Abstract
Background
More than twenty years following the end of the 1990–1991 Gulf War it is estimated that approximately 300,000 veterans of this conflict suffer from an unexplained chronic, multi-system disorder known as Gulf War Illness (GWI). The etiology of GWI may be exposure to chemical toxins, but it remains only partially defined, and its case definition is based only on symptoms. Objective criteria for the diagnosis of GWI are urgently needed for diagnosis and therapeutic research.
Objective
This study was designed to determine if blood biomarkers could provide objective criteria to assist diagnosis of GWI.
Design
A surveillance study of 85 Gulf War Veteran volunteers identified from the Department of Veterans Affairs Minnesota Gulf War registry was performed. All subjects were deployed to the Gulf War. Fifty seven subjects had GWI defined by CDC criteria, and 28 did not have symptomatic criteria for a diagnosis of GWI. Statistical analyses were performed on peripheral blood counts and assays of 61 plasma proteins using the Mann-Whitney rank sum test to compare biomarker distributions and stepwise logistic regression to formulate a diagnostic model.
Results
Lymphocyte, monocyte, neutrophil, and platelet counts were higher in GWI subjects. Six serum proteins associated with inflammation were significantly different in GWI subjects. A diagnostic model of three biomarkers—lymphocytes, monocytes, and C reactive protein—had a predicted probability of 90% (CI 76–90%) for diagnosing GWI when the probability of having GWI was above 70%.
Significance
The results of the current study indicate that inflammation is a component of the pathobiology of GWI. Analysis of the data resulted in a model utilizing three readily measurable biomarkers that appears to significantly augment the symptom-based case definition of GWI. These new observations are highly relevant to the diagnosis of GWI, and to therapeutic trials.
Introduction
From August 2, 1990 to July 31, 1991 approximately 697,000 United States military personnel were deployed to the Kuwaiti Theater of Operations during Operation Desert Shield and Operation Desert Storm (Gulf War)[1]. Many veterans of this conflict—estimated to be approximately 300,000 based on data from a recent survey of Gulf War veterans [2]—now suffer from an unexplained chronic multi-symptom disorder known as Gulf War Illness (GWI). The symptoms most frequently associated with GWI are widespread pain, unexplained fatigue, and cognitive difficulties. Comprehensive reviews of GWI have been published by the Research Advisory Committee on Gulf War Veterans’ Illnesses [3, 4] and the Institute of Medicine [1, 5, 6].
The Department of Veterans Affairs (VA) Office of Public Health (OPH) has conducted survey studies of the mental and physical health of a population-based cohort of 30,000 Gulf War and Gulf War era Veterans [2, 7–9]. The most significant health-related difference revealed by these studies was the higher prevalence of unexplained chronic multi-symptom illnesses in the deployed veterans group. Ten years post-deployment the difference was 28.9% vs 15.8% (adjusted odds ratio = 2.16) [8]. Fourteen years post-deployment the difference was 36.5% vs 11.7% (adjusted risk ratio = 3.05) [9]. Twenty years post-deployment the difference was 43.9% vs 20.3% (adjusted odds ratio = 3.06) [2]. Thus a chronic unexplained multi-symptom illness is the signature health-related outcome of the 1990–1991 Gulf War and the incidence of GWI continues to increase.
Multiple studies of Gulf War and Gulf War era Veterans from several countries consistently found higher rates and greater severity of symptoms in deployed than in non-deployed veterans. However, the variability of symptoms among the deployed, the presence of the same symptoms in non-deployed veterans, and multiple biases, prompted skepticism by authors of an Institute of Medicine review [1] regarding the representativeness of these studies. This review concluded that the data available did not support definition of a unique syndrome. They further cautioned that it was unlikely that factor analysis or cluster analysis of symptoms could produce a satisfactory case definition of GWI [1]. Therefore, we searched for objective parameters to augment current symptomatic diagnostic criteria.
Evidence of immunological aberrations in veterans with GWI has been published by other research groups [10–19]. However, no consensus has been reached regarding immunological criteria for the diagnosis of GWI [17]. Kelsall, et al [20] reported evidence of inflammation (elevated erythrocyte sedimentation rate, C-reactive protein, or leucocyte count) in Australian Veterans of the Gulf War who had multisymptom illness, but only pooled biomarker data was published. A previous study carried out in our laboratory also found higher blood CRP, plus elevated platelet counts and thromboxane analog-stimulated platelet aggregation, in deployed veterans with GWI compared to deployed veterans without GWI [21]. The results of this study stimulated evaluation of a larger cohort of Gulf War Veterans to determine if significant blood biomarker differences exist between veterans with GWI and without GWI as defined by accepted symptomatic criteria [22].
Methods
IRB Approval
The study was approved by the Human Studies Subcommittee of the Research Committee of the Minneapolis Veterans Affairs Health Care System (MVAHCS), and by the U.S. Army Medical Research and Materiel Command IRB.
Subjects
Five hundred deployed veterans of the Gulf War enrolled in the Department of Veterans Affairs Minnesota Gulf War Registry were invited to participate in the study by means of an IRB–approved letter. Eighty six veterans replied, and 85 entered the study between 2010 and 2013. Veterans who volunteered for the study were interviewed in person. Written informed consent was obtained. A structured interview assessed their health status, and blood samples were obtained.
Inclusion and Exclusion Criteria
Deployed Gulf War Veterans were eligible for entry into the study unless they had cancer, liver disease, acute or chronic inflammatory states, or other major chronic illness including chronic fatigue syndrome and fibromyalgia. Post-traumatic stress disorder (PTSD) did not exclude subjects. All 85 of the Gulf War Veterans who volunteered for the study were eligible; so none were excluded.
Subject Classification
All subjects completed a symptom questionnaire that contained the elements of the case definition of the chronic multisystem illness characteristic of GWI developed by Fukuda and colleagues [22], also referred to as the CDC-10 survey. This survey included three categories with nine subcategories: 1) fatigue; 2) mood-cognition (depression, anxiety, moodiness, memory problems, difficulty with words, difficulty sleeping); 3) musculoskeletal (muscle pain, joint pain, joint stiffness). Subjects were considered to have GWI, and were classified as GWI+ if, a) they had one or more chronic symptoms from at least 2 of 3 of the case-defining symptom categories—fatigue, mood-cognition, and musculoskeletal pain; b) the symptoms began during or after the 1990–1991 Gulf War; and c) symptoms were present for at least 6 months. Subjects without case-defining symptoms or symptoms in only one category were considered not to have GWI, and were classified as GWI-.
Blood Sample Collection and Preparation
Non-fasting, peripheral venous blood was collected into 4.0 ml Vacutainer® vacuum tubes (BD, Franklin Lakes, NJ, USA) containing 7.2 mg K2 EDTA for evaluation of blood cell and plasma protein components. One tube was sent to the MVAHCS Clinical Laboratory for a complete blood count with differential leucocyte count. A second tube was used to prepare platelet-free plasma (PFP). Platelet-poor plasma (PPP) was isolated from whole blood by centrifugation at 3,200 rpm for 15 min (1,770g) at room temperature (RT). The PPP layer was carefully removed, and the PPP was centrifuged at 3,200 rpm for 15 min at RT repeatedly if necessary until the platelet count was ≤1/μl (Beckman Coulter AcTdiff 2 counter). PFP aliquots were snap frozen on dry ice, and stored at -80°C.
Plasma Proteomic Analysis
Frozen PFP samples were sent to Myriad RBM, Austin, TX, for proteomic analysis performed by quantitative multiplexed immunoassays using a Multi-Analyte Profile (MAP) platform [23, 24]. HumanMAP® v.1.6 analysis was programmed to measure 88 plasma proteins (Table 1). Twenty-seven of the antigens were not included in the statistical analyses because measurable protein values were not detected in at least 90% of the subjects (Table 1).
Table 1. HumanMAP® v.1.6 analysis by Myriad RBM, Austin TX, USA.
| ANTIGEN | N* | ANTIGEN | N |
|---|---|---|---|
| Alpha-2-macroglobulin | 85 | Interleukin-4 | 36 |
| Alpha-1-Antitrypsin | 85 | Interleukin-5 | 67 |
| Adiponectin | 85 | Interleukin-6 | 55 |
| Alpha-Fetoprotein | 85 | Interleukin-7 | 56 |
| Apolipoprotein(a) | 84 | Interleukin-8 | 84 |
| Apolipoprotein A-I | 85 | Interleukin-10 | 79 |
| Apolipoprotein C-III | 85 | Interleukin-12p40 | 48 |
| Apolipoprotein H | 85 | Interleukin-12p70 | 14 |
| Beta-2-Microglobulin | 85 | Interleukin-13 | 71 |
| Brain-Derived Neurotrophic Factor | 85 | Interleukin-15 | 60 |
| Complement C3 | 85 | Interleukin-16 | 85 |
| Cancer Antigen 125 | 66 | Interleukin-18 | 85 |
| Cancer Antigen 19–9 | 81 | Insulin | 84 |
| Calcitonin | 82 | Leptin | 85 |
| CD 40 antigen | 85 | Lymphotactin | 2 |
| CD40 Ligand | 85 | Monocyte Chemotactic Protein 1 | 85 |
| Carcinoembryonic Antigen | 85 | Macrophage-Derived Chemokine | 85 |
| Creatine Kinase-MB | 84 | MIP-1 alpha | 85 |
| C-Reactive Protein | 84 | MIP-1 beta | 85 |
| Epidermal Growth Factor | 41 | Matrix Metalloproteinase-2 | 85 |
| ENA-78 | 85 | Matrix Metalloproteinase-3 | 27 |
| EN-RAGE | 60 | Matrix Metalloproteinase-9 | 85 |
| Eotaxin-1 | 73 | Myeloperoxidase | 78 |
| Erythropoietin | 34 | Myoglobin | 85 |
| Endothelin-1 | 43 | Plasminogen Activator Inhibitor 1 | 85 |
| Fatty Acid-Binding Protein 3 | 79 | Prostatic Acid Phosphatase | 85 |
| Factor VII | 85 | PAPPA | 72 |
| Fibroblast Growth Factor Basic | 16 | Prostate-Specific Antigen, Free | 85 |
| Fibrinogen | 85 | RANTES | 85 |
| Ferritin | 85 | Serum Amyloid P-Component | 85 |
| GCSF | 73 | Stem Cell Factor | 85 |
| Growth Hormone | 69 | SGOT | 84 |
| GM-CSF | 23 | Sex Hormone-Binding Globulin | 85 |
| Haptoglobin | 84 | Thyroxine-Binding Globulin | 85 |
| Intercellular Adhesion Molecule 1 | 85 | Tissue Factor | 29 |
| Interferon gamma | 29 | Tissue Inhibitor of Metalloproteinases 1 | 85 |
| Immunoglobulin A | 84 | Tumor Necrosis Factor alpha | 77 |
| Immunoglobulin E | 83 | Tumor Necrosis Factor beta | 27 |
| Immunoglobulin M | 85 | Tumor Necrosis Factor Receptor 2 | 85 |
| Interleukin-1 alpha | 52 | Thrombopoietin | 80 |
| Interleukin-1 beta | 24 | Thyroid-Stimulating Hormone | 84 |
| Interleukin-1 receptor antagonist | 77 | Vascular Cell Adhesion Molecule-1 | 85 |
| Interleukin-2 | 6 | VEGF | 85 |
| Interleukin-3 | 80 | vonWillebrand Factor | 85 |
* number of subjects which yielded protein concentrations above the lower limit of detection
Statistical Analyses
The Mann-Whitney rank sum test was used to compare the distribution of each biomarker between the GWI+ and GWI- groups. The c-statistic is reported as a measure of discrimination.
Stepwise logistic regression was employed first to identify cell counts that may provide independent discriminatory information. Other biomarkers were then tested one by one to see if they provided additional discriminatory information. Given the modest sample, a p-value <0.10 was considered significant without correction for the number of comparisons in this effort to identify diagnostic biomarkers.
Results
Demographic Data
Characteristics of the 57 (67%) GWI+ and 28 (33%) GWI- veterans enrolled in this study are presented in Table 2. The subjects were predominantly male (95%), white (93%), and middle aged (median 46–48 years). The median weights (p = 0.249) and BMIs (p = 0.236) of the GWI+ and GWI- groups were not significantly different. PTSD had been diagnosed in 17.5% of GWI+ subjects (all male), but in none of the GWI- subjects. Fourteen percent of the GWI+ subjects and 4% of GWI- subjects were being treated with antidepressants, or other psychotropic medications at the time of study.
Table 2. Characteristics of Subjects at Time of Study.
| Characteristics | GWI+ | GWI- |
|---|---|---|
| Number of Participants (n) | 57 | 28 |
| Age | ||
| Age, years (median) | 46 | 48 |
| Age, years (range) | (38–68) | (38–70) |
| BMI | ||
| BMI (median) | 31 | 28 |
| BMI (range) | (19–46) | (22–47) |
| BMI <30 (median) | 27 | 26 |
| BMI ≥30 (median) | 34 | 36 |
| Weight | ||
| Weight, lbs. (median) | 220 | 200 |
| Weight, lbs. (range) | (125–360) | (130–320) |
| Gender | ||
| Female | 3 | 1 |
| Male | 54 | 27 |
| Ethnic Origin | ||
| Black | 2 | 2 |
| White | 53 | 26 |
| Hispanic | 2 | 0 |
| Symptoms | ||
| None | 0 | 13 |
| Single | 0 | 15 |
| Multiple | 57 | 0 |
| Cognition | 57 | 6 |
| Fatigue | 52 | 6 |
| Pain | 48 | 1 |
| Use of Nicotine | 42% | 21% |
| Concomitant Medications | ||
| NSAIDS | 58% | 57% |
| OTC supplements | 32% | 61% |
| Statins | 18% | 32% |
| Antidepressants/other psych meds | 14% | 4% |
| Opiates | 7% | 4% |
Symptomatology
By definition, 100% of GWI+ subjects had multiple symptoms compared to none of the GWI- subjects (Table 2). Forty six percent of GWI- subjects had no relevant case- defining symptoms, and 53% had a single symptom. Among GWI+ subjects cognitive disturbances were present in 100%, fatigue in 91%, and pain in 84%. Twenty one per cent of GWI- subjects had cognitive dysfunction, 21% had fatigue, and 4% had pain.
Hematological Data
Compared to GWI- subjects, the distributions of peripheral blood lymphocyte, monocyte, neutrophil, and platelet counts were higher in GWI+ subjects (Table 3).
Table 3. Blood Cell Counts for GWI+ and GWI- Veterans.
| Blood Cell Type | GWI+ (n = 57), Cells x103/μl | GWI- (n = 28), Cells x 103/μl | c-statistic* | p-value† |
|---|---|---|---|---|
| White Blood Cells | 6.54 (5.55–7.74)‡ | 5.66 (4.33–6.56) | 0.69 | 0.005 |
| Lymphocytes | 1.82 (1.49–2.39) | 1.50 (1.35–1.76) | 0.70 | 0.004 |
| Monocytes | 0.51 (0.42–0.66) | 0.40 (0.35–0.52) | 0.68 | 0.009 |
| Neutrophils | 3.91 (3.27–4.87) | 3.29 (2.58–3.96) | 0.67 | 0.010 |
| Platelets | 222 (201–259) | 205 (164–240) | 0.65 | 0.030 |
* A value of 0.50 would indicate no discrimination, 1.0 would be perfect discrimination of those with GWI.
† Mann-Whitney test
‡ Median (interquartiles)
The lymphocyte counts were slightly better at discriminating the GWI groups. The distributions of neutrophil to lymphocyte ratios were not significantly different.
Proteomic Analysis
The most discriminating plasma proteomic variables are summarized in Table 4.
Table 4. Proteomic Analysis of Plasmas from GWI+ and GWI- Veterans.
| Plasma Proteins | GWI+ (n = 57) | GWI- (n = 28) | c-statistic* | p-value† |
|---|---|---|---|---|
| C-Reactive Protein (CRP), μg/ml | 2.1‡ | 1.2 | 0.65 | 0.03 |
| C-Reactive Protein (CRP), μg/ml (range) | (1.0–4.2) | (0.7–2.8) | ||
| Leptin, ng/ml | 9 | 5.4 | 0.63 | 0.05 |
| Leptin, ng/ml (range) | (4.5–13.8) | (2.8–11.1) | ||
| Matrix Metalloproteinase-9 (MMP-9), ng/ml | 103 | 90 | 0.64 | 0.03 |
| Matrix Metalloproteinase-9 (MMP-9), ng/ml (range) | (79–141) | (66–116) | ||
| Brain-Derived Neurotrophic Factor (BDNF), ng/ml | 1.3 | 0.81 | 0.66 | 0.02 |
| Brain-Derived Neurotrophic Factor (BDNF), ng/ml (range) | (0.83–1.86) | (0.36–1.38) | ||
| Matrix Metalloproteinase-2 (MMP-2), ng/ml | 2130 | 2430 | 0.69 | 0.004 |
| Matrix Metalloproteinase-2 (MMP-2), ng/ml (range) | (1770–2590) | (2250–3175) | ||
| Heart-Fatty Acid-Binding Protein (H-FABP), ng/ml | 1.8 | 2.8 | 0.66 | 0.02 |
| Heart-Fatty Acid-Binding Protein (H-FABP), ng/ml (range) | (0.9–3.1) | (1.9–3.7) |
* A value of 0.50 would indicate no discrimination, 1.0 would be perfect discrimination of those with GWI.
† Mann-Whitney test
‡ Median (interquartiles)
Six statistically significant differences (p<0.05) between GWI+ and GWI- subjects were identified. Plasma C-reactive protein (CRP), leptin, brain-derived neurotrophic factor (BDNF), and matrix metalloproteinase-9 (MMP-9) were significantly higher in the GWI+ group. Heart-type fatty acid binding protein (H-FABP) and matrix metalloproteinase-2 (MMP-2) were significantly lower in the blood of GWI+ subjects. The level of detection of cytokines, such as IL-1α, IL-1β, IL-2, IL-6 and interferon γ, was well below the criteria for analysis (<10% of subjects with undetectable levels).
Diagnostic Model
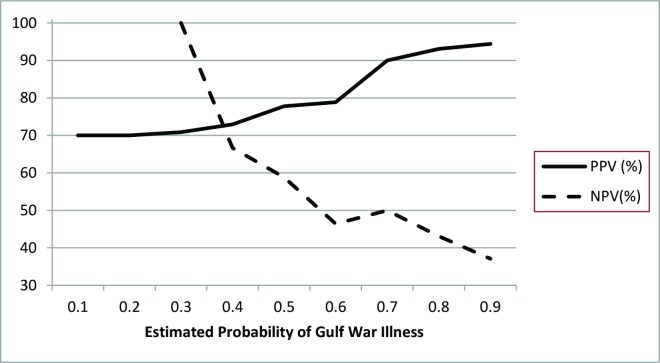
Stepwise multivariable logistic regression led to a diagnostic model comprised of three biomarkers—lymphocytes, monocytes, and CRP. We estimated the probability of having GWI using this fitted model. A positive diagnosis of GWI was defined as a model-estimated probability exceeding 0.70 (70%). For subjects with a model-predicted probability of >70% [a criterion met by 40/80 (50%) of the sample] the positive predictive value of the confirmatory diagnostic model was 90% (95% confidence interval 76–97%) for diagnosing GWI. The corresponding negative predictive value for ruling out GWI was 50% (95% confidence interval 34–66%; Fig 1). The model c-statistic was 0.77 (95% confidence interval 0.67–0.88; p = 0.05).
Fig 1. The positive predictive value (PPV) and negative predictive value (NPV) of a multivariable model of GWI.
Since obesity occurred in GWI+ and GWI- groups, a characteristic of Gulf War era veterans described previously [2, 20, 25], the potential influence of obesity on the diagnostic model was analysed. BMI was weakly correlated with lymphocytes (r = 0.22) and monocytes (r = 0.13), and moderately correlated with CRP (r = 0.43); however, BMI did not reach the specified p value of 0.1 to enter the model after the addition of lymphocytes and monocytes. Therefore, BMI did not add to the discrimination of the model. Similarly, leptin was highly correlated with BMI (r = 0.78), but the addition of leptin did not add discriminating information to the lymphocyte, monocyte model.
Discussion
Long after the end of the 1990–1991 Gulf War many veterans of this conflict are ill with an unexplained chronic, multi-system disorder recognized by the Department of Veterans Affairs as GWI [2, 26]. This disorder is characterized by an incomplete understanding of its etiology and pathophysiology, and a case definition based only on symptoms [22, 27]. The absence of objective diagnostic criteria is a substantial barrier to clinical diagnosis and research. Despite research by multiple investigators, readily measurable parameters that would permit an objective diagnosis of GWI have not previously been identified. The results of the current study provide evidence of alterations in a number of blood parameters that are readily measurable in routine clinical laboratories. We found white blood cell counts and blood biomarkers related to inflammation could discriminate groups that did or did not meet the current symptom-based criteria for case definition of GWI. Our observations are consistent with conclusions expressed in recent literature reviews that immune dysregulation/neuroinflammation are components of the pathobiology of GWI [28, 29]. Appropriate assays for the presence of chronic inflammation could provide objective evidence that would facilitate the diagnosis of GWI+.
Alterations of leucocyte counts, particularly the neutrophil to lymphocyte ratio, have recently been reported to have prognostic significance in a wide range of diseases [30–33]. An elevated neutrophil to lymphocyte ratio, often found to correlate with CRP or IL6 levels [30, 34, 35] has been interpreted as evidence of an inflammatory component of the disorder studied. In contrast to other inflammatory conditions, we did not observe an increase in the neutrophil to lymphocyte ratio because the lymphocyte count was elevated rather than decreased as described in other studies.
Support for an inflammatory component of GWI is provided by the significantly higher levels of plasma CRP detected in GWI+ veterans. CRP is an acute phase plasma protein synthesized in the liver which rises rapidly in response to infection or tissue injury [36]. CRP is frequently employed as a biomarker of IL-6-mediated inflammation, and it may also augment inflammation. CRP exists in two distinct protein confirmations. Native pentameric CRP is the circulating precursor of monomeric CRP which is strongly proinflammatory [37].
In some inflammatory disorders, CRP is highly elevated, but in other disorders modest elevations of CRP have been found to be indicators of chronic inflammation with prognostic significance [38–41]. In coronary artery disease CRP concentrations found in the general population (1–3 μg/ml) predict increased cardiovascular mortality [41].
Leptin, an adipokine produced primarily by white fat tissue, is another biomarker linked to inflammation, and found in the current study to be elevated in GWI+ veterans. Leptin production is elevated in experimental inflammation and in human autoimmune diseases [42, 43]. Leptin is known to cross the blood-brain barrier and to interact with cells in the hypothalamus, arcuate nucleus, and endothelium, and with leucocytes. These interactions have been shown to result in prolonged neuroinflammation with behavioral changes in experimental animals [43]. A leptin antagonist mutant demonstrated benefit in experimental autoimmune inflammatory bowel disease [44]. Leptin also affects hematopoiesis [45–49]. It may have contributed to the elevated lymphocyte counts observed in GWI+ subjects, and thus explain why leptin was not an independent correlate of GWI+. Leptin has also been demonstrated to interact with CRP. In vitro studies found leptin to stimulate the expression of CRP by human hepatocytes. In addition, CRP bound to leptin and directly inhibited its binding to its receptors [50].
BDNF, another plasma protein elevated in GWI+ subjects, may also be a biomarker of inflammation in GWI. BDNF is a neurotrophin which functions as a major regulator of synaptic plasticity and neurogenesis in the central nervous system [51]. Blood BDNF derives primarily from the brain [52], and has been observed in several diseases accompanied by inflammation—rapid cycling bipolar disorder [53], Alzheimer’s disease [54], and fibromyalgia [55]. Multiple animal studies indicate that overexpression of BDNF following nerve injury or peripheral inflammation stimulates synaptic changes that contribute to chronic pain [56]. Thus, inflammation-induced BDNF could be a mediator of cognitive impairment and chronic pain in GWI. Neither serum nor plasma BDNF correlate with body weight [57] or obesity [58].
Matrix metalloproteinases (MMPs) are endopeptidases that participate in tissue extracellular matrix degradation and remodeling. MMP activation has been observed in inflammatory and neurodegenerative disorders [59, 60]. Elevation of plasma MMP-2, MMP-9 or both have been observed in coronary heart disease [61], polypoidal choroidal vasculopathy [62] and Japanese encephalitis [63]. We observed higher median MMP-9, but lower median MMP-2 in GWI+ subjects than in GWI- subjects. The reason for the opposing direction of change is unexplained, but precedent exists for differential patterns of MMP-2 and MMP-9 gene expression in coronary heart disease [61], chronic obstructive pulmonary disease [64], and thoracic aortic aneurysm [65].
H-FABP is a fatty acid binding protein expressed primarily in the heart. Release of H-FABP into the blood occurs during cardiac ischemia, strenuous exercise, and neurodegenerative disorders, but low levels have been reported to occur in patients with Down syndrome [66]. Decreased H-FABP has been postulated to protect against atherosclerosis. In the current study median plasma H-FABP was significantly lower in GWI+ subjects than in GWI- subjects. Possibly relevant to the lower blood levels in GWI+ subjects is the observation that fatty acid binding protein mRNA was substantially decreased in hamster skeletal muscle and heart muscle by LPS-induced inflammation [67]. It is possible that blood H-FABP is suppressed by inflammation in GWI, and that the suppression is modulated by elevated blood leptin [68].
Although inflammatory mediators are implicated, the precise stimuli for the elevations of blood cell counts, CRP, leptin and the alterations in other blood proteins could not be determined by the current study, and the relationship of these parameters to the symptoms experienced by the subjects with GWI can only be hypothesized. However, observations of others noted above suggest that some of the patient’s symptoms may be caused by or accentuated by CRP, leptin, BDNF or other inflammation-related blood proteins.
This study has strengths and limitations that must be considered in assessing its significance. A strength of the study is the classification of subjects deployed to the Gulf War based on accepted case-definition criteria. Another strength of the study is the evaluation of blood parameters that are readily available in clinical laboratories. The limitations of the study include small sample size, restricted geographic, ethnic, and sex composition of the study subjects, assay of blood parameters only once, some plasma protein assays, including cytokines, considered inevaluable due to a high percentage of assays below the level of detection, overlap of biomarker distributions within the normal range, absence of correction for multiple comparisons, a limited number of blood proteins found to be positively related to GWI+ status, and the absence of a confirmation cohort study.
Despite these limitations, the diagnostic model yielded a high positive predictive value for the 50% of the study participants who had an estimated probability of GWI of 70%, although it is recognized that this estimate may be optimistic because the diagnostic model was fit to these particular data. Although the positive association of 6/61 plasma proteins with GWI+ subjects may have occurred by chance, the close functional and biochemical relationships of these proteins suggest that the differences were not random events. Also, the observation that the subsequent addition of other inflammation-related proteins did not improve the predictive probability of the model above that provided by CRP is consistent with a functional relationship of these parameters.
Although not a deficiency of the study, the issue of obesity is a confounding variable for interpretation of the results of the current study as well as other studies of GWI. The median body weights and BMIs of GWI+ and GWI- subjects were not significantly different, and BMI did not enter into the multivariable diagnostic model. Both groups included obese subjects. Similar obesity observations were found in studies of American [2, 25] and Australian Gulf War Veterans [20]. These studies found no differences in BMI of subjects with GWI and those in comparison groups of veterans who were either non-deployed or deployed to areas other than the Persian Gulf. Coughlin, et al [25] found no associations between BMI and unexplained multisystem illness in multivariate analysis. Kelsall, et al [20] found a relationship between laboratory parameters of inflammation and multisystem illness, but BMI was not related to multisystem illness [20]. Dursa, et al [2] reported average BMIs of 29.8 for Gulf War Veterans and 29.7 for Gulf War era Veterans 20 years after the Gulf War. These figures are quite similar to those we observed in GWI+ subjects (BMI 31) and GWI-subjects (BMI 28). Therefore, elevation of some of the inflammatory parameters observed in the current study may be attributable to obesity, but obesity does not explain the differences between the GWI+ and GWI- groups, and does not diminish the predictive value of the diagnostic model.
In summary, the results of the current study support the hypothesis that chronic inflammation is a component of the pathophysiology of GWI. Multivariable logistic regression analysis resulted in a model with a high positive predictive value for GWI in subjects with symptoms considered to be significant by current case definition criteria. This diagnostic model requires validation in other samples of Gulf War Veterans. A clinical trial that will further evaluate inflammatory parameters and the efficacy of anti-inflammatory therapy in GWI is in progress at our institution (Gulf War Illness Inflammation Reduction Trial, ClinicalTrials.gov #NCT02506192). The results of this clinical trial will provide valuable data to further evaluate the utility of measuring inflammatory biomarkers in the diagnosis of GWI, but validation by studies of other cohorts of veterans with GWI is required.
Acknowledgments
Disclaimers
The views expressed in this article are the author’s and not an official position of the United States Department of Veterans Affairs or the United States Department of Defense.
Data Accountability
Dr. Ronald Bach, Dr. Gerhard Johnson, Co-Principal Investigators, plus Dr. Thomas Rector, and Ms. Billie Slater had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Data Availability
Analyses were performed using raw data that are only available within the US Department of Veterans Affairs firewall in a secure research environment. VA privacy and data security policies and regulatory constraints provide that only aggregate summary data may be removed from the VA for publication. The authors have provided detailed results of these analyses in the paper. These restrictions are in place in order to maintain patient privacy and confidentiality. Access to these data can be granted to persons who are not an employee of the VA; however, there is an official protocol that must be followed for doing so. The authors invite those wishing to access the raw data that were used for this analysis to contact Ronald Bach (Ronald.bach@va.gov) to discuss the details of the VA data access approval process.
Funding Statement
This work was supported by Department of Defense Congressionally Directed Medical Research Programs/Gulf War Illness Research Program Grant Number W81XWH-09-2-0047.
References
- 1.Institute of Medicine. Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined Washington, DC: The National Academies Press; 2014. [PubMed] [Google Scholar]
- 2.Dursa EK, Barth SK, Schneiderman AI, Bossarte RM. Physical and Mental Health Status of Gulf War and Gulf Era Veterans: Results From a Large Population-Based Epidemiological Study. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2016;58(1):41–6. Epub 2015/12/31. 10.1097/JOM.0000000000000627 [DOI] [PubMed] [Google Scholar]
- 3.Research Advisory Committee on Gulf War Veterans' Illnesses. Gulf War Illness and the Health of Gulf War Veterans: Scientific Findings and Recommendations Washington D.C., U.S. Government Printing Office; 2008. [Google Scholar]
- 4.Research Advisory Committee on Gulf War Veterans' Illnesses. Gulf War Illness and the Health of Gulf War Veterans: Scientific Findings and Recommendations 2009–2013. Washington D.C. April 2014.
- 5.National Resource Council. Gulf War and Health: Volume 8: Update of Health Effects of Serving in the Gulf War Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 6.Institute of Medicine of the National academies Committee on Gulf War and Health. Gulf War and Health: Treatment for Chronic Multisymptom Illness Washington D.C.: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 7.Kang HK, Mahan CM, Lee KY, Magee CA, Murphy FM. Illnesses among United States Veterans of the Gulf War: a population-based survey of 30,000 veterans. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2000;42(5):491–501. Epub 2000/05/29. [DOI] [PubMed] [Google Scholar]
- 8.Blanchard MS, Eisen SA, Alpern R, Karlinsky J, Toomey R, Reda DJ, et al. Chronic multisymptom illness complex in Gulf War I Veterans 10 years later. American journal of epidemiology. 2006;163(1):66–75. Epub 2005/11/19. [DOI] [PubMed] [Google Scholar]
- 9.Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US Veterans of 1991 Gulf War: a follow-up survey in 10 years. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2009;51(4):401–10. Epub 2009/03/27. 10.1097/JOM.0b013e3181a2feeb [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Zhou XD, Denny T, Ottenweller JE, Lange G, LaManca JJ, et al. Changes in immune parameters seen in Gulf War Veterans but not in civilians with chronic fatigue syndrome. Clinical and diagnostic laboratory immunology. 1999;6(1):6–13. Epub 1999/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skowera A, Hotopf M, Sawicka E, Varela-Calvino R, Unwin C, Nikolaou V, et al. Cellular immune activation in Gulf War Veterans. Journal of clinical immunology. 2004;24(1):66–73. Epub 2004/03/05. [DOI] [PubMed] [Google Scholar]
- 12.Vojdani A, Thrasher JD. Cellular and humoral immune abnormalities in Gulf War Veterans. Environmental health perspectives. 2004;112(8):840–6. Epub 2004/06/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whistler T, Fletcher MA, Lonergan W, Zeng XR, Lin JM, Laperriere A, et al. Impaired immune function in Gulf War Illness. BMC medical genomics. 2009;2:12 Epub 2009/03/07. 10.1186/1755-8794-2-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broderick G, Fletcher MA, Gallagher M, Barnes Z, Vernon SD, Klimas NG. Exploring the diagnostic potential of immune biomarker coexpression in Gulf War Illness. Methods Mol Biol. 2012;934:145–64. Epub 2012/08/31. 10.1007/978-1-62703-071-7_8 [DOI] [PubMed] [Google Scholar]
- 15.Maloney CD, Jensen S, Gil-Rivas V, Goolkasian P. Latent viral immune inflammatory response model for chronic multisymptom illness. Medical hypotheses. 2013;80(3):220–9. Epub 2012/12/26. 10.1016/j.mehy.2012.11.024 [DOI] [PubMed] [Google Scholar]
- 16.Smylie AL, Broderick G, Fernandes H, Razdan S, Barnes Z, Collado F, et al. A comparison of sex-specific immune signatures in Gulf War illness and chronic fatigue syndrome. BMC immunology. 2013;14:29 Epub 2013/06/27. 10.1186/1471-2172-14-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khaiboullina SF, DeMeirleir KL, Rawat S, Berk GS, Gaynor-Berk RS, Mijatovic T, et al. Cytokine expression provides clues to the pathophysiology of Gulf War illness and myalgic encephalomyelitis. Cytokine. 2015;72(1):1–8. Epub 2014/12/17. 10.1016/j.cyto.2014.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parkitny L, Middleton S, Baker K, Younger J. Evidence for abnormal cytokine expression in Gulf War Illness: A preliminary analysis of daily immune monitoring data. BMC immunology. 2015;16:57 Epub 2015/10/01. 10.1186/s12865-015-0122-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broderick G, Ben-Hamo R, Vashishtha S, Efroni S, Nathanson L, Barnes Z, et al. Altered immune pathway activity under exercise challenge in Gulf War Illness: an exploratory analysis. Brain, behavior, and immunity. 2013;28:159–69. Epub 2012/12/04. 10.1016/j.bbi.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 20.Kelsall HL, McKenzie DP, Sim MR, Leder K, Forbes AB, Dwyer T. Physical, psychological, and functional comorbidities of multisymptom illness in Australian male Veterans of the 1991 Gulf War. American journal of epidemiology. 2009;170(8):1048–56. Epub 2009/09/19. 10.1093/aje/kwp238 [DOI] [PubMed] [Google Scholar]
- 21.Johnson GJ, Leis LA, Slater BC, Bach RR. Elevated platelet count, C-reactive protein and thromboxane analog-induced platelet aggregation in patients with Gulf War Veterans' illnesses: evidence of a chronic inflammatory state? Blood coagulation & fibrinolysis: an international journal in haemostasis and thrombosis. 2013;24(7):736–41. Epub 2013/06/12. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, et al. Chronic multisymptom illness affecting Air Force Veterans of the Gulf War. Jama. 1998;280(11):981–8. Epub 1998/09/28. [DOI] [PubMed] [Google Scholar]
- 23.Thrailkill KM, Moreau CS, Cockrell G, Simpson P, Goel R, North P, et al. Physiological matrix metalloproteinase concentrations in serum during childhood and adolescence, using Luminex Multiplex technology. Clinical chemistry and laboratory medicine: CCLM / FESCC. 2005;43(12):1392–9. Epub 2005/11/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luchting B, Rachinger-Adam B, Zeitler J, Egenberger L, Mohnle P, Kreth S, et al. Disrupted TH17/Treg balance in patients with chronic low back pain. PloS one. 2014;9(8):e104883 Epub 2014/08/15. 10.1371/journal.pone.0104883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coughlin SS, Kang HK, Mahan CM. Selected Health Conditions Among Overweight, Obese, and Non-Obese Veterans of the 1991 Gulf War: Results from a Survey Conducted in 2003–2005. The open epidemiology journal. 2011;4:140–6. Epub 2011/07/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Academy of Science. Gulf War and Health: Volume 10: Update of Health Effects of Serving in the Gulf War. Cory-Slechta DA, Wedge R, editors, 2016. [PubMed]
- 27.Steele L. Prevalence and patterns of Gulf War illness in Kansas Veterans: association of symptoms with characteristics of person, place, and time of military service. American journal of epidemiology. 2000;152(10):992–1002. Epub 2000/11/25. [DOI] [PubMed] [Google Scholar]
- 28.Craddock TJ, Del Rosario RR, Rice M, Zysman JP, Fletcher MA, Klimas NG, et al. Achieving Remission in Gulf War Illness: A Simulation-Based Approach to Treatment Design. PloS one. 2015;10(7):e0132774 Epub 2015/07/21. 10.1371/journal.pone.0132774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White RF, Steele L, O'Callaghan JP, Sullivan K, Binns JH, Golomb BA, et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex; a journal devoted to the study of the nervous system and behavior. 2016;74:449–75. Epub 2015/10/24. 10.1016/j.cortex.2015.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsson L, Wieringa WG, Pundziute G, Gjerde M, Engvall J, Swahn E, et al. Neutrophil/Lymphocyte ratio is associated with non-calcified plaque burden in patients with coronary artery disease. PloS one. 2014;9(9):e108183 Epub 2014/10/01. 10.1371/journal.pone.0108183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Journal of the National Cancer Institute. 2014;106(6):dju124 Epub 2014/05/31. 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 32.Balta S, Celik T, Mikhailidis DP, Ozturk C, Demirkol S, Aparci M, et al. The Relation Between Atherosclerosis and the Neutrophil-Lymphocyte Ratio. Clinical and applied thrombosis/hemostasis: official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2015. Epub 2015/02/11. [Google Scholar]
- 33.Benites-Zapata VA, Hernandez AV, Nagarajan V, Cauthen CA, Starling RC, Tang WH. Usefulness of neutrophil-to-lymphocyte ratio in risk stratification of patients with advanced heart failure. The American journal of cardiology. 2015;115(1):57–61. Epub 2014/12/03. 10.1016/j.amjcard.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akpek M, Kaya MG, Lam YY, Sahin O, Elcik D, Celik T, et al. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. The American journal of cardiology. 2012;110(5):621–7. Epub 2012/05/23. 10.1016/j.amjcard.2012.04.041 [DOI] [PubMed] [Google Scholar]
- 35.Chen ZY, Raghav K, Lieu CH, Jiang ZQ, Eng C, Vauthey JN, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. British journal of cancer. 2015;112(6):1088–97. Epub 2015/02/18. 10.1038/bjc.2015.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. The Journal of clinical investigation. 2003;111(12):1805–12. Epub 2003/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thiele JR, Zeller J, Bannasch H, Stark GB, Peter K, Eisenhardt SU. Targeting C-Reactive Protein in Inflammatory Disease by Preventing Conformational Changes. Mediators of inflammation. 2015;2015:372432 Epub 2015/06/20. 10.1155/2015/372432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99(2):237–42. Epub 1999/01/20. [DOI] [PubMed] [Google Scholar]
- 39.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321(7255):199–204. Epub 2000/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. The New England journal of medicine. 2002;347(20):1557–65. Epub 2002/11/15. [DOI] [PubMed] [Google Scholar]
- 41.Yeh ET, Willerson JT. Coming of age of C-reactive protein: using inflammation markers in cardiology. Circulation. 2003;107(3):370–1. Epub 2003/01/29. [DOI] [PubMed] [Google Scholar]
- 42.Procaccini C, Pucino V, Mantzoros CS, Matarese G. Leptin in autoimmune diseases. Metabolism: clinical and experimental. 2015;64(1):92–104. Epub 2014/12/04. [DOI] [PubMed] [Google Scholar]
- 43.Aguilar-Valles A, Inoue W, Rummel C, Luheshi GN. Obesity, adipokines and neuroinflammation. Neuropharmacology. 2015;96(Pt A):124–34. Epub 2015/01/15. 10.1016/j.neuropharm.2014.12.023 [DOI] [PubMed] [Google Scholar]
- 44.Singh UP, Singh NP, Guan H, Busbee B, Price RL, Taub DD, et al. The emerging role of leptin antagonist as potential therapeutic option for inflammatory bowel disease. International reviews of immunology. 2014;33(1):23–33. Epub 2013/07/12. 10.3109/08830185.2013.809071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Claycombe K, King LE, Fraker PJ. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(6):2017–21. Epub 2008/02/06. 10.1073/pnas.0712053105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trottier MD, Naaz A, Li Y, Fraker PJ. Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(20):7622–9. Epub 2012/04/28. 10.1073/pnas.1205129109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dias CC, Nogueira-Pedro A, Barbosa CM, Ribeiro-Filho AC, Wasinski F, Araujo RC, et al. Hematopoietic stem cell expansion caused by a synthetic fragment of leptin. Peptides. 2013;50:24–7. Epub 2013/10/05. 10.1016/j.peptides.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 48.Dias CC, Nogueira-Pedro A, Tokuyama PY, Martins MN, Segreto HR, Buri MV, et al. A Synthetic Fragment of Leptin Increase Hematopoietic Stem Cell Population and Improve Its Engraftment Ability. Journal of cellular biochemistry. 2015. Epub 2015/03/05. [DOI] [PubMed] [Google Scholar]
- 49.Hirose H, Saito I, Kawai T, Nakamura K, Maruyama H, Saruta T. Serum leptin level: possible association with haematopoiesis in adolescents, independent of body mass index and serum insulin. Clin Sci (Lond). 1998;94(6):633–6. Epub 1998/12/17. [DOI] [PubMed] [Google Scholar]
- 50.Chen K, Li F, Li J, Cai H, Strom S, Bisello A, et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nature medicine. 2006;12(4):425–32. Epub 2006/04/04. [DOI] [PubMed] [Google Scholar]
- 51.Leal G, Afonso PM, Salazar IL, Duarte CB. Regulation of hippocampal synaptic plasticity by BDNF. Brain research. 2014. Epub 2014/12/03. [DOI] [PubMed] [Google Scholar]
- 52.Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? The Journal of experimental medicine. 1999;189(5):865–70. Epub 1999/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Munkholm K, Pedersen BK, Kessing LV, Vinberg M. Elevated levels of plasma brain derived neurotrophic factor in rapid cycling bipolar disorder patients. Psychoneuroendocrinology. 2014;47:199–211. Epub 2014/07/09. 10.1016/j.psyneuen.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 54.Faria MC, Goncalves GS, Rocha NP, Moraes EN, Bicalho MA, Gualberto Cintra MT, et al. Increased plasma levels of BDNF and inflammatory markers in Alzheimer's disease. Journal of psychiatric research. 2014;53:166–72. Epub 2014/03/01. 10.1016/j.jpsychires.2014.01.019 [DOI] [PubMed] [Google Scholar]
- 55.Haas L, Portela LV, Bohmer AE, Oses JP, Lara DR. Increased plasma levels of brain derived neurotrophic factor (BDNF) in patients with fibromyalgia. Neurochemical research. 2010;35(5):830–4. Epub 2010/02/02. 10.1007/s11064-010-0129-z [DOI] [PubMed] [Google Scholar]
- 56.Thibault K, Lin WK, Rancillac A, Fan M, Snollaerts T, Sordoillet V, et al. BDNF-dependent plasticity induced by peripheral inflammation in the primary sensory and the cingulate cortex triggers cold allodynia and reveals a major role for endogenous BDNF as a tuner of the affective aspect of pain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34(44):14739–51. Epub 2014/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gajewska E, Sobieska M, Lojko D, Wieczorowska-Tobis K, Suwalska A. Obesity itself does not influence BDNF serum levels in adults. European review for medical and pharmacological sciences. 2014;18(21):3246–50. Epub 2014/12/10. [PubMed] [Google Scholar]
- 58.Huang CJ, Mari DC, Whitehurst M, Slusher A, Wilson A, Shibata Y. Brain-derived neurotrophic factor expression ex vivo in obesity. Physiology & behavior. 2014;123:76–9. Epub 2013/10/22. [DOI] [PubMed] [Google Scholar]
- 59.Singh D, Srivastava SK, Chaudhuri TK, Upadhyay G. Multifaceted role of matrix metalloproteinases (MMPs). Frontiers in molecular biosciences. 2015;2:19 Epub 2015/05/20. 10.3389/fmolb.2015.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hopps E, Caimi G. Matrix metalloproteases as a pharmacological target in cardiovascular diseases. European review for medical and pharmacological sciences. 2015;19(14):2583–9. Epub 2015/07/30. [PubMed] [Google Scholar]
- 61.Wang KF, Huang PH, Chiang CH, Hsu CY, Leu HB, Chen JW, et al. Usefulness of plasma matrix metalloproteinase-9 level in predicting future coronary revascularization in patients after acute myocardial infarction. Coronary artery disease. 2013;24(1):23–8. Epub 2012/11/16. 10.1097/MCA.0b013e32835aab4a [DOI] [PubMed] [Google Scholar]
- 62.Zeng R, Wen F, Zhang X, Su Y. Serum levels of matrix metalloproteinase 2 and matrix metalloproteinase 9 elevated in polypoidal choroidal vasculopathy but not in age-related macular degeneration. Molecular vision. 2013;19:729–36. Epub 2013/04/06. [PMC free article] [PubMed] [Google Scholar]
- 63.Shukla V, Shakya AK, Dhole TN, Misra UK. Matrix metalloproteinases and their tissue inhibitors in serum and cerebrospinal fluid of children with Japanese encephalitis virus infection. Archives of virology. 2013;158(12):2561–75. Epub 2013/07/10. 10.1007/s00705-013-1783-7 [DOI] [PubMed] [Google Scholar]
- 64.Gosselink JV, Hayashi S, Elliott WM, Xing L, Chan B, Yang L, et al. Differential expression of tissue repair genes in the pathogenesis of chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2010;181(12):1329–35. Epub 2010/01/16. 10.1164/rccm.200812-1902OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rabkin SW. Differential expression of MMP-2, MMP-9 and TIMP proteins in thoracic aortic aneurysm—comparison with and without bicuspid aortic valve: a meta-analysis. VASA Zeitschrift fur Gefasskrankheiten. 2014;43(6):433–42. Epub 2014/10/24. 10.1024/0301-1526/a000390 [DOI] [PubMed] [Google Scholar]
- 66.Vianello E, Dogliotti G, Dozio E, Corsi Romanelli MM. Low heart-type fatty acid binding protein level during aging may protect down syndrome people against atherosclerosis. Immunity & ageing: I & A. 2013;10(1):2. Epub 2013/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Memon RA, Feingold KR, Moser AH, Fuller J, Grunfeld C. Regulation of fatty acid transport protein and fatty acid translocase mRNA levels by endotoxin and cytokines. The American journal of physiology. 1998;274(2 Pt 1):E210–7. Epub 1998/03/05. [DOI] [PubMed] [Google Scholar]
- 68.Yan GT, Lin J, Hao XH, Xue H, Zhang K, Wang LH. Heart-type fatty acid-binding protein is a useful marker for organ dysfunction and leptin alleviates sepsis-induced organ injuries by restraining its tissue levels. European journal of pharmacology. 2009;616(1–3):244–50. Epub 2009/07/07. 10.1016/j.ejphar.2009.06.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Analyses were performed using raw data that are only available within the US Department of Veterans Affairs firewall in a secure research environment. VA privacy and data security policies and regulatory constraints provide that only aggregate summary data may be removed from the VA for publication. The authors have provided detailed results of these analyses in the paper. These restrictions are in place in order to maintain patient privacy and confidentiality. Access to these data can be granted to persons who are not an employee of the VA; however, there is an official protocol that must be followed for doing so. The authors invite those wishing to access the raw data that were used for this analysis to contact Ronald Bach (Ronald.bach@va.gov) to discuss the details of the VA data access approval process.