Abstract
BACKGROUND
Early detection of subclinical left ventricular (LV) systolic dysfunction in hypertensive patients is important for the prevention of progression of hypertensive heart disease.
METHODS
We studied 60 hypertensive patients (age ranged from 21 to 49 years, the duration of hypertension ranged from 1 to 18 years) and 30 healthy controls, all had preserved left ventricular ejection fraction (LVEF), detected by two-dimensional speckle tracking echocardiography (2D-STE).
RESULTS
There was no significant difference between the two groups regarding ejection fraction (EF) by Simpson’s method. Systolic velocity was significantly higher in the control group, and global longitudinal strain was significantly higher in the control group compared with the hypertensive group. In the hypertensive group, 23 of 60 patients had less negative global longitudinal strain than −19.1, defined as reduced systolic function, which is detected by 2D-STE (subclinical systolic dysfunction), when compared with 3 of 30 control subjects.
CONCLUSION
2D-STE detected substantial impairment of LV systolic function in hypertensive patients with preserved LVEF, which identifies higher risk subgroups for earlier medical intervention.
Keywords: speckle tracking, hypertension, global longitudinal strain (GLS)
Introduction
Hypertension is one of the most common diseases among population. It is a well-recognized risk factor for cardiovascular diseases and causes left ventricular (LV) pressure overload, which results in various geometric changes that progress to diastolic heart failure and/or heart failure, with LV systolic dysfunction.1
While conventional echocardiography can detect changes in LV diastolic function associated with left ventricular hypertrophy (LVH), global LV systolic function often remains preserved until late in the course of the disease, making subtle changes in LV contractile function difficult to interpret in the early stages.2
Early detection of LV dysfunction before the development of LVH may represent a clinical finding that would justify aggressive treatment aimed at reducing cardiovascular morbidity and mortality; therefore, it has to be considered in the assessment of global cardiovascular risk.3
Subclinical changes in LV function can be identified by quantifying myocardial strain, a dimensionless measurement of deformation, expressed as a fractional or percentage change from an object’s original dimension.4
Two-dimensional (2D) speckle tracking has recently emerged as a novel echocardiographic technique for rapid, offiine, bedside analysis of regional LV strains in the longitudinal, radial, and circumferential directions.5 This technique analyzes myocardial motion by tracking natural acoustic reflections and interference patterns seen in 2D echocardiographic images and has been validated with measurements obtained by sonomicrometry and magnetic resonance imaging.6
The aim of the present study was to detect subclinical LV dysfunction in hypertensive patients with apparently normal LV systolic function, using speckle tracking echocardiography (longitudinal strain pattern).
Patients and Methods
Patients
Hypertensive patients were selected from the cardiology outpatient clinic. Full history taking and general and cardiac clinical examinations were done before selection. Written informed consent was obtained from all patients, and the study protocol was approved by the Faculty of Medicine Ain Shams University Research Ethics Committee. Our research complies with the principles of the Declaration of Helsinki.
This study was carried out on 60 hypertensive patients and 30 age- and sex-matched healthy subjects as a control group. Control subjects had no detectable cardiovascular risk factors and not receiving any medications, who were volunteers recruited from among the hospital staff, medical and nursing students, and members of the local community.
Exclusion criteria
Patients aged above 50 years, with ejection fraction (EF) <55% or symptoms or signs of heart failure, diabetes mellitus, known coronary artery disease echocardiographic evidence of either regional or global wall motion abnormalities, significant valvular disease or any structural heart disease, and with atrial fibrillation or other rhythm disturbances were excluded.
Methods
All patients were subjected to proper history taking including clinical examination, measurements of heart rate, weight, and height. Body surface area and body mass index (BMI) were calculated. Measurements of blood pressure (BP) were taken according to American Heart Association and American College of Cardiology 2013 guidelines for the management of BP.7
Transthoracic echocardiography
Conventional echocardiographic Doppler study, tissue Doppler imaging, and 2D speckle tracking imaging were performed using Vivid 9 (General Electric Healthcare), equipped with harmonic M4S variable frequency phased-array transducer and echo Pac software for offiine analysis.
Images were obtained with patients in the left lateral decubitus position at end-expiration according to the recommendations of the American Society of Echocardiography and connected to single-lead electrocardiography (ECG).8 All standard measurements were obtained in the parasternal long-and short-axis views, apical four-chamber view, two-chamber view, and apical long-axis view.
Quantification of the LV dimensions was done using M-mode echocardiography, and then using the biplane (modified Simpson’s method). LV mass in grams was calculated according to Devereux et al.9, and then, LV mass was normalized for body surface area to obtain LV mass index (LVMI in g/m2). Relative wall thickness (RWT) was calculated as 2PWT/LVEDD (where PWT is the posterior wall thickness and LVEDD is the left ventricle end diastolic diameter).
Mitral annular plane systolic excursion (MAPSE) was assessed by placing M-mode cursor through medial and lateral mitral annulus; maximum systolic displacement was measured, and then, the mean of medial and lateral mitral annular excursion was calculated.
LA volume was calculated by applying the Simpson biplane method to the apical four- and two-chamber views. Volume was indexed to body surface area.
Transmitral pulsed-wave Doppler was recorded, the peaks of both early diastolic filling (E) and late diastolic filling (A) were measured, and the E/A ratio and E wave deceleration time were calculated.
Offiine color-coded tissue Doppler imaging was done in the apical four-chamber view by placing the sample volume over the septal and lateral mitral annuli, and then, the peak systolic velocity (S′), early diastolic velocity (E′), and late diastolic velocity (A′) were measured. The average E′ and S′ velocities at the sepal and lateral mitral annuli were estimated, and the E/E′ ratio was calculated.
2D speckle tracking echocardiography
Longitudinal strain imaging by 2D-speckle tracking echocardiography (2D-STE) was done with high-quality ECG-gated images from the apical four-chamber, two-chamber, and three-chamber views; all were obtained at nearly identical heart rates. The gain settings were optimized. The depth was reduced so that the LV occupied most of the image sector. Care was taken to avoid foreshortening of the LV, the gray-scale frame rate was kept between 50 and 90 frames/s; minimum three cardiac cycles were obtained for each loop. All the images were obtained in breath-hold to avoid any breathing artifacts. All images were stored in cine-loop format, and data were transferred to a workstation for further offiine analysis using the Echopac software (General Electric version 1.8.1.X-Vingmed). In order to measure the timing of cardiac events, LV inflow (mitral) and outflow (aortic) velocities were recorded using pulsed-wave Doppler.
Image analysis
In the end-systolic frame, endocardial border was traced manually in the three apical views. Then, the software generated a region of interest (ROI) to include the entire myocardial thickness. The width of the ROI was manually adjusted as required. Care was taken to avoid including bright, echogenic pericardium in the ROI. Then, the software tracked the myocardial speckles frame by frame and generated moving images, displaying the tracking. Visual inspection of the moving image allowed the operator to determine the adequacy of the tracking. When the tracking was not accurate, the operator returned back and readjusted the ROI or an altogether new ROI was selected.
The software then divided the LV myocardium into six segments in each view and generated segmental and global longitudinal strain (GLS). As the myocardium usually shortened in longitudinal direction during systole, the longitudinal strain was displayed below the baseline. From these curves, peak systolic longitudinal strain was recorded for each of the myocardial segments.
The strain values for all the segments were recorded and averaged to obtain the GLS, and also Bull’s eye display of the regional longitudinal strain and GLS was generated. Lower level of normal GLS is −19.1%. So, reduced GLS (subclinical LV systolic dysfunction) is defined when GLS is less negative than −19.1%.10
Statistical analysis
Statistical Package for Social Sciences (SPSS version 15.0) was used. Categorical data were expressed as frequencies and percentages, while continuous data were expressed as mean ± SD. Comparison between categorical data was done using chi-square or Fisher’s exact test as appropriate. Comparison between continuous variables was done using unpaired t-test. Correlations between continuous data were done using Pearson’s correlation coefficient. Predictors of subclinical systolic dysfunction among the whole study sample and among the hypertensive group were tested in a univariate model. Variables with P-values <0.2 on univariate analysis or variables with clinical relevance were listed in a multiple logistic regression model. 95% confidence intervals were used to detect significant predictors. P-value was considered significant if <0.05.
Results
The study population consisted of 90 subjects, including 60 patients of systemic hypertension (42 ± 7 years, 16 male) and 30 age- and sex-matched healthy controls (40 ± 6 years, 4 male).
Clinical and demographic data of patients with systemic hypertension and controls are listed in Table 1. No significant differences were found between the two groups in terms of age or sex. Patients with systemic hypertension had significantly higher BMI than the control group (P = 0.03). There was a significant higher systolic and diastolic blood pressure in patients with systemic hypertension than the control group (P < 0.001).
Table 1.
Demographic and clinical data of the two groups.
| HYPERTENSIVE GROUP (N = 60) | CONTROL GROUP (N = 30) | P-VALUE | |
|---|---|---|---|
| Age (yrs) | 42.11 ± 7.41 | 40.43 ± 6.38 | NS |
| Sex (male)% (females)% |
16 (26.7%) 44 (73.3%) |
4 (13.3%) 26 (86.7%) |
NS |
| BMI (mean) | 32.11 ± 6.32 | 29.32 ± 4.77 | 0.03 |
| SBP | 137.33 ± 33 | 115 ± 82 | <0.001 |
| DBP | 84.91 ± 9.54 | 73.33 ± 7.11 | <0.001 |
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Conventional echocardiography
Conventional echocardiographic data of patients with systemic hypertension and controls are listed in Table 2, which revealed significantly higher septal wall thickness (interventricular septum), PWT, and RWT in hypertensive patients (P < 0.001). Also, the LVMI was significantly higher in the hypertensive group. There was no significant difference in the global LV ejection fraction (LVEF) between the two groups. However, the MAPSE was significantly higher in the control group.
Table 2.
Conventional echocardiographic parameters of the two groups.
| HYPERTENSIVE GROUP (N = 60) | CONTROL GROUP (N = 30) | P-VALUE | |
|---|---|---|---|
| IVS (cm) | 0.96 ± 0.13 | 0.86 ± 0.8 | <0.001 |
| PWT (cm) | 0.95 ± 0.139 | 0.87 ± 0.08 | <0.001 |
| LVEED (cm) | 5.02 ± 0.37 | 4.77 ± 0.85 | 0.04 |
| LVESD (cm) | 3.2 ± 0.35 | 3.15 ± 0.58 | NS |
| RWT | 0.37 ± 0.35 | 0.35 ± 0.02 | <0.001 |
| LV MI(g/m2) | 89.02 ± 19.90 | 80.01 ± 17.29 | 0.03 |
| EDV (ml) | 108.8 ± 33.70 | 94.8 ± 26.66 | 0.05 |
| ESV (ml) | 41.58 ± 16.1 | 36.9 ± 17.04 | NS |
| EF Simpson (%) | 63.21 ± 4.81 | 63.53 ± 8.13 | NS |
| MAPSE (mm) | 15.48 ± 2.01 | 16.45 + 2.03 | 0.03 |
| Aorta (cm) | 2.93 ± 0.39 | 2.66 ± 0.35 | 0.002 |
| LA diameter (cm) | 3.7 ± 0.435 | 3.44 ± 0.38 | 0.008 |
| LA volume index (ml/m2) | 22.36 ± 3.99 | 19.87 ± 3.61 | 0.005 |
| E (cm/s) | 82.7 ± 16.06 | 87 ± 15.57 | NS |
| A (cm/s) | 74.18 ± 17.36 | 71.8 ± 18.87 | NS |
| DT (ms) | 199.56 ± 29.06 | 200.2 ± 29.04 | NS |
| E/A | 1.15 ± 0.3 | 1.26 ± 0.33 | NS |
Abbreviations: A, atrial kick mitral inflow velocity; DT, deceleration time; E, early mitral inflow velocity; EDV, end diastolic volume; ESV, end-systolic volume; EF, ejection fraction; IVS, interventricular septum; LA, left atrium; LVEDD, left ventricle end diastolic dimension; LVESD, left ventricle end-systolic dimension; LVMI, left ventricular mass index; MAPSE, mitral annular plane systolic excursion; PWT, posterior wall thickness; RWT, relative wall thickness.
Tissue Doppler imaging
Mitral annular velocities of the two groups are presented in Table 3. E′ and S′ velocities were significantly lower in the hypertensive patients. However, E/E′ ratio was significantly higher in the hypertensive group.
Table 3.
Tissue Doppler parameters in the two groups.
| HYPERTENSIVE GROUP (N = 60) | CONTROL GROUP (N = 30) | P-VALUE | |
|---|---|---|---|
| E` (cm/s) | 9.17 ± 1.92 | 10.64 ± 1.99 | 0.001 |
| A` (cm/s) | 7.31 ± 1.83 | 7.34 ± 1.61 | NS |
| S` (cm/s) | 7.73 ± 1.75 | 8.2 ± 1.56 | 0.01 |
| E`/À | 1.29 ± 0.37 | 1.48 ± 0.29 | 0.02 |
| E/E` | 9.31 ± 2.19 | 8.22 ± 1.56 | 0.01 |
Abbreviations: A, atrial kick mitral inflow velocity; A′, atrial annular velocity; E, early mitral inflow velocity; E′, early diastolic annular velocity; S`, systolic velocity of mitral annulus.
2D speckle tracking
In comparison with normal controls, GLS was significantly attenuated in patients with systemic hypertension (−20.75 ± 1.56 in the control group vs. −19.54 ± 2.43 in the hypertensive group) as shown in Figure 1 and Table 4.
Figure 1.
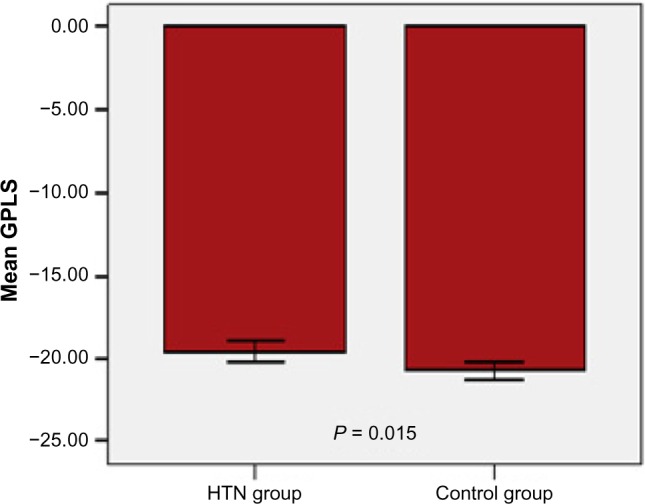
Comparison between hypertensive group and control group in mean GLS (global longitudinal strain).
Table 4.
2D speckle tracking data in the two groups.
| HYPERTENSIVE GROUP (N = 60) | CONTROL GROUP (N = 30) | P-VALUE | |
|---|---|---|---|
| GLS (%) | −19.5 ± 2.43 | −20.75 ± 1.56 | 0.015 |
| Normal systolic function Subclinical dysfunction |
37 (61.7%) 23 (38.3%) |
27 (90%) 3 (10%) |
0.005 |
Abbreviation: GLS, global longitudinal strain.
We found that 23 of 60 patients (38.3%) in the hypertensive group had subclinical LV systolic dysfunction defined as GLS less negative than −19.1%, but only 3 of 30 controls (10%) had subclinical LV dysfunction as listed in Table 4.
There was no significant difference between hypertensive patients with normal GLS and hypertensive patients with reduced GLS (subclinical LV systolic dysfunction) in terms of age and sex distribution or duration of hypertension. BMI was significantly higher among hypertensive patients with reduced GLS than those with normal GLS, as listed in Table 5.
Table 5.
Comparison between hypertensive patients with normal GLS and hypertensive patients with reduced GLS.
| NORMAL GLS (N = 37) | REDUCED GLS (N = 23) | P-VALUE | |
|---|---|---|---|
| Age (years) | 42.62 ± 7.13 | 41.3 ± 7.93 | NS |
| Sex (%): Males Females |
8 (21.6%) 29 (78.37%) |
8 (34.7%) 15 (65.21%) |
NS |
| BMI | 30.36 ± 6.32 | 34.92 ± 5.34 | 0.006 |
| Duration of HTN (years) | 3.75 ± 3.92 | 3.56 ± 4.48 | NS |
| RWT | 0.37 ± 0.46 | 0.38 ± 0.39 | 0.17 |
| LVMI (gm/m2) | 86.12 ± 19.57 | 93.67 ± 19.98 | 0.15 |
| LAvol.index (ml/m2) | 21.88 ± 3.8 | 23.15 ± 4.25 | 0.23 |
| S’ (cm/sec) | 7.84 ± 1.82 | 7.55 ± 1.67 | 0.54 |
| MAPSE (mm) | 15.89 ± 1.7 | 14.8 ± 2.31 | 0.06 |
RWT, LVMI, and LA volume index were higher among hypertensive patients with reduced GLS than those with normal GLS, despite being statistically nonsignificant. Also patients having reduced GLS had lower S′ wave velocity and lower MAPSE values than those patients with normal GLS (despite being statistically nonsignificant), as listed in Table 5.
Among the whole studied sample (90 patients), a significant positive correlation was evident between GLS and BMI (r = 0.43, P < 0.0001). Similarly, a significant positive correlation was evident between GLS and LVMI (r = 0.27, P = 0.009), eg, as BMI or LVMI increased, GLS became less negative (worsen), as shown in Figure 2.
Figure 2.
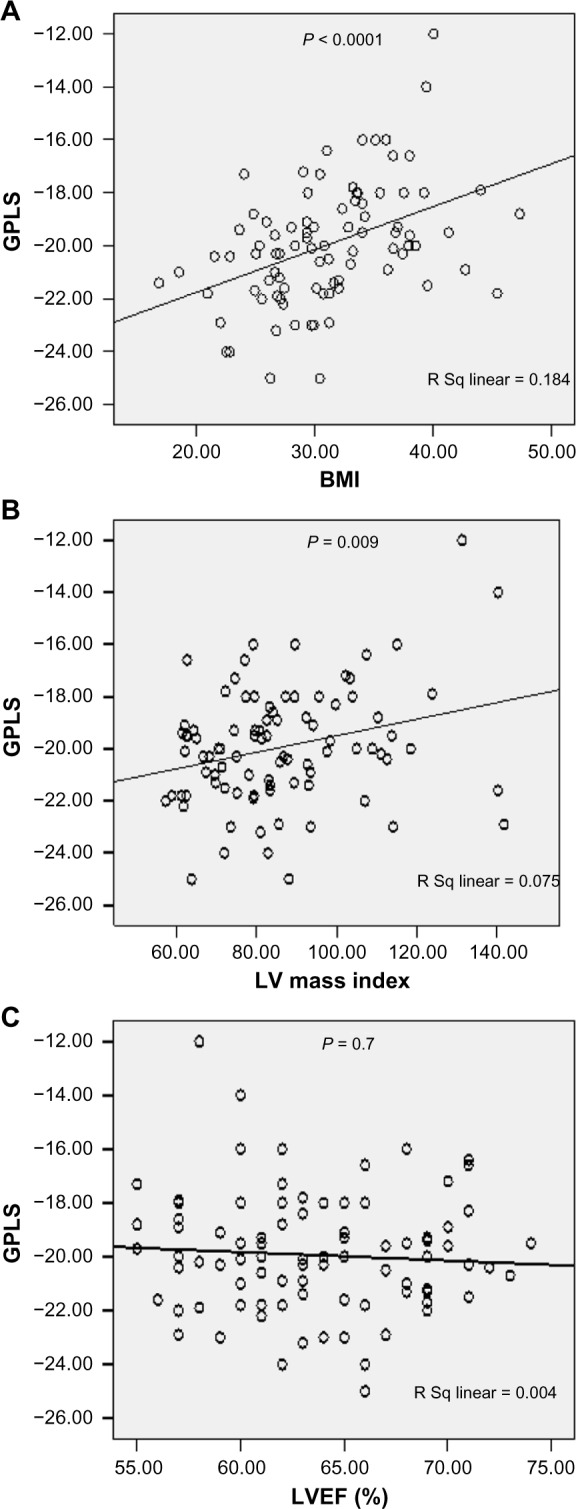
Relationship between global peak longitudinal strain (GPLS) in the whole study sample to body mass index (BMI) (A), to left ventricular mass index (LVMI) (B), and to left ventricular ejection fraction (LVEF) (C).
A significantly negative correlation was found between GLS and MAPSE (r = −0.33, P = 0.002). Negative but statistically nonsignificant correlation was evident between GLS and S′ wave mitral annular velocity by tissue Doppler imaging (TDI) (r = −0.18, P = 0.08). Similarly, negative statistically nonsignificant correlation was noticed between GLS and LVEF by Simpson’s method (r = −0.04, P = 0.7), eg, as MAPSE diminished or S′ velocity decreased, GLS became less negative (worsen), as shown in Figure 2.
Discussion
In hypertensive patients, LV systolic function is commonly considered normal if the global EF and fractional shortening (FS) are normal. However, the EF and FS reflect only the global cardiac contractile function and do not take regional systolic abnormalities into consideration.11
The above results show that 2D speckle tracking is able to detect subclinical myocardial dysfunction in hypertensive patients, despite normal global systolic parameters by conventional 2D echocardiography.
In our results, GLS was significantly attenuated in patients with systemic hypertension compared with normal controls. We also found that 38.3% of hypertensive patients had subclinical systolic LV dysfunction defined as 2D derived GLS less negative than −19.1%.
Our results are in concordance with the findings by Imbalzano et al, who revealed that 2D-STE showed an impairment of systolic longitudinal strain in all hypertensive patients including those without LVH.12
A study was conducted by Tulika et al in 2014 on 72 hypertensive patients with preserved EF. They postulated that the early features of LV systolic dysfunction in hypertension with apparently normal LV systolic function was in the axial axis, while systolic shortening in circumferential and radial axes was preserved.13
In our study, LVMI was higher in hypertensive patients with subclinical LV systolic dysfunction (reduced GLS) than hypertensive patients with normal GLS, despite being nonsignificant. However, a significant positive correlation was evident between GLS and LVMI in all the studied patients. This may be explained by our small study sample, especially when subdivided into three groups (control, hypertensive with normal GLS, and hypertensive with reduced GLS).
A study done by Saghir et al revealed that hypertensive individuals with LVH had significantly decreased systolic longitudinal strain and strain rate values compared with control subjects.14 Also in the study by Tulika et al, LVMI and DBP were found to be independent predictors of reduced global strain in longitudinal axis.13 Narayanan et al reported that LVMI and DBP were independent predictors of reduced global strain in longitudinal axis.15 Also Schillaci et al demonstrated that there is a continuous relationship between increased LV mass and cardiovascular risk in essential HTN, even in the absence of target organ damage.16
With regard to the effect of BMI on GLS in our study, increasing BMI showed a greater likelihood of developing subclinical LV dysfunction. BMI was significantly higher among hypertensive patients with reduced GLS than those with normal GLS. This result is similar to that of the study by Ballo et al, which included 112 hypertensive subjects and showed independent negative association between BMI and myocardial contractility.17
In Matos et al study, a retrospective analysis of echocardiogram was done to 600 patients. It concluded that MAPSE measured by an untrained observer is a viable surrogate for expert determined LVEF. They established upper and lower thresholds for normal and severely reduced EFs for each gender and a simple gender-specific equation to calculate EF from intermediate MAPSE values. They believed that MAPSE should be routinely acquired in all echocardiography studies and used as a surrogate for global LVEF.18
According to our study, hypertensive patients had significantly lower MAPSE values compared with control subjects. Also patients with subclinical LV dysfunction had lower MAPSE values compared with those without subclinical LV dysfunction, despite being nonsignificant and all within the normal range. However, there was a significant negative correlation between MAPSE and GLS.
In our study, it was found that hypertensive patients had higher BMI and higher LVMI than normal subjects. Lavie et al stated that HTN occurs in nearly 50% of obese patients and more so in class III obesity (35 < BMI < 40). The effect of coexistent HTN and obesity on LV morphology depends largely on the relative severity and duration of both disorders; HTN alone promotes the development of concentric LVH or concentric remodeling, whereas when long-standing HT is combined with chronic severe obesity, a hybrid form of LVH develops. Previously characterized as eccentric–concentric LVH, this morphology is now characterized as a form of concentric LVH.19
We excluded patients older than 50 years to avoid aging effect on myocardial systolic and diastolic function and hence affecting GLS, as stated by Fernandes et al that even patients with subclinical atherosclerosis presented by greater carotid intima media thickness has associated alternations of myocardial strain parameters.20
We detected 10% of healthy individuals with subclinical LV dysfunction, and we attribute this to smoking,21 passive smoking, dyslipidemia, unknown genetic causes, or premature atherosclerosis. And this high rate may also be attributed to the small sample size.
Study limitations include small sample size, so the trends of reduced regional systolic strain observed in longitudinal axis might be truly reduced but due to the limited power of the study. The reproducibility of the estimates of systolic strain using the speckle tracking method was not recorded; thus, there could have been measurement bias, especially in the small sample size study population. The operator was not double blinded about hypertensive patients and control subjects, having the whole study done in a single center. Hence, our study should be considered a preliminary study.
Conclusion
We concluded that 2D-STE detected subclinical LV systolic dysfunction in 38.3% of our patients (patients with GLS less negative than −19.1%), despite preserved LVEF and independent LV structural changes. This suggests that earlier intervention in these patients may be beneficial.
We also concluded that increased BMI is an independent predictor of reduced global longitudinal systolic strain in hypertensive patients, which raises the importance of its modification in hypertensive patients, ie, weight loss can potentially prevent LV systolic dysfunction in hypertensive patients.
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
PEER REVIEW: Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 595 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: AMA, VWK, YAA. Analyzed the data: VWK, YAA, RAE. Wrote the first draft of the manuscript: VWK, YAA. Contributed to the writing of the manuscript: VWK, YAA. Agreed with manuscript results and conclusions: AMA, VWK, YAA. Jointly developed the structure and arguments for the paper: AMA, VWK, YAA. Made critical revisions and approved the final version: VWK, YAA. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Cameli M, Lisi M, Righini FM, Massoni A, Mondillo S. Left ventricular remodeling and torsion dynamics in hypertensive patients. Int J Cardiovasc Imaging. 2013;29(1):79–86. doi: 10.1007/s10554-012-0054-0. [DOI] [PubMed] [Google Scholar]
- 2.Edvardsen T, Rosen BD, Pan L, et al. Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imaging – the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2006;151:109–14. doi: 10.1016/j.ahj.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Cuspidi C, Meani S, Valerio C, Fusi V, Sala C, Zanchetti A. Left ventricular hypertrophy and cardiovascular risk stratification: impact and cost effectiveness of echocardiography in recently diagnosed essential hypertensives. J Hypertens. 2006;24(8):1671–7. doi: 10.1097/01.hjh.0000239305.01496.ca. [DOI] [PubMed] [Google Scholar]
- 4.Rovner A, de las Fuentes L, Waggoner AD, Memon N, Chohan R, Dávila-Román VG. Characterization of left ventricular diastolic function in hypertension by use of Doppler tissue imaging and color M-mode techniques. J Am Soc Echocardiogr. 2006;19:872–9. doi: 10.1016/j.echo.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004;17:630–3. doi: 10.1016/j.echo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–69. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Mancia G, Fagard R, Krzysztof N, et al. ESH/ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 10.Takigiku K, Takeuchi M, Izumi C, et al. Normal range of left ventricular 2-dimensional strain – Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) Study. Circ J. 2012;76:2623–32. doi: 10.1253/circj.cj-12-0264. [DOI] [PubMed] [Google Scholar]
- 11.Sengupta SP, Caracciolo G, Thompson C, Abe H, Sengupta PP. Early impairment of left ventricular function in patients with systemic hypertension: new insights with 2-dimensional speckle tracking echocardiography. Indian Heart J. 2013;65:48–52. doi: 10.1016/j.ihj.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imbalzano E, Zito C, Carerj S, et al. Left ventricular function in hypertension: new insight by speckle tracking echocardiography. Echocardiography. 2011;28:649–57. doi: 10.1111/j.1540-8175.2011.01410.x. [DOI] [PubMed] [Google Scholar]
- 13.Tulika M, Prakash N, Anita P, Urmil G. Subclinical systolic dysfunction among newly diagnosed hypertensive’s with preserved left ventricular ejection fraction using two dimensional strain imaging method: hospital based observational study. Natl J Med Res. 2014;4:2277–8810. [Google Scholar]
- 14.Saghir M, Areces M, Makan M. Strain rate imaging differentiates hypertensive cardiac hypertrophy from physiologic cardiac hypertrophy (athlete’s heart) J Am Soc Echocardiogr. 2007;20(2):151–7. doi: 10.1016/j.echo.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE, Tighe DA. Cardiac mechanics in mild hypertensive heart disease. Circ Cardiovasc Imaging. 2009;2(5):382–90. doi: 10.1161/CIRCIMAGING.108.811620. [DOI] [PubMed] [Google Scholar]
- 16.Schillaci G, Verdecchia P, Porcellati C, et al. Continuous relation between ventricular mass and cardiovascular risk in essential hypertension. Hypertension. 2000;35:580–6. doi: 10.1161/01.hyp.35.2.580. [DOI] [PubMed] [Google Scholar]
- 17.Ballo P, Zaca V, Giacomin E, Galderisi M, Mondillo S. Impact of obesity on left ventricular systolic function in hypertensive subjects with normal ejection fraction. Int J Cardiol. 2010;141(3):316–20. doi: 10.1016/j.ijcard.2008.11.129. [DOI] [PubMed] [Google Scholar]
- 18.Matos J, Kronzon I, Panagopoulos G, Perk G. Mitral annular plane systolic excursion as a surrogate for left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25(9):969–74. doi: 10.1016/j.echo.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;2:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes VRS, Polak JF, Edvardsen T, et al. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals. J Am Coll Cardiol. 2006;47(12):2420–8. doi: 10.1016/j.jacc.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 21.Rosen BD, Saad MF, Shea S, et al. Hypertension and smoking are associated with reduced regional left ventricular function in asymptomatic individuals. J Am Coll Cardiol. 2006;47(6):1150–8. doi: 10.1016/j.jacc.2005.08.078. [DOI] [PubMed] [Google Scholar]


