G-protein receptor kinase and arrestin 1 are required for inactivation of photoactivated vertebrate rhodopsin. Frederiksen et al. show that they additionally regulate the subsequent decay of inactive rhodopsin into opsin and all-trans retinal and therefore dark adaptation.
Abstract
Photoactivation of vertebrate rhodopsin converts it to the physiologically active Meta II (R*) state, which triggers the rod light response. Meta II is rapidly inactivated by the phosphorylation of C-terminal serine and threonine residues by G-protein receptor kinase (Grk1) and subsequent binding of arrestin 1 (Arr1). Meta II exists in equilibrium with the more stable inactive form of rhodopsin, Meta III. Dark adaptation of rods requires the complete thermal decay of Meta II/Meta III into opsin and all-trans retinal and the subsequent regeneration of rhodopsin with 11-cis retinal chromophore. In this study, we examine the regulation of Meta III decay by Grk1 and Arr1 in intact mouse rods and their effect on rod dark adaptation. We measure the rates of Meta III decay in isolated retinas of wild-type (WT), Grk1-deficient (Grk1−/−), Arr1-deficient (Arr1−/−), and Arr1-overexpressing (Arr1ox) mice. We find that in WT mouse rods, Meta III peaks ∼6 min after rhodopsin activation and decays with a time constant (τ) of 17 min. Meta III decay slows in Arr1−/− rods (τ of ∼27 min), whereas it accelerates in Arr1ox rods (τ of ∼8 min) and Grk1−/− rods (τ of ∼13 min). In all cases, regeneration of rhodopsin with exogenous 11-cis retinal is rate limited by the decay of Meta III. Notably, the kinetics of rod dark adaptation in vivo is also modulated by the levels of Arr1 and Grk1. We conclude that, in addition to their well-established roles in Meta II inactivation, Grk1 and Arr1 can modulate the kinetics of Meta III decay and rod dark adaptation in vivo.
INTRODUCTION
The initiation of light sensation in rod photoreceptors in the vertebrate retina begins with the absorption of photons by rhodopsin, which triggers photoisomerization of its chromophore 11-cis retinal to the all-trans configuration. This leads to the formation of metarhodopsin II (Meta II), which activates the G-protein transducin. Transducin activates cGMP phosphodiesterase, which leads to a reduction in cytosolic cGMP and hyperpolarization of rod membrane potential to produce the light response. Rhodopsin inactivation is achieved by phosphorylation of Meta II by rhodopsin kinase 1 (Grk1) and the subsequent binding of visual arrestin 1 (Arr1; Wilden and Kühn, 1982; Wilden et al., 1986; Makino et al., 2003).
Once inactivated, Meta II is no longer capable of efficiently triggering the phototransduction cascade and eventually decays via several long-lived photoproducts to all-trans retinal and opsin (Hagins, 1956; Cone and Cobbs, 1969; Reuter, 2011). Rod photosensitivity can only be restored by pigment regeneration when opsin recombines with fresh 11-cis retinal recycled in the retinal pigment epithelium (RPE) adjacent to rod outer segments in a process known as the visual cycle (Lamb and Pugh, 2004; Reuter, 2011; Saari, 2012).
The visual cycle involves several regulatory steps. One of the most notable bottlenecks in rhodopsin regeneration in intact vertebrate eyes is thought to be associated with the RPE, which forms 11-cis chromophore and regulates its delivery to rods (Lamb and Pugh, 2004; Imai et al., 2007; Wang et al., 2014; Xue et al., 2015). Another bottleneck is the reduction of all-trans retinal to retinol in rods (Saari et al., 1998). The latter reaction might depend on the rate of liberation of opsin and release of all-trans retinal from the decaying photoproducts or the metabolic supply of retinol dehydrogenases (Bartl et al., 2001; Kolesnikov et al., 2003, 2006, 2007; Ala-Laurila et al., 2006; Adler et al., 2014). It is also possible that the Meta III decay is modulated by additional cellular mechanisms, such as rhodopsin phosphorylation, Arr1 binding, and interaction with transducin (Hofmann et al., 1992; Palczewski et al., 1999; Zimmermann et al., 2004; Sommer et al., 2005; Sommer and Farrens, 2006). However, the physiological regulation of Meta III decay in the context of in vivo dark adaptation of mammalian rods has not been examined.
Here, we tested the hypothesis that the decay of long-lived rhodopsin photoproducts within bleached mouse rods can modulate the speed of regeneration of rhodopsin and, ultimately, the recovery of rod sensitivity after pigment bleaching. We found that the genetic ablation of rhodopsin kinase accelerates the Meta III decay and speeds up the regeneration of rhodopsin and dark adaptation of rods. Conversely, the elimination of arrestin greatly extends the lifetime of Meta III and slows down rhodopsin regeneration and rod dark adaptation. Thus, phosphorylation and arrestin binding can modulate the decay of photoactivated rhodopsin to free opsin, regulating in this way its availability for recombination with 11-cis retinal for pigment regeneration. We conclude that, in addition to their well-established roles in quenching photoactivated rhodopsin, Grk1 and Arr1 can modulate the recovery of rod responsiveness to light in the course of dark adaptation after extensive pigment bleaching.
MATERIALS AND METHODS
Animals and preparation
Four mouse lines were used in the experiments: wild-type (WT) C57BL/6J, rhodopsin kinase 1 knockout (Grk1−/−), Arr1 knockout (Arr1−/−), and Arr1ox, in which Arr1 was overexpressed to 220% its normal level. All the genetically modified mice were maintained on a C57BL/6J background and were homozygous for the Met-450 variant of RPE65. WT mice were purchased from The Jackson Laboratory. Arr1−/− (Xu et al., 1997) and Grk1−/− (Chen et al., 1999) mice were gifts from J. Chen (University of Southern California, Los Angeles, Los Angeles, CA) and J. Chen (Baylor University, Waco, TX), respectively. Arr1ox animals were provided by V. Gurevich (Vanderbilt University Medical Center, Nashville, TN).
All experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Boston University School of Medicine and Washington University Animal Studies Committee according to the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act. Mice of both sexes were maintained on a 12-h day–night cycle and adapted to the dark for at least 4 h before experimentation. At the beginning of each experiment, mice were euthanized in dim red light by cervical dislocation followed by decapitation. After euthanasia, eyes were removed and transferred to a piece of filter paper (EMD Millipore). From this point, all dissection and manipulation of tissue were performed under infrared illumination with the aid of infrared image converters (BE Meyers & Co.) attached to a conventional dissecting microscope. Eyes were hemisected, and eyecups were transferred to a 35-mm Petri dish containing HEPES-buffered (10 mM at pH 7.4) Ames medium. The cornea and lens were removed, and the retina was dissected free. The retina was then transferred intact to a recording chamber for microspectrophotometry (MSP).
MSP
Absorbance of rod outer segments was assessed using a custom designed microspectrophotometer (Frederiksen et al., 2012; Nymark et al., 2012). The dark-adapted retina was oriented photoreceptor side up on a quartz coverslip window located in the bottom of a 2-mm-deep Plexiglas recording chamber. Retinas were gently flattened on this window with forceps and a slice anchor (Warner Instruments). The tissue was superfused at a rate of 4 ml/min with Ames medium (Sigma-Aldrich) buffered with sodium bicarbonate and equilibrated with a gas mixture of 95% O2 and 5% CO2. Temperature was maintained at 35–37°C, except where otherwise stated.
The recording chamber was placed on a microscope stage located in the beam path of the microspectrophotometer, and visual pigment absorption spectra (optical density vs. wavelength) were obtained from a region of the retina along its edge where isolated outer segments were visually identified. The measuring area contained predominantly rod photoreceptor outer segments, as evinced by the absorption spectrum that peaked at ∼500 nm (Fig. 1 A). Measurements were made over the wavelength range of 300–700 nm with 2-nm resolution. The absorbance spectrum was calculated from Beer’s law, as follows: OD = log10(Ii/It), where OD is the optical density or absorbance, Ii is the light transmitted through a cell free space adjacent to the outer segments, and It is the light transmitted through the tissue. Absorption spectra were measured with the polarization of the incident measuring beam parallel to the plane of the intracellular disks (T polarization). Generally, 10 complete sample scans and 10 baseline scans were averaged to increase the signal to noise ratio of the data. Dark-adapted spectra of the visual pigment were then compared with spectra recorded at different times after exposure to a bright 505-nm LED light. Light intensity at the plane of the retina was calibrated with a silicon photodiode (UDT Instruments) to bleach a specified fraction of the visual pigment. Such recordings of postbleach absorption spectra were continued for up to 120 min after bleaching, after which the retina was exposed again to a 505-nm light, calculated to bleach in excess of 99%. This last measurement was used to calculate the total bleaching fraction that had been produced with any given set of bleaching conditions.
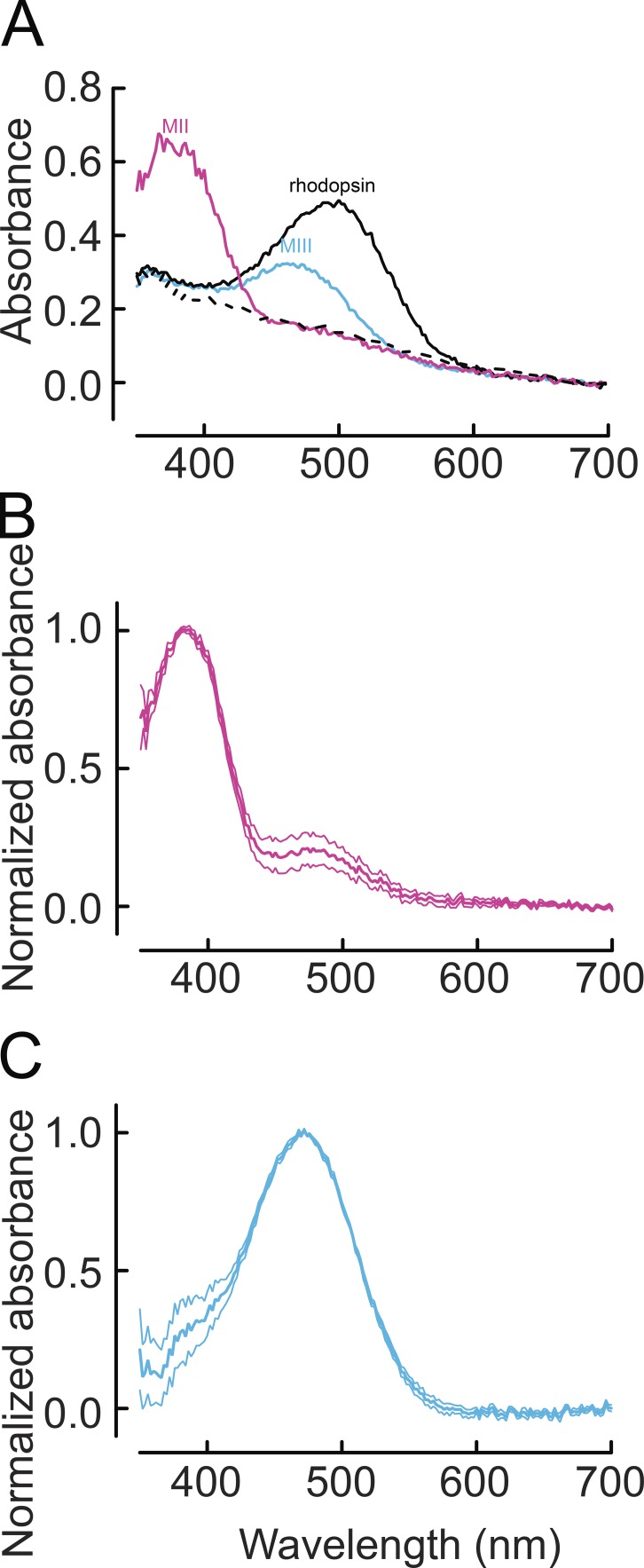
Figure 1.
Spectral absorbance of rhodopsin and its photoproducts Meta II and Meta III. (A) Transverse polarization absorbance spectra of rhodopsin, Meta II, and Meta III from a typical MSP recording. Raw spectra were recorded from dark-adapted WT mouse retina (black), immediately after near-complete bleaching of the visual pigment with 500-nm light (purple), and 6 min after bleaching (blue). Dashed line represents spectrum after 60 min when all photoproducts have decayed. (B) Mean ± SEM normalized spectrum of Meta II (n = 10; λmax = 384 nm). (C) Mean ± SEM normalized spectrum of Meta III (n = 13; λmax = 472 nm). All recordings were made at 37°C.
Preparation of 11-cis retinal and pigment regeneration
In experiments where rhodopsin regeneration was measured, we treated bleached retinas with 11-cis retinal. The 11-cis retinal was obtained as a gift from the National Eye Institute provided through the laboratory of R. Crouch (Medical University of South Carolina, Charleston, SC). Working solutions containing 11-cis retinal were prepared for exogenous delivery to bleached photoreceptors in Ames medium. Retinoid in Ames solution was prepared in dim red light by adding 1 µl of a stock solution of 11-cis retinal (30 mM dissolved in ethanol) to a conical vial. Then HEPES-buffered Ames medium containing 1% delipidated BSA (Sigma-Aldrich) was added, at first in small amounts (9 × 5 µl) and then in increasing amounts (1 × 50 µl and 2 × 450 µl), until the final volume was ∼1 ml. The peak absorbance (OD) of retinoid in this solution was measured using a conventional spectrophotometer, and its concentration was calculated as: c = (OD380)/(l ε380), where l is a 1-cm path length and ε380 = 24,900 M−1 cm−1, the extinction coefficient of 11-cis retinal in ethanol (Wald et al., 1955). The concentration of 11-cis retinal in the working solution was adjusted to 30 µM. During treatment, the effective concentration of retinal in the recording chamber was diluted to 10 µM, unless otherwise stated.
Electroretinography
Mice were adapted to the dark overnight and anesthetized with an intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg). Pupils were dilated with 1% atropine sulfate. Mouse body temperature was maintained at 37°C with a heating pad. Electroretinogram (ERG) responses were measured from both eyes using corneal contact lens electrodes held in place by a drop of Gonak solution. Full-field ERGs were recorded with a UTAS BigShot system (LKC Technologies) using Ganzfeld-derived stimuli of calibrated green LED light with a maximum intensity of 23.5 cd·s·m−2 (1.37 log cd·s·m−2) for test flashes that saturated rods but barely activated cones. Rod ERG a-wave maximal amplitude (Amax) was first determined in darkness (up to eight measurements were averaged). Then, >90% rod pigment was bleached by a 30-s exposure to bright light (∼1.3 × 108 photons µm−2 s−1) delivered by a 520-nm LED focused at the surface of mouse eye cornea. After bleaching, the recovery of rod Amax was followed in darkness (two to eight measurements were averaged, depending on the postbleach time point). At 20 and 60 min after bleaching, animals were reanesthetized with a smaller dose of ketamine (approximately half of the initial dose), and a 1:1 mixture of PBS and Gonak solutions was gently applied to the eyes using a plastic syringe to protect the eyes from drying and to maintain good electrical contact.
RESULTS
Rhodopsin photointermediates in mouse rods
To determine the physiological significance of Meta III decay for rod pigment regeneration, we first measured the time course of production and decay of rhodopsin photolysis products. Previous in vitro and in situ studies of amphibian and mammalian rhodopsin have demonstrated that rhodopsin photoproducts differ widely in their lifetimes (Hagins, 1956; Cone and Cobbs, 1969; Arnis and Hofmann, 1995; Bartl et al., 2001; Ritter et al., 2004; Vogel et al., 2004; Kolesnikov et al., 2006, 2011a). A typical sequence of observed mouse rhodopsin photointermediates is illustrated in Fig. 1, which shows absorption spectra recorded before and after a 12-s exposure to bright light calculated to bleach more than 90% of rhodopsin. The solid black trace in Fig. 1 A shows the absorbance spectrum of dark-adapted mouse rhodopsin, with the absorption maximum near 500 nm. A spectrum recorded 30–40 s after bleaching is illustrated by the purple trace labeled Meta II, with the peak absorbance at ∼380 nm. A longer wavelength shoulder visible in the Meta II spectrum near 480 nm (Fig. 1 B) likely represents a mixture of Meta I that is in fast equilibrium with Meta II and photoregenerated rhodopsin (Kolesnikov et al., 2011a). However, given that our spectral scans required up to 30 s to complete, there may also be contamination with a small amount of Meta III, to which Meta II rapidly decays. As we have shown previously, this decay in darkness is bi-exponential, with time constants of 3.6 min and 16 min (Nymark et al., 2012). A spectrum of the much longer lived Meta III intermediate recorded 6 min after bleaching is indicated as the blue trace in Fig. 1 A. Meta II and Meta III then progressively decayed to all-trans retinal and opsin and completely disappeared 60 min after bleaching (Fig. 1 A, dashed black trace). Fig. 1 (B and C) illustrates mean normalized difference spectra of Meta II (λmax = 380 nm) and Meta III (λmax = 472 nm), respectively. These spectra were obtained by subtracting the spectrum of fully bleached rods recorded at the end of each experiment (Fig. 1 A, dashed black trace) from the raw spectra recorded when these intermediates reached their maximal concentration.
Altered rates of Meta III decay in rods lacking Grk1 or Arr1
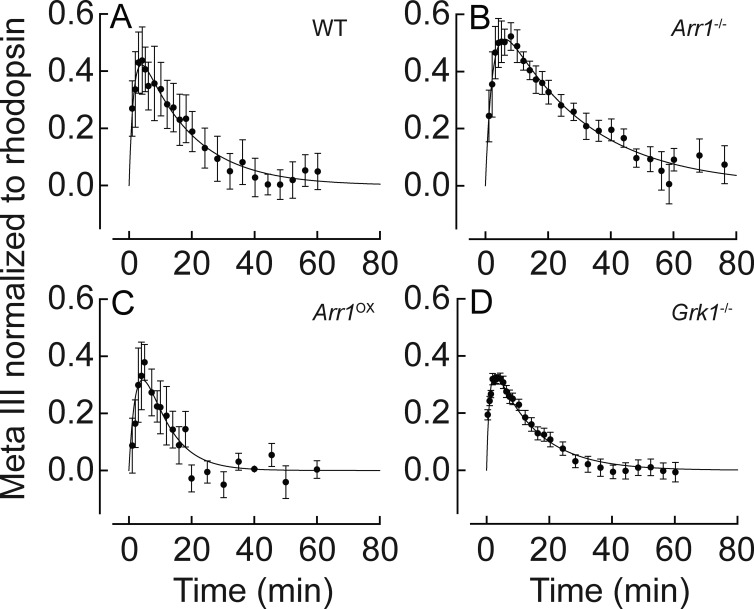
Once we had characterized the basic spectral properties of mouse rod Meta III, we aimed to determine whether the rate of Meta III production and thermal decay can be controlled in intact mouse rods, and, if so, by what mechanisms. It is well established that the termination of rhodopsin photoactivation in intact photoreceptors is mediated by multiple-pigment phosphorylation by Grk1 and subsequent binding of Arr1, each greatly reducing the catalytic activity of Meta II toward transducin (Xu et al., 1997; Mendez et al., 2000). Based on previous biochemical experiments establishing the relationship between Meta II/III and Arr1 (Sommer and Farrens, 2006), we reasoned that phosphorylation and/or Arr1 binding might introduce significant structural changes in photoactivated rhodopsin that could modify the kinetics of Meta III production and decay and eventually impact the rate of dark adaptation of rods after bright light exposure. Therefore, we compared the rates of production and decay of Meta III in WT mouse rods, rods lacking rhodopsin kinase (Grk1−/−) or arrestin 1 (Arr1−/−), and rods overexpressing arrestin 1 (Arr1ox). Fig. 2 shows the time course of Meta III production (from Meta II) and decay after 50% pigment bleaching. Fig. 2 A shows the time course and decay of Meta III in WT rods. Meta III absorbance was normalized to that of rhodopsin in the dark, using its relative extinction coefficient of 1.16 in situ (Kolesnikov et al., 2003). The time course of Meta III in WT rods could be well described by the combination of two exponential functions: τ1 = 1.5 ± 0.2 min and τ2 = 17 ± 1.2 min (±SEM). Both the production and the decay of Meta III were substantially slowed in Arr1-deficient rods (bi-exponential; τ1 = 2.3 ± 0.2 min and τ2 = 27 ± 1.5 min) compared with WT rods (Fig. 2 B). This result is consistent with previous in vitro findings on bovine rhodopsin (Sommer and Farrens, 2006) showing that arrestin can accelerate the rate of Meta III decay. Consistent with the arrestin-induced acceleration of Meta III decay, Meta III decay from Arr1ox rods appeared to be faster than that of controls (bi-exponential; τ1 = 2.7 ± 1.1 min and τ2 = 7.6 ± 0.8 min; Fig. 2 C). However, retinal degeneration resulted in fewer and smaller rod outer segments in Arr1ox mice (Song et al., 2011), thus rendering measurements of Meta III kinetics challenging. The smaller MSP spectral signal reduced the signal to noise ratio. Thus, the MSP results from this model should be interpreted with caution. Finally, we found that in Grk1-deficient rods, the rate of Meta III production and decay were faster (bi-exponential; τ1 = 0.98 min ± 0.1 and τ2 = 13 ± 0.9 min) compared with those in WT photoreceptors (Fig. 2 D). Together, these results indicate that blocking phosphorylation of rhodopsin accelerates the production and thermal decay of Meta III in mouse rods. Conversely, the lack of arrestin binding to phosphorylated rhodopsin slows down the turnover of Meta III. Most likely, these changes in the overall Meta III time course are at least partially related to the shift of Meta II/III equilibrium toward either slower decaying Meta III (in Arr1−/− rods) or faster decaying Meta II (in Grk1−/− rods). This assumption is supported by the substantially increased relative fraction of Meta III at its peak time in Arr1-deficient rods (>50%) and, conversely, by its reduced peak amount in Grk1-deficient cells (∼30%), as compared with that in WT rods (∼40%). However, a close overlap of the Meta II spectrum with that of released all-trans retinal (its product) did not allow us to reliably determine the correlation of Meta III production rate with the rate of Meta II decay in our experiments (unpublished data). This issue requires further investigation.
Figure 2.
Phosphorylation and arrestin binding modulate the production and decay of Meta III in mouse rods. The time course of Meta III was measured as its normalized absorbance (Kolesnikov et al., 2003) after 50% rhodopsin bleach. Fits are bi-exponential functions, f(x) = A(−e-x/τ1 + e-x/τ2), with the following time constants ± SEM. (A) WT: τ1 = 1.5 ± 0.2 min and τ2 = 17 ± 1.2 min (n = 6). (B) Arr1−/−: τ1 = 2.3 ± 0.2 min and τ2 = 27 ± 1.5 min (n = 5). (C) Arr1Ox: τ1 = 2.7 ± 1.1 min and τ2 = 7.6 ± 0.8 min (n = 4). (D) Grk1−/−: τ1 = 0.98 ± 0.1 min and τ2 = 13 ± 0.9 min (n = 6). The error bars represent SEM. All recordings were made at 37°C.
Meta III decay and pigment regeneration in WT and Grk1- and Arr1-deficient rods
The different rates of Meta III decay that we observed in WT, Arr1−/−, and Grk1−/− mouse rods allowed us to test whether Meta III decay in these mutants sets the amount of opsin available for regeneration in intact rods. To address this issue, we treated bleached retinas with 10 µM 11-cis retinal for 10 min to promote pigment regeneration. We did this in the presence of Meta III shortly after bleaching, 1 h after bleaching, or after the complete decay of Meta III 2 h after bleaching.
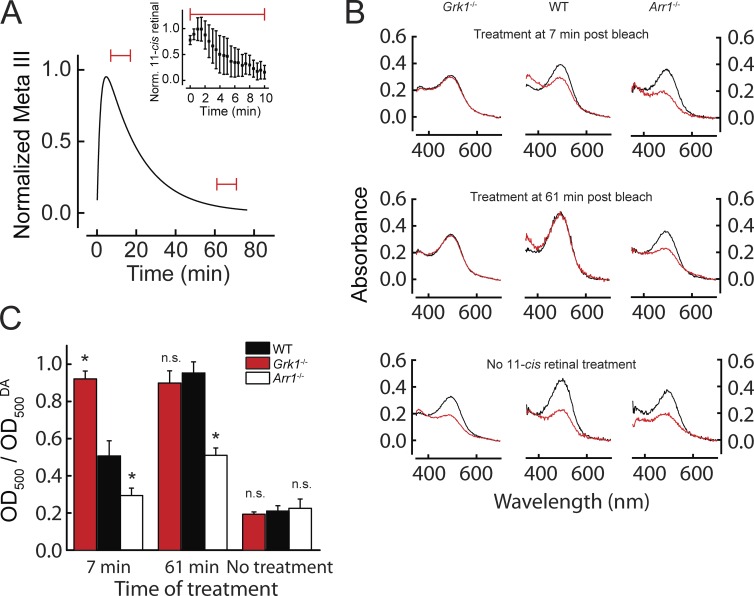
First, we determined whether the altered rates of Meta III decay observed in WT, Arr1−/−, and Grk1−/− mouse rods would influence the availability of free opsin and thus the extent of pigment regeneration. Fig. 3 A illustrates the method used to determine the extent of pigment regeneration in these models. The time course of Meta III in WT rods is reproduced from Fig. 2 A, together with two red horizontal bars that indicate short (10 min) periods, during one of which the retina was treated with exogenous 11-cis retinal (either when Meta III content in rods was maximal, 7 min after bleaching, or when it was minimal upon its near complete decay 60 min after bleaching). The time course of 11-cis retinal treatment is shown in the inset of Fig. 3 A, with the relative concentration of 11-cis retinal estimated from its absorbance at 380 nm. The chromophore reached its peak concentration in the bath in 1 min and was effectively washed out of the bath within 10 min. Fig. 3 B illustrates rhodopsin spectra in WT, Arr1−/−, and Grk1−/− rods that were recorded either in dark-adapted state at the beginning of the experiment (black traces) or at the end of the experiment (120 min after 80% pigment bleaching and treatment with 11-cis retinal [red traces]). The final amount of rhodopsin in WT retina after the 7-min postbleach treatment with chromophore was 54%. Consistent with the slower decay of Meta III in Arr1-deficient rods (Fig. 2 B), the amount of pigment regenerated in Arr1−/− rods under the same conditions was only 18%. In contrast, in Grk1−/− rods with faster-decaying Meta III (Fig. 2 D), the corresponding pigment level was 89% (Fig. 3 B, top row). Treatment with 11-cis retinal 61 min after bleaching resulted in a total amount of rhodopsin that approached 100% of its dark-adapted level in WT and Grk1−/− retinas. In the Arr1−/− retina at the same time, the rhodopsin content was still only 42% of its prebleach value (Fig. 3 B, middle row). In contrast, when Arr1−/− retinas were treated with 11-cis retinal after the complete decay of Meta III at 120 min, the regeneration of rhodopsin was complete (not depicted). Control experiments performed in the absence of exogenous 11-cis retinal treatment demonstrated that no rhodopsin was regenerated in isolated retinas for up to 120 min after bleaching in any of the mouse strains (Fig. 3 B, bottom row). Together, these findings demonstrate that Meta III decay is a requisite for efficient pigment regeneration in mouse rods and that this process can be modulated by rhodopsin kinase and arrestin. Fig. 3 C summarizes our findings displayed in Fig. 3 B, to provide averaged fractions of pigment regenerated with exogenous 11-cis retinal in retinas of WT, Grk1−/−, and Arr1−/− mice.
Figure 3.
Pigment regeneration depends on Meta III decay in Grk1−/− and Arr1−/− mouse rods. (A) Typical experiment in which retinas were exposed to exogenous 11-cis retinal for 10 min (red bars) at either 7 min after bleaching (when Meta III reached its maximum) or 61 min after bleaching (Meta III decayed). The inset shows the change in concentration of 11-cis retinal in the recording chamber over the time of treatment normalized to its maximal concentration (∼10 µM). Data were reproduced from Fig. 4. (B) Spectra of Grk1−/−, WT, and Arr1−/− retinas measured 120 min after 80% pigment bleaching. The retinas were exposed to 10 µM exogenous 11-cis retinal for 10 min either at 7 min after bleaching when Meta III had reached its maximum (top) or 61 min after bleaching when there was no Meta III left in WT and Grk1−/− rods but a substantial amount left in Arr1−/− rods (middle). Bottom panels show untreated controls. Black traces represent prebleach spectra. Red traces show spectra measured after bleaching and/or treatment with 11-cis retinal. (C) Summary of the averaged data ± SEM from experiments as in B. The bars show the amount of pigment regenerated in WT (7 min, n = 4; 61 min, n = 4; no treatment, n = 7), Grk1−/− (7 min, n = 6; 61 min, n = 4; no treatment, n = 2), and Arr1−/− (7 min, n = 5; 61 min, n = 6; no treatment, n = 6) retinas when treated with 11-cis retinal 7 or 61 min after bleaching or not treated. Asterisks indicate a significant difference (P < 0.05) from WT; n.s., not significant (one-way ANOVA with Tukey’s test for means comparisons; F = 41; P = 1.3 × 10−15). Additionally, all models at both 7 and 61 min were significantly different from the untreated control, except Arr1−/− treated at 7 min. All recordings were made at 37°C.
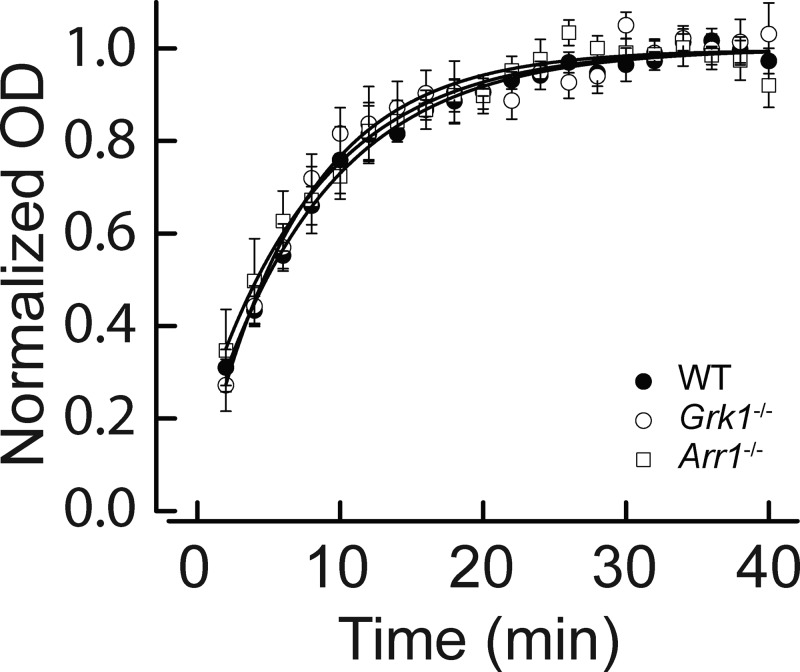
To rule out the possibility that the data were biased by different rhodopsin regeneration rates in these models, we measured the rates of pigment regeneration when the retinas where treated with exogenous 11-cis retinal after the complete decay of Meta III. The data presented in Fig. 4 were acquired 120 min after bleaching. Here, retinas of WT, Grk1−/−, and Arr1−/− mice were treated with 10 µM 11-cis retinal after 70% bleaching and full decay of Meta III. The data could be fitted with single exponential functions with the similar time constants of τ = 8.4 ± 0.4 min (WT), τ = 7.0 ± 0.4 min (Grk1−/−), and τ = 8.2 ± 0.8 min (Arr1−/−). These results demonstrate that the rate of rhodopsin regeneration per se does not depend on the presence of rhodopsin kinase or arrestin. The results presented in Figs. 3 and 4 reveal that the Meta III decay in intact WT, Grk1-deficient, and Arr1-deficient mouse rods determines the availability of free opsin and sets the overall kinetics of pigment regeneration in the three models.
Figure 4.
Time course of rhodopsin regeneration. Rhodopsin regeneration measured as normalized OD at 500 nm after completion of Meta III decay in WT (n = 5), Grk1−/− (n = 5), and Arr1−/− (n = 7) mouse rods after 70% pigment bleaching and exogenous treatment with 10 µM 11-cis retinal. Fitting with single saturation exponential functions yielded regeneration time constants (τ ± SEM) of 8.4 ± 0.4 min (WT), 7.0 ± 0.4 min (Grk1−/−), and 8.2 ± 0.8 min (Arr1−/−). Error bars represent SEM in all panels. All recordings were made at 27°C.
Rod dark adaptation in mice with varying expression levels of Grk1 and Arr1
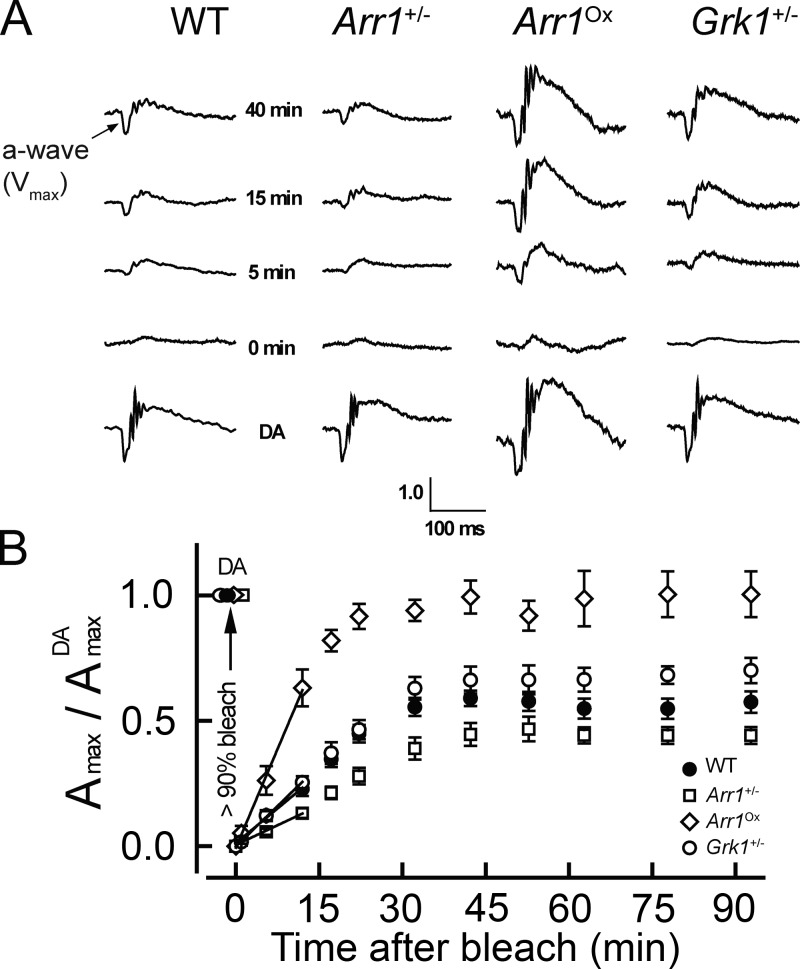
Finally, we sought to understand the physiological significance of Meta III decay for the kinetics of mouse rod dark adaptation in live animals. In these conditions, the rate of rod recovery is believed to be determined by the speed of 11-cis chromophore production in the RPE cells and its subsequent delivery to photoreceptors, where it then recombines with opsin liberated by the decay of photoproducts (Lamb and Pugh, 2004; Kolesnikov et al., 2011b; Frederiksen et al., 2012; Wang et al., 2014; Lamb et al., 2015). Specifically, we measured the recovery of the rod maximal photoresponse after exposure to bright bleaching light by performing in vivo full-field ERG recordings (Fig. 5). First, we determined the dark-adapted rod ERG Amax (AmaxDA). We then applied green light to bleach >90% of the rod pigment and monitored the subsequent recovery of the rod response amplitude (Amax) in darkness in WT mice and animals with altered levels of Grk1 and Arr1 in their rod outer segments. Grk1−/− and Arr1−/− mouse rods have been shown to have extremely prolonged saturation of their rod responses caused by persistent activation of the phototransduction cascade (Xu et al., 1997; Chen et al., 1999), especially after exposure to intense bleaching light. This slow rod response inactivation in homozygous Grk1- and Arr1-deficient mice makes it impossible to follow the recovery of rod responsiveness during dark adaptation. Thus, to determine the role of Grk1 and Arr1 in modulating rod dark adaptation, we performed recordings from heterozygous animals (Grk1+/− or Arr1+/−), whose rods express ∼30% Grk1 or ∼50% Arr1 compared with their WT counterparts and whose photoresponse kinetics are only slightly different from those of WT rods (Xu et al., 1997; Doan et al., 2009; Song et al., 2011).
Figure 5.
Dependence of mouse rod dark adaptation on Arr1 and GRK1 levels in vivo. (A) Representative scotopic ERG responses in the dark (dark adapted [DA], bottom) and at indicated time points after bleaching >90% of the rod pigment in WT, Arr1+/−, Arr1ox, and Grk1+/− mice. For each time point, the Amax values were normalized to the corresponding AmaxDA values (hence the vertical scale bar is without units). (B) Averaged results for the recovery of scotopic ERG Amax (measured as described in A) after bleaching >90% of the rod pigment in WT (n = 8), Arr1+/− (n = 7), Arr1ox (n = 5), and Grk1+/− (n = 5) mice. Bleaching was achieved by illumination with green light at time 0. Values are means ± SEM. Initial rates of Vmax recovery were determined from linear fits (straight lines) that yielded the following slopes: 0.018 min−1 (WT), 0.010 min−1 (Arr1+/−), 0.052 min−1 (Arr1ox), and 0.021 min−1 (Grk1+/−).
Fig. 5 A shows representative dark-adapted ERG traces (bottom) as well as responses recorded at various times after bleaching (as indicated) from WT, Arr1+/−, Arr1ox, and Grk1+/− mice. All traces were normalized to the corresponding prebleach ERG Amax values. As previously shown (Song et al., 2011), the 2.2-fold overexpression of arrestin in Arr1ox mice did not affect the rod dim flash and saturated response inactivation kinetics. Although Amax recovered to a plateau within ∼40 min for all strains, the initial recovery was notably faster for Arr1ox rods compared with the other three strains (Fig. 5 B). The Arr1+/− rods, on the other hand, recovered approximately two times slower than Grk1+/− and WT rods. Interestingly, the final fraction of response recovery at the end of our 90-min recordings also correlated with the relative kinetics of Meta III decay induced by altering the expression of Grk1 and Arr1 (Fig. 2) and was most substantial in Arr1ox mice and most suppressed in Arr1+/− animals (Fig. 5 B). However, the mechanistic reason for these different levels of final recovery remains unclear. In summary, these results establish a link between the speed of Meta III decay and the rate of dark adaptation in intact mouse rod photoreceptors. Our results also reveal the important role of Arr1 (and, to a lesser degree, Grk1) in modulating Meta III decay and rod dark adaptation in vivo.
DISCUSSION
Phosphorylation by Grk1 and binding of Arr1 have long been established as playing critical roles in quenching photoactivated rhodopsin and controlling the termination of the flash response in rods (Makino et al., 2003). However, whether the quenching of rhodopsin by Grk1 and Arr1 also affects the eventual decay of its photointermediates to free opsin in the intact photoreceptors had not been investigated. Equally important, it was unclear whether the interaction of Grk1 and Arr1 with rhodopsin modulates the regeneration of visual pigment and the resulting dark adaptation of rods. Here, we addressed these questions by investigating the kinetics of photoactivated rhodopsin decay and dark adaptation in intact rods from mice with altered expression levels of Grk1 and Arr1. Our results clearly demonstrate that the decay of photoactivated rhodopsin is slowed in Arr1-deficient rods but accelerated in Grk1-deficient rods. Furthermore, dark adaptation of rods in vivo is also slowed in Arr1-deficient rods and accelerated in Grk1-deficient rods. Together, our findings reveal a novel role for Grk1 and Arr1 in modulating the photochemistry of rhodopsin and the function of mammalian rod photoreceptors.
Arr1 and Grk1 modulate the decay of photoactivated rhodopsin
After rhodopsin is activated by light, its R* (Meta II) state is quickly phosphorylated and binds arrestin. The phosphorylated and arrestin-bound Meta II decays via Meta III (and directly) to all-trans retinal and free opsin ready for pigment regeneration (Kolesnikov et al., 2003; Vogel et al., 2004). Meta III– and Meta III–like species of bleached rhodopsin are protonated forms of visual pigment photointermediates that contain all-trans retinal chromophore. They have a lifetime of minutes and activate transducin only marginally (Kibelbek et al., 1991; Leibrock and Lamb, 1997; Bartl et al., 2001), most likely because of reversion back to a Meta II–like state. As a result of its slow decay, Meta III accumulates in rods after moderate and high bleaching, effectively acting as a repository for all-trans retinal in rods (Bartl et al., 2001; Heck et al., 2003). Thus, despite its negligible activity on a single-molecule level, the excess of slowly decaying Meta III can greatly prolong rod recovery from saturation (Firsov et al., 2005). The resulting slow release of all-trans retinal would also delay pigment regeneration by making opsin unavailable in the rod outer segment.
Several previous studies have suggested that the time course of production and decay of rhodopsin photoproducts can be affected by basic components of phototransduction. For instance, the transition of unphosphorylated Meta III back to Meta II and its eventual spontaneous decay can be accelerated in vitro in the presence of substoichiometric amounts of exogenous G-protein transducin (Zimmermann et al., 2004). In regard to another phototransduction component, visual arrestin, there is still no consensus about its effects on rhodopsin photointermediates in the literature. Previous in vitro work has suggested that recombinant arrestin can stabilize Meta II, block the Meta III formation, or shift the Meta II/III equilibrium toward Meta II and inhibit the overall rate of all-trans retinal release (Sommer and Farrens, 2006). However, other findings instead suggest the stabilization of Meta III by either full-length arrestin (Burns et al., 2006) or its truncated form, ArrTr, which is similar to physiologically relevant splice variant p44 (Chatterjee et al., 2015). A major caveat of these biochemical experiments is that they were performed with either detergent-solubilized rhodopsin or purified rod outer segment membranes, in which concentrations of added protein components (and the overall metabolic state of the system) are substantially different from those in intact cells. To circumvent these issues, here we examined the role of arrestin in intact mouse rods. We observed a substantially slower decay of Meta III intermediates in Arr1-deficient photoreceptors compared with control rods (Fig. 2). This effect was mirrored in the slower release of free opsin available for regeneration with exogenous 11-cis retinal (Fig. 3). Our results suggest that, in in vivo conditions, the binding of endogenous Arr1 shifts the equilibrium between Meta II and Meta III to the more rapidly decaying Meta II (Kolesnikov et al., 2003). This, in turn, accelerates the overall decay of metarhodopsins to free opsin and all-trans retinal, releasing the chromophore for recycling and making opsin available for regeneration back to rhodopsin sooner. Increasing Arr1 expression accelerates the decay of Meta III (Fig. 2), making more opsin available for regeneration with exogenous chromophore at a set time after bleaching (Fig. 3) and ultimately accelerating rod dark adaptation (Fig. 5). The slower release of retinal from photoactivated rhodopsin in Arr1-deficient rods may be expected to result in slower formation of retinol. The kinetics of retinol formation in whole retinas and retinal slices from Arr1-deficient mice is indeed slower compared with WT mice (Blakeley et al., 2011). In single isolated rod photoreceptors, however, this difference in retinol formation kinetics was not evident, presumably because of the complicating effect of retinol diffusing out of the rod outer segment.
On the other hand, the situation in Grk1−/− rods, in which the decay of Meta III (Fig. 2) and the production of free opsin (Fig. 3) are accelerated, is likely more complicated. First, the lack of pigment phosphorylation could itself increase the decay rate of Meta III, which would suggest that phosphorylation extends the lifetime of Meta III. Alternatively, the loss of Grk1 could facilitate the binding of Arr1 to unphosphorylated pigment (e.g., because of relief of its competition with the kinase; Doan et al., 2009). Such interactions between unphosphorylated opsin and the long arrestin splice variant p48 have been proposed in vivo (Burns et al., 2006). It should be noted, however, that high-affinity Arr1 binding to unphosphorylated rhodopsin has not been detected in biochemical experiments (Gurevich and Benovic, 1992; Krupnick et al., 1997). Although a recent study reported the ability of arrestins to interact with photoactivated unphosphorylated pigments in carp rods and cones (Tomizuka et al., 2015), the binding was rather transient and disappeared by 5 min after bleaching. This is substantially shorter than the lifetime of Meta III in mouse rods under physiological conditions (Fig. 2). In any case, such a possible lower affinity binding of native arrestin to unphosphorylated pigment would not be expected to accelerate Meta III decay to a greater extent than its normal high-affinity binding to phosphorylated rhodopsin in WT rods. Therefore, we favor a scenario in which substoichiometric amounts of transducin interact more readily with the unphosphorylated Meta III in Grk1−/− rods compared with its phosphorylated form in WT rods. This could shift the Meta II/III equilibrium toward Meta II (Zimmermann et al., 2004) and, in a fashion similar to the effect of arrestin in WT rods, could lead to the faster decay of metarhodopsins in the absence of Grk1. Furthermore, the Meta III production and decay rates in Grk1-deficient rods would be expected to be similar to those for rhodopsin in purified rod outer segment membranes, which would also not be subject to phosphorylation by Grk1. Indeed, these rates are in close agreement with the rates for biphasic retinal release from photoactivated rhodopsin in purified mouse rod outer segment membranes (0.77- and 14.7-min time constants; Blakeley et al., 2011). Although more work is needed to elucidate the exact mechanism of regulation of Meta III decay by rhodopsin kinase, our present findings unravel a novel physiological role of Arr1 and Grk1 in modulating the lifetime of long-lived Meta III and the regeneration of visual pigment in mammalian rod photoreceptors.
Arr1 and Grk1 modulate the dark adaptation of mammalian rods after bleaching
Previous studies have suggested that the stage limiting the rate of visual pigment regeneration does not reside in the rod photoreceptors themselves, but rather lies in the supply of 11-cis retinal from the RPE to opsin (Chen et al., 1999; Lamb and Pugh, 2004; Maeda et al., 2005; Imai et al., 2007; Kessler et al., 2014; Wang et al., 2014; Lamb et al., 2015). The newly found modulation of Meta III decay by Arr1 and Grk1 allowed us to investigate the role of this process in rod pigment regeneration and dark adaptation. Our results presented here demonstrate that reactions involving the visual pigment in rods can also modulate the visual cycle and the overall rate of rod dark adaptation in the intact eye. Specifically, we found that the recovery of rod maximal photoresponse amplitude after bleaching >90% of the pigment accelerated substantially with increasing expression of Arr1 (Fig. 5). However, rods in Grk1+/− mice, which express Grk1 at ∼30% of WT levels, displayed a milder phenotype. This is consistent with our finding that Grk1 deletion accelerates the decay of Meta III only moderately (Fig. 2). Thus, the altered dark adaptation of rods in these mouse models in vivo was consistent with the change in their respective Meta III decay rates measured in isolated retinas. This reveals a regulation of rod dark adaptation by Meta III decay that has heretofore been unappreciated and demonstrates a novel role for arrestin and rhodopsin kinase in modulating this process.
Pigment phosphorylation, arresting binding, and Oguchi disease
Our results demonstrate that the deletion of arrestin results in slower decay of Meta III (Fig. 2), which in turn delays the release of free opsin needed for pigment regeneration (Fig. 3) and rod dark adaptation in vivo (Fig. 5). These results provide insight into one long-standing question: Why do patients with deficient arrestin linked to Oguchi disease suffer from delayed rod dark adaptation? In dark-adapted and dim light conditions, most of Arr1 is localized outside the outer segments of rods (Strissel et al., 2006) and would be expected to exert a minimal effect on Meta III decay. At the same time, the level of pigment bleaching in such light conditions would be negligible, again preventing its modulation from affecting the function of rod photoreceptors. However, after exposure to bright bleaching light and throughout most of the day, a significant fraction of rhodopsin will be bleached, triggering the translocation of arrestin from the inner to the outer segments of rods (Strissel et al., 2006). In the past, it had been difficult to interpret such massive arrestin translocation as a mechanism of adaptation of the rods to bright light, because rods are saturated in such conditions. However, our finding that Arr1 binding accelerates the decay of Meta III sheds new light onto this process and suggests that arrestin translocation to the rod outer segments after bright bleaching light will help accelerate the subsequent regeneration of rhodopsin, effectively speeding up the dark adaptation of rod photoreceptors. In contrast, in patients with Ogushi disease who lack a functional arrestin, the decay of Meta III will be substantially slower, delaying the regeneration of rhodopsin and dark adaptation. Thus, our results indicate that the lack of effective acceleration of Meta III decay by arrestin in patients with Oguchi disease could explain their abnormally slow dark adaptation. As for Oguchi patients with mutations in their rhodopsin kinase, it is possible that the slight acceleration of Meta III decay in GRK1-deficient rods is offset by the substantially delayed shutoff of the phototransduction cascade in the absence of GRK1, which dominates the overall kinetics of rod dark adaptation. Finally, as arrestin binding can enhance rhodopsin shutoff even in the absence of functional Grk1 (Song et al., 2009), its translocation to the outer segments would compensate partially for the lack of rhodopsin phosphorylation, again enhancing the shutoff of the rods and accelerating their dark adaptation. Together, these results indicate that treatments that accelerate Meta III decay could have therapeutic potential in accelerating dark adaptation and ameliorating the visual deficiency of patients with Oguchi disease.
ACKNOWLEDGMENTS
We thank Jeannie Chen for the Arr1−/− mice, Jason Chen for the Grk1−/− mice, and Vsevolod Gurevich for the Arr1ox mice. We thank Howard Cohen for help building electronics equipment.
This work was supported by National Institutes of Health grants EY01157 (to C. Cornwall), EY019312 and EY021126 (to V.J. Kefalov), EY014850 (to Y. Koutalos), and EY002687 (to the Department of Ophthalmology and Visual Sciences, Washington University) and by the Research to Prevent Blindness and the Academy of Finland grant 260375 (to S. Nymark).
The authors declare no competing financial interests.
Angus C. Nairn served as editor.
Footnotes
Abbreviations used in this paper:
- ERG
- electroretinogram
- MSP
- microspectrophotometry
- RPE
- retinal pigment epithelium
- WT
- wild type
References
- Adler L. IV, Chen C., and Koutalos Y.. 2014. Mitochondria contribute to NADPH generation in mouse rod photoreceptors. J. Biol. Chem. 289:1519–1528. 10.1074/jbc.M113.511295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Laurila P., Kolesnikov A.V., Crouch R.K., Tsina E., Shukolyukov S.A., Govardovskii V.I., Koutalos Y., Wiggert B., Estevez M.E., and Cornwall M.C.. 2006. Visual cycle: Dependence of retinol production and removal on photoproduct decay and cell morphology. J. Gen. Physiol. 128:153–169. 10.1085/jgp.200609557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnis S., and Hofmann K.P.. 1995. Photoregeneration of bovine rhodopsin from its signaling state. Biochemistry. 34:9333–9340. 10.1021/bi00029a008 [DOI] [PubMed] [Google Scholar]
- Bartl F.J., Ritter E., and Hofmann K.P.. 2001. Signaling states of rhodopsin: Absorption of light in active metarhodopsin II generates an all-trans-retinal bound inactive state. J. Biol. Chem. 276:30161–30166. 10.1074/jbc.M101506200 [DOI] [PubMed] [Google Scholar]
- Blakeley L.R., Chen C., Chen C.K., Chen J., Crouch R.K., Travis G.H., and Koutalos Y.. 2011. Rod outer segment retinol formation is independent of Abca4, arrestin, rhodopsin kinase, and rhodopsin palmitylation. Invest. Ophthalmol. Vis. Sci. 52:3483–3491. 10.1167/iovs.10-6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns M.E., Mendez A., Chen C.K., Almuete A., Quillinan N., Simon M.I., Baylor D.A., and Chen J.. 2006. Deactivation of phosphorylated and nonphosphorylated rhodopsin by arrestin splice variants. J. Neurosci. 26:1036–1044. 10.1523/JNEUROSCI.3301-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D., Eckert C.E., Slavov C., Saxena K., Fürtig B., Sanders C.R., Gurevich V.V., Wachtveitl J., and Schwalbe H.. 2015. Influence of arrestin on the photodecay of bovine rhodopsin. Angew. Chem. Int. Ed. Engl. 54:13555–13560. 10.1002/anie.201505798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.K., Burns M.E., Spencer M., Niemi G.A., Chen J., Hurley J.B., Baylor D.A., and Simon M.I.. 1999. Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc. Natl. Acad. Sci. USA. 96:3718–3722. 10.1073/pnas.96.7.3718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R.A., and Cobbs W.H. III. 1969. Rhodopsin cycle in the living eye of the rat. Nature. 221:820–822. 10.1038/221820a0 [DOI] [PubMed] [Google Scholar]
- Doan T., Azevedo A.W., Hurley J.B., and Rieke F.. 2009. Arrestin competition influences the kinetics and variability of the single-photon responses of mammalian rod photoreceptors. J. Neurosci. 29:11867–11879. 10.1523/JNEUROSCI.0819-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firsov M.L., Kolesnikov A.V., Golobokova E.Y., and Govardovskii V.I.. 2005. Two realms of dark adaptation. Vision Res. 45:147–151. 10.1016/j.visres.2004.08.005 [DOI] [PubMed] [Google Scholar]
- Frederiksen R., Boyer N.P., Nickle B., Chakrabarti K.S., Koutalos Y., Crouch R.K., Oprian D., and Cornwall M.C.. 2012. Low aqueous solubility of 11-cis-retinal limits the rate of pigment formation and dark adaptation in salamander rods. J. Gen. Physiol. 139:493–505. 10.1085/jgp.201110685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich V.V., and Benovic J.L.. 1992. Cell-free expression of visual arrestin. Truncation mutagenesis identifies multiple domains involved in rhodopsin interaction. J. Biol. Chem. 267:21919–21923. [PubMed] [Google Scholar]
- Hagins W.A. 1956. Flash photolysis of rhodopsin in the retina. Nature. 177:989–990. 10.1038/177989b0 [DOI] [PubMed] [Google Scholar]
- Heck M., Schädel S.A., Maretzki D., Bartl F.J., Ritter E., Palczewski K., and Hofmann K.P.. 2003. Signaling states of rhodopsin. Formation of the storage form, metarhodopsin III, from active metarhodopsin II. J. Biol. Chem. 278:3162–3169. 10.1074/jbc.M209675200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K.P., Pulvermüller A., Buczyłko J., Van Hooser P., and Palczewski K.. 1992. The role of arrestin and retinoids in the regeneration pathway of rhodopsin. J. Biol. Chem. 267:15701–15706. [PubMed] [Google Scholar]
- Imai H., Kefalov V., Sakurai K., Chisaka O., Ueda Y., Onishi A., Morizumi T., Fu Y., Ichikawa K., Nakatani K., et al. . 2007. Molecular properties of rhodopsin and rod function. J. Biol. Chem. 282:6677–6684. 10.1074/jbc.M610086200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler C., Tillman M., Burns M.E., and Pugh E.N. Jr. 2014. Rhodopsin in the rod surface membrane regenerates more rapidly than bulk rhodopsin in the disc membranes in vivo. J. Physiol. 592:2785–2797. 10.1113/jphysiol.2014.272518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibelbek J., Mitchell D.C., Beach J.M., and Litman B.J.. 1991. Functional equivalence of metarhodopsin II and the Gt-activating form of photolyzed bovine rhodopsin. Biochemistry. 30:6761–6768. 10.1021/bi00241a019 [DOI] [PubMed] [Google Scholar]
- Kolesnikov A.V., Golobokova E.Y., and Govardovskii V.I.. 2003. The identity of metarhodopsin III. Vis. Neurosci. 20:249–265. 10.1017/S0952523803203047 [DOI] [PubMed] [Google Scholar]
- Kolesnikov A.V., Shukolyukov S.A., Cornwall M.C., and Govardovskii V.I.. 2006. Recombination reaction of rhodopsin in situ studied by photoconversion of “indicator yellow.” Vision Res. 46:1665–1675. 10.1016/j.visres.2005.07.032 [DOI] [PubMed] [Google Scholar]
- Kolesnikov A.V., Ala-Laurila P., Shukolyukov S.A., Crouch R.K., Wiggert B., Estevez M.E., Govardovskii V.I., and Cornwall M.C.. 2007. Visual cycle and its metabolic support in gecko photoreceptors. Vision Res. 47:363–374. 10.1016/j.visres.2006.08.024 [DOI] [PubMed] [Google Scholar]
- Kolesnikov A.V., Korenyak D.A., Shukolyukov S.A., and Govardovskii V.I.. 2011a Photoreactions of metarhodopsin II. Sensory Systems (Russian). 25:55–64. [Google Scholar]
- Kolesnikov A.V., Tang P.H., Parker R.O., Crouch R.K., and Kefalov V.J.. 2011b The mammalian cone visual cycle promotes rapid M/L-cone pigment regeneration independently of the interphotoreceptor retinoid-binding protein. J. Neurosci. 31:7900–7909. 10.1523/JNEUROSCI.0438-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick J.G., Gurevich V.V., and Benovic J.L.. 1997. Mechanism of quenching of phototransduction. Binding competition between arrestin and transducin for phosphorhodopsin. J. Biol. Chem. 272:18125–18131. 10.1074/jbc.272.29.18125 [DOI] [PubMed] [Google Scholar]
- Lamb T.D., and Pugh E.N. Jr. 2004. Dark adaptation and the retinoid cycle of vision. Prog. Retin. Eye Res. 23:307–380. 10.1016/j.preteyeres.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Lamb T.D., Corless R.M., and Pananos A.D.. 2015. The kinetics of regeneration of rhodopsin under enzyme-limited availability of 11-cis retinoid. Vision Res. 110(Pt A):23–33. 10.1016/j.visres.2015.02.014 [DOI] [PubMed] [Google Scholar]
- Leibrock C.S., and Lamb T.D.. 1997. Effect of hydroxylamine on photon-like events during dark adaptation in toad rod photoreceptors. J. Physiol. 501:97–109. 10.1111/j.1469-7793.1997.00097.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A., Maeda T., Imanishi Y., Kuksa V., Alekseev A., Bronson J.D., Zhang H., Zhu L., Sun W., Saperstein D.A., et al. . 2005. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J. Biol. Chem. 280:18822–18832. 10.1074/jbc.M501757200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino C.L., Wen X.H., and Lem J.. 2003. Piecing together the timetable for visual transduction with transgenic animals. Curr. Opin. Neurobiol. 13:404–412. 10.1016/S0959-4388(03)00091-6 [DOI] [PubMed] [Google Scholar]
- Mendez A., Burns M.E., Roca A., Lem J., Wu L.W., Simon M.I., Baylor D.A., and Chen J.. 2000. Rapid and reproducible deactivation of rhodopsin requires multiple phosphorylation sites. Neuron. 28:153–164. 10.1016/S0896-6273(00)00093-3 [DOI] [PubMed] [Google Scholar]
- Nymark S., Frederiksen R., Woodruff M.L., Cornwall M.C., and Fain G.L.. 2012. Bleaching of mouse rods: Microspectrophotometry and suction-electrode recording. J. Physiol. 590:2353–2364. 10.1113/jphysiol.2012.228627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K., Van Hooser J.P., Garwin G.G., Chen J., Liou G.I., and Saari J.C.. 1999. Kinetics of visual pigment regeneration in excised mouse eyes and in mice with a targeted disruption of the gene encoding interphotoreceptor retinoid-binding protein or arrestin. Biochemistry. 38:12012–12019. 10.1021/bi990504d [DOI] [PubMed] [Google Scholar]
- Reuter T. 2011. Fifty years of dark adaptation 1961-2011. Vision Res. 51:2243–2262. 10.1016/j.visres.2011.08.021 [DOI] [PubMed] [Google Scholar]
- Ritter E., Zimmermann K., Heck M., Hofmann K.P., and Bartl F.J.. 2004. Transition of rhodopsin into the active metarhodopsin II state opens a new light-induced pathway linked to Schiff base isomerization. J. Biol. Chem. 279:48102–48111. 10.1074/jbc.M406857200 [DOI] [PubMed] [Google Scholar]
- Saari J.C. 2012. Vitamin A metabolism in rod and cone visual cycles. Annu. Rev. Nutr. 32:125–145. 10.1146/annurev-nutr-071811-150748 [DOI] [PubMed] [Google Scholar]
- Saari J.C., Garwin G.G., Van Hooser J.P., and Palczewski K.. 1998. Reduction of all-trans-retinal limits regeneration of visual pigment in mice. Vision Res. 38:1325–1333. 10.1016/S0042-6989(97)00198-3 [DOI] [PubMed] [Google Scholar]
- Sommer M.E., and Farrens D.L.. 2006. Arrestin can act as a regulator of rhodopsin photochemistry. Vision Res. 46:4532–4546. 10.1016/j.visres.2006.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M.E., Smith W.C., and Farrens D.L.. 2005. Dynamics of arrestin-rhodopsin interactions: Arrestin and retinal release are directly linked events. J. Biol. Chem. 280:6861–6871. 10.1074/jbc.M411341200 [DOI] [PubMed] [Google Scholar]
- Song X., Vishnivetskiy S.A., Gross O.P., Emelianoff K., Mendez A., Chen J., Gurevich E.V., Burns M.E., and Gurevich V.V.. 2009. Enhanced arrestin facilitates recovery and protects rods lacking rhodopsin phosphorylation. Curr. Biol. 19:700–705. 10.1016/j.cub.2009.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Vishnivetskiy S.A., Seo J., Chen J., Gurevich E.V., and Gurevich V.V.. 2011. Arrestin-1 expression level in rods: Balancing functional performance and photoreceptor health. Neuroscience. 174:37–49. 10.1016/j.neuroscience.2010.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strissel K.J., Sokolov M., Trieu L.H., and Arshavsky V.Y.. 2006. Arrestin translocation is induced at a critical threshold of visual signaling and is superstoichiometric to bleached rhodopsin. J. Neurosci. 26:1146–1153. 10.1523/JNEUROSCI.4289-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizuka J., Tachibanaki S., and Kawamura S.. 2015. Phosphorylation-independent suppression of light-activated visual pigment by arrestin in carp rods and cones. J. Biol. Chem. 290:9399–9411. 10.1074/jbc.M114.634543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel R., Lüdeke S., Radu I., Siebert F., and Sheves M.. 2004. Photoreactions of metarhodopsin III. Biochemistry. 43:10255–10264. 10.1021/bi049182q [DOI] [PubMed] [Google Scholar]
- Wald G., Brown P.K., Hubbard R., and Oroshnik W.. 1955. Hindered cis isomers of vitamin A and retinene: The structure of the neo-B isomer. Proc. Natl. Acad. Sci. USA. 41:438–451. 10.1073/pnas.41.7.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.S., Nymark S., Frederiksen R., Estevez M.E., Shen S.Q., Corbo J.C., Cornwall M.C., and Kefalov V.J.. 2014. Chromophore supply rate-limits mammalian photoreceptor dark adaptation. J. Neurosci. 34:11212–11221. 10.1523/JNEUROSCI.1245-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden U., and Kühn H.. 1982. Light-dependent phosphorylation of rhodopsin: number of phosphorylation sites. Biochemistry. 21:3014–3022. 10.1021/bi00541a032 [DOI] [PubMed] [Google Scholar]
- Wilden U., Hall S.W., and Kühn H.. 1986. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc. Natl. Acad. Sci. USA. 83:1174–1178. 10.1073/pnas.83.5.1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Dodd R.L., Makino C.L., Simon M.I., Baylor D.A., and Chen J.. 1997. Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature. 389:505–509. 10.1038/39068 [DOI] [PubMed] [Google Scholar]
- Xue Y., Shen S.Q., Corbo J.C., and Kefalov V.J.. 2015. Circadian and light-driven regulation of rod dark adaptation. Sci. Rep. 5:17616 10.1038/srep17616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K., Ritter E., Bartl F.J., Hofmann K.P., and Heck M.. 2004. Interaction with transducin depletes metarhodopsin III: A regulated retinal storage in visual signal transduction? J. Biol. Chem. 279:48112–48119. 10.1074/jbc.M406856200 [DOI] [PubMed] [Google Scholar]