Abstract
Summary
A comparison of the association of different forms of 25-hydroxyvitamin D [25(OH)D] with parathyroid hormone (PTH) and with areal and volumetric bone mineral density (BMD) demonstrated that bioavailable and free 25(OH)D do not provide a better index of vitamin D status in terms of bone health compared to total 25(OH)D.
Introduction
This study aims to compare measures of vitamin D-binding protein (DBP) using a monoclonal versus polyclonal ELISA and assess correlations of total versus estimated free and bioavailable 25(OH)D with BMD and PTH concentrations.
Methods
DXA and peripheral quantitative CT (pQCT) scans were obtained in 304 adults (158 black, 146 white), ages 21–80 years. Free and bioavailable 25(OH)D were calculated from total 25(OH)D, DBP, and albumin concentrations. Multivariable linear regression with standardized beta coefficients was used to evaluate associations of bone measures and PTH with total, free, and bioavailable 25(OH)D.
Results
Measures of DBP obtained using a monoclonal versus polyclonal ELISA were not correlated (rs=0.02, p=0.76). Free and bioavailable 25(OH)D based on the polyclonal assay were lower in black versus white participants (p<0.0001); this race difference was not evident using the monoclonal assay. Adjusted for age, sex, calcium intake, and race, all forms of 25(OH)D were negatively associated with PTH, but the absolute coefficient was greatest for total 25(OH)D (−0.34, p<0.001) versus free/bioavailable 25(OH)D (−0.18/−0.24 depending on DBP assay, p≤0.003). In analyses stratified on race, none of the measures of 25(OH)D were associated with BMD across DXA and pQCT sites.
Conclusions
The monoclonal versus polyclonal ELISA yielded highly discrepant measures of DBP, particularly among black individuals, likely related to established race differences in DBP polymorphisms. Contrary to prior studies, our findings indicate that using DBP to estimate bioavailable and free 25(OH)D does not provide a better index of vitamin D status in terms of bone health.
Keywords: Bone mineral density, Parathyroid hormone, Vitamin D, Vitamin D-binding protein
Introduction
Studies of the relationship between circulating concentrations of serum 25-hydroxyvitamin D [25(OH)D], the conventional index of vitamin D status, and bone mineral density (BMD) have yielded inconsistent results. Several observational studies have demonstrated an association of higher 25(OH)D concentrations with greater BMD [1–3] and lower fracture risk [4, 5]. Randomized controlled trials have shown a beneficial effect of vitamin D supplementation on BMD and/or facture risk [6–10]. In contrast, other observational studies and trials have been equivocal [11, 12], found no association between 25(OH)D and BMD [13, 14], showed no benefit of vitamin D supplementation on BMD and fracture risk [15, 16], or showed an increased risk of fracture following high-dose supplementation [17]. Studies have also suggested that racial differences exist in the relationship between 25(OH)D and BMD [18, 19], findings which are underscored by the fact that black individuals, compared to whites, have significantly lower fracture risk [20–24] despite consistently lower 25(OH)D concentrations [25, 26].
Recent evidence highlights the importance of the vitamin D-binding protein (DBP) in the assessment of racial differences in vitamin D status. Both 25(OH)D and 1,25-dihydroxyvitamin D [1,25(OH)2D] circulate bound to DBP (85–90 %) or albumin (10–15 %), with <1 % in their free forms. The binding affinity of DBP for vitamin D metabolites is >1000-fold stronger than that of albumin [7×108 vs. 6×105 M−1 for 25(OH)D and 3.7×107 vs. 5.4×104 M−1 for 1, 25(OH)2D], and therefore, the albumin-bound and free fractions together are considered bioavailable [27–31]. Combinations of two single-nucleotide polymorphisms, rs4588 and rs7041, produce three major polymorphic forms of DBP (Gc1F, Gc1S, and Gc2) which differ substantially in their binding affinity, circulating concentration, and racial distribution [32–34]. In a recent study, 93 % of homozygous black individuals had the high affinity Gc1F variant as compared to only 6 % of white homozygotes [34]. Pre-clinical studies have established a role for DBP in regulating the availability of 25(OH)D to certain cell types [35, 36]. For example, greater concentrations of DBP and higher affinity forms of DBP attenuated 25(OH)D-induced monocyte antimicrobial responses, suggesting that bioavailable 25(OH)D is biologically relevant for monocytes.
A recent study in 49 healthy young adults (63 % white) showed that lumbar spine areal BMD (aBMD), measured by dual-energy x-ray absorptiometry (DXA), was positively associated with free (r=0.413, p=0.003) and bioavailable (r= 0.441, p=0.002), but not total 25(OH)D (r=0.172, p=0.236) [30]. Serum DBP concentrations varied widely and were inversely associated with black race. The authors concluded that bioavailable 25(OH)D may be a better measure of vitamin D status with regards to bone health. Concern has since been raised about the validity of the monoclonal antibody enzyme-linked immunosorbent assay (ELISA) used in this study and in widespread use [37–39]. This concern centered on discrepant findings from prior studies using polyclonal DBP assays and the potential of binding affinities and glycosylation patterns of the polymorphic DBP variants leading to differential and biased performance of the monoclonal assay.
Expanding on this prior work, this study is the first to examine the comparative associations of total, free, and bio-available 25(OH)D with aBMD, measured at multiple sites by DXA, and trabecular volumetric BMD (vBMD), measured by peripheral quantitative computed tomography (pQCT), in a large cohort of adults. The racial distribution in this cohort was approximately 50 % black and 50 % white by design, facilitating the assessment of racial differences in these associations. DBP was measured by both monoclonal and polyclonal ELISA methods. The primary objective was to examine the comparative associations of total versus free and bioavailable 25(OH)D with aBMD of the lumbar spine, proximal femur, and radius, trabecular vBMD in the tibia, and parathyroid hormone (PTH) concentrations as another index of vitamin D status. The secondary objective was to compare measures of DBP and estimates of free and bioavailable 25(OH)D based on a monoclonal as compared to a polyclonal ELISA.
Methods
Study population
Adults, ages 21 to 80 years, were enrolled as healthy reference participants for bone studies at the Children’s Hospital of Philadelphia (CHOP) and University of Pennsylvania (UPENN) between March of 2004 and June of 2008 as previously described [40, 41]. The study protocols were approved by the Institutional Review Boards at CHOP and UPENN, and informed consent was obtained from all participants. The study targeted equal numbers of participants in each decade of life, with 50 % black and 50 % female participants. Exclusion criteria comprised a history of chronic diseases or medications that impact skeletal health or nutrition, including diabetes, malabsorption, malignancy, and chronic kidney, liver, and thyroid disease. A total of 304 participants with complete measures of 25(OH)D, DBP, albumin, who self-identified as black (n=158) or white (n=146), and who had DXA and/or pQCT data were included here.
Anthropometrics and dietary calcium
Height was measured using a stadiometer (Holtain Ltd., Crymych, UK), and weight was measured using a digital scale (Scaltronix, White Plains, NY). Dietary information was collected using the validated National Cancer Institute Dietary History Questionnaire (DHQ) [42]. After manual review for completeness, DHQs were analyzed for daily nutrient intake using the National Cancer Institute’s DietCalc software. DHQ results with extreme values for total daily energy intake were excluded (<600 or >4000 kcal for women and <800 or >5000 kcal for men), so DHQ data was available in 257 participants (85 %).
Bone outcomes
Measures of aBMD (g/cm2) at five sites (lumbar spine, total hip, femoral neck, 1/3 radius, and ultra-distal radius) were obtained by DXA using a Hologic densitometer (Delphi Systems; Hologic, Inc., Bedford, MA). Measures of vBMD (mg/cm3) were obtained in the left tibia by pQCT (Stratec XCT2000 12-detector unit; Orthometrix, Inc.) with a voxel size of 0.4 mm, slice thickness of 2.3 mm, and scan speed of 25 mm/s as previously described [40]. Measurements for trabecular vBMD were obtained at 3 % of tibia length proximal to the reference line at the distal endplate. The associations of pQCT outcomes with age, sex, race, and body composition have been described [40].
Vitamin D, DBP, and PTH measures
Serum 25(OH)D and 1,25(OH)2D concentrations were measured by I125 radioimmunoassay (RIA; DiaSorin Inc., Stillwater, MN) [43]. Intra-assay CVs were 2.2 and 7–11 %, respectively [44]. Serum DBP was measured in duplicate (CV< 10 %) using a monoclonal antibody ELISA (R&D Systems, Minneapolis, MN). The inter-assay CV was 1.6–3.6 %, and recovery was 98–103 %. Serum DBP was also measured in duplicate using a polyclonal ELISA (ALPCO Diagnostics, Salem, NH). The intra-assay variation of this ELISA was <10 %, and recovery was 99–100 %.
Intact PTH concentrations were measured by RIA with CVs of 3–10 % (Scantibodies Laboratory, Santee, CA) [45]. Serum albumin was quantified using a colorimetric method based on a bromcresol purple performed on a COBAS c501 analyzer (Roche Diagnostics, Indianapolis, IN). Total imprecision ranged from 1.0 to 1.3 %, and limit of detection was 0.2 g/dl.
Serum free and bioavailable 25(OH)D were calculated using total 25(OH)D, DBP, and albumin concentrations, according to the equations of Powe et al. [30]. Powe et al. adapted validated equations for free and bioavailable testosterone, by replacing testosterone, sex hormone-binding globulin, and albumin and their respective binding constants with those of 25(OH)D, DBP, and albumin. The equations used to calculate free and bioavailable 25(OH)D are described in detail in the Supplementary Material of that report and summarized below:
where
| a | Kdbp*Kalb*albumin+Kdbp |
| b | (Kdbp*DBP)−(Kdbp*25(OH)D)+(Kalb*albumin)+1 |
| c | −[25(OH)D] |
| Kdbp | affinity constant between 25(OH)D and DBP |
| Kalb | affinity constant between 25(OH)D and albumin |
Concentrations of albumin, DBP, and 25(OH)D in these equations are in moles per liter, and free and bioavailable 25(OH)D were then converted to picograms per milliliter and nanograms per milliliter, respectively, for ease of interpretation of results.
Statistical analysis
All analyses were performed using STATA 13.0 (Stata Corporation, College Station, TX). A two-sided p value of <0.05 was considered statistically significant. Distributions of all variables were assessed for normality. All serum measures were skewed, so descriptive statistics for these continuous variables were reported as median and inter-quartile range (IQR). Group differences were assessed using Student’s t, Wilcoxon rank sum, or chi-square tests as appropriate. Serum 25(OH)D concentrations were categorized as deficient (<20 ng/ml) or not, in accordance with the 2011 Institute of Medicine report [46]. Correlations were assessed by Spearman’s rank correlations. The corcor program was used to test the equality of dependent correlations.
Multivariable linear regression analysis was used to compare the association of the total, free, and bioavailable 25(OH)D measures with PTH, adjusted for age, sex, race, and calcium intake. Multivariable linear regression analysis was used to evaluate the association of 25(OH)D measures with trabecular vBMD in the tibia and with aBMD in the lumbar spine, femoral neck, total hip, 1/3 radius, and ultra-distal radius. Skewed bone outcome variables (lumbar spine aBMD) were natural log transformed to achieve normality. Base models were first established for each bone outcome, examining independent associations with age, sex, race, and BMI as well as a two-way interaction between age and sex. Once a base model was established for each outcome, models were compared, with each model including a different form of 25(OH)D (total, free, or bioavailable) as an independent variable. Models were also stratified by race. Model assumptions were assessed by graphical checks, particularly checking the normality of residuals. To facilitate comparison of the individual vitamin D variables with their different units of measurement and their respective associations with bone outcomes, standardized regression coefficients were presented in each model rather than conventional beta coefficients. The value of a standardized regression coefficient can be interpreted as follows: one standard deviation greater value of the variable is associated with that magnitude of difference in standard deviation(s) of the outcome.
Results
Participant characteristics
Participant characteristics are summarized by sex and race in Table 1. By design, participants were evenly distributed across race and sex, and age distribution did not differ by race. BMI was significantly higher among black versus white females, but there was no racial difference in males. Black participants of both sexes had significantly lower total 25(OH)D concentrations. There were no sex differences in total 25(OH)D concentrations. The prevalence of vitamin D deficiency [25(OH)D<20 ng/ml] was 63 % among black males versus 21 % among white males (p<0.001), and 57 % compared to 13 % (p<0.001) among black and white females, respectively. Black participants had significantly higher PTH concentrations, consistent with prior publications [47]. The 13 values of PTH concentration ≥70 pg/ml were observed only among black participants (10 women and 3 men). Among black (p=0.01) but not white (p=0.25) participants, women had higher PTH concentrations than men, potentially related to their lower calcium intake. No racial difference was found in 1, 25(OH)2D concentrations, but women had higher concentrations than men among both black (p=0.02) and white (p<0.001) participants. Black participants of both sexes had significantly lower average dietary calcium intake.
Table 1.
Participant characteristics according to sex and race
| Male
|
Female
|
|||||
|---|---|---|---|---|---|---|
| Black | White | p valuea | Black | White | p valuea | |
| N | 71 (48.3 %) | 76 (51.7 %) | 87 (55.4 %) | 70 (44.6 %) | ||
| Age, years | 47.7 (37.2, 61.2) | 49.4 (27.9, 65.4) | 0.63 | 48.5 (38.2, 62.1) | 45.0 (35.0, 56.7) | 0.10 |
| BMI, kg/m2 | 26.1 (22.7, 29.3) | 24.9 (22.9, 28.0) | 0.51 | 28.1 (24.8, 33.0) | 23.6 (20.6, 27.2) | <0.0001 |
| Total 25D, ng/ml | 16.0 (10.3, 23.6) | 28.4 (21.2, 35.9) | <0.0001 | 18.6 (13.0, 25.0) | 30.6 (23.6, 37.2) | <0.0001 |
| Vitamin D-binding protein, mg/dl | ||||||
| Polyclonal | 41.6 (35.4, 47.1) | 38.4 (33.0, 44.2) | 0.14 | 48.1 (40.1, 56.0) | 40.9 (37.5, 48.9) | 0.002 |
| Monoclonal | 7.5 (5.9, 9.8) | 22.2 (17.4, 28.6) | <0.0001 | 9.1 (6.4, 16.9) | 21.5 (16.9, 27.1) | <0.0001 |
| Albumin | 4.1 (4.0, 4.3) | 4.3 (4.0, 4.5) | 0.03 | 4.0 (3.8, 4.2) | 4.0 (3.9, 4.3) | 0.12 |
| Free 25(OH)D, pg/ml | ||||||
| Polyclonal | 3.1 (2.0, 4.4) | 6.1 (4.0, 7.4) | <0.0001 | 2.9 (2.1, 4.0) | 5.3 (4.4, 6.7) | <0.0001 |
| Monoclonal | 11.8 (7.7, 19.1) | 10.0 (6.7, 13.0) | 0.02 | 12.4 (6.8, 16.7) | 9.7 (8.1, 12.9) | 0.16 |
| Bioavailable 25(OH)D, ng/ml | ||||||
| Polyclonal | 1.2 (0.7, 1.7) | 2.2 (1.5, 2.8) | <0.0001 | 1.0 (0.7, 1.5) | 2.0 (1.6, 2.5) | <0.0001 |
| Monoclonal | 4.4 (2.7, 7.1) | 3.7 (2.6, 5.0) | 0.06 | 4.3 (2.6, 5.9) | 3.5 (2.9, 4.8) | 0.18 |
| 1,25D, pg/ml | 37.0 (31.2, 43.9) | 36.4 (28.7, 45.9) | 0.86 | 41.6 (33.1, 51.1) | 45.7 (35.2, 53.1) | 0.22 |
| Parathyroid hormone, pg/ml | 31.0 (24.0, 46.0) | 25.0 (19.4, 34.0) | 0.004 | 37.0 (29.0, 52.0) | 28.0 (22.0, 35.2) | <0.0001 |
| Dietary calcium intake, mg/dayb | 709 (447, 890) | 857 (526, 1132) | 0.01 | 530 (389, 837) | 768 (551, 1014) | 0.0003 |
Results are presented as median (inter-quartile range)
Refers to a rank-sum test between blacks and whites
N=355
Measures of DBP concentrations differed markedly between the monoclonal and polyclonal assays, with the greatest discrepancies between assay results observed among black participants. Results from the two assays were not correlated overall (rs =0.02, p=0.76) and poorly correlated when stratified by race (black, rs =0.19, p=0.02; white, rs =0.22, p=0.01). Using the monoclonal assay, black participants of both sexes had DBP concentrations that were more than 50 % lower than white participants. In contrast, using the polyclonal assay, DBP concentrations were significantly higher in black females compared to white females, with no race difference among males. Not surprisingly, given the discrepant results of the monoclonal versus polyclonal DBP assay, the estimation of free and bioavailable 25(OH)D concentrations differed considerably depending on which DBP assay was used (Fig. 2). Using the monoclonal assay results, no statistically significant race difference was found in free or bioavailable 25(OH)D concentrations except for slightly greater free 25(OH)D in black males compared to white males (Table 1). In stark contrast, based on DBP results from the polyclonal assay and in keeping with their lower total 25(OH)D concentrations, black participants of both sexes had significantly lower free and bioavailable 25(OH)D compared to white participants (p<0.001).
Fig. 2.
Estimation of free and bioavailable 25(OH)D concentrations
Correlations between PTH and total, free, and bioavailable 25(OH)D
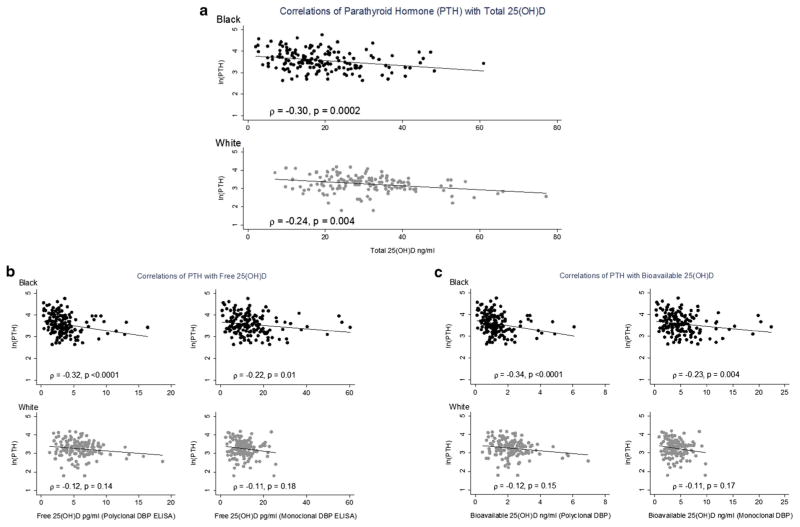
Figure 1 summarizes the Spearman correlation matrix for PTH with each form of 25(OH)D. Among black participants, total, free, and bioavailable 25(OH)D were all significantly and inversely associated with PTH. Correlations were greatest for total 25(OH)D (rs=−0.30) and free and bioavailable 25(OH)D estimated from the polyclonal DBP assay (rs = −0.32 and −0.34, respectively), as compared to those for free and bio-available 25(OH)D estimated from the monoclonal DBP assay (rs=−0.22 and −0.23, respectively), though this difference in correlations was not statistically significant. Among white participants, only total 25(OH)D was significantly correlated with PTH. There was no association between PTH and 1, 25(OH)2D concentrations. In multivariable linear regression analysis adjusted for age, sex, calcium intake, and race (Table 2), all forms of 25(OH)D were negatively associated with PTH, but the absolute standardized beta coefficient was greatest for total 25(OH)D (−0.34, p<0.001) as compared to free/bioavailable 25(OH)D, and the standardized beta coefficients were greater for estimates of free and bioavailable 25(OH)D based on the polyclonal (−0.24, p<0.001) as compared to the monoclonal DBP assay (−0.18/−0.19, p≤0.003).
Fig. 1.
a Correlations of parathyroid hormone (PTH) with total 25(OH)D. b Correlations of PTH with free 25(OH)D. c Correlations of PTH with bioavailable 25(OH)D
Table 2.
Multivariable regression analysis of the association between parathyroid hormone (PTH) and total, free, and bioavailable 25(OH)D concentrations
| Model R2 | 0.25 | 0.21 | 0.19 | 0.21 | 0.19 |
|---|---|---|---|---|---|
| Age | 0.27 (<0.001) | 0.26 (<0.001) | 0.24 (<0.001) | 0.25 (<0.001) | 0.24 (<0.001) |
| Female | 0.14 (0.01) | 0.10 (0.08) | 0.12 (0.03) | 0.09 (0.11) | 0.12 (0.04) |
| Black | 0.14 (0.02) | 0.20 (0.002) | 0.34 (<0.001) | 0.20 (0.002) | 0.34 (<0.001) |
| Dietary calcium intake, mg/day | −0.02 (0.77) | −0.03 (0.56) | −0.02 (0.73) | −0.03 (0.60) | −0.02 (0.78) |
| Vitamin D measure | Total 25(OH)D | Free 25(OH)D (Polyclonal) | Free 25(OH)D (Monoclonal) | Bioavailable 25(OH)D (Polyclonal) | Bioavailable 25(OH)D (Monoclonal) |
| −0.34 (<0.001) | −0.24 (<0.001) | −0.18 (0.003) | −0.24 (<0.001) | −0.19 (0.002) |
Data presented are standardized regression coefficients with p value in parentheses. PTH natural log-transformed
Associations between BMD and total, free, and bioavailable 25(OH)D
Table 3 summarizes the multivariable regression models relating DXA aBMD at several sites and trabecular vBMD to the multiple forms of 25(OH)D. Among black participants, only 5 of the 25 DXA models summarized in the table achieved statistical significance, but no consistent pattern of association with a form of 25(OH)D emerged. Within the group of white participants, none of the forms of 25(OH)D was associated with aBMD at any DXA site. Among black participants, total and bioavailable 25(OH)D (based on the monoclonal DBP assay) were independently and positively associated with trabecular vBMD. Among white participants, there was no association between any form of 25(OH)D and trabecular vBMD.
Table 3.
Multivariable regression analysis of the association of DXA areal bone mineral density (aBMD) and trabecular volumetric BMD (vBMD) with total, free, and bioavailable 25(OH)D concentrations
| Site | Measure of 25(OH)D | Black | White |
|---|---|---|---|
| Standardized regression coefficient (p value) | |||
| Lumbar spine aBMDa | Total 25(OH)D | 0.09 (0.25) | −0.04 (0.62) |
| Free 25(OH)D (polyclonal) | 0.13 (0.10) | 0.02 (0.78) | |
| Free 25(OH)D (monoclonal) | 0.15 (0.06) | 0.02 (0.73) | |
| Bioavailable 25(OH)D (polyclonal) | 0.15 (0.06) | 0.02 (0.78) | |
| Bioavailable 25(OH)D (monoclonal) | 0.17 (0.03) | 0.02 (0.76) | |
| Femoral neck aBMD | Total 25(OH)D | 0.12 (0.09) | 0.02 (0.75) |
| Free 25(OH)D (polyclonal) | 0.15 (0.03) | 0.01 (0.88) | |
| Free 25(OH)D (monoclonal) | 0.10 (0.18) | 0.08 (0.20) | |
| Bioavailable 25(OH)D (polyclonal) | 0.17 (0.02) | 0.01 (0.84) | |
| Bioavailable 25(OH)D (monoclonal) | 0.12 (0.10) | 0.08 (0.20) | |
| Total hip aBMD | Total 25(OH)D | 0.11 (0.14) | 0.005 (0.94) |
| Free 25(OH)D (polyclonal) | 0.12 (0.10) | −0.004 (0.95) | |
| Free 25(OH)D (monoclonal) | 0.08 (0.30) | 0.05 (0.50) | |
| Bioavailable 25(OH)D (polyclonal) | 0.14 (0.06) | 0.0005 (0.99) | |
| Bioavailable 25(OH)D (monoclonal) | 0.09 (0.20) | 0.05 (0.49) | |
| Ultra-distal radius aBMD | Total 25(OH)D | 0.14 (0.04) | −0.05 (0.49) |
| Free 25(OH)D (polyclonal) | 0.07 (0.29) | 0.01 (0.93) | |
| Free 25(OH)D (monoclonal) | 0.12 (0.07) | −0.01 (0.93) | |
| Bioavailable 25(OH)D (polyclonal) | 0.08 (0.23) | −0.002 (0.98) | |
| Bioavailable 25(OH)D (monoclonal) | 0.13 (0.047) | −0.01 (0.91) | |
| 1/3 radius aBMD | Total 25(OH)D | 0.10 (0.12) | 0.004 (0.96) |
| Free 25(OH)D (polyclonal) | 0.06 (0.40) | −0.002 (0.98) | |
| Free 25(OH)D (monoclonal) | 0.05 (0.47) | −0.02 (0.72) | |
| Bioavailable 25(OH)D (polyclonal) | 0.06 (0.40) | 0.0002 (1.00) | |
| Bioavailable 25(OH)D (monoclonal) | 0.05 (0.42) | −0.02 (0.75) | |
| Trabecular vBMD | Total 25(OH)D | 0.17 (0.04) | 0.03 (0.65) |
| Free 25(OH)D (polyclonal) | 0.13 (0.11) | 0.03 (0.67) | |
| Free 25(OH)D (monoclonal) | 0.16 (0.05) | 0.11 (0.12) | |
| Bioavailable 25(OH)D (polyclonal) | 0.15 (0.07) | 0.03 (0.65) | |
| Bioavailable 25(OH)D (monoclonal) | 0.17 (0.04) | 0.11 (0.11) | |
All models were adjusted for BMI, age, and sex, and the lumbar spine and 1/3 radius models also included an age-by-sex interaction term. Bolded values refer to point estimates with p<0.05
Log transformed to achieve normality
Discussion
This is the first and largest study to investigate the comparative associations of total, free, and bioavailable 25(OH)D with both aBMD, measured at multiple sites by DXA, and trabecular vBMD, measured by pQCT. The primary objective was to determine if estimates of free and bioavailable 25(OH)D provide a better index of vitamin D status in terms of bone health, as suggested by the prior report that lumbar spine aBMD was positively associated with free and bioavailable, but not total 25(OH)D in 49 healthy young adults [30]. Our results did corroborate this finding in terms of bioavailable 25(OH)D at the lumbar spine; however, this pattern was only present in black participants and only based on the monoclonal DBP assay. Furthermore, this finding was not observed at any other DXA site, nor was it supported by pQCT measures of vBMD. Indeed, among black participants, findings were highly inconsistent across aBMD sites, and total and bioavailable 25(OH)D were similarly associated with trabecular vBMD. Among white participants, no association was found between any form of 25(OH)D and any measure of aBMD or trabecular vBMD.
Similarly, there was no evidence that free and bioavailable 25(OH)D were more strongly associated with PTH compared to total 25(OH)D. In fact, the magnitude of the correlation of total 25(OH)D with PTH was greater than the correlations of free or bioavailable 25(OH)D. This is in contrast to the prior finding of Bhan et al. [27] that only bioavailable 25(OH)D was significantly associated with PTH in 94 hemodialysis patients. However, as expected, the dialysis patients had considerably higher and more variable PTH and lower albumin levels than our healthy control population. Furthermore, consistent with the findings of this study, in our prior study of 148 children with chronic kidney disease, PTH was inversely and comparably associated with total, free, and bioavailable 25(OH)D (Spearman’s rho of −0.48, −0.38, and −0.41, respectively) [48].
The primary limitations of this study are the lack of data on DBP polymorphisms and the cross-sectional design. Compared to the Gc1F allele, the relative affinities of the Gc1S and Gc2 alleles for 25(OH)D are 0.536 and 0.321, respectively [32]. Therefore, the six allelic combinations differ considerably in their affinity for 25(OH)D and 1,25(OH)2D. Circulating concentrations of 25(OH)D and racial distribution also differ by allelic combination [33]. In a recent population-based cohort study of healthy adults, Powe et al. [34] showed that 54 % of black participants were homozygous for the Gc1F allele. In contrast, only 2.6 % of white participants were homozygous for the Gc1F allele. The equations used to estimate free and bioavailable 25(OH)D in our study used the affinity constants of the Gc1F allele, as did the equations in the 2011 study by Powe et al. [30] reporting that DXA spine aBMD was correlated with free and bioavailable 25(OH)D, but not total 25(OH)D. This results in a systematic underestimation of the bioavailable and free forms of 25(OH)D for all five allelic combinations other than the Gc1F homozygote and particularly for those combinations with no Gc1F allele, which predominate in non-black populations. Indeed, 63 % of the 49 participants in the 2011 study by Powe et al. [30] were white. Given that young white adults have lower spine aBMD compared with young black adults (as confirmed in our study here), this under-estimation of free and bioavailable 25(OH)D among white participants may have contributed to the observed positive associations with aBMD. Furthermore, even with available genotypic data and the ability to apply genotype-specific affinity constants to homozygous individuals, the relative circulating concentrations of different allelic forms of DBP in heterozygotes is unknown. The stratified analyses performed in this study begin to address confounding by race. DXA aBMD at the lumbar spine, femoral neck, and hip were significantly lower in white versus black participants (all p ≤0.002). Thus, under-estimation of free and bioavailable 25(OH)D in the white participants could potentially have driven an association between these measures and BMD in the overall cohort, making the stratified analyses critical. Last, the cross-sectional nature of the study limits our ability to infer causality from the associations observed.
The direct comparison of findings based on a monoclonal versus polyclonal assay for DBP highlights an issue of profound importance, namely the lack of a gold standard measure of DBP and concern about differential performance of the monoclonal assay by genotype [37–39]. The primary monoclonal antibody of the R&D ELISA binds to a single peptide fragment of DBP and may bind differently to Gc1F and Gc1S variants, yielding underestimated concentrations in black individuals [37, 39]. Consistent with this concern, DBP results between the two assays were most discrepant among black participants in our study, with concentrations >50 % lower than among white participants based on the monoclonal assay. In keeping with prior studies which did not find racial differences in circulating DBP using polyclonal assays [37, 49–51], the only difference we observed using the polyclonal ELISA was actually higher DBP concentrations in black versus white females and no racial difference among males. While there may be cross-reactivity of polyclonal antibodies with other proteins [52, 53] which likely explains the systematically higher DBP results with the polyclonal assay, this is non-differential by race. The markedly discrepant DBP measures had a considerable impact on interpretation of racial differences in free and bioavailable 25(OH)D. Using the monoclonal assay would lead to the conclusion that despite lower total 25(OH)D concentrations, black individuals do not have lower free and bioavailable 25(OH)D, as put forth in the Powe et al. study [34]. As shown in Fig. 2, when not stratified by sex, black participants actually had higher free and bioavailable 25(OH)D estimated using the monoclonal assay. However, using the polyclonal assay, black participants had significantly (approximately 50 %) lower free and bioavailable 25(OH)D. The impact in multivariable models comparing associations of total, free, and bioavailable 25(OH)D with measures of BMD and PTH was mitigated by the fact that much of the variability in these outcomes was explained by other factors in the model and the associations were driven primarily by the total 25(OH)D.
In summary, this study does not support the prior suggestion that using total 25(OH)D, DBP, and albumin to calculate bioavailable or free 25(OH)D provides a better index of vitamin D status in terms of bone health. Furthermore, our findings substantiate the concern about differential performance of the monoclonal ELISA according to genotype and, therefore, race. The polymorphism of DBP challenges its accurate measurement, and until the methodologic issues of specificity and differential performance by genotype are addressed, cautious interpretation of results is warranted. Whether direct measurement of free or bioavailable 25(OH)D will provide better indices of vitamin D-related bone health remains to be seen.
Acknowledgments
Funding sources This project was supported by NIH Grants K23 DK093556 (MRD), K24 DK076808 (MBL and JS), R01DK064966 (MBL), and T32DK060455 (TJ). Dr. Denburg was also funded by The Nephcure Foundation–American Society of Nephrology Research Grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Baker is supported by a Veterans Affairs Clinical Science Research & Development Career Development Award. The contents of this work do not represent the views of the Department of Veterans Affairs or the United States Government. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
Footnotes
Conflict of interest Thomas Jemielita, Joshua Baker, Samir Sayed, Babette Zemel, Justine Shults, and Rita Herskovitz declare that they have no conflict of interest.
Disclosures Dr. Denburg receives/received funding (not related to this work) from Genentech, Inc, Mallinckrodt Pharmaceuticals, and the National Kidney Foundation/Amgen KDOQI Research Fellowship. Dr. Denburg has a consultancy agreement with Infiniti Medical. Dr. Leonard’s consultancy agreements include Amgen, Inc., Johnson & Johnson, and Novartis. She also serves/served as an Associate Editor for the Journal of the American Society of Nephrology and Journal of Bone and Mineral Research, on the American Society of Pediatric Nephrology Council, on the Scientific Advisory Board of Marodyne Medical, and an NIH Data Safety and Monitoring Board.
References
- 1.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116(9):634–639. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff-Ferrari HA, Kiel DP, Dawson-Hughes B, Orav JE, Li R, Spiegelman D, Dietrich T, Willett WC. Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J Bone Miner Res. 2009;24(5):935–942. doi: 10.1359/jbmr.081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valimaki VV, Alfthan H, Lehmuskallio E, Loyttyniemi E, Sahi T, Stenman UH, Suominen H, Valimaki MJ. Vitamin D status as a determinant of peak bone mass in young Finnish men. J Clin Endocrinol Metab. 2004;89(1):76–80. doi: 10.1210/jc.2003-030817. [DOI] [PubMed] [Google Scholar]
- 4.Cauley JA, Lacroix AZ, Wu L, Horwitz M, Danielson ME, Bauer DC, Lee JS, Jackson RD, Robbins JA, Wu C, Stanczyk FZ, LeBoff MS, Wactawski-Wende J, Sarto G, Ockene J, Cummings SR. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149(4):242–250. doi: 10.7326/0003-4819-149-4-200808190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int. 2005;16(11):1425–1431. doi: 10.1007/s00198-005-1860-1. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, Meyer HE, Pfeifer M, Sanders KM, Stahelin HB, Theiler R, Dawson-Hughes B. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40–49. doi: 10.1056/NEJMoa1109617. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, Thoma A, Kiel DP, Henschkowski J. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169(6):551–561. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 9.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 10.Prentice RL, Pettinger MB, Jackson RD, Wactawski-Wende J, Lacroix AZ, Anderson GL, Chlebowski RT, Manson JE, Van Horn L, Vitolins MZ, Datta M, LeBlanc ES, Cauley JA, Rossouw JE. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24(2):567–580. doi: 10.1007/s00198-012-2224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Group D. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010;340:b5463. doi: 10.1136/bmj.b5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O’Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D Women’s Health Initiative I. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 13.Kremer R, Campbell PP, Reinhardt T, Gilsanz V. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab. 2009;94(1):67–73. doi: 10.1210/jc.2008-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherman SS, Tobin JD, Hollis BW, Gundberg CM, Roy TA, Plato CC. Biochemical parameters associated with low bone density in healthy men and women. J Bone Miner Res. 1992;7(10):1123–1130. doi: 10.1002/jbmr.5650071003. [DOI] [PubMed] [Google Scholar]
- 15.Aloia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. 2005;165(14):1618–1623. doi: 10.1001/archinte.165.14.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, Anderson FH, Cooper C, Francis RM, Donaldson C, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA, Group RT. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365(9471):1621–1628. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 17.Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22(6):1745–1753. doi: 10.1007/s00198-010-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannan MT, Litman HJ, Araujo AB, McLennan CE, McLean RR, McKinlay JB, Chen TC, Holick MF. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008;93(1):40–46. doi: 10.1210/jc.2007-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20(2):185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 21.Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, Cummings SR. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293(17):2102–2108. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 22.Fang J, Freeman R, Jeganathan R, Alderman MH. Variations in hip fracture hospitalization rates among different race/ethnicity groups in New York City. Ethn Dis. 2004;14(2):280–284. [PubMed] [Google Scholar]
- 23.Finkelstein JS, Lee ML, Sowers M, Ettinger B, Neer RM, Kelsey JL, Cauley JA, Huang MH, Greendale GA. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87(7):3057–3067. doi: 10.1210/jcem.87.7.8654. [DOI] [PubMed] [Google Scholar]
- 24.Greendale GA, Sowers M, Han W, Huang MH, Finkelstein JS, Crandall CJ, Lee JS, Karlamangla AS. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN) J Bone Miner Res. 2012;27(1):111–118. doi: 10.1002/jbmr.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. 2000;85(11):4125–4130. doi: 10.1210/jcem.85.11.6962. [DOI] [PubMed] [Google Scholar]
- 26.Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 27.Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, Thadhani RI. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82(1):84–89. doi: 10.1038/ki.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63(4):954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 29.Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab. 1985;61(5):969–975. doi: 10.1210/jcem-61-5-969. [DOI] [PubMed] [Google Scholar]
- 30.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26(7):1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol Metab. 2000;11(8):320–327. doi: 10.1016/s1043-2760(00)00317-9. [DOI] [PubMed] [Google Scholar]
- 32.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP) Hum Genet. 1993;92(2):183–188. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 33.Constans J, Hazout S, Garruto RM, Gajdusek DC, Spees EK. Population distribution of the human vitamin D binding protein: anthropological considerations. Am J Phys Anthropol. 1985;68(1):107–122. doi: 10.1002/ajpa.1330680110. [DOI] [PubMed] [Google Scholar]
- 34.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bikle DD, Gee E. Free, and not total, 1,25-dihydroxyvitamin D regulates 25-hydroxyvitamin D metabolism by keratinocytes. Endocrinology. 1989;124(2):649–654. doi: 10.1210/endo-124-2-649. [DOI] [PubMed] [Google Scholar]
- 36.Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, Modlin RL, Martineau AR, Wilkinson RJ, Adams J, Hewison M. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010;95(7):3368–3376. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouillon R, Jones K, Schoenmakers I. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370(9):879. doi: 10.1056/NEJMc1315850#SA3. [DOI] [PubMed] [Google Scholar]
- 38.Carter GD, Phinney KW. Assessing vitamin D status: time for a rethink? Clin Chem. 2014;60(6):809–811. doi: 10.1373/clinchem.2013.219386. [DOI] [PubMed] [Google Scholar]
- 39.Hollis BW, Bikle DD. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370(9):879–880. doi: 10.1056/NEJMc1315850#SA4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker JF, Davis M, Alexander R, Zemel BS, Mostoufi-Moab S, Shults J, Sulik M, Schiferl DJ, Leonard MB. Associations between body composition and bone density and structure in men and women across the adult age spectrum. Bone. 2013;53(1):34–41. doi: 10.1016/j.bone.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leonard MB, Elmi A, Mostoufi-Moab S, Shults J, Burnham JM, Thayu M, Kibe L, Wetzsteon RJ, Zemel BS. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metabol. 2010;95(4):1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 43.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–186. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 44.Clive DR, Sudhaker D, Giacherio D, Gupta M, Schreiber MJ, Sackrison JL, MacFarlane GD. Analytical and clinical validation of a radioimmunoassay for the measurement of 1,25 dihydroxy vitamin D. Clin Biochem. 2002;35(7):517–521. doi: 10.1016/s0009-9120(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 45.Gao P, Scheibel S, D’Amour P, John MR, Rao SD, Schmidt-Gayk H, Cantor TL. Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1–84: implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res. 2001;16(4):605–614. doi: 10.1359/jbmr.2001.16.4.605. [DOI] [PubMed] [Google Scholar]
- 46.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary reference intakes for calcium and vitamin D. National Academies Press; Washington DC: 2011. [PubMed] [Google Scholar]
- 47.Cosman F, Nieves J, Dempster D, Lindsay R. Vitamin D economy in blacks. J Bone Miner Res. 2007;22(Suppl 2):V34–V38. doi: 10.1359/jbmr.07s220. [DOI] [PubMed] [Google Scholar]
- 48.Denburg MR, Kalkwarf HJ, de Boer IH, Hewison M, Shults J, Zemel BS, Stokes D, Foerster D, Laskin B, Ramirez A, Leonard MB. Vitamin D bioavailability and catabolism in pediatric chronic kidney disease. Pediatr Nephrol. 2013;28(9):1843–1853. doi: 10.1007/s00467-013-2493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouillon R, van Baelen H, de Moor P. The measurement of the vitamin D-binding protein in human serum. J Clin Endocrinol Metab. 1977;45(2):225–231. doi: 10.1210/jcem-45-2-225. [DOI] [PubMed] [Google Scholar]
- 50.M’Buyamba-Kabangu JR, Fagard R, Lijnen P, Bouillon R, Lissens W, Amery A. Calcium, vitamin D-endocrine system, and parathyroid hormone in black and white males. Calcif Tissue Int. 1987;41(2):70–74. doi: 10.1007/BF02555247. [DOI] [PubMed] [Google Scholar]
- 51.Winters SJ, Chennubhatla R, Wang C, Miller JJ. Influence of obesity on vitamin D-binding protein and 25-hydroxy vitamin D levels in African American and white women. Metabolism. 2009;58(4):438–442. doi: 10.1016/j.metabol.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 52.Christiansen M, Jorgensen CS, Laursen I, Hirschberg D, Hojrup P, Houen G. Protein chemical characterization of Gc globulin (vitamin D-binding protein) isoforms; Gc-1f, Gc-1s and Gc-2. Biochim Biophys Acta. 2007;1774(4):481–492. doi: 10.1016/j.bbapap.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 53.Powe CE, Karumanchi SA, Thadhani R. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370(9):880–881. doi: 10.1056/NEJMc1315850. [DOI] [PubMed] [Google Scholar]