic targets in glioblastoma in preclinical models [3, 4]. These data supported the clinical development program for the pentapeptide, first-in-class integrin inhibitor, cilengitide [5, 6]. In phase I, dose-limiting toxicity was not seen at doses up to 2400 mg/m2, whereas clinical activity was seen at both low and high levels [7]. A randomized phase II trial in recurrent glioblastoma comparing two different doses of cilengitide noted a moderate radiological response rate, interpreted to reflect biological activity, and a trend towards better outcome with the higher dose of cilengitide [8]. Improved outcome at higher dose was also observed in a randomized phase II trial in the newly diagnosed setting in combination with the standard of care, temozolomide chemoradiotherapy (TMZ/RT→TMZ) [9]. An earlier uncontrolled phase II trial indicated preferential benefit from cilengitide in newly diagnosed glioblastoma patients with, as opposed to without, O6-methylguanine DNA methyltransferase (MGMT) promoter methylation [10].
Based on these data, separate trials were designed for patients with (“CENTRIC”) and without (“CORE”) MGMT promoter methylation. The phase III CENTRIC trial was designed to verify the activity of cilengitide in newly diagnosed patients with MGMT promoter methylation. The exploratory phase II CORE trial explored, in addition, whether intensified the dose of cilengitide during radiotherapy might provide a signal of activity in patients with tumors lacking MGMT promoter methylation, too. Neither trial demonstrated biological activity of cilengitide defined by the primary endpoints of the trials [11, 12], resulting in the discontinuation of the clinical development of cilengitide.
Detailed analysis of the expression of the target integrins of cilengitide, αvβ3 and αvβ5 in tumor and endothelial cells, might result in a better understanding of these disappointing trial results. Unfortunately, tumor tissues were not systematically collected in the earlier trials, and appropriate antibodies have only recently been generated [13].
While αvβ3 and αvβ5 expression in glioblastoma have been related mainly to angiogenesis, αvβ8 has been attributed roles in migration and invasion [14-16]. Among the multiple effects of integrin signaling, we have recently delineated how the target integrins of cilengitide, αvβ3 and αvβ5, as well as αvβ8 integrin, may control activity of the transforming growth factor (TGF)-β pathway [17-19], which has been linked to the malignant phenotype of glioblastoma. Specifically, we observed that either exposure to cilengitide or gene silencing of αvβ3, αvβ5 or αvβ8, or neutralizing antibodies to these integrins reduced (TGF)-β1/2 mRNA expression, protein release and pSMAD2 phosphorylation, a surrogate marker of canonical TGF-β pathway activation [20], in glioma cells [17]. Conversely, integrin αvβ3 expression had previously been reported to be induced by TGF-β [21], potentially constituting a positive feedback loop. These data indicated that pSmad2 levels could serve as a biomarker to identify integrin signaling-dependent tumors. While the prognostic role of elevated pSMAD2 levels has remained controversial [20, 22], TGF-β itself is also a candidate therapeutic target in glioblastoma [23]. Accordingly, here we studied integrin expression profiles in tumors of patients enrolled in the CENTRIC and CORE trials and explored whether these expression profiles were related to levels of pSMAD2 and outcome.
RESULTS
Tumor and patient characteristics
We studied the levels of integrins αvβ3, αvβ5 and αvβ8 and of pSMAD2 by immunocytochemistry in tissues obtained at study entry from patients randomized into the CENTRIC or CORE trials. Tissue samples from 498 patients were analyzed, representing 61% of the patient cohorts. For 39% of the patients, no or insufficient tumor tissue was submitted or available for ancillary biological investigations. Samples were received from 106 and 52 centers in CENTRIC and CORE, respectively, whereof 35 centers were in common. Patient characteristics, treatment received and outcome by trial and in the biomarker cohort are summarized in Table 1. Patients in all groups received a median of 6 cycles of TMZ. That PFS in CORE is still only 6 months from randomization, can be explained by the recommendation to consider the possibility of pseudoprogression in the adjuvant treatment phase and not to stop adjuvant TMZ too early unless there was unequivocal PD. There was no significant outcome difference between the patients in the biomarker cohort and those where biomarkers were not evaluated (Supplementary Table S1).
Table 1. Summary of patient characteristics, treatment delivery and outcome.
| CENTRIC All Patients N=545 |
CENTRIC Biomarker Cohort n=274 |
CORE All Patients N=265 |
CORE Biomarker Cohort n=224 |
|
|---|---|---|---|---|
| Age at baseline | ||||
| Median (years) | 57.9 | 58.8 | 56.2 | 56.6 |
| Range (years) | 21.7 - 81.0 | 21.7-81.0 | 20.8 - 77.5 | 20.8 - 76.5 |
| Gender | ||||
| Male | 291 (53.4) | 148 (54.0%) | 155 (58.5) | 131 (58.5) |
| Female | 254 (46.6) | 126(46.0%) | 110 (41.5) | 93 (41.5) |
| Histological subtype | ||||
| Glioblastoma | 496 (91.0) | 248 (90.5) | 251 (94.7) | 211 (94.2) |
| Gliosarcoma | 21 (3.9) | 10 (3.6) | 10 (3.8) | 9 (4.0) |
| Giant cell | 17 (3.1) | 12 (4.4) | 3 (1.1) | 3 (1.3) |
| Other | 11 (2.0) | 4 (1.5) | 1 (0.4) | 1 (0.4) |
| ECOG Performance Status at baseline | ||||
| PS 0 | 309 (56.7) | 163 (59.5) | 131 (49.4) | 111 (49.6) |
| PS 1 | 236 (43.3) | 111 (40.5) | 132 (49.8) | 111 (49.6) |
| No data | 0 (0.0) | 0 (0.0) | 2 (0.8) | 2 (0.9) |
| Surgery | ||||
| Subtotal resection (partial/biopsy) | 274 (50.3) | 128 (46.7) | 128 (48.3) | 102 (45.5) |
| Gross total resection | 269 (49.4) | 144 (52.6) | 136 (51.3) | 121 (54.0) |
| No data | 2 (0.4) | 2 (0.7) | 1 (0.4) | 1 (0.4) |
| Treatment received | ||||
| TMZ (n=273) | TMZ (n=137) | TMZ (n=89) | TMZ (n=71) | |
| Received study intervention | 258 (94.5) | 130 (94.9) | 85(95.5) | 68 (95.8) |
| Started RTX | 256 (93.8) | 129 (94.2) | 85(95.5) | 68 (95.8) |
| Started maintenance TMZ | 211 (77.3) | 106 (77.4) | 71(80.0) | 58 (81.7) |
| Number of TMZ maintenance cycles | ||||
| Median | 6 | 6 | 6 | 6 |
| Range | 1-32 | 1-21 | 1-11 | 1-8 |
| Cilengitide (n=272) | Cilengitide (n=137) | Cilengitide (n=176) | Cilengitide (n=153) | |
| Received study intervention | 263 (96.7) | 133 (97.0) | 170(96.6) | 147(96.1) |
| Started Pre-RTX phase | 259 (95.2) | 131 (95.6) | 168(95.5) | 146(95.4) |
| Started RTX phase | 260 (95.6) | 131 (95.6) | 168(95.5) | 146(95.4) |
| Started maintenance TMZ | 221 (81.3) | 111 (81.0) | 144(81.8) | 125(81.7) |
| Number of TMZ maintenance cycles | ||||
| Median | 6 | 6 | 6 | 6 |
| Range | 1-21 | 1-21 | 1-19 | 1-19 |
| Started cilengitide monotherapy phase | 168 (61.8) | 77 (56.2) | 85(48.3) | 74(48.4) |
| Total number of cilengitide infusions | ||||
| Median | 90 | 72 | 69 | 69 |
| Range | 1-388 | 1-317 | 2-224 | 2-224 |
| Outcome | ||||
| Median PFS (months, 95% CI) | 12.3 (10.6, 13.6) | 12.1 (10.4,13.6) | 6.2 (5.9, 7.7) | 6.3 (5.9, 7.7) |
| Median OS (months, 95% CI) | 26.3 (24.4, 29.3) | 25.4 (23.3,30.9) | 14.4 (13.4, 15.6) | 14.1 (12.9, 15.5) |
Integrin and pSMAD2 staining patterns
The expression of the integrins within most tumor samples was heterogeneous. Staining was localized to the cytoplasm with sparing of the nuclei and without membranous accentuation. Representative staining patterns illustrating the H scores are depicted in Figure 1. Antigen expression was evaluated separately in the tumor and endothelial compartments. The quantitative assessments are summarized in Table 2 and Figure 2. In CENTRIC, αvβ3 levels in tumor cells correlated weakly with αvβ3 in endothelial cells (SSC=0.26, p<0.0001), with αvβ5 in tumor cells (SSC=0.18, p=0.002) and with αvβ5 levels in endothelial cells (SSC=0.16, p=0.006). αvβ5 levels in tumor and endothelial cells were also weakly correlated (SSC=0.17, p=0.003) whereas pSMAD2 levels in both compartments showed strong correlation (SSC=0.50, p<0.0001). In CORE, αvβ3 levels in tumor cells weakly correlated with αvβ3 levels in endothelial cells (SSC=0.26, p<0.001) and with αvβ5 levels in endothelial cells (SSC=0.29, p<0.001). αvβ5 levels in tumor and endothelial cells were weakly correlated (SSC=0.29, p<0.001). αvβ5 levels in endothelial cells correlated weakly with αvβ8 in tumor cells (SSC=0.21, p=0.002). There was good correlation between pSMAD2 levels in both compartments (SSC=0.55, p<0.001) (Supplementary Table S2). Investigation of the relationship among the different markers by PCA illustrated in Figure 3A underlines the correlations detailed above. It does not support a direct relationship between the integrins and pSMAD2 levels as measured by immunohistochemistry. The markers analyzed do not segregate the tumors into different subgroups (Figure 3B). Further, exploration of the major sources of variation among tumors did not indicate any difference by gender (p=0.088, not shown), or age (Figure 3C, p=0.380), but a significant difference between the two studies, CENTRIC and CORE (p=0.001) was observed (Figure 3D). However, the analysis of the variation fraction revealed that the variable “center” explained 40% of the total variation (between-group ratio=0.403, p-value < 0.001), and only 2% were attributed to differences between the studies (between-group ratio=0.022, p-value< 0.001). Nevertheless, the overall structure of the relationships among markers between CORE and CENTRIC, when analyzed separately, is preserved (not shown).
Figure 1. Immunohistochemical assessment of integrins and pSmad2 in glioblastoma.
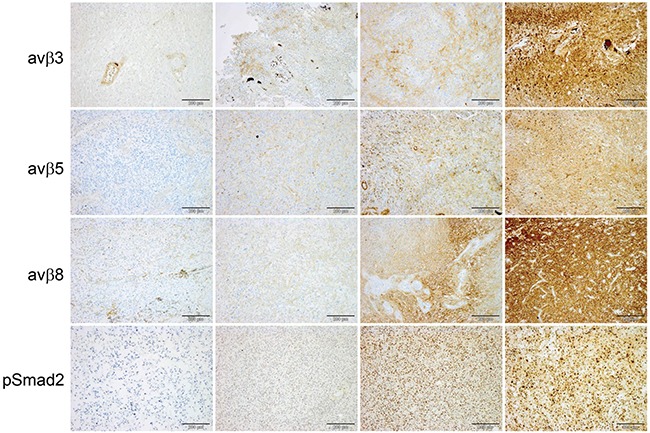
Representative sections immunostained for αvβ3, αvβ5, αvβ8 and pSMAD2. Negative staining of tumor tissue with immunolabeled vasculature as internal positive control (left column), H score (tumor) < 100 second column, H score (tumor) 101-200 third column, H score (tumor) >200 (right column), size bars correspond to 200 μm.
Table 2. Quantitative assessment of immunohistochemistry data.
| CENTRIC biomarker cohort n=274* |
CORE biomarker cohort n=224* |
|
|---|---|---|
| αvβ3 tumor cells | ||
| Median | 0 | 0 |
| Range | 0–220 | 0–300 |
| N | 294 | 241 |
| αvβ3 endothelial cells | ||
| Median | 10 | 30 |
| Range | 0-300 | 0-300 |
| N | 294 | 241 |
| αvβ5 tumor cells | ||
| Median | 60 | 90 |
| Range | 0-285 | 0-300 |
| N | 294 | 237 |
| αvβ5 endothelial cells | ||
| Median | 125 | 140 |
| Range | 0-280 | 0-300 |
| N | 292 | 236 |
| αvβ8 tumor cells | ||
| Median | 180 | 200 |
| Range | 0-300 | 0-300 |
| N | 283 | 231 |
| αvβ8 endothelial cells | ||
| Median | 0 | 0 |
| Range | 0-120 | 0-150 |
| N | 283 | 231 |
| pSMAD2 tumor cells | ||
| Median | 70 | 79 |
| Range | 0-270 | 0-260 |
| N | 281 | 227 |
| pSMAD2 endothelial cells | ||
| Median | 30 | 46 |
| Range | 0-145 | 0-190 |
| N | 281 | 227 |
Note that n in the table may be higher for individual markers since the biomarker cohorts were defined as patients with tumors where all markers were assessed.
Figure 2. Quantitative assessment of immunohistochemistry data.
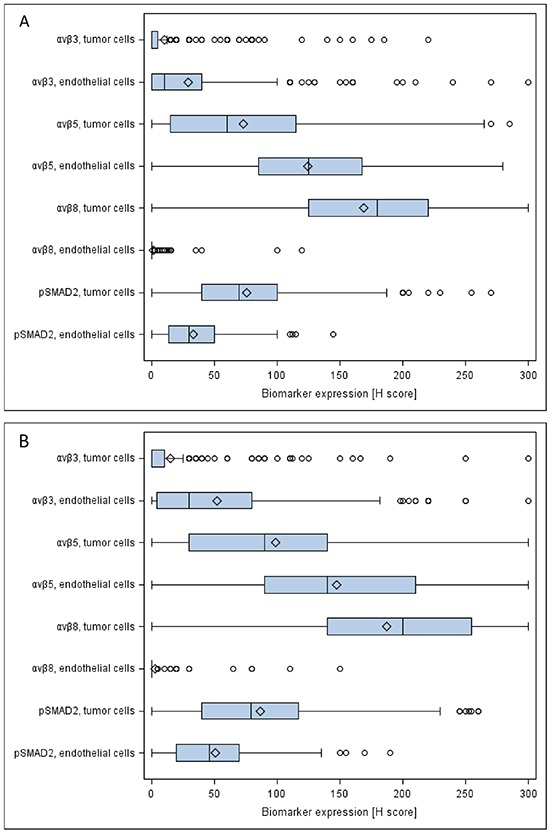
Boxplots of biomarker expression in the CENTRIC A. and CORE B. biomarker cohorts are depicted. The boxes represent the interquartile range split by the medians. Diamonds represent the means. Lines extending horizontally from the boxes indicate variability outside the range (lowest value within 1.5 IQR of Q1, and highest value within 1.5 IQR of Q3). Outliers beyond 1.5 IQR are plotted as individual points.
Figure 3. Principal component analysis (PCA) of biomarker analyses.
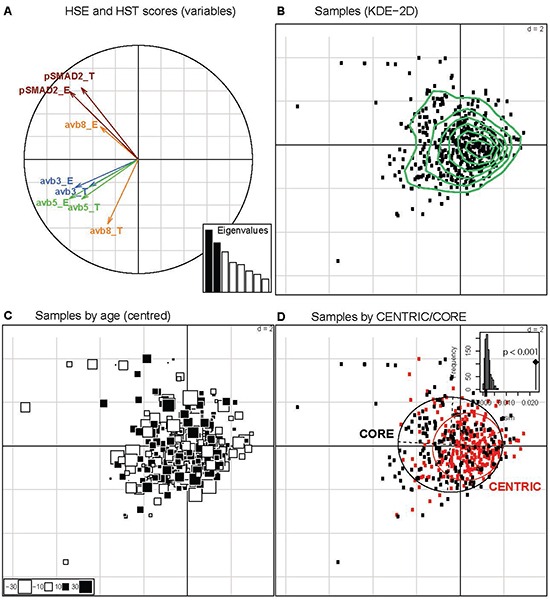
A. The histoscores of the markers for tumor (HST) and endothelial cells (HSE) are represented on the first vectorial plan of the PCA. The two first distinct eigenvalues (Histogram of Eigenvalues, in black) explain 42.6% of the total variation. B. The patient samples are projected onto the two first axes of the PCA and patterns were explored by Kernel Density Estimation (KDE) in these two dimensions (green curves). No indications for marker driven subgroups are observed. C. Each patient sample is represented by a square, with proportional size to the distance to the mean age (55.3 years). The white and black squares identify the patients with age inferior or superior to the mean age, respectively. D. The impact of the study (CENRTIC/CORE) was investigated on the two first axes visualized by the inertia ellipses for CENTRIC (red) and CORE (black). A significant difference is observed (P < 0.001, between-group permutation tests) illustrated by a histogram, where the observed value is given by a black vertical line.
Clinical pathological correlations
In the CENTRIC cohort, there was no significant interaction between the biomarkers and treatment for the prediction of PFS determined by central review (Figure 4A) or investigator assessment (Supplementary Figure S1A) or for the prediction of OS (Figure 5A). In contrast, in CORE, higher αvβ3 levels in tumor cells were associated with improved PFS by central review (Figure 4B, p=0.036) and improved OS (Figure 5B, p=0.02) in patients treated with cilengitide. This effect persisted when analysed stratified for prognostic factors, including age, RPA score, extent of surgery, MMSE, or ECOG PS (data on shown). However, the PFS effect was not confirmed when exploring investigator-assessed PFS (investigator PFS interaction test p=0.345, IRC PFS p=0.036) (Supplementary Figure S1B).
Figure 4. Forest plots: predictive value of biomarkers for the efficacy of cilengitide for PFS assessed by central review.
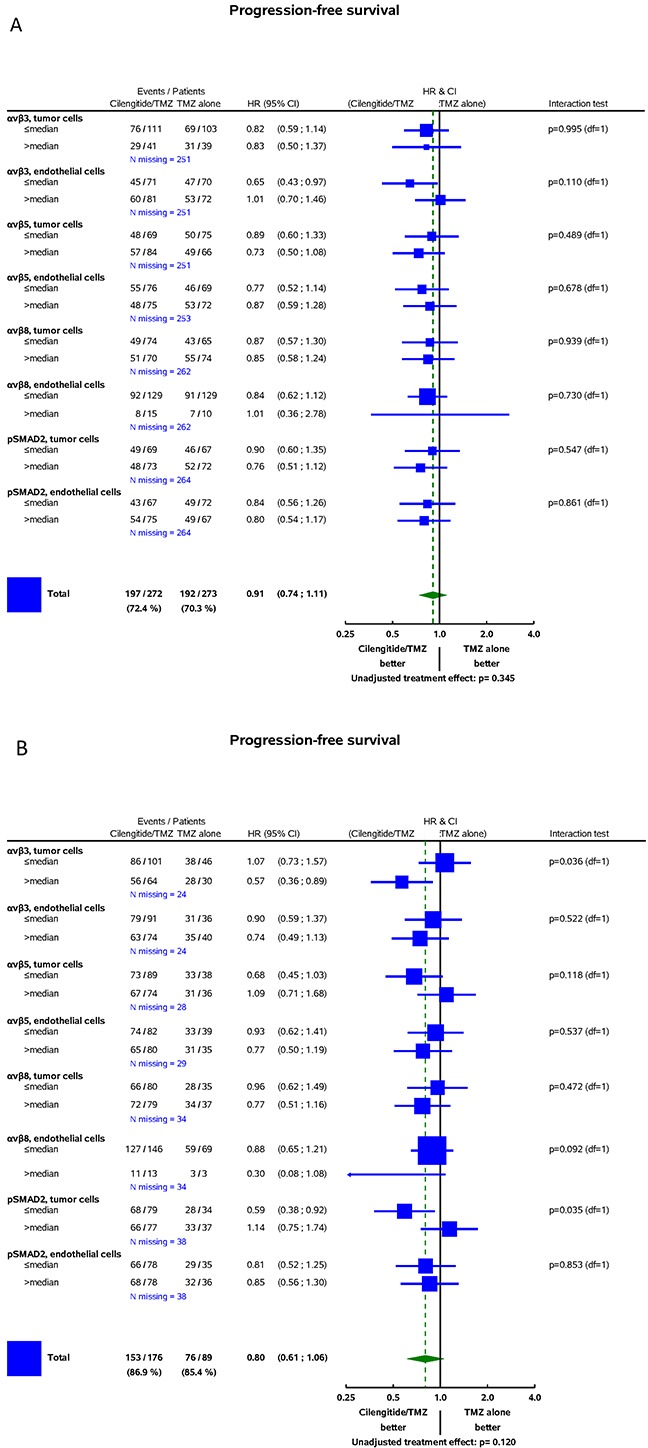
A. CENTRIC. B. CORE. On the left-hand, the integrin subgroups with numbers of events/sample size by treatment arm, number of missing data, and hazard ratios (HR) with 95% confidence intervals are shown. The vertical line represents the absence of differential effects between the two treatments, i.e., if for an integrin subgroup, the 95% confidence intervals overlap with this line, it indicates that treatment effects are not different. The square represents the Cilengitide/TMZ hazard ratio in the integrin subgroup. The area of each square is proportional to the number of events. The diamond indicates a differential effect of treatment in the whole cohort. Diamond overlapping the vertical lines indicates in-significantly different treatment effects at 5% significance. On the right-hand, interaction tests are presented. They assess the significance of a differential treatment effect between two integrin subgroups, i.e., tests have one degree of freedom (df=1).
Figure 5. Forest plots: predictive value of biomarkers for the efficacy of cilengitide for OS.
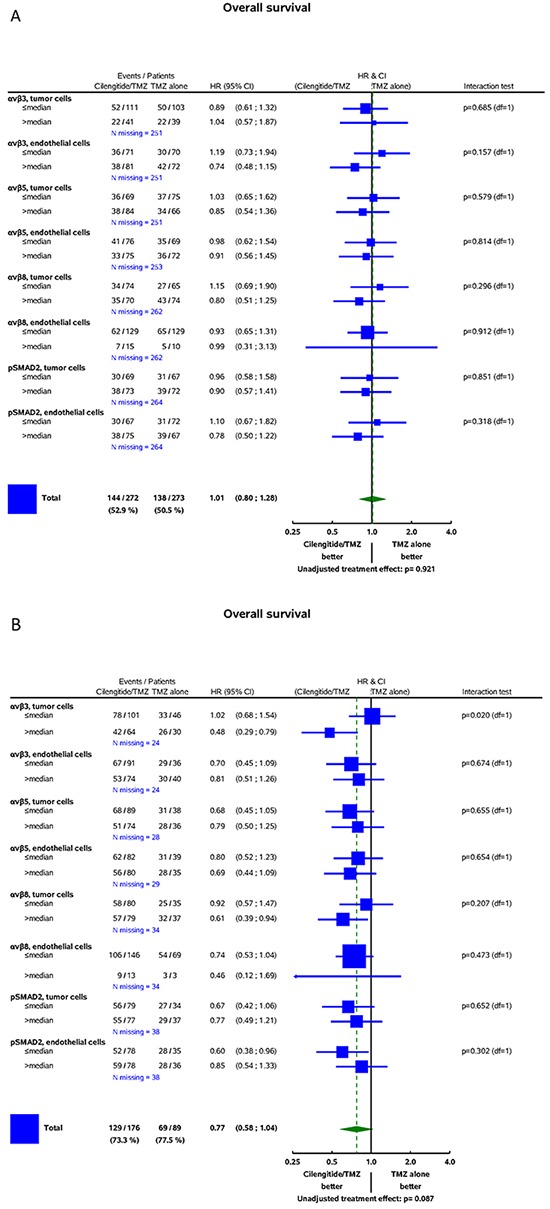
A. CENTRIC B. CORE (for detailed explanations, see Figure 4).
In order to maximize the statistical power, the univariate prognostic value of the biomarkers was assessed in the whole cohort. No significant association of integrin expression in tumor or endothelial cells with outcome was revealed in either trial cohort. Similarly, no prognostic role for pSMAD2 levels became apparent (Supplementary Table S3 and S4).
DISCUSSION
The main goal of this study was to explore whether the expression patterns of the putative target integrins of cilengitide, αvβ3 and aβ5, might shed light on the results of the CENTRIC and CORE clinical trials. We find that the target integrins are differentially expressed in tumor and endothelial cells within glioblastomas (Figures 1 and 2, Table 2). In contrast to previous analyses of smaller, non-clinical trial cohorts, [24] integrin expression was neither prognostic in the CENTRIC nor in the CORE biomarker cohort. The differential expression of target integrins either in tumor or in endothelial cells was unrelated to outcome with cilengitide treatment in the CENTRIC trial. In contrast, higher αvβ3 levels in tumor cells were associated with improved PFS by central review and with improved OS in cilengitide-treated patients in the CORE cohort (Figure 4B and 5B). Patient cohorts from both trials were analysed separately because of the powerful prognostic role of MGMT promoter methylation, the biomarker that determined patient enrolment in either CENTRIC or CORE.
The levels of αvβ3 and αvβ5 correlated weakly, both in tumor and endothelial cells, suggesting a common upstream regulatory pathway regulating integrin expression in both compartments in glioblastoma. In contrast, the levels of αvβ3 and αvβ5 showed almost no relation to the levels of αvβ8. While αvβ8 is not a target of cilengitide, it may be involved in the activation of latent TGF-β [17, 25]. Importantly, there is no surrogate biomarker reflecting integrin activity as opposed to mere expression which may be heterogeneous within a tumor [26], and defining a biomarker of integrin pathway activation may hαve been more informative, e.g., levels of focal adhesion kinase or other focal adhesion-associated proteins. Importantly, however, the present analysis suggests that enrichment for patients with tumors with high levels of target integrin expression would not hαve altered the negative outcome at least in the CENTRIC trial.
A second goal of this study was to verify whether the involvement of αv integrin in controlling TGF-β pathway activity [17] is reflected by correlated integrin expression and Smad2 phosphorylation, a marker for TGF-β pathway activity [20]. This was not confirmed in either trial cohort (Figure 3; Supplementary Table S2), suggesting that expression of integrins alone is not an adequate surrogate marker for integrin activity in situ. Alternatively, pSMAD2 levels in glioblastoma and the TGF-β pathway may not be controlled by αv integrins to a relevant extent. Interestingly, however, there was strong correlation between pSMAD2 levels in tumor and in endothelial cells, suggesting that both compartments are equally responsive to TGF-β activity in the tumor microenvironment. Thus, escape from the inhibitory signaling activity of TGF-β as seen in other cancers, e.g., colon cancer, is not required in glioblastoma, at least not at the level of Smad2 canonical signaling.
In summary, the expression patterns of the integrin targets of cilengitide, αvβ3 and αvβ5, did not provide prognostic information and did not reveal glioblastoma patient populations that were more or less responsive to cilengitide in the phase III CENTRIC trial. The weak association with improved outcome with cilengitide in patients with glioblastoma lacking MGMT promoter methylation in the phase II CORE trial may justify patient enrichment based on αvβ3 expression in tumor cells in future trials. Yet, agents more potent than cilengitide will be needed to explore whether integrins are a relevant target in glioblastoma, and indicators of integrin pathway activation may be superior biomarkers over integrin expression levels for patient enrichment.
MATERIALS AND METHODS
Patients
We examined tumor tissues obtained at study entry of patients with newly diagnosed glioblastoma enrolled in the CENTRIC (NCT00689221) and CORE (NCT00813943) trials [11, 12]. Accurate immunohistochemical detection of antigen in tissue was based on procedures published by Vogetseder et al. [27] which analyzed integrin expression in a TMA containing 152 cores of non-neoplastic tissue in our laboratory. The biomarker cohorts were defined as patients in each trial where all markers could be assessed. All patients provided written informed consent for the clinical investigation and correlative science reported here. The protocols were approved by the local ethics committees or institutional review boards, and appropriate regulatory authorities.
Immunohistochemistry
Four μm sections of tumor tissue from paraffin-embedded blocks were deparaffinized and immunostained for the expression of integrin αvβ3 (clone EM22703, 5 μg ml−1), αvβ5 (clone EM09902, 1 μg ml−1) and αvβ8 (clone EM13309, 1.3 μg ml−1) [13, 17, 27] as well as pSMAD2 expression (Cell Signaling clone 138D4, 1:200), according to the Ventana protocols (Ventana Medical System). The semiquantitative expression level and area of staining on each section for αvβ3, αvβ5 and αvβ8 integrins and for pSMAD2 were assessed by two neuropathologists, independently in glioma cells and endothelial cells within the tumor using the semiquantitative histoscore (H-Score) method [28, 29]. Briefly, staining intensity is scored as absent (0), mild (1), moderate (2) or strong (3) expression. The staining intensity value is multiplied by the percentage of cells showing each grade of positivity, for a maximum total score of 300. The neuropathologists were blinded with regard to patient allocation to treatment arm and outcome. The concordance between the two neuropathologists was 100% since any differences in the initial evaluation, which were always minor, were sorted out until a consensus was reached. Also noteworthy is the fact that even though the absolute percentages of the various components differed somewhat among the two pathologists, the H-score proved to be the same.
Statistical analysis
Continuous variables (including biomarkers) were presented using median and range (minimum, maximum). Boxplots were drawn to visualize biomarker distributions. Frequency tables were tabulated (by whole trial and biomarker cohort) for all categorical variables. Spearman Correlation Coefficients (SCC) were computed to quantify the relationships between biomarkers. Correlations with p values less than 1% are summarized. SSC less than or equal to 0.3 was considered a weak correlation. SCC between 0.3 and 0.49 was considered fair correlation and SSC equal to or above 0.5 a good correlation.
Principal component analysis (PCA) was performed to explore and to illustrate the correlation pattern among the markers. A Monte-Carlo test on between-group inertia (global test) based on the percentage of explained variation was used to test the overall difference between gender, centers and CENTRIC and CORE trials [30]. The PCA, between-group test analyses and graphic representations were performed using R package ade4 [31].
For progression-free survival (PFS) and overall survival (OS), Kaplan Meier curves were computed for each biomarker split by their median in each trial. Score tests obtained from univariate Cox regression models were used to assess the prognostic value of each biomarker in the biomarker cohort. Predictive value for treatment efficacy was assessed by Cox regression including treatment (TMZ/RT→TMZ versus TMZ/RT→TMZ+Cilengitide), biomarker (≤ median versus > median) and treatment by biomarker interaction score tests. All outcome analyses were exploratory and performed without adjustment for multiplicity at 5% significance, and outcome parameters (i.e. medians, hazard ratios) were presented with 95% confidence intervals. In the CORE trial, data of both cilengitide arms pooled.
SUPPLEMENTARY FIGURES AND TABLES
Footnotes
FUNDING
The immunohistochemical analyses were supported by Merck & Co (Darmstadt, Germany). PB was supported by the Swiss National Science Foundation (31003A-138116 to MH).
CONFLICTS OF INTEREST
Michael Weller has received research grants from Acceleron, Bayer, Isarna, MSD, Merck Serono, PIQUR and Roche and honoraria for lectures or advisory board participation from Celldex, Isarna, Magforce, MSD, Merck Serono, Pfizer, Roche and Teva.
Louis Burt Nabors serves in an uncompensated advisory board role for Merck Serono.
Thierry Gorlia reports no disclosures.
Henning Leske reports no disclosures.
Elisabeth Rushing reports no disclosures.
Pierre Bady reports no disclosures.
Christine Hicking is an employee and stockowner of Merck KGaA.
James Perry has received honoraria for lectures or advisory board participation from Merck, MSD, Midatech, and Roche.
Yong-Kil Hong reports no disclosures.
Patrick Roth has received honoraria for advisory boards or lectures from Roche, MSD, Novartis and Molecular Partners.
Wolfgang Wick has received research grants from Apogenix, Boehringer Ingelheim, Eli Lilly, immatics, MSD and Roche as well as honoraria for lectures or advisory board participation from MSD and Roche.
Simon Goodman is a Merck KGaA employee and holds patents on the integrin antibodies used in this study.
Monika Hegi has received an honorary for advisory board participation from MSD, Merck Serono, Roche, and MDxHealth.
Martin Picard is a Merck KGaA employee.
Holger Moch has received research grants from Merck Serono.
Josef Straub is a Merck KGaA employee.
Roger Stupp has served on advisory boards for Roche/Genentech, MSD and EMD-Serono/Merck.
REFERENCES
- 1.Gladson CL, Cheresh DA. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J Clin Invest. 1991;88:1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnell O, Krebs B, Carlsen J, Miederer I, Goetz C, Goldbrunner RH, Wester HJ, Haubner R, Popperl G, Holtmannspotter M, Kretzschmar HA, Kessler H, Tonn JC, et al. Imaging of integrin alpha(v)beta(3) expression in patients with malignant glioma by [18F] Galacto-RGD positron emission tomography. Neuro Oncol. 2009;11:861–870. doi: 10.1215/15228517-2009-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mikkelsen T, Brodie C, Finniss S, Berens ME, Rennert JL, Nelson K, Lemke N, Brown SL, Hahn D, Neuteboom B, Goodman SL. Radiation sensitization of glioblastoma by cilengitide has unanticipated schedule-dependency. Int J Cancer. 2009;124:2719–2727. doi: 10.1002/ijc.24240. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald TJ, Taga T, Shimada H, Tabrizi P, Zlokovic BV, Cheresh DA, Laug WE. Preferential susceptibility of brain tumors to the antiangiogenic effects of an alpha(v) integrin antagonist. Neurosurgery. 2001;48:151–157. doi: 10.1097/00006123-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 5.Dechantsreiter MA, Planker E, Matha B, Lohof E, Holzemann G, Jonczyk A, Goodman SL, Kessler H. N-Methylated cyclic RGD peptides as highly active and selective alpha(V)beta(3) integrin antagonists. J Med Chem. 1999;42:3033–3040. doi: 10.1021/jm970832g. [DOI] [PubMed] [Google Scholar]
- 6.Goodman SL, Picard M. Integrins as therapeutic targets. Trends in pharmacological sciences. 2012;33:405–412. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, Cloud GA, Zhang Y, Carson K, Wittemer SM, Colevas AD, Grossman SA. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25:1651–1657. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O'Neill A, Plotkin S, Glantz M, Ravin P, Raizer JJ, Rich KM, Schiff D, Shapiro WR, Burdette-Radoux S, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26:5610–5617. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 9.Nabors LB, Mikkelsen T, Hegi ME, Ye X, Batchelor T, Lesser G, Peereboom D, Rosenfeld MR, Olsen J, Brem S, Fisher JD, Grossman SA, New Approaches to Brain Tumor Therapy Central Nervous System C A safety run-in and randomized phase 2 study of cilengitide combined with chemoradiation for newly diagnosed glioblastoma (NABTT 0306) Cancer. 2012;118:5601–5607. doi: 10.1002/cncr.27585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stupp R, Hegi ME, Neyns B, Goldbrunner R, Schlegel U, Clement PM, Grabenbauer GG, Ochsenbein AF, Simon M, Dietrich PY, Pietsch T, Hicking C, Tonn JC, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:2712–2718. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 11.Nabors LB, Fink KL, Mikkelsen T, Grujicic D, Tarnawski R, Nam DH, Mazurkiewicz M, Salacz M, Ashby L, Zagonel V, Depenni R, Perry JR, Hicking C, et al. Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study. Neuro Oncol. 2015;17:708–717. doi: 10.1093/neuonc/nou356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, Aldape KD, Lhermitte B, Pietsch T, Grujicic D, Steinbach JP, Wick W, Tarnawski R, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100–1108. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 13.Goodman SL, Grote HJ, Wilm C. Matched rabbit monoclonal antibodies against αv-series integrins reveal a novel αvβ3-LIBS epitope, and permit routine staining of archival paraffin samples of human tumors. Biol Open. 2012;1:329–340. doi: 10.1242/bio.2012364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riemenschneider MJ, Mueller W, Betensky RA, Mohapatra G, Louis DN. In situ analysis of integrin and growth factor receptor signaling pathways in human glioblastomas suggests overlapping relationships with focal adhesion kinase activation. Am J Pathol. 2005;167:1379–1387. doi: 10.1016/S0002-9440(10)61225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tchaicha JH, Reyes SB, Shin J, Hossain MG, Lang FF, McCarty JH. Glioblastoma angiogenesis and tumor cell invasiveness are differentially regulated by beta8 integrin. Cancer Res. 2011;71:6371–6381. doi: 10.1158/0008-5472.CAN-11-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reyes SB, Narayanan AS, Lee HS, Tchaicha JH, Aldape KD, Lang FF, Tolias KF, McCarty JH. alphavbeta8 integrin interacts with RhoGDI1 to regulate Rac1 and Cdc42 activation and drive glioblastoma cell invasion. Mol Biol Cell. 2013;24:474–482. doi: 10.1091/mbc.E12-07-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth P, Silginer M, Goodman SL, Hasenbach K, Thies S, Maurer G, Schraml P, Tabatabai G, Moch H, Tritschler I, Weller M. Integrin control of the transforming growth factor-beta pathway in glioblastoma. Brain. 2013;136:564–576. doi: 10.1093/brain/aws351. [DOI] [PubMed] [Google Scholar]
- 18.Silginer M, Weller M, Ziegler U, Roth P. Integrin inhibition promotes atypical anoikis in glioma cells. Cell death & disease. 2014;5:e1012. doi: 10.1038/cddis.2013.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silginer M, Burghardt I, Gramatzki D, Bunse L, Leske H, Rushing EJ, Hao N, Platten M, Weller M, Roth P. The aryl hydrocarbon receptor links integrin signaling to the TGF-beta pathway. Oncogene. 2015 Oct 26; doi: 10.1038/onc.2015.387. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Bruna A, Darken RS, Rojo F, Ocana A, Penuelas S, Arias A, Paris R, Tortosa A, Mora J, Baselga J, Seoane J. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11:147–160. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Platten M, Wick W, Wild-Bode C, Aulwurm S, Dichgans J, Weller M. Transforming growth factors beta(1) (TGF-beta(1)) and TGF-beta(2) promote glioma cell migration via Up-regulation of alpha(V)beta(3) integrin expression. Biochem Biophys Res Commun. 2000;268:607–611. doi: 10.1006/bbrc.2000.2176. [DOI] [PubMed] [Google Scholar]
- 22.Frei K, Gramatzki D, Tritschler I, Schroeder JJ, Espinoza L, Rushing EJ, Weller M. Transforming growth factor-β pathway activity in glioblastoma. Oncotarget. 2015;6:5963–5977. doi: 10.18632/oncotarget.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodon J, Carducci MA, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J, Brana I, Sicart E, Gueorguieva I, Cleverly AL, Pillay NS, Desaiah D, Estrem ST, et al. First-in-human dose study of the novel transforming growth factor-beta receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res. 2015;21:553–560. doi: 10.1158/1078-0432.CCR-14-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schittenhelm J, Schwab EI, Sperveslage J, Tatagiba M, Meyermann R, Fend F, Goodman SL, Sipos B. Longitudinal expression analysis of alphav integrins in human gliomas reveals upregulation of integrin alphavbeta3 as a negative prognostic factor. J Neuropathol Exp Neurol. 2013;72:194–210. doi: 10.1097/NEN.0b013e3182851019. [DOI] [PubMed] [Google Scholar]
- 25.Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schittenhelm J, Klein A, Tatagiba MS, Meyermann R, Fend F, Goodman SL, Sipos B. Comparing the expression of integrins alphavbeta3, alphavbeta5, alphavbeta6, alphavbeta8, fibronectin and fibrinogen in human brain metastases and their corresponding primary tumors. International journal of clinical and experimental pathology. 2013;6:2719–2732. [PMC free article] [PubMed] [Google Scholar]
- 27.Vogetseder A, Thies S, Ingold B, Roth P, Weller M, Schraml P, Goodman SL, Moch H. alphav-Integrin isoform expression in primary human tumors and brain metastases. Int J Cancer. 2013;133:2362–2371. doi: 10.1002/ijc.28267. [DOI] [PubMed] [Google Scholar]
- 28.Kraus JA, Dabbs DJ, Beriwal S, Bhargava R. Semi-quantitative immunohistochemical assay versus oncotype DX((R)) qRT-PCR assay for estrogen and progesterone receptors: an independent quality assurance study. Mod Pathol. 2012;25:869–876. doi: 10.1038/modpathol.2011.219. [DOI] [PubMed] [Google Scholar]
- 29.Kinsel LB, Szabo E, Greene GL, Konrath J, Leight GS, McCarty KS., Jr Immunocytochemical analysis of estrogen receptors as a predictor of prognosis in breast cancer patients: comparison with quantitative biochemical methods. Cancer Res. 1989;49:1052–1056. [PubMed] [Google Scholar]
- 30.Romesburg HC. Exploring, confirming and randomization tests. Computers and Geosciences. 1985;11:19–37. [Google Scholar]
- 31.Dray S, Dufour AB. The ade4 package: implementing the duality diagrom for ecologists. Journal of statistical software. 2007;22:1–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


