Abstract
Stroke, a devastating complication of sickle cell anemia (SCA), can cause irreversible brain injury with physical and cognitive deficits. Transcranial Doppler ultrasonography (TCD) is a non-invasive tool for identifying children with SCA at highest risk of stroke. National guidelines recommend that TCD screening begin at age 2 years, yet there is research to suggest less than half of young children undergo screening. The purpose of this project was to use quality improvement methods to improve the proportion of patients aged 24–27 months who successfully completed their initial TCD from 25% to 75% by December 31, 2013. Quality improvement methods (e.g., process mapping, simplified failure mode effect analysis, and plan–do–study–act cycles) were used to develop and test processes for identifying eligible patients, scheduling TCDs, preparing children and families for the first TCD, and monitoring outcomes (i.e., TCD protocol). Progress was tracked using a report of eligible patients and a chart showing the age in months for the first successful TCD (population metric). As of December 2013, 100% of eligible patients successfully completed their initial TCD screen; this improvement was maintained for the next 20 months. In November 2014, a Welch’s one-way ANOVA was conducted. Results showed a statistically significant difference between the average age of first TCD for eligible patients born in 2009 and eligible patients born during the intervention period (2010–2013; F[1,11.712]=16.03, p=0.002). Use of quality improvement methods to implement a TCD protocol was associated with improved TCD screening rates in young children with SCA.
Introduction
Stroke, a devastating complication of sickle cell anemia (SCA), can cause irreversible brain injury with physical and cognitive deficits.1 Without primary prevention, 10% of children with SCA will experience an overt ischemic stroke by age 20 years, with the highest incidence at age 2–5 years.2–4 Given the irreversible brain damage that a single stroke can cause, prevention is essential5 and ongoing efforts are needed to improve the availability and implementation of stroke prevention programs. Transcranial Doppler ultrasonography (TCD) is a non-invasive tool that can identify children with SCA at highest risk of overt stroke.6 Use of chronic blood transfusion therapy in these children significantly reduces the first stroke incidence.5–8
National SCA guidelines recommend that TCD screening should begin at age 2 years, continuing annually until age 16 years.9–11 Magnetic resonance imaging/angiographic abnormalities have been reported in children as young as 7–48 months, reinforcing the need to begin screening at this age. In 2011, the authors reviewed all cases of new overt stroke in SCA patients during the preceding 10 years at Cincinnati Children’s Hospital Medical Center (CCHMC) and found that the frequency of stroke had significantly decreased after the implementation of routine TCD screening in 2005. However, the last two overt strokes occurred in children who were younger than 3 years and had not yet had an initial TCD examination, although one had been scheduled. At the time, the average age at initial TCD was 33.2 months, and only 25% of patients had successfully completed a TCD by age 27 months. This is not surprising because the mere availability of evidence-based recommendations does not guarantee implementation.12 An analysis of the failures (children aged 24–36 months who had not received an initial TCD screen) revealed variability in processes related to identifying eligible patients, educating parents, scheduling TCDs, tracking TCD completion, and acting upon results. Moreover, the clinical team did not systematically prepare children and families for the procedure, but only asked families whether they thought their child could complete the TCD examination.
A multidisciplinary quality improvement (QI) team convened to develop a reliable process for TCD screening consistent with national recommendations. The primary aim was to increase the proportion of eligible children with SCA (hemoglobin [Hb]SS or sickle-β0-thalassemia) aged 24–27 months who successfully completed their initial TCD from 25% (baseline) to 75% by December 31, 2013. This paper describes the QI methods used to develop and implement a process for obtaining initial TCDs within a busy pediatric sickle cell clinic.
Methods
Setting
A non-profit, 587-bed children’s hospital, CCHMC serves Southern Ohio, Northern Kentucky, and Southeastern Indiana. The Cincinnati Comprehensive Sickle Cell clinic at CCHMC is the regional coordinating center for the hemoglobinopathy newborn screening program and cares for all children with sickle cell disease from birth to age 21 years (N=280). Most patients (>75%) reside within a 15-mile radius; therefore, the center provides acute and chronic care for these patients and maintains an electronic medical record (EMR) patient registry.
Study Sample
Participants were included in analyses if they met the following criteria: (1) SCA (HbSS or HbSβ0-thalassemia) diagnosis documented via ICD-9 Code in the EMR (Epic Systems) registry; and (2) born between January 1, 2009 (aged 5 years 4 months), and December 31, 2011 (aged 1 year 5 months). Older children were not targeted because data indicated that the majority of children aged ≥5 years 5 months had successfully completed initial TCDs.
Procedures
The TCD exams at CCHMC are conducted by certified radiologic technologists following the Stroke Prevention Trial in Sickle Cell Anemia (STOP) protocol with modifications for an imaging TCD technique.13,14 All exams were performed in the Radiology department. To address the challenges of conducting full TCD exams in younger patients, a multidisciplinary core QI team consisting of two physicians, two psychologists, four nurse practitioners, three SCA nurse care managers, a QI consultant, and a data analyst began meeting weekly with regular input from radiologic technologists, Child Life specialists, social workers, school interventionists, design and technology students, and consultants. The team’s first step was to develop a key driver diagram that described the project aim, measures, and drivers for change. Next, a process map or pictorial representation of all of the steps in the process of scheduling and following up on initial TCDs was developed.15 This was followed by a simplified failure mode and effect analysis (SFMEA), where each step in the process was identified as well as potential failures and then the failures were evaluated and ranked.16,17 The final step was to develop potential strategies to address high-ranking failures. The SFMEA revealed failures related to provider awareness and education, family education and preparation, and scheduling and follow-up. From February 2012 to December 2013, the team conducted a series of plan–do–study–act cycles (PDSAs) to develop and test strategies to address identified failures. PDSA cycles use a “trial-and-learning” approach to test a strategy on a small scale.18,19 Test results then guide the next steps (i.e., Should the strategy be adapted, adopted or abandoned?). Once PDSA data indicated stable screening completion rates, the process for the initial TCD was finalized. Because the purpose of this project was to improve care locally, the IRB deemed it QI, exempted it from review, and waived written informed consent.
Statistical Analysis
To monitor progress, a report listing SCA patients needing an initial TCD and their status was used. An accompanying chart showing the age in months for the initial TCD (population metric) for patients born between 2009 and 2011 was developed.
To examine whether TCD screening performance differed before and after the intervention, the average age of initial TCD for eligible patients with SCA born in 2009 (pre-intervention group) was compared with the average age of initial TCD for eligible patients with SCA born in 2010–2011 (intervention group). Because the sample size of the groups differed (pre-intervention group had 15 patients [80% male, 100% HbSS, 73.3% public insurance], whereas the intervention group had nine patients [44.4% male, 100% HbSS, 77.8% public insurance]), a Welch’s one-way ANOVA was conducted using SAS, version 9.3. Analyses were conducted in November 2014.
Results
Development and Implementation of the Process for Initial Transcranial Doppler Ultrasonography
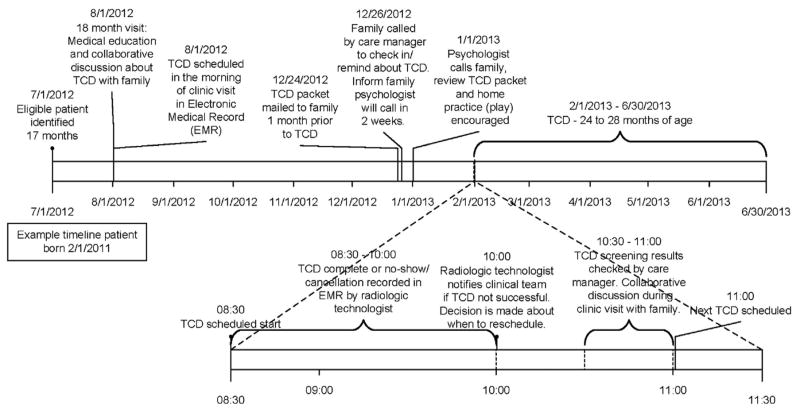
A review of data showed variability in scheduling initial TCDs due to a lack of (1) provider awareness that initial TCDs were not occurring as close to age 2 years as possible; (2) team motivation to change the process because of doubt that screening rates would improve; and (3) the perception that TCDs would not be successful in less cooperative young children. Thus, the improvement work began with a series of PDSAs focused on provider awareness, education, and motivation. Specifically, educational presentations on evidence-based recommendations, baseline TCD screening rates, and the gap in performance were tested to see if scheduling rates would improve. The scheduling rate increased briefly and then returned to baseline, which was attributed to education alone not being enough (i.e., families continued to no-show and TCD completion was not monitored). The next series of PDSAs tested interactive meetings where providers completed a key driver diagram, a process map, and the SFMEA. Following this, the scheduling rate improved briefly but this was not maintained. The third set of PDSAs focused on demonstrating that with proper preparation patients could successfully undergo TCD at around age 24 months. The team identified two potential patients aged 24–27 months, provided education during their clinic visits, and scheduled TCDs. The successful completion of TCDs in these patients generated provider consensus for changing the TCD process. Figure 1 shows a timeline for the initial TCD process.
Figure 1.
Timeline for initial transcranial Doppler ultrasonography (TCD) process.
Although providers were now hopeful that changing the process could improve the initial TCD screening rate, there were barriers related to family education and preparation. Young children had difficulty lying still and staying awake for the 45 to 60–minute procedure. Hence, a series of PDSAs tested a “prep book” of pictures and short descriptions to walk families through the TCD exam. Testing revealed providers had difficulty remembering to review the prep book with families in the absence of reminders. Once these were in place, providers (usually the nurse) reliably reviewed the prep book with families; parents reported the prep book was useful but requested two additional tools: (1) a video of the actual TCD exam and (2) written materials to share with others at home. Consequently, team members from Child Life and Psychology developed a video of a TCD exam with a young child, which parents reported was helpful. To address the desire for written materials, PDSAs shifted to developing two brochures, one to explain the rationale for TCD screening (TCD Educational brochure) and one to help parents prepare the child (TCD Parenting Tips). Two brochures were developed, as parents described having one brochure with all of the information as overwhelming. Families suggested all of the educational materials (prep book, video, and TCD Educational and TCD Parenting Tips brochures) were useful, so a parent education toolkit or bundle was created.
With the toolkit complete, PDSAs focused on implementation (i.e., how and when families should receive the toolkit). A series of tests revealed families wanted the toolkit mailed to them at least 2 weeks prior to the TCD appointment and a phone call 48 hours after the mailing. To accommodate this change in process, care managers began systematically collecting updated contact information from families during clinic visits. Phone calls revealed that the majority of families’ questions were related to preparing their child for the TCD; thus, the team tested having the psychologist call families. The care manager documented the parent follow-up call and then routed it to a psychologist. Two weeks later, the psychologist called and reviewed the toolkit information and documented this in the EMR. Families were readily available and engaged well in conversation with the psychologist during the 10-minute phone call; hence, this process change was adopted.
The next series of PDSAs focused on parent education during clinic visits. After several cycles, the team adopted a process where the care manager initiated a TCD discussion at the 18-month visit, provided a copy of the TCD Knowing Note (plain-language educational brochure designed for patients and families) to the family, and documented the education in the EMR.
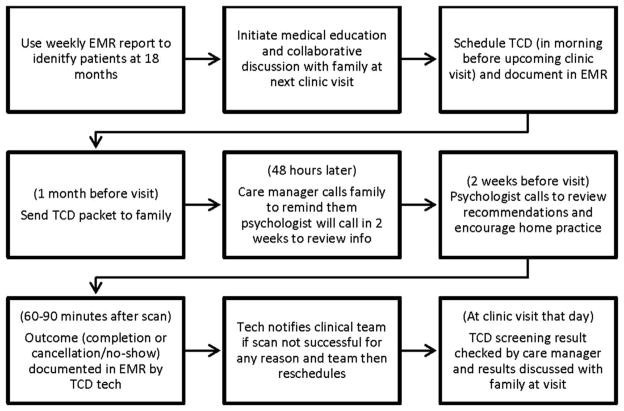
The SFMEA revealed no systematic way of identifying eligible patients or tracking TCD scheduling or completion. The data analyst then developed an EMR report to identify patients aged ≥17 months without a completed TCD. During pre-visit planning, the team reviewed this list and implemented the process beginning with family education at the 18-month visit (Figure 2 shows the final process). Once this part of the process was stable, the EMR report was automated.
Figure 2.
Final process for initial transcranial Doppler ultrasonography (TCD).
EMR, electronic medical record.
The team wanted TCDs scheduled on the same day as an SCD clinic visit for timely review of results with the family. PDSAs tested scheduling the procedure at various times relative to child’s developmental needs (e.g., after breakfast, after nap) and the clinic visit (before physical and labs drawn). Results indicated the optimal time was 8:00AM or 8:30AM, as children had eaten, were alert, and could attend a clinic visit afterwards. PDSAs tested TCDs with and without a Child Life specialist present and found they were essential to the process (i.e., provided reinforcement and distractions like movies to help children stay still).
The follow-up process became the focus of a series of PDSAs. Care managers tested a phone script to determine the reason for missed appointments. If the TCD was missed because the child was ill, could not lay still, or the family showed signs of low motivation, the care manager repeated the TCD education provided at the 18-month visit and mailed the TCD toolkit again. Every effort was made to reschedule any TCDs within 1–2 months of the original appointment.
Initial Transcranial Doppler Ultrasonography Screening Rate
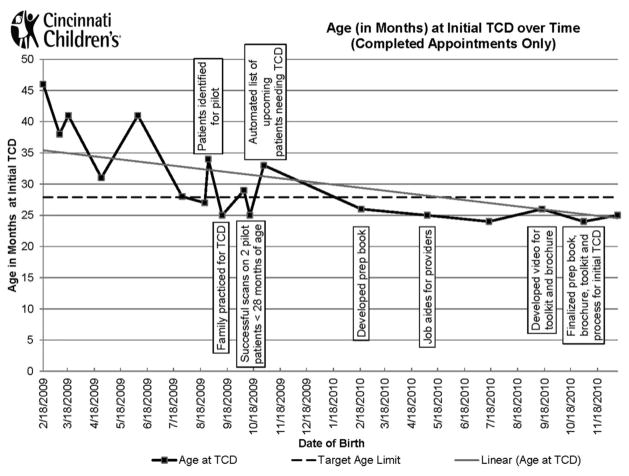
As of December 2013, after the implementation of the new process for the initial TCD, 100% of eligible patients who needed initial TCD screening successfully received it, and this performance was maintained for the next 20 months (n=6) (Figure 3). Three patients after December 2013 did not receive their initial TCD on time. All TCDs were scheduled within the appropriate timeframe but they were unsuccessful because of one child being ill, another’s inability to lie still, and a no-show.
Figure 3.
Age (in months) at initial transcranial Doppler ultrasonography (TCD) over time (completed appointments only).
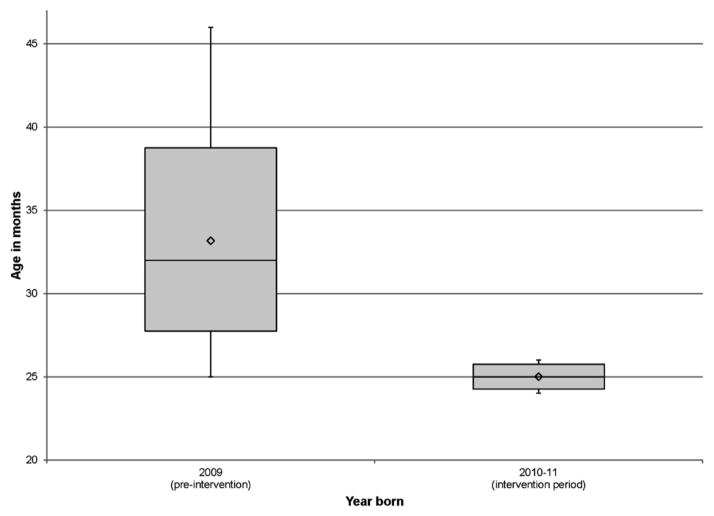
By December 2013, the average age of initial TCD decreased to 27.2 months. A Welch’s one-way ANOVA found a statistically significant difference between the pre-and post-intervention groups (F[1,11.712]=16.03, p=0.002; Figure 4). For eligible patients born in 2009, the average age at initial TCD was 33.2 compared with 25.0 months for eligible patients born between 2010 and 2011.
Figure 4.
Distribution of age pre and post implementation of the initial transcranial Doppler ultrasonography (TCD) process.
Discussion
Stroke is a devastating complication of SCA.1 TCD screening has been shown to identify children with SCA at highest risk of stroke.6 National guidelines recommend screening children beginning at age 2 years9–11; however, successfully performing TCD can be challenging in children that young. Using QI methods, a multidisciplinary team developed and implemented a process for obtaining initial TCDs for young children with SCA in a busy outpatient clinic. Following implementation of the new process, the team exceeded its goal: One hundred percent of eligible patients received an initial TCD screen, and this performance was maintained for the next 20 months. The average age for initial TCD screening was significantly different before and after the intervention (33.2 vs 25.0 months). This finding is consistent with other studies demonstrating that TCD screening can be performed successfully in children younger than age 3 years.20,21 The final TCD process included several effective TCD screening strategies supported in the literature (e.g., telephone support, scheduling TCDs to coincide with clinic visits).22,23 However, this study differed in important ways. A previous study found higher rates of TCD screen adherence in patients with private insurance.24 The current intervention improved the initial TCD screen rate in a sample of patients with primarily public insurance, suggesting it may have broad clinical utility. Similar to McCarville and colleagues,23 conducing TCDs in conjunction with a clinic visit was effective; however, the current project differed in that technologists conducted the TCD screens in the Radiology department. This approach is not without limitations such as the potential for increased costs and resources (e.g., psychologists, Child Life specialists) but has some advantages (e.g., comprehensive examination, improved clinic flow). The Child Life specialist might have been obviated with the use of an abbreviated TCD examination protocol (e.g., BabyHUG abbreviated TCD20), but this was not possible because the institution’s Radiology department specifies that all patients have complete examinations by a radiologic technologist.
The QI methods were essential for increasing providers’ awareness and motivation to change their practice around scheduling initial TCD screens. Educational presentations on current screening rates as well as reasons for failures and successes were not sufficient. Only when a small test of change showed TCD screens completed with a small number of patients aged 24–27 months (i.e., two) did providers come to consensus on trialing a change in the process.
Process mapping and conducting the SFMEA were extremely useful. Developing the map aided the team in examining the flow and identifying barriers across the entire process. This comprehensive, systematic approach uncovered key drivers of the process (e.g., SCA nurse care managers, parental need for tips to prepare their child for the TCD) that could have been missed using a different approach. For example, in order for the process for the initial TCD to be implemented successfully, SCA nurse care managers’ workflow and responsibilities had to be changed. Care managers expanded their workflow to include preparing TCD eligibility information for pre-visit planning meetings, mailing TCD toolkits, calling families to follow-up on toolkit and missed appointments, routing phone calls to the psychologist in the EMR, and scheduling TCDs at a specific time with the radiologic technologist and a Child Life specialist. They also took on the new role of updating family contact information.
Because QI methods incorporate contextual factors that influence a process,25 the team was able to develop a TCD process that addressed salient patient/family barriers identified as important for adherence in prior TCD screening studies. Had patient factors not been considered in a systematic way, the team may have failed to develop educational materials parents felt were necessary (e.g., TCD video, TCD Parenting Tips brochure).
Limitations
First, this study did not include a control or usual care group against which to compare TCD screening rates, so it is possible that the described changes were influenced by other factors. However, other interventions appeared to have little influence over TCD screening rates at the beginning of this project (2010). Furthermore, no other stroke prevention project or changes in transfusion guidelines were launched from 2010 to 2012, decreasing the likelihood that improvements were due to external factors. Second, it is possible there were residual confounders to the improvement given that the team was working on multiple clinical outcomes projects (e.g., home pain management plan).26 It is also possible that clinical outcomes improved because of other factors such as general SCD quality care initiatives. Third, the team used all available data in comparing the average age of initial TCD pre- and post-intervention; however, the post-intervention period was twice as long as the pre-intervention period because fewer patients with HbSS were born during the post-intervention period. This may have biased results. Last, this project took place at a single center, included a small numbers of patients, and was dependent upon documentation in the EMR by the SCA nurse care manager.
Conclusions
Methods for QI were an effective means to implement an evidence-based practice guideline for young children with SCA. The average age at initial TCD decreased from 33.2 to 25.0 months after the new process for initial TCDs was implemented. Although the direct causes for improvements (e.g., parent education versus follow-up phone call) were not examined, this study contributes to the limited literature on QI in pediatric SCA11,26–28 and addresses a critical gap: the implementation of a TCD screening program for young children with SCA in a real-world setting.
Supplementary Material
Acknowledgments
Publication of this article was supported by the Centers for Disease Control and Prevention.
The authors would like to thank the SCD clinic team and the patients and families for their contributions to this project. This project was funded in part by U.S. Health Resources and Services Administration (HRSA) Grant Number U38MC22218 and NIH Grant Number K07HL108720-04. The research presented in this paper is that of the authors and does not reflect the official policy of the NIH or HRSA. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis. All authors made a substantial contribution to the conception and design of study, wrote or revised the article for important intellectual content, and read and approved the final version of the submitted manuscript.
Appendix. Supplementary data
Supplementary data associated with this article can be found at http://dx.doi.org/10.1016/j.amepre.2016.01.021.
Footnotes
No financial disclosures were reported by the authors of this paper.
This article is part of the supplement issue titled Developing a Unified Approach for Sickle Cell Disease.
References
- 1.DeBaun MR, Armstrong FD, McKinstry RC, Ware RE, Vichinsky E, Kirkham FJ. Silent cerebral infarcts: a review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Blood. 2012;119(20):4587–4596. doi: 10.1182/blood-2011-02-272682. http://dx.doi.org/10.1182/blood-2011-02-272682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11. doi: 10.1056/NEJM199807023390102. http://dx.doi.org/10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 3.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. [PubMed] [Google Scholar]
- 4.Arkuszewski M, Krejza J, Chen R, et al. Sickle cell disease: reference values and interhemispheric differences of nonimaging transcranial Doppler blood flow parameters. AJNR Am J Neuroradiol. 2011;32(8):1444–1450. doi: 10.3174/ajnr.A2529. http://dx.doi.org/10.3174/ajnr.A2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams RJ, McKie VC, Brambilla D, et al. Stroke prevention trial in sickle cell anemia. Controlled Clin Trials. 1998;19(1):110–129. doi: 10.1016/s0197-2456(97)00099-8. http://dx.doi.org/10.1016/S0197-2456(97)00099-8. [DOI] [PubMed] [Google Scholar]
- 6.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11. doi: 10.1056/NEJM199807023390102. http://dx.doi.org/10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 7.Vichinsky EP, Neumayr LD, Gold JI, et al. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA. 2010;303(18):1823–1831. doi: 10.1001/jama.2010.562. http://dx.doi.org/10.1001/jama.2010.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vichinsky EP. Current issues with blood transfusions in sickle cell disease. Sem Hematol. 2001;38(1 suppl 1):14–22. doi: 10.1016/s0037-1963(01)90056-3. [DOI] [PubMed] [Google Scholar]
- 9.Adams RJ. TCD in sickle cell disease: an important and useful test. Pediatr Radiol. 2005;35(3):229–234. doi: 10.1007/s00247-005-1409-7. http://dx.doi.org/10.1007/s00247-005-1409-7. [DOI] [PubMed] [Google Scholar]
- 10.Nichols FT, Jones AM, Adams RJ. Stroke prevention in sickle cell disease (STOP) study guidelines for transcranial Doppler testing. J Neuroimaging. 2001;11(4):354–362. doi: 10.1111/j.1552-6569.2001.tb00063.x. http://dx.doi.org/10.1111/j.1552-6569.2001.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang CJ, Kavanagh PL, Little AA, Holliman JB, Sprinz PG. Quality-of-care indicators for children with sickle cell disease. Pediatrics. 2011;128(3):484–493. doi: 10.1542/peds.2010-1791. http://dx.doi.org/10.1542/peds.2010-1791. [DOI] [PubMed] [Google Scholar]
- 12.Francke AL, Smit MC, de Veer AJ, Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak. 2008;8(1):38. doi: 10.1186/1472-6947-8-38. http://dx.doi.org/10.1186/1472-6947-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones AM, Seibert JJ, Nichols FT, et al. Comparison of transcranial color Doppler imaging (TCDI) and transcranial Doppler (TCD) in children with sickle-cell anemia. Pediatr Radiol. 2001;31(7):461–469. doi: 10.1007/s002470100427. http://dx.doi.org/10.1007/s002470100427. [DOI] [PubMed] [Google Scholar]
- 14.Jones A, Granger S, Brambilla D, et al. Can peak systolic velocities be used for prediction of stroke in sickle cell anemia? Pediatr Radiol. 2005;35(1):66–72. doi: 10.1007/s00247-004-1282-9. http://dx.doi.org/10.1007/s00247-004-1282-9. [DOI] [PubMed] [Google Scholar]
- 15.Colligan L, Anderson JE, Potts HW, Berman J. Does the process map influence the outcome of quality improvement work? A comparison of a sequential flow diagram and a hierarchical task analysis diagram. BMC Health Serv Res. 2010;10(1):7. doi: 10.1186/1472-6963-10-7. http://dx.doi.org/10.1186/1472-6963-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McElroy LM, Khorzad R, Nannicelli AP, Brown AR, Ladner DP, Holl JL. Failure mode and effects analysis: a comparison of two common risk prioritisation methods. BMJ Qual Safe. 2015 doi: 10.1136/bmjqs-2015-004130. bmjqs-2015-004130. [DOI] [PubMed] [Google Scholar]
- 17.Johnson CD, Miranda R, Aakre KT, Roberts CC, Patel MD, Krecke KN. Process improvement: what is it, why is it important, and how is it done? AJR Am J Roentgenol. 2010;194(2):461–468. doi: 10.2214/AJR.09.3213. http://dx.doi.org/10.2214/AJR.09.3213. [DOI] [PubMed] [Google Scholar]
- 18.Berwick DM. Developing and testing changes in delivery of care. Ann Intern Med. 1998;128(8):651–656. doi: 10.7326/0003-4819-128-8-199804150-00009. http://dx.doi.org/10.7326/0003-4819-128-8-199804150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Varkey P, Reller MK, Resar RK. Basics of quality improvement in health care. Mayo Clin Proc. 2007;82(6):735–739. doi: 10.4065/82.6.735. [DOI] [PubMed] [Google Scholar]
- 20.Pavlakis SG, Rees RC, Huang X, et al. Transcranial Doppler ultrasonography (TCD) in infants with sickle cell anemia: baseline data from the BABY HUG trial. Pediatr Blood Cancer. 2010;54(2):256–259. doi: 10.1002/pbc.22282. http://dx.doi.org/10.1002/pbc.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts L, O’Driscoll S, Dick MC, et al. Stroke prevention in the young child with sickle cell anaemia. Ann Hematol. 2009;88(10):943–946. doi: 10.1007/s00277-009-0757-z. http://dx.doi.org/10.1007/s00277-009-0757-z. [DOI] [PubMed] [Google Scholar]
- 22.Patik M, Phillips L, Kladny B, Captain A, Gettig E, Krishnamurti L. Structured telephone-based outreach using nonmedical personnel can improve adherence to comprehensive care in families of children with sickle cell disease. Am J Hematol. 2006;81(6):462–464. doi: 10.1002/ajh.20605. http://dx.doi.org/10.1002/ajh.20605. [DOI] [PubMed] [Google Scholar]
- 23.McCarville MB, Goodin GS, Fortner G, et al. Evaluation of a comprehensive transcranial Doppler screening program for children with sickle cell anemia. Pediatr Blood Cancer. 2008;50(4):818–821. doi: 10.1002/pbc.21430. http://dx.doi.org/10.1002/pbc.21430. [DOI] [PubMed] [Google Scholar]
- 24.Raphael JL, Shetty PB, Liu H, Mahoney DH, Mueller BU. A critical assessment of transcranial Doppler screening rates in a large pediatric sickle cell center: opportunities to improve healthcare quality. Pediatr Blood Cancer. 2008;51(5):647–651. doi: 10.1002/pbc.21677. http://dx.doi.org/10.1002/pbc.21677. [DOI] [PubMed] [Google Scholar]
- 25.Batalden PB, Davidoff F. What is “quality improvement” and how can it transform healthcare? Qual Saf Health Care. 2007;16(1):2–3. doi: 10.1136/qshc.2006.022046. http://dx.doi.org/10.1136/qshc.2006.022046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crosby LE, Simmons K, Kaiser P, et al. Using quality improvement methods to implement an electronic medical record (EMR) supported individualized home pain management plan for children with sickle cell disease. J Clin Outcomes Manag. 2014;21(5):210–217. [PMC free article] [PubMed] [Google Scholar]
- 27.Oyeku SO, Wang CJ, Scoville R, et al. Hemoglobinopathy Learning Collaborative: using quality improvement (QI) to achieve equity in health care quality, coordination, and outcomes for sickle cell disease. J Health Care Poor Underserved. 2012;23(3):34–48. doi: 10.1353/hpu.2012.0127. http://dx.doi.org/10.1353/hpu.2012.0127. [DOI] [PubMed] [Google Scholar]
- 28.Sobota AE, Kavanagh PL, Adams WG, McClure E, Farrell D, Sprinz PG. Improvement in influenza vaccination rates in a pediatric sickle cell disease clinic. Pediatr Blood Cancer. 2015;62(4):654–657. doi: 10.1002/pbc.25390. http://dx.doi.org/10.1002/pbc.25390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.