Abstract
Biochemical and/or physical communication between the conceptus and the uterine endometrium is required for conceptus implantation to the maternal endometrium, leading to placentation and the establishment of pregnancy. We previously reported that in vitro co-culture system with bovine trophoblast CT-1 cells, primary uterine endometrial epithelial cells (EECs), and uterine flushings (UFs) mimics in vivo conceptus attachment process. To identify molecules in UFs responsible for this change, we first characterized protein contents of UFs from day 17 cyclic (C17) and pregnant (P17) ewes through the use of two dimensional-Polyacrylamide Gel Electrophoresis (2D-PAGE), followed by Liquid Chromatography-tandem Mass Spectrometry (LC-MS/MS) analysis. These analyses identified 266 proteins specific for P17 UFs, from which 172 proteins were identified as exosomal proteins. Among 172 exosomal proteins, 8 proteins that had been identified as exosomal proteins were chosen for further analysis, including macrophage-capping protein (CAPG), aldo-keto reductase family 1, member B1 protein (AKR1B1), bcl-2-like protein 15 (BCL2L15), carbonic anhydrase 2 (CA2), isocitrate dehydrogenase 2 (IDH2), eukaryotic translation elongation factor 2 (EEF2), moesin (MSN), and ezrin (EZR). CAPG and AKR1B1 were again confirmed in P15 and P17 UFs, and more importantly CAPG and AKR1B1, mRNA and protein, were found only in P15 and P17 conceptuses. Moreover, exosomes were isolated from C15, C17, P15, or P17 UFs. Only P15 and P17 exosomes, originated from the conceptus, contained interferon tau (IFNT) as well as CAPG and AKR1B1, and up-regulated STAT1, STAT2, MX1, MX2, BST2, and ISG15 transcripts in EECs. These observations indicate that in addition to endometrial derived exosomes previously described, conceptus-derived exosomes are present in UFs and could function to modify endometrial response. These results suggest that exosomes secreted from conceptuses as well as endometria are involved in cell to cell interactions for conceptus implantation to the maternal endometrium.
Introduction
Numerous studies have been conducted to elucidate molecular mechanisms by which conceptuses of ruminants attach to uterine epithelial cells and form the placenta. It is well documented that efficient biochemical communication between the conceptus and the uterine endometrium is required for conceptus implantation and placentation, leading to the establishment of pregnancy.
On day 8 of ovine pregnancy, the blastocyst hatches from the zona pellucida and begins to secrete a cytokine, interferon tau (IFNT), which peaks on day 16 and declines soon after the conceptus attaches to the uterine epithelium. It has been shown that along with maternal progesterone secretion, IFNT regulates many endometrial gene expressions, including chemokine (C-X-C motif) ligand 10 (CXCL10) and galactoside-binding, soluble, 15 (LGALS15) [1–3]. These proteins are required for conceptus attachment to the uterine epithelium. Despite the data extensively accumulated, low pregnancy rate has not been improved, resulting from early embryonic losses. These observations suggest that a factor or a mechanism, which, along with progesterone and/or IFNT, plays a major role in pregnancy establishment, has not been well-characterized.
Recently, exosomes have gained much attention because they have been implicated in various events such as cancer growth and/or metastasis. In fact, cancer cells uptake exosomes to protect them from genotoxic stress-induced cell death [4]. Bone marrow mesenchymal stromal cells from multiple myeloma (MM) patients release exosomes that express increased levels of oncogenic proteins, cytokines, and adhesion molecules to facilitate the growth of MM cells [5]. Moreover, it is reported that exosomal integrins, α6β4, α6β1 and αvβ5, are associated with lung and liver metastasis [6]. Exosomes have also been identified in many body fluids including cerebrospinal fluids [7], urine [8], and blood [9] as well as uterine flushings (UFs) [10]. The presence of exosomes in ovine UFs has been demonstrated and these exosomes are thought to be involved in conceptus-endometrial interactions during the pre-attachment period [11]. It was recently shown that endometrial exosomes released into UFs act on the trophectoderm via the toll-like receptors family to induce the secretion of IFNT during the pre-attachment phase of pregnancy [12]. These studies focused on the functions of endometrial exosomes on the conceptuses; however, the effects of conceptus exosomes on the endometrium have not been characterized.
Our laboratory has established an in vitro co-culture system with bovine trophoblast CT-1 cells and primary uterine endometrial epithelial cells (EECs) to study conceptus attachment processes; however, this co-culture system requires UFs from pregnant ewes to mimic the in vivo attachment process [13, 14]. Compared to UFs from cyclic animals, UFs recovered from pregnant animals during the attachment period dramatically changed various gene expressions in CT-1 and EECs. These results suggest that UF components during the conceptus attachment period, including various cytokines and/or exosomes, are essential for biochemical and/or physical interactions between the conceptus and the endometrium. It should be noted that the source/origin of exosomes in UFs could be conceptuses and/or endometrium.
We therefore hypothesized that the UFs during conceptus attachment period contain important molecules of conceptus and/or endometrial origins, which may regulate the uterine environment, facilitating conceptus attachment to the uterine epithelium. To examine this hypothesis, we first characterized the protein content of UFs from day 17 cyclic and pregnant ewes using two dimensional-Polyacrylamide Gel Electrophoresis (2D-PAGE) and Liquid Chromatography-tandem Mass Spectrometry (LC-MS/MS). From the results of LC-MS/MS study, we then used in silico analysis to identify exosomal proteins and further investigated the presence and potential function of conceptus exosomes in UFs during the attachment period.
Materials and Methods
Collection of Ovine Conceptuses/Endometrial Tissues, Uterine Fixation, and Uterine Flushing Preparation
All animal maintenance, care, breeding and surgical procedures were reviewed and approved by the University of Tennessee Institutional Animal Care and Use Committee (IACUC). Whiteface crossbred ewes were maintained at the farm of the University of Tennessee (UT), Knoxville, TN, USA and animal care, estrous synchronization procedures, and tissue collections were performed in accordance with the guidelines set forth by IACUC [15]. Following hysterectomy was performed in the large animal surgical suite of the Johnson Research and Teaching Unit of the University on days 15, and 17 (day 0 = day of estrus), uteri from days 15 and 17 cyclic and pregnant animals (C15, C17, P15, and P17, respectively; n = 3 each day) were flushed with 20 ml sterile PBS (pH 7.2), from which approximately 19 ml uterine flushings (UFs) including elongated conceptuses were collected. UFs were then centrifuged at 1,000 rpm for 5 min, and UFs and conceptuses were collected separately. Endometrial tissues were sampled from the uterine horns ipsilateral to the corpus luteum. All samples were frozen immediately in liquid nitrogen, and were transferred to the Laboratory of Animal Breeding at The University of Tokyo.
For uterine fixation, uteri from pregnant ewes on day 17 of gestation (n = 3) were removed and subjected to whole uterus fixation immediately after slaughter [16, 17]. Fixed, whole uteri were serially dissected into proximal to distal uterine segments, and each section was then paraffin-embedded. These paraffin-embedded tissue samples had been transported to the Laboratory of Animal Breeding at The University of Tokyo. These fixed-tissue blocks were sectioned (5 μm) and evaluated for the presence of conceptus microscopically after hematoxylin-eosin staining [16–18].
Isolation of Exosomes from Uterine Flushings
Exosomes were isolated from 1 ml of C15, C17, P15 and P17 filtered UFs (n = 3) by adding 200 μl exosome precipitation solution (Exo-Quick-TC, System Biosciences, Mountain View, CA). The UFs with Exo-Quick-TC were incubated overnight at 4°C and then centrifuged at 1,500 x g for 30 min at 4°C to pellet exosomes. Exosomes were suspended and their protein concentration adjusted in PBS (1 μg/μl) for transmission electron microscopy analysis, nanoparticle tracking analysis, and in vitro culture experimentation or in mammalian protein extraction reagent (M-PER, Thermo Fisher Scientific, Inc., Waltham, MA) for western blot analysis.
Cell Preparation and Culture Conditions
EECs were isolated and cultured as previously described [19]. In brief, uteri of healthy Holstein cows were obtained from a local abattoir in accordance with protocols approved by the local Institutional Animal Care, Use and Ethics Committee at Okayama University, Okayama, Japan. Uteri of the early luteal phase (Days 2–5) were excised and immediately transported to the laboratory. To detach EECs, the uterine lumen was trypsinized (0.3% w/v), from which EECs were isolated [19]. The isolated EECs were cultured on collagen type IA-coated six-well plates in Dulbecco-modified Eagle medium/F12 (DMEM/F12) (1:1) medium (Wako Pure Chemical Industries, Ltd., Osaka, Japan) supplemented with 10% (v/v) newborn calf serum (Invitrogen, Tokyo, Japan), 2 mM glutamine (Invitrogen), and antibiotic/antimycotic solution (Invitrogen) at 37°C under 5% CO2 in humidified air. EECs were used within four passages to avoid changes in cell characteristics, specifically down-regulation of steroid receptor expression [13]. In the in vitro cultures, EECs (1 × 105 cells/well) placed onto collagen type IA-coated six-well dishes were incubated with 10 μg proteins from P17 UFs, or C15, C17, P15, or P17 exosomes in serum-free DMEM/F12 for 48 h, all of which treatments were similar to those described previously [20].
Transmission Electron Microscopy
The exosomes in PBS were placed on carbon-film grids for 2 min, from which excess PBS was removed by touching one end of the grid with the filter paper. After grids were partially dried, the staining solution, 2% uranyl acetate in water, was added to grids and allowed to settle for 2 min. The excess liquid was blotted off by filter paper, and grids were allowed to dry overnight at room temperature. Grids were analyzed by using a HITACHI H-7600 Transmission Electron Microscopy (TEM, Hitachi High-Technologies Corporation, Tokyo, Japan), of which analysis was carried out at Hanaichi UltraStracture Research, Inc., Aichi, Japan.
Nanoparticle Tracking Analysis
Nanoparticle tracking analysis of exosomes, isolated from P17 UF and suspended in PBS, was performed using NanoSight NS300 (NanoSight Ltd, Amesbury, UK) instrument with a 488 nm laser and a complementary metal-oxide-semiconductor (CMOS) camera (Andor Technology, Belfast, UK) and NanoSight NTA 3.2 software calibrated with 100 nm polystyrene beads (Thermo Fisher Scientific, Inc.). Particle suspensions were diluted with PBS to adjust a concentration of 2–6 x 108 particles per milliliter. Videos were recorded for 30 seconds during which the nanoparticle tracking analysis software (NanoSight Ltd) tracked each visible particle. The Stokes-Einstein equation was employed to determine the size distribution and number of particles (concentration) within the sample.
2D-PAGE
10 μl of C17 and P17 UFs (1 μg/μl) were dissolved in 90 μl urea buffer (0.06 M Tris hydroxymethyl aminomethane, 1 M thiourea, 6 M urea, 3% CHAPS, 1% Triton X-100) and centrifuged at 15,000 × g for 30 min at 4°C. Supernatant was recovered and protein concentrations were measured using Benchmark Plus microplate spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA).
After measuring the protein concentrations, UFs were mixed with 1 M acrylamide solution and were separated in agar gel (pH range: 3–10) (ATTO, Tokyo, Japan) and 5–20% SDS-polyacrylamide gradient gel (ATTO) according to the protocol provided by the manufacturer. After 2D-PAGE, the gel was stained with SYPRO Ruby (Thermo Fisher Scientific, Inc.) overnight and washed with distilled water. The images were then captured using an LAS-3000 camera (FUJIFILM, Tokyo, Japan).
Analysis by LC-MS/MS
Proteins especially up-regulated in P17 UFs were identified by LC-MS/MS. After SDS-PAGE, the spots of these proteins were cut out and treated with trypsin. The tryptic digest was directly analyzed by nanoscale HPLC (MAGIC 2002, Michrom BioResources Inc., Auburn, CA) on a C18 column (0.1 × 50 mm, Michrom BioResources Inc.) coupled to a tandem mass spectrometer (Q-Tof2, Micromass, London, UK) equipped with a nanoelectrospray ionization source. Tandem mass spectra were analyzed using Mascot, which allows for the correlation of experimental data with theoretical spectra generated from known protein sequences. The data were used to search a compiled protein database, NCBInr, which is publicly available (http://www.ncbi.nlm.nih.gov/).
RNA Extraction and Quantitative RT-PCR
Using the ISOGEN reagent (Nippon Gene, Tokyo, Japan), total RNAs were extracted from days 15 and 17 conceptuses and endometria and cultured EECs according to the manufacturer’s protocol. For quantitative real-time PCR (qPCR) analyses, isolated RNA (total 250 ng) was reverse-transcribed to cDNA using ReverTra Ace qRNA RT Kit (Toyobo, Osaka, Japan), and the resulting cDNA (RT template) was stored at 4°C until use [21].
Reverse-transcribed cDNA was subjected to qPCR amplification using Thunderbird SYBR qPCR Mix Kit (Toyobo) with 0.3 μM of the oligonucleotide primers listed in S1 Table, and qPCR amplification was carried out on an Applied Biosystems STEP One Plus real-time PCR System (Applied Biosystems, Foster City, CA) [22]. Amplification efficiencies of each target gene and two reference genes, bovine beta-action (ACTB) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), were examined through their calibration curves and found to be comparable [14]. The thermal profile for qPCR consisted of 40 cycles at 95°C for 10 sec and annealing and extension at 60°C for 45 sec. Average threshold (Ct) values for each target were determined by Sequence Detection System software v2.2 (Applied Biosystems). Each run was completed with melting curve analysis to confirm the specificity of amplification and the absence of primer dimer.
Western Blot Analysis
To determine expressions of macrophage-capping protein (CAPG), aldo-keto reductase family 1, member B1 protein (AKR1B1), tetraspanin protein 63 (CD63), heat shock protein 70 (HSP70), or IFNT protein in frozen samples, conceptuses or endometrial tissues were prepared in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton X-100, 0.1% sodium dodecyl sulfate, 1 mM Na3VO4, and 50 mM NaF). Tissue lysates, UFs or exosomes prepared in M-PER (10 μg/lane) were separated through 12.5% SDS-PAGE and were then transferred onto polyvinylidene difluoride (PVDF) membranes (n = 3) (Millipore, Milford, MA). After blocking with Block Ace reagent (DS Pharma Biomedical, Osaka, Japan), membranes were incubated with a goat polyclonal anti-human CAPG polyclonal antibody(2 μg/ml, sc-33084, Santa Cruz Biotechnology, Inc., Dallas, TX), a rabbit polyclonal anti-human AKR1B1 antibody (1 μg/ml, sc-33219, Santa Cruz Biotechnology, Inc.), rabbit monoclonal anti-bovine IFNT antibody (1:1000 dilution, Eurofins Genomics, Inc., Ebersberg, Germany), rabbit monoclonal anti-human ACTB antibody (for internal control, 1:1000, ab1801, Abcam, Tokyo, Japan), rabbit polyclonal anti-human CD63 antibody (for exosome marker, 0.25 μg/ml, EXOAB-CD63A-1, System Biosciences), or rabbit polyclonal anti-human HSP70 antibody (for exosome marker, 0.25 μg/ml, EXOAB-HSP70A-1, System Biosciences). Immunoreactive bands were detected using enhanced chemiluminescence (Millipore) after incubation with horseradish peroxidase-labeled SAP solution (APRO life Science, Inc., Tokushima, Japan).
Immunohistochemistry
Immunohistochemical analyses were performed on 5 μm paraffin sections of day 17 uterine tissue blocks. Paraffin sections were rehydrated and boiled for 20 min in 10 mM citrate buffer (pH 6.0), and endogenous peroxidase was quenched by immersing in 0.3% (v/v) hydrogen peroxide/methanol, as described previously [17, 18]. A streptavidin/biotin blocking kit (Vector Laboratories, Burlingame, CA) was used to block endogenous biotin according to the manufacturer’s instructions. After 30 min incubation with 10% normal goat or donkey serum, the sections were incubated at 4°C overnight with a goat anti-human CAPG polyclonal antibody (2 μg/ml, sc-33084, Santa Cruz Biotechnology, Inc.), or a rabbit anti-human AKR1B1 polyclonal antibody (4 μg/ml, sc-33219, Santa Cruz Biotechnology, Inc.), or the respective negative controls; normal goat IgG (10 μg/ml, sc-2028, Santa Cruz Biotechnology, Inc.) or normal rabbit IgG (10 μg/ml, sc-2027, Santa Cruz Biotechnology, Inc.). Subsequently, the sections were incubated at room temperature for 1 h with either a donkey anti-goat IgG-HRP (1 μg/ml, sc-2020, Santa Cruz Biotechnology, Inc) or a goat anti-rabbit IgG biotin conjugate (1:400 dilution, B8895, Sigma-Aldrich, St. Louis, MO). The immunoreactivity was visualized by means of avidin-peroxidase (1:400 dilution, E2886, Sigma-Aldrich) and AEC substrate kits (Invitrogen) according to the manufacturer’s instructions and then examined under light microscope (BX-51, Olympus, Tokyo, Japan).
Statistical Analysis
Data are expressed as the mean ± SEM. Significance was assessed using t-test or the Tukey-Kramer test. A P-value < 0.05 was considered statistically significant.
Results
UFs from Day 17 Pregnant Ewes Contain Up-Regulated Proteins
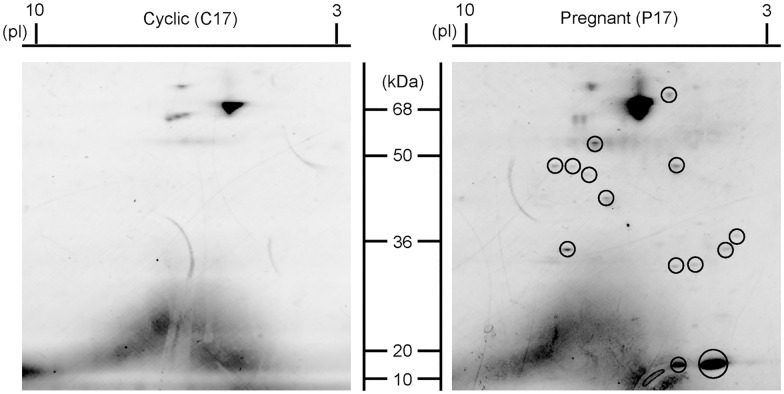
Results from 2D-PAGE revealed that although similar protein migration patterns between C17 and P17 UFs were recognized, P17 UFs contain increased proteins compared to those of C17 (Fig 1). Spots especially up-regulated in P17 UFs were cut off and subjected to LC-MS/MS analysis, which revealed that a total of 266 proteins were identified from the P17 up-regulated spots (see S2 Table). These proteins were further analyzed through the use of Database for Annotation, Visualization and Integrated Discovery (DAVID) (https://david.ncifcrf.gov/) and ExoCarta (http://exocarta.ludwig.edu.au), resulting in the identification of 13 secretory proteins and 172 exosomal proteins. Based on the previous results of UF analyses [23, 24], the 8 exosomal proteins CAPG, AKR1B1, bcl-2-like protein 15 (BCL2L15), carbonic anhydrase 2 (CA2), isocitrate dehydrogenase 2 (IDH2), eukaryotic translation elongation factor 2 (EEF2), moesin (MSN), and ezrin (EZR) were selected for further analysis.
Fig 1. Use of 2D-PAGE to identify proteins specific to day 17 pregnant UFs.
Representative SYPRO Ruby stained 2D gel images of UFs from day 17 cyclic (left) or pregnant (right) ewes. Several spots designated by circle in day 17 pregnant UFs were up-regulated compared to those in day 17 cyclic UFs, which were subjected to LC-MS/MS analysis.
Up-Regulation of CAPG and AKR1B1 mRNAs are Specific to Day 15 and 17 Conceptuses
To identify the source of exosomal proteins unique to P17 UFs, real-time PCR was executed to examine transcripts of CAPG, AKR1B1, BCL2L15, CA2, IDH2, EEF2, MSN, and EZR in the RNAs extracted from C15, C17, P15, and P17 endometrial tissues, and P15 and P17 conceptus tissues. CAPG and AKR1B1 mRNAs were up-regulated in conceptus tissues (Fig 2A and 2B), whereas BCL2L15, CA2, and IDH2 mRNAs were expressed in C15, C17, P15, and P17 endometrial tissues (Fig 2C, 2D and 2E). Expression of EEF2, MSN, and EZR transcripts was similar between endometrium and conceptus tissues (Fig 2F, 2G and 2H).
Fig 2. Levels of 8 exosomal transcripts in endometria and conceptuses.
The relative mRNA expression of 8 exosomal transcripts, CAPG (A), AKR1B1 (B), BCL2L15 (C), CA2 (D), IDH2 (E), EEF2 (F), MSN (G), EZR (H), in C15, C17, P15, or P17 endometria (grey bar) and P15 or P17 conceptuses (solid bar) (n = 3 each day). ACTB and GAPDH were used as internal controls for RNA integrity. Values represent mean ± SEM. *Statistically significant difference between conceptus and endometrium in each day (P<0.05).
CAPG and AKR1B1 Proteins Are Expressed by Conceptuses
Since CAPG and AKR1B1 mRNAs were found only in P15 and P17 conceptus tissues, the expression and localizations of these proteins were further examined in C15, C17, P15, and P17 endometrial tissues, and P15 and P17 conceptus tissues. Western blot analysis revealed that similar to CAPG and AKR1B1 transcripts, CAPG protein was specifically expressed in P15 and P17 conceptuses. Minute expression of AKR1B1 protein was found in P15 and P17 endometrium, but definitive expression was found in P15 and P17 conceptuses (Fig 3A). To confirm the localizations of CAPG and AKR1B1 proteins associated with its transcript expression in utero, immunohistochemical analysis was carried out with paraffin sections from P17 uterine tissues. Consistent with the results from western blot analysis, CAPG and AKR1B1 proteins were localized to the conceptuses, but minute expression of AKR1B1 was also found in the endometrial glandular epithelia (Fig 3B).
Fig 3. Expression and localization of CAPG and AKR1B1 proteins in the conceptuses.
(A) The presence of CAPG or AKR1B1 proteins in C15, C17, P15, or P17 ovine endometria and P15 or P17 conceptuses was examined by western blot analysis. ACTB was used as an internal control. A representative data from three independent experiments containing protein samples from different animals was shown. Endo, endometrium; Con, conceptus. (B) Immunohistochemical detection of CAPG (a, c, and e) and AKR1B1 (b, d, and f) in P17 ovine uteri. Tissue sections were immunostained for CAPG using anti-CAPG antibody (a and c) or normal goat IgG (e) as a negative control. Boxed area in (a) is shown at a higher magnification (c). Tissue sections were immunostained for AKR1B1 using anti- AKR1B1 antibody (b and d) or normal rabbit IgG (f) as a negative control. Boxed area in (b) is shown at a higher magnification (d). Con, conceptus; LE, luminal epithelium; GE, glandular epithelium. Scale bar = 250 μm (a, b, e, and f), or 50μm (c and d), respectively.
Exosomes Are Present in Cyclic and Pregnant UFs, but CAPG and AKR1B1 Proteins Are Found in Exosomes from P15 and P17 UFs
Western blot analysis revealed that CAPG and AKR1B1 proteins were present in P15 and P17 UFs (Fig 4A). Transmission electron microscopy detected vesicles of approximately 150 nm in diameter in the isolated pellets (Fig 4B), and separated exosome preparations from P17 UF were subjected to nanoparticle tracking analysis, revealing a mean of 131.8 nm, standard deviation of 61.9 nm, mode of 102.1 nm, and a range of 50 to 200 nm (Fig 4C). While exosomal protein markers CD63 and HSP70 were positive in all isolated pellets, CAPG and AKR1B1 proteins were only identified in exosomes isolated from P15 and P17 UFs (Fig 4D). These results indicated that exosomes containing CAPG and AKR1B1 were secreted from days 15 and 17 conceptuses during the attachment period.
Fig 4. Identification of exosomal CAPG and AKR1B1 in days 15 and 17 pregnant UFs.
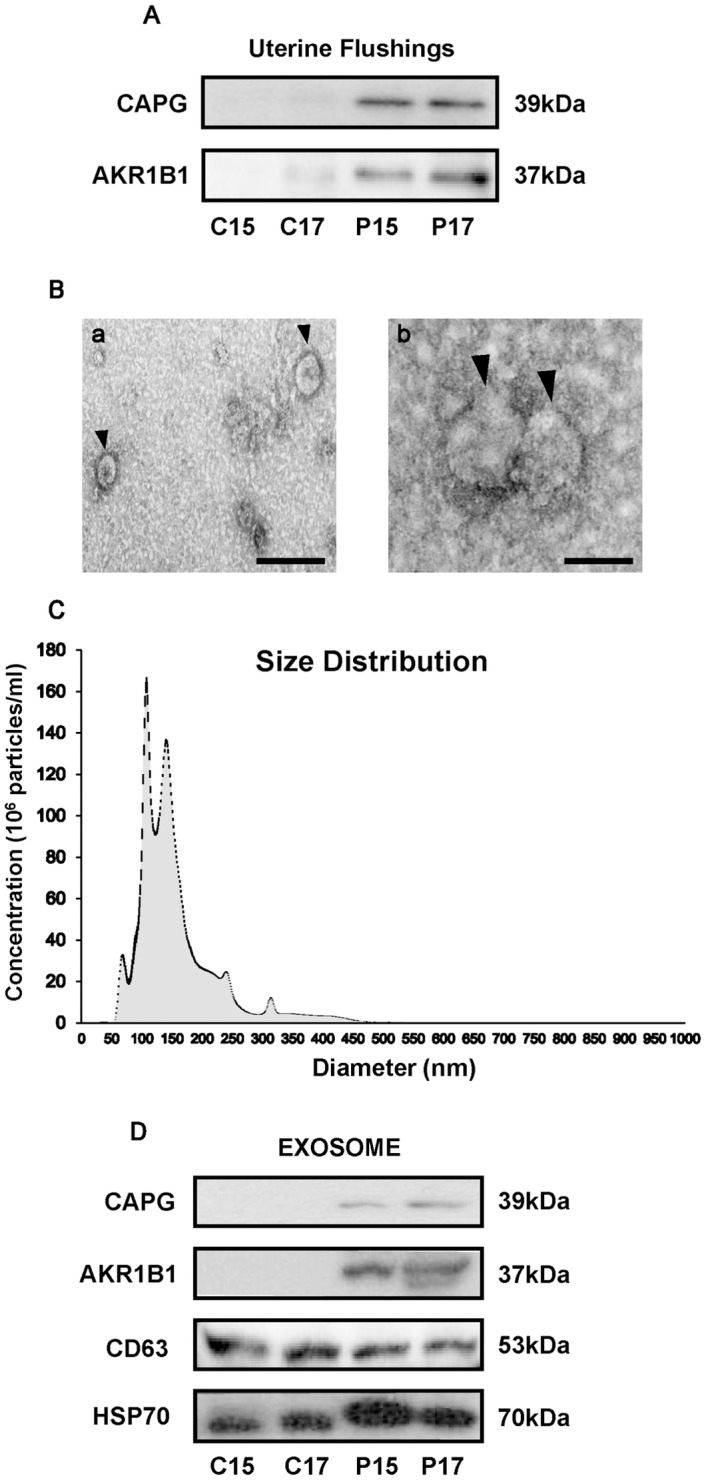
(A) The presence of CAPG and AKR1B1 in C15, C17, P15, or P17 UFs (10 μg each) was examined by western blot analysis (n = 3 each day). (B) Transmission electron microscopy analysis revealed the presence of approximately 150 nm vesicles in UFs, consistent with exosomes. Scale bar = 200 nm (a) or 100 nm (b). (C) Nanoparticle tracking analysis (n = 3, triplet analysis in each sample) of P17 UF revealed that a range of exosomal size is 50 to 150 nm. Gray area in (C) represents an average from three samples, whereas black area represents SEM. (D) Western blot analysis showed the presence of CD63 and HSP70 in exosomes isolated from C15, C17, P15, or P17 UFs, and the presence of CAPG and AKR1B1 in exosomes isolated from days 15 and 17 pregnant UFs (n = 3 each day). In (A), (B), and (D), a representative one is shown.
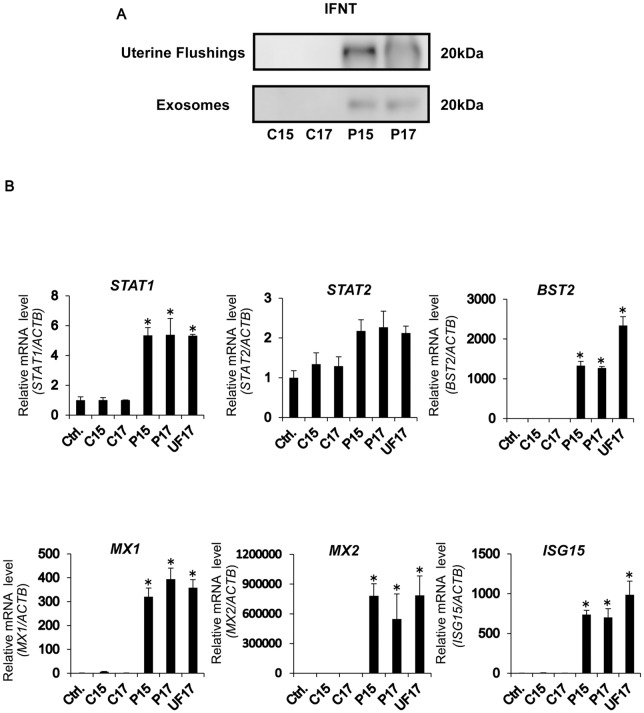
Exosomes Isolated from P15 and P17 UFs Contain IFNT, Which Up-Regulates Interferon Stimulated Genes (ISGs) in EECs
Consistent with the study by Ruiz-González et al. [12], P15 and P17 UFs contained IFNT, and exosomes isolated from P15 and P17 UFs contained IFNT (Fig 5A). To study potential functions of these exosomes, UFs or isolated exosomes were added to the cultured EECs, from which RNA was extracted and subjected to qPCR analysis to determine changes in ISGs, STAT1, STAT2, MX1, MX2, BST2, and ISG15 transcripts. The results revealed that ISG transcripts in EECs treated with P17 UFs, and P15 or P17 exosomes were up-regulated (Fig 5B). These results indicated that up-regulation of ISGs expression in EECs could result from the IFNT-containing exosomes released from the conceptuses.
Fig 5. Presence of IFNT in UFs and exosomes from days 15 and 17 pregnant ewes and the effect of those exosomes on EECs.
(A) The presence of IFNT in C15, C17, P15, or P17 UFs and exosomes was examined by western blot analysis (n = 3 each day), and IFNT was detected in P15 and P17 UFs. A representative of three independent experiments is shown. (B) Effects of exosomes on EECs. EECs were treated with or without C15, C17, P15, or P17 exosome (10 μg each), or P17 UF (10 μg proteins) for 48 h. RNA was extracted from EECs and subjected to real-time PCR analysis for STAT1, STAT2, BST2, MX1, MX2, and ISG15 transcript levels. ACTB and GAPDH mRNA were used as internal controls for RNA integrity. Values were from three independent experiments, each containing duplicates. Values represent mean ± SEM. *Statistically significant difference in mRNA levels vs. control (Ctrl) without exosomes or UFs treatment (P<0.05).
Discussion
The results from this study provide the evidence that conceptus-derived exosomes are present in P15 and P17 UFs and could potentially function to modify endometrial response during the attachment period. Compared to C17, proteins unique to the existence of conceptuses are predominantly present in P17 UFs, and many of them are associated with extracellular vesicular exosomes and extracellular region/matrix. In addition, CAPG and AKR1B1 predominantly expressed by conceptuses are present in isolated exosomes during the attachment period. Moreover, ISGs mRNA expressions in EECs are up-regulated in response to the exosomes containing IFNT, suggesting that exosomes secreted from conceptuses are involved in the generation of uterine environment required for pregnancy establishment.
Results of 2D-PAGE followed by LC-MS/MS analysis revealed that P17 UFs contained 267 specific proteins, from which DAVID and ExoCarta revealed 13 secretory proteins and 172 exosomal proteins, respectively. These observations, together with the reports that exosomes are present in UFs during the pre-attachment period in ewe [11, 12], indicate that exosomes present in pregnant UFs during the attachment period could be responsible for the regulation of genes in bovine trophoblast CT1 cells and EECs in our previous studies [13, 14].
Conceptus and cancer cells possess various characteristics in common. Both, for instance, undergo an epithelial-mesenchymal transition (EMT) to acquire adhesion and/or invasive competence [22, 25]. In addition, hypoxia promotes cancer cells and conceptus cells for proliferation and angiogenesis [26, 27]. Furthermore, a number of recent evidence indicates that cancer cell-derived exosomes mediate the interaction between cancer cells and their neighboring cells, which play a role in cancer development and invasion [28–30]. These findings support a notion that conceptuses produce exosomes to facilitate conceptus-uterine interaction in a manner similar to that whereby cancer cells secrete exosomes to serve as mediators with target cells. Based on this idea, exosomal proteins derived from conceptuses during the attachment period were re-evaluated, and 8 exosomal proteins, CAPG, AKR1B1, BCL2L15, CA2, IDH2, EEF2, MSN, EZR, were selected as more reliable exosomal proteins. Selection of the eight exosomal proteins, out of 172 classified by ExoCarta, was based upon the observations of UFs from pregnant animals as described by other investigators [23, 24]. In addition to the endometrium derived exosomes, CAPG and AKR1B1 expressions found from this study suggest that exosomes derived from conceptuses are present in UFs during the attachment period.
In recent years, several proteomic studies demonstrated that CAPG is overexpressed in various types of cancer [31–33], which enhances cellular motility/chemotaxis [34], and are associated with increased invasion into collagen type I or chick heart fragments [35]. Moreover, AKR1B1 has been shown as a molecule associated with EMT [36] and angiogenesis [37]. Because conceptus and cancer cells possess various characteristics in common such as EMT and angiogenesis, it is considered that CAPG and AKR1B1 included in conceptus-derived exosomes could be involved in the conceptus attachment similar to those demonstrated in cancer adhesion and/or invasion. However, the elucidation of exact function exhibited by CAPG and AKR1B1 in exosomes, particularly within the context of conceptus attachment to the uterine epithelium requires further experimentation.
Nanoparticles isolated from UFs were confirmed to be exosomes through their size and morphology as seen by TEM and nanoparticle tracking analysis. In addition, CD63 and HSP70 were present in all exosomes isolated from C15, C17, P15, or P17 UFs, indicating that exosomes are present within the uterine lumen in cyclic ewes as well as pregnant ewes [38, 39]. However, CAPG and AKR1B1, which were mainly expressed in P15 and P17 conceptuses, were present only in exosomes isolated from P15 and P17 UFs during the attachment period. This study supports the hypothesis that exosomes containing CAPG and AKR1B1, secreted by conceptuses, are released into the uterine lumen during the attachment period. In the previous analysis by other investigators, 30 conceptus-derived proteins were identified in UFs during the pre-attachment period and were thought to facilitate biochemical and/or physical interactions between the conceptus and the endometrium via a micro-vesicular transport mechanism [24]. This and our results suggest that exosomes released from the conceptus could play a role in carrying these proteins.
IFNT has been considered to be the main trophoblast factor which acts on uterine endometrium and abrogates luteolytic mechanisms [40], resulting in the continued production of progesterone. Recently, however, extra-uterine or endocrine effects of IFNT have been noted. For instance, MX gene expression predominantly increases in peripheral blood cells (PBMC) in response to initial IFNT signaling in pregnant ewes [41]. Compared with the corpus luteum (CL) in non-pregnant ewes, the CL in pregnant ewes has a higher number of neutrophils, which are influenced by IFNT [42]. In addition, IFNT induces ISG15 gene expression in jugular blood and liver tissue and several ISGs including ISG15, STAT1, and STAT2 in CL via uterine veins [43, 44]. It has been reported that blood also contains exosomes for organ-to-organ communications [45, 46]. Results from our studies together with these observations suggest that exosomes including IFNT could be involved in the regulation of ISGs in extra-uterine tissues as well. However, the exact mechanisms by which IFNT directly or indirectly functions on the extra-uterine tissues such as CL or PBMC remain unknown. Further experiment is required to clarify how trophoblast IFNT reaches CL or effects on extra-uterine tissues.
In conclusion, we propose that IFNT-containing exosomes derived from conceptus may facilitate conceptus-endometrium interaction, resulting in the generation of uterine environment required for conceptus attachment to the uterine endometrium.
Supporting Information
(XLSX)
(XLSX)
Acknowledgments
We wish to thank Drs. Kiyoshi Okuda (Okayama University, Okayama, Japan) and Ronald K. Christenson (Clay Center, NE, USA) for the generous gifts of EECs and paraffin embedded sheep uterine samples, respectively. We would like to thank Mr. Yasuaki Fukuta (APRO life Science Inc., Tokushima, Japan) for the execution of LC-MS/MS analysis and advice in protein analysis methodology throughout the course of the study. Our appreciation is extended to Prof. Seiichi Oshita (The University of Tokyo) and Mr. Yoichi Noguchi and Dr. Kazue Okubo (Genostaff, Tokyo, Japan) for the execution of nanoparticle analysis and the validation and execution of immunohistochemical analysis, respectively. We also thank Mr. Robert Moriarty for his English editing of the manuscript, and Mr. Yuta Matsuno (The University of Tokyo) for his advice on experiments involving exosome isolation and analysis.
Data Availability
All data are available in the text and its Supporting Information files.
Funding Statement
This work was supported by the Science and Technology Research Promotion Program for Ministry of Agriculture, Forestry and Fisheries (25030AB, http://www.maff.go.jp/e/index.html) (to KI), Grant-in-Aid for Young Scientists (B) from Japan Society for the Promotion of Science (15K18767, https://www.jsps.go.jp/english/index.html) (to KK), and Grants-in-Aid for Scientific Research (C) from Japan Society for the Promotion of Science (15K07697, https://www.jsps.go.jp/english/index.html) (to TS). The funders had no roles in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nagaoka K, Sakai A, Nojima H, Suda Y, Yokomizo Y, Imakawa K, et al. A chemokine, interferon (IFN)-gamma-inducible protein 10 kDa, is stimulated by IFN-tau and recruits immune cells in the ovine endometrium. Biol Reprod. 2003; 68: 1413–1421. [DOI] [PubMed] [Google Scholar]
- 2.Nagaoka K, Nojima H, Watanabe F, Chang KT, Christenson RK, Sakai S, et al. Regulation of blastocyst migration, apposition, and initial adhesion by a chemokine, interferon gamma-inducible protein 10 kDa (IP-10), during early gestation. J Biol Chem. 2003; 278: 29048–29056. [DOI] [PubMed] [Google Scholar]
- 3.Gray CA, Adelson DL, Bazer FW, Burghardt RC, Meeusen EN, Spencer TE. Discovery and characterization of an epithelial-specific galectin in the endometrium that forms crystals in the trophectoderm. Proc Natl Acad Sci U S A. 2004; 101: 7982–7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan S, Aspe JR, Asumen MG, Almaguel F, Odumosu O, Acevego-Martinez S, et al. Extracellular, cell-permeable surviving inhibits apoptosis while promoting proliferative and metastatic potential. Br J Cancer. 2009; 100: 1073–1086. 10.1038/sj.bjc.6604978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013; 123: 1542–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015; 527: 329–335. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS, et al. Idetification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med. 2012; 10: 5 10.1186/1479-5876-10-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prunotto M, Farina A, Lane L, Pernin A, Schifferli J, Hochstrasser DF, et al. Proteomic analysis of podocyte exosome-enriched fraction from normal human urine. J Proteomics. 2013; 82: 193–229. 10.1016/j.jprot.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 9.Cleys ER, Halleran JL, McWhorter E, Hergenreder J, Enriquez VA, da Silveira JC, et al. Identification of microRNAs in exosomes isolated from serum and umbilical cord blood, as well as placentomes of gestational day 90 pregnant sheep. Mol Reprod Dev. 2014; 81: 983–993. 10.1002/mrd.22420 [DOI] [PubMed] [Google Scholar]
- 10.Ng YH, Rome S, Jalabert A, Forterre A, Singh H, Hincks CL, et al. Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS One. 2013; 8: e58502 10.1371/journal.pone.0058502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns G, Brooks K, Wildung M, Navakanitworakul R, Christenson LK, Spencer TE. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS One. 2014; 9: e90913 10.1371/journal.pone.0090913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz-González I, Xu J, Wang X, Burghardt RC, Dunlap KA, Bazer FW. Exosomes, endogenous retroviruses and toll-like receptors: pregnancy recognition in ewes. Reproduction. 2015;149: 281–291. 10.1530/REP-14-0538 [DOI] [PubMed] [Google Scholar]
- 13.Sakurai T, Bai H, Bai R, Arai M, Iwazawa M, Zhang J, et al. Coculture system that mimics in vivo attachment processes in bovine trophoblast cells. Biol Reprod. 2012; 87: 60 10.1095/biolreprod.112.100180 [DOI] [PubMed] [Google Scholar]
- 14.Bai R, Kusama K, Sakurai T, Bai H, Wang C, Zhang J, et al. The Role of Endometrial Selectins and Their Ligands on Bovine Conceptus Attachment to the Uterine Epithelium During Peri-Implantation Period. Biol Reprod. 2015; 93: 46 10.1095/biolreprod.115.128652 [DOI] [PubMed] [Google Scholar]
- 15.Sakurai T, Bai H, Konno T, Ideta A, Aoyagi Y, Godkin JD, et al. Function of a transcription factor CDX2 beyond its trophectoderm lineage specification. Endocrinology. 2010; 151: 5873–5881. 10.1210/en.2010-0458 [DOI] [PubMed] [Google Scholar]
- 16.Imakawa K, Tamura K, Lee RS, Ji Y, Kogo H, Sakai S, et al. Temporal expression of type I interferon receptor in the peri-implantation ovine extra-embryonic membranes: demonstration that human IFNalpha can bind to this receptor. Endocr J. 2002; 49: 195–205. [DOI] [PubMed] [Google Scholar]
- 17.McGuire WJ, Imakawa K, Tamura K, Meka CS, Christenson RK. Regulation of endometrial granulocyte macrophage-colony stimulating factor (GM-CSF) in the ewe. Domest Anim Endocrinol. 2002; 23: 383–396. [DOI] [PubMed] [Google Scholar]
- 18.Imakawa K, Kim MS, Matsuda-Minehata F, Ishida S, Iizuka M, Suzuki M, et al. Regulation of the ovine interferon-tau gene by a blastocyst-specific transcription factor, Cdx2. Mol Reprod Dev. 2006; 73: 559–567. [DOI] [PubMed] [Google Scholar]
- 19.Skarzynski DJ, Miyamoto Y, Okuda K. Production of prostaglandin f (2alpha) by cultured bovine endometrial cells in response to tumor necrosis factor alpha: cell type specificity and intracellular mechanisms. Biol Reprod. 2000; 62: 1116–1120. [DOI] [PubMed] [Google Scholar]
- 20.Bai R, Bai H, Kuse M, Ideta A, Aoyagi Y, Fujiwara H, et al. Involvement of VCAM1 in the bovine conceptus adhesion to the uterine endometrium. Reproduction. 2014; 148: 119–127. 10.1530/REP-13-0655 [DOI] [PubMed] [Google Scholar]
- 21.Bai H, Sakurai T, Kim MS, Muroi Y, Ideta A, Aoyagi Y, et al. Involvement of GATA transcription factors in the regulation of endogenous bovine interferon-tau gene transcription. Mol Reprod Dev. 2009; 76: 1143–1152. 10.1002/mrd.21082 [DOI] [PubMed] [Google Scholar]
- 22.Yamakoshi S, Bai R, Chaen T, Ideta A, Aoyagi Y, Sakurai T, et al. Expression of mesenchymal-related genes by the bovine trophectoderm following conceptus attachment to the endometrial epithelium. Reproduction. 2012; 143: 377–387. 10.1530/REP-11-0364 [DOI] [PubMed] [Google Scholar]
- 23.Koch JM, Ramadoss J, Magness RR. Proteomic profile of uterine luminal fluid from early pregnant ewes. J Proteome Res. 2010; 9: 3878–3885. 10.1021/pr100096b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forde N, Bazer FW, Spencer TE, Lonergan P. 'Conceptualizing' the Endometrium: Identification of Conceptus-Derived Proteins During Early Pregnancy in Cattle. Biol Reprod. 2015; 92: 156 10.1095/biolreprod.115.129296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan X. Cancer Metastases: challenges and opportunities. Acta Pharm Sin B. 2015; 5: 402–418. 10.1016/j.apsb.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong J, Xu J, Wang X, Jin B. Influence of the interaction between long noncoding RNAs and hypoxia on tumorigenesis. Tumour Biol. 2015; in press. [DOI] [PubMed] [Google Scholar]
- 27.Jeong W, Bazer FW, Song G, Kim J. Expression of hypoxia-inducible factor-1 by trophectoderm cells in response to hypoxia and epidermal growth factor. Biochem Biophys Res Commun. 2015; in press. [DOI] [PubMed] [Google Scholar]
- 28.Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015; 219: 278–294. 10.1016/j.jconrel.2015.06.029 [DOI] [PubMed] [Google Scholar]
- 29.Kharaziha P, Ceder S, Li Q, Panaretakis T. Tumor cell-derived exosomes: a message in a bottle. Biochim Biophys Acta. 2012; 1826: 103–111. 10.1016/j.bbcan.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 30.Soung YH, Nguyen T, Cao H, Lee J, Chung J. Emerging roles of exosomes in cancer invasion and metastasis. BMB Rep. 2015; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang S, Kim MJ, An H, Kim BG, Choi YP, Kang KS, et al. Proteomic molecular portrait of interface zone in breast cancer. J Proteome Res. 2010; 9: 5638–5645. 10.1021/pr1004532 [DOI] [PubMed] [Google Scholar]
- 32.Xu SG, Yan PJ, Shao ZM. Differential proteomic analysis of a highly metastatic variant of human breast cancer cells using two-dimensional differential gel electrophoresis. J Cancer Res Clin Oncol. 2010; 136: 1545–1556. 10.1007/s00432-010-0812-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voisin SN, Krakovska O, Matta A, DeSouza LV, Romaschin AD, Colgan TJ, et al. Identification of novel molecular targets for endometrial cancer using a drill-down LC-MS/MS approach with iTRAQ. PLoS One. 2011; 6: e16352 10.1371/journal.pone.0016352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun HQ, Kwiatkowska K, Wooten DC, Yin HL. Effects of CapG overexpression on agonist-induced motility and second messenger generation. J Cell Biol. 1995; 129: 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Corte V, Van Impe K, Bruyneel E, Boucherie C, Mareel M, Vandekerckhove J, et al. Increased importin-beta-dependent nuclear import of the actin modulating protein CapG promotes cell invasion. J Cell Sci. 2004; 117: 5283–5292. [DOI] [PubMed] [Google Scholar]
- 36.Zablocki GJ, Ruzycki PA, Overturf MA, Palla S, Reddy GB, Petrash JM. Aldose reductase-mediated induction of epithelium-to-mesenchymal transition (EMT) in lens. Chem Biol Interact. 2011; 191: 351–356. 10.1016/j.cbi.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tammali R, Reddy AB, Srivastava SK, Ramana KV. Inhibition of aldose reductase prevents angiogenesis in vitro and in vivo. Angiogenesis. 2011; 14: 209–221. 10.1007/s10456-011-9206-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200: 373–383. 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010; 73: 1907–1920. 10.1016/j.jprot.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 40.Bazer FW, Ying W, Wang X, Dunlap KA, Zhou B, Johnson GA, et al. The many faces of interferon tau. Amino Acids. 2015; 47: 449–460. 10.1007/s00726-014-1905-x [DOI] [PubMed] [Google Scholar]
- 41.Yankey SJ, Hicks BA, Carnahan KG, Assiri AM, Sinor SJ, Kodali K, et al. Expression of the antiviral protein Mx in peripheral blood mononuclear cells of pregnant and bred, non-pregnant ewes. J Endocrinol. 2001; 170: R7–11. [DOI] [PubMed] [Google Scholar]
- 42.Shirasuna K, Matsumoto H, Matsuyama S, Kimura K, Bollwein H, Miyamoto A. Possible role of interferon tau on the bovine corpus luteum and neutrophils during the early pregnancy. Reproduction. 2015; 150: 217–225. 10.1530/REP-15-0085 [DOI] [PubMed] [Google Scholar]
- 43.Oliveira JF, Henkes LE, Ashley RL, Purcell SH, Smirnova NP, Veeramachaneni DN, et al. Expression of interferon (IFN)-stimulated genes in extrauterine tissues during early pregnancy in sheep is the consequence of endocrine IFN-tau release from the uterine vein. Endocrinology. 2008; 149: 1252–1259. [DOI] [PubMed] [Google Scholar]
- 44.Romero JJ, Antoniazzi AQ, Nett TM, Ashley RL, Webb BT, Smirnova NP, et al. Temporal Release, Paracrine and Endocrine Actions of Ovine Conceptus-Derived Interferon-Tau During Early Pregnancy. Biol Reprod. 2015; 93: 146 10.1095/biolreprod.115.132860 [DOI] [PubMed] [Google Scholar]
- 45.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005; 17: 879–887. [DOI] [PubMed] [Google Scholar]
- 46.Berrone E, Corona C, Mazza M, Vallino Costassa E, Lo Faro M, Properzi F, et al. Detection of cellular prion protein in exosome derived from ovine plasma. J Gen Virol. 2015; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All data are available in the text and its Supporting Information files.