Abstract
Background:
Intra-articular injection of hyaluronic acid (HA) for knee osteoarthritis (OA) effectively reduces pain and delays total knee replacement (TKR) surgery; however, little is known about relative differences in clinical and cost outcomes among different HA products.
Objective:
To compare disease-specific costs and risk of TKR among patients receiving different HA treatments in a commercially insured cohort of patients with knee OA in the USA.
Method:
Retrospective analyses using IMS Health’s PharMetrics Plus Health Plan Claims Database were conducted by identifying knee OA patients with claims indicating initiation of HA treatment at an ‘index date’ during the selection period (2007–2010). Patients were required to be continuously enrolled in the database for 12 months preindex to 36 months postindex. A generalized linear model (GLM) with a gamma distribution and log-link function was used to model aggregate patient-based changes in disease-specific costs. A Cox proportional hazards model (PHM) was used to model the risk of TKR. Both multivariate models included covariates such as age, gender, comorbidities, and preindex healthcare costs.
Results:
50,389 patients with HA treatment for knee OA were identified. 18,217 (36.2%) patients were treated with HA products indicated for five injections per treatment course (Supartz and Hyalgan). The remainder were treated with HA products indicated for fewer than five injections per treatment course, with 20,518 patients (40.7%) receiving Synvisc; 6,263 (12.4%), Euflexxa; and 5,391 (10.7%), Orthovisc. Synvisc- and Orthovisc-injected patients had greater disease-specific costs compared to Supartz/Hyalgan (9.0%, p<0.0001 and 6.8%, p=0.0050, respectively). Hazard ratios (HRs) showed a significantly higher risk of TKR for patients receiving Synvisc compared to Supartz/Hyalgan (HR=1.069, p=0.0009). Patients treated with Supartz/Hyalgan, Euflexxa, and Orthovisc had longer delays to TKR than those treated with Synvisc.
Conclusion:
Analysis of administrative claims data provides real-world evidence that meaningful differences exist among some HA products in disease-specific cost and time to knee replacement surgery.
Keywords: intra-articular, hyaluronic acid, viscosupplementation, knee replacement, health economics, outcomes research
Introduction
Osteoarthritis (OA) is a common chronic illness in older adults characterized by deterioration of joint cartilage, accompanied by joint inflammation, pain, and loss of physical function. OA ranks as the fifth leading cause of disability among US adults [1]. Because age is a major risk factor for OA [2], the prevalence of OA is expected to increase as the US population ages. In addition to age, previous joint injury and obesity are considered major risk factors for knee OA; previous joint injury is a common cause of knee OA among young adults [3], whereas high body mass index (BMI) is associated with increased risk of knee OA, particularly among old adults [4]. Indeed, research shows that the annual estimated number of people in the USA with OA was approximately 30.8 million for 2008–2011 [5]. Knee OA is one of the most common forms of arthritis, with an estimated 644,000 total knee replacement (TKR) surgeries performed in 2011, 97% of which were due to osteoarthritis [6].
Viscosupplementation, in which hyaluronic acid (HA) is injected into the knee joint for the symptomatic relief of pain, has been available for treatment of knee OA in the USA since 1997. Various mechanisms of action have been suggested to explain the clinical effects of intra-articular injection of HA (IAHA) [7]: IAHA provides extra lubrication and cushioning within affected knee joints [8] and has been shown to induce direct anti-inflammatory, chondroprotective [9–11], and analgesic effects [12]. Hyaluronic acid injections are recognized as safe and effective for the alleviation of joint pain and improvement of joint function in patients with knee OA [13], with positive clinical evidence demonstrated in clinical trials [14,15]. However, after the American Academy of Orthopedic Surgeons (AAOS) revised its treatment guidelines in 2013 to issue a recommendation against the use of IAHA [16], there has been a debate over the clinical impact of these injections. Evidence from meta-analyses has been mixed. For example, one meta-analysis showed that effects of viscosupplementation were only marginally different from placebo injections [17], whereas another meta-analysis showed that viscosupplementation was more effective than any oral medication for knee OA pain [18,19]. Treatment guidelines issued by different professional medical societies do not point in a single direction either. AAOS recommends against the use of IAHA, but in 2014, the American College of Rheumatology (ACR) made conditional recommendation for the use of IAHA to treat pain in patients who have failed to respond adequately to conservative nonpharmacologic therapy and simple analgesics in its position statement on viscosupplementation [20,21]; Osteoarthritis Research Society International (OARSI) rates the benefit of IAHA as uncertain [22], whereas American Medical Society for Sport Medicine (AMSSM) recommends the use of IAHA for the appropriate patients with knee OA [23].
Given such conflicting views on the clinical value of IAHA, recent research attention has turned to the real-world evidence that has been accumulating to suggest that IAHA injections result in clinical effectiveness culminating in a delay to total knee replacement [24,25]. One study that utilized US health plan administrative claims retrospectively looked at the data of knee OA patients who ultimately underwent TKR. It showed that the HA cohort had a median 1.0-year longer time to TKR surgery than the non-HA cohort [26]; another retrospective study with a different US health claims database has demonstrated a delay of median 1.6-year difference between the HA cohort and the non-HA cohort [27]. Populations in both studies were relatively similar in age and gender distribution; more than 70% of patients were aged 55 or older and more than 50% of patients were females. In both studies, age distributions between HA and non-HA cohorts were similar, with HA cohorts having had a slightly greater proportion of females than non-HA cohorts. When a Cox proportional hazards model (PHM) was fit to adjust for age and gender, the TKR-delaying effect of repeated HA treatments still remained. As for the duration of knee OA, neither study provided clear information other than the time between the first diagnosis of OA and TKR, so it is uncertain whether HA and non-HA cohorts were clearly identical in disease duration at study entry. However, both studies used the first diagnosis of knee OA as the index date, implying that included patients were unlikely to be at an advanced stage of OA at study entry, and both studies also required the presence of TKR within the study window as an inclusion criterion, implying that included patients were unlikely to be at an early stage of OA at study entry due to chronic nature of knee OA. Thus, it is likely that data for both HA and non-HA cohorts were included and tracked from a relatively moderate stage of disease and thus HA and non-HA cohorts were largely comparable with each other to support valid inference with time-to-TKR analysis.
In light of these findings, additional research on the differences in clinical and cost outcomes among different HA products may be of interest to key decision makers. In the USA, various IAHA injection products, with different molecular properties, production processes, and number of injections per treatment course, are currently FDA approved for use. While a cost-effectiveness analysis of a high molecular weight, bioengineered HA with data obtained from a clinical trial [28] concluded that the HA product was less costly and more effective than conventional care with NSAID and analgesics, previous research has not examined cost and clinical effectiveness data across different HA products in the real-world setting. This study compares different US FDA-approved HA viscosupplements using real-world evidence from an administrative claims database.
Methods
Data source
For this retrospective, observational cohort study, integrated medical and pharmacy claims data were extracted from IMS Health’s PharMetrics Plus Health Plan Claims Database, which comprises adjudicated claims for more than 150 million unique patients across the USA and has diverse representation of geography, employers, and payers. The PharMetrics Plus database is thought to be representative of the commercially insured US national population in terms of age and gender. However, the population in the database is slightly younger than the entire US population because all members in the database have commercial insurance coverage with limited Medicare data. As all data are deidentified to protect patient privacy and are compliant with the Health Insurance Portability and Accountability Act, no informed consent was required.
Study design
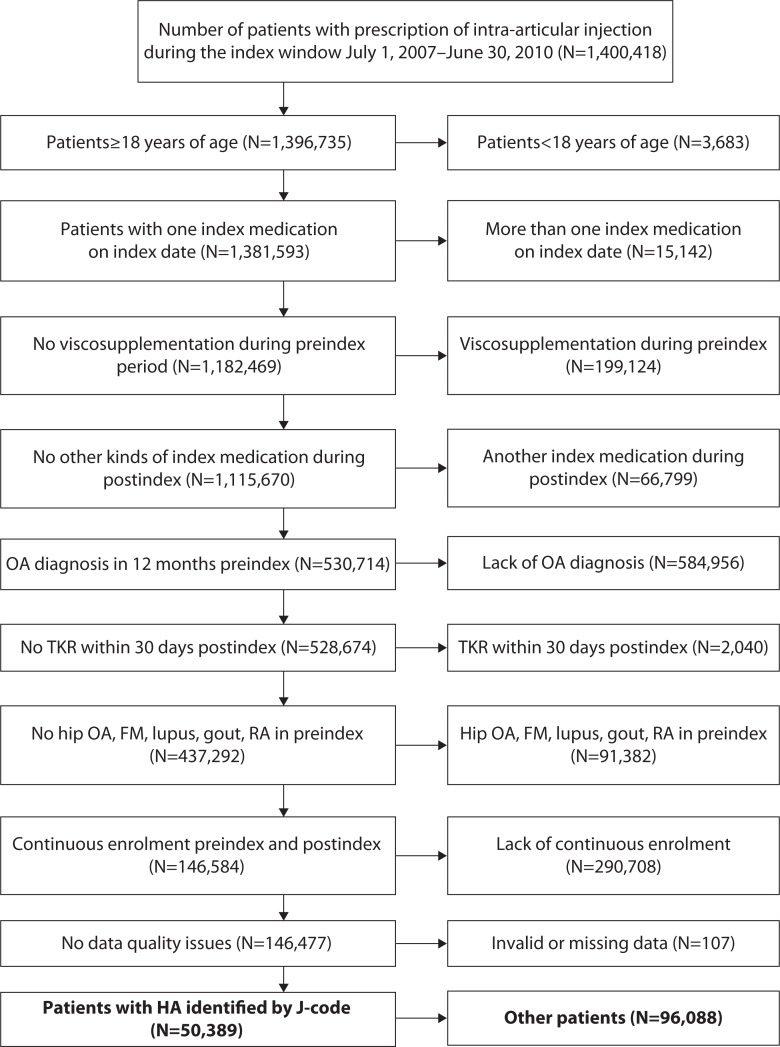
Claims data for knee OA patients with outpatient claims were indicating initiation of HA injection during the selection period (July 1, 2007–June 30, 2010). Product J-codes were used to select all common HA agents in the US market during this period (Euflexxa, Hyalgan, Orthovisc, Supartz, Synvisc; Table 1). Because two HA agents (Supartz and Hyalgan) share the same J-code and thus could not be distinguished by J-code, they were categorized as a single group of Supartz/Hyalgan. Likewise, Synvisc-One, which is chemically identical to Synvisc and began to be sold in the USA in 2009 as a single-dose regimen per treatment course, shares the same J-code as Synvisc and was classified together with Synvisc. Thus, four patient cohorts were created (Euflexxa, Orthovisc, Supartz/Hyalgan, Synvisc). The date of the first such claim within the selection window was defined as the ‘index date’. This set the stage for a comparative effectiveness study, as different HA products were assumed to be used at the same stage in knee OA treatment pathway and assumed to be relatively similar in disease severity at patient selection. Patients had to meet the following inclusion criteria to be eligible: ≥18 years of age in the year of their index date, at least one clinical knee OA diagnosis during the 12-month preindex period, and continuous enrolment from 12 months preindex to 36 months postindex date. Although lengthier pre- and post-index periods would have been ideal to establish the duration and severity of OA, the ability to follow patients longitudinally for a longer period of time in the data asset was limited. Exclusion criteria included the following: any HA use in the preindex period; a different kind of HA index medication (from the index date/prescription) in the postindex period; a TKR within 30 days following the index event; two different kinds of HA index medications on the index date; and diagnosis of hip OA, fibromyalgia, rheumatoid arthritis, lupus, or gout during the preindex period. Clinical and health economic outcomes were measured over the 36-month postindex period. Baseline information such as demographic characteristics, health plan type, physician specialty, comorbidities, and medications of interest were obtained during the 12-month preindex baseline period.
Table 1.
Characteristics of different FDA-approved HA viscosupplements.
| PMA number | P010029 | P950027 | P030019 | P980044** | P940015*** |
|---|---|---|---|---|---|
| Current J-code | J7323 | J7321 | J7324 | J7321 | J7325 |
| Trade name | Euflexxa | Hyalgan | Orthovisc | Supartz† | Synvisc |
| Number of injections per treatment course | 3 | 3 to 5* | 3 or 4 | 3 to 5* | 3 |
| HA source | Bacterial cells | Avian | Bacterial cells | Avian | Avian |
| HA concentration (mg/mL) | 10 | 10 | 15 | 10 | 8 |
| Volume per injection | 2 mL | 2 mL | 2 mL | 2.5 mL | 2 mL |
| Molecular weight (MDa) | 2.4–3.6 | 0.5–0.73 | 1.0–2.9 | 0.62–1.17 | >6†† |
Indicated for five weekly injections, but product labelling indicates that some patients may receive pain relief with only three injections per course, leading some healthcare professionals to administer these products as three to five injections per treatment course.
PMA supplement approved by FDA in December 2015 for three injections per treatment course in addition to five injections per treatment course.
PMA supplement approved by FDA in February 2009 for single 6 mL injection per treatment course in addition to three 2 mL injections per treatment course.
Trademark used in the US market changed to Supartz FX since October 2015.
Hylan A’s molecular weight is 6 MDa. Hylan B’s molecular weight is not displayed in package insert.
Outcome measures
The primary outcome measures were disease-specific costs associated with knee OA and time from the index date to TKR surgery. For calculation of disease-specific costs, disease-specific claims were denoted by those claims with an OA of the knee diagnosis code while disease-specific drugs included NSAIDs, Cox-2 inhibitors, non-narcotic analgesics, opioids, and other anti-inflammatory analgesics. All index medications administered in the postindex period were considered disease-specific whether they had an OA of the knee diagnosis or not.
Statistical analysis
Descriptive statistics were reported for continuous and categorical variables. T-tests were used for continuous variables and chi-square tests were used for categorical variables. All statistical tests were two-tailed tests. For statistical significance, we used the conventional alpha level of 0.05. A generalized linear model (GLM) with a gamma distribution and log-link function was fit to model aggregate patient-based changes in OA-related costs. Survival analysis was carried out with time-to-TKR data via the Kaplan–Meier method. A Cox PHM was used to model risk of TKR. Both GLM and PHM used a prespecified set of covariates such as age, gender, geographic region, health plan type, comorbidities, preindex corticosteroid use, and preindex healthcare costs to adjust for baseline differences among different HA cohorts. Statistical analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC).
Results
Patient characteristics
Following the application of the inclusion/exclusion criteria, a total of 50,389 patients were included for statistical analysis (Figure 1). Among the 50,389 patients who received HA for treatment of knee OA, 6,263 (12.4%) patients received Euflexxa; 5,391 (10.7%) patients, Orthovisc; 18,217 (36.2%) patients, Supartz/Hyalgan; and 20,518 (40.7%) patients, Synvisc. The four different HA cohort groups were similar in baseline characteristics (Table 2), with only relatively minor differences among them. The Euflexxa cohort had a greater proportion of patients insured through commercial health plans (61.2%) compared to the other cohorts (55.9–57.2%). In terms of physician specialty, the Orthovisc cohort had a greater proportion of patients who saw orthopedic surgeons (54.9%) compared to the other cohorts (46.3–49.2%). The four groups were similar in terms of comorbidities of interest at baseline, but for medications of interest, the Euflexxa cohort had a lower proportion of patients receiving corticosteroids (58.8%) compared to the other three cohorts (61.4–61.8%). With regards to healthcare costs, the Synvisc and Orthovisc cohorts had slightly greater preindex healthcare costs ($11,118 and $11,356) compared to the Euflexxa and Supartz/Hyalgan cohorts ($10,732 and $10,747).
Figure 1.
Selection of study patients.
Table 2.
Patient demographics and characteristics at baseline.
| Euflexxa only | Synvisc only | Supartz/Hyalgan | Orthovisc only | p value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Supartz/Hyalgan compared with Euflexxa | Supartz/Hyalgan compared with Synvisc | Supartz/Hyalgan compared with Orthovisc | |||||||||
| Total patients | 6,263 | 20,518 | 18,217 | 5,391 | |||||||
|
| |||||||||||
| Age (years) (n, %) | |||||||||||
| 18–44 | 658 | 10.5% | 1,725 | 8.4% | 1,645 | 9.0% | 569 | 10.6% | 0.0001* | 0.1353 | <0.0001* |
| 45–54 | 1,842 | 29.4% | 5,991 | 29.2% | 5,264 | 28.9% | 1,636 | 30.3% | |||
| 55–64 | 2,542 | 40.6% | 8,417 | 41.0% | 7,373 | 40.5% | 2,135 | 39.6% | |||
| 65+ | 1,221 | 19.5% | 4,385 | 21.4% | 3,935 | 21.6% | 1,051 | 19.5% | |||
| Mean | 57.0 | 57.7 | 57.7 | 56.8 | <0.0001* | 0.4798 | <0.0001* | ||||
| SD | 10.6 | 10.4 | 10.6 | 10.5 | |||||||
| Median | 57 | 57 | 57 | 57 | 0.0002* | 0.8306 | <0.0001* | ||||
| First quartile | 51 | 51 | 51 | 51 | |||||||
| Third quartile | 62 | 63 | 63 | 62 | |||||||
|
| |||||||||||
| Gender (n, %) | |||||||||||
| Male | 2,463 | 39.3% | 8,450 | 41.2% | 7,314 | 40.1% | 2,194 | 40.7% | 0.2512 | 0.0387* | 0.4710 |
| Female | 3,800 | 60.7% | 12,068 | 58.8% | 10,903 | 59.9% | 3,197 | 59.3% | |||
|
| |||||||||||
| Plan type (n, %) | |||||||||||
| Consumer-directed healthcare product | 17 | 0.3% | 47 | 0.2% | 52 | 0.3% | 7 | 0.1% | <0.0001* | <0.0001* | <0.0001* |
| HealthMaintenance Organization (HMO) | 1,169 | 18.7% | 2,950 | 14.4% | 2,509 | 13.8% | 543 | 10.1% | |||
| Indemnity plan | 473 | 7.6% | 1,443 | 7.0% | 1,374 | 7.5% | 338 | 6.3% | |||
| Point of service (POS) | 331 | 5.3% | 1,111 | 5.4% | 754 | 4.1% | 267 | 5.0% | |||
| Preferred Provider Organization (PPO) | 4,182 | 66.8% | 14,728 | 71.8% | 13,372 | 73.4% | 4,197 | 77.9% | |||
| Unknown | 91 | 1.5% | 239 | 1.2% | 156 | 0.9% | 39 | 0.7% | |||
|
| |||||||||||
| Payer type (n, %) | |||||||||||
| Commercial plan | 3,830 | 61.2% | 11,470 | 55.9% | 10,425 | 57.2% | 3,041 | 56.4% | <0.0001* | 0.0002* | <0.0001* |
| Medicaid | 23 | 0.4% | 81 | 0.4% | 37 | 0.2% | 6 | 0.1% | |||
| Medicare risk | 107 | 1.7% | 476 | 2.3% | 348 | 1.9% | 79 | 1.5% | |||
| Medicare cost | 107 | 1.7% | 435 | 2.1% | 383 | 2.1% | 44 | 0.8% | |||
| Self-insured | 2,135 | 34.1% | 7,943 | 38.7% | 6,934 | 38.1% | 2,200 | 40.8% | |||
| Unknown | 61 | 1.0% | 113 | 0.6% | 90 | 0.5% | 21 | 0.4% | |||
|
| |||||||||||
| Physician specialty (n, %) | |||||||||||
| Orthopedic surgery | 3,084 | 49.2% | 9,660 | 47.1% | 8,430 | 46.3% | 2,957 | 54.9% | <0.0001* | <0.0001* | <0.0001* |
| GP/FP/IM | 395 | 6.3% | 1,528 | 7.4% | 1,678 | 9.2% | 251 | 4.7% | |||
| Orthopedics | 382 | 6.1% | 1,339 | 6.5% | 1,089 | 6.0% | 173 | 3.2% | |||
| Physical medicine and rehabilitation | 180 | 2.9% | 611 | 3.0% | 653 | 3.6% | 76 | 1.4% | |||
| Rheumatology | 183 | 2.9% | 393 | 1.9% | 284 | 1.6% | 108 | 2.0% | |||
| Other | 2,039 | 32.6% | 6,987 | 34.1% | 6,083 | 33.4% | 1,826 | 33.9% | |||
|
| |||||||||||
| Charlson Comorbidity Score (n, %) | |||||||||||
| 0 | 3,854 | 61.5% | 12,502 | 60.9% | 11,104 | 61.0% | 3,301 | 61.2% | 0.5022 | 0.9211 | 0.8228 |
| 1 | 1,259 | 20.1% | 4,066 | 19.8% | 3,585 | 19.7% | 1,067 | 19.8% | |||
| 2 | 685 | 10.9% | 2,323 | 11.3% | 2,086 | 11.5% | 609 | 11.3% | |||
| 3 | 255 | 4.1% | 884 | 4.3% | 805 | 4.4% | 243 | 4.5% | |||
| 4+ | 210 | 3.4% | 743 | 3.6% | 637 | 3.5% | 171 | 3.2% | |||
|
| |||||||||||
| Medications of interest (n, %) | |||||||||||
| Corticosteroids | 3,685 | 58.8% | 12,591 | 61.4% | 11,267 | 61.8% | 3,318 | 61.5% | <0.0001* | 0.3291 | 0.6887 |
| NSAIDS | 2,386 | 38.1% | 7,864 | 38.3% | 6,920 | 38.0% | 2,221 | 41.2% | 0.8768 | 0.4907 | <0.0001* |
| Cox-2 inhibitors | 461 | 7.4% | 1,792 | 8.7% | 1,611 | 8.8% | 517 | 9.6% | 0.0003* | 0.7037 | 0.0926 |
| Analgesics non-narcotic | 76 | 1.2% | 348 | 1.7% | 333 | 1.8% | 102 | 1.9% | 0.0011* | 0.3242 | 0.7586 |
| Opioids | 2,933 | 46.8% | 10,131 | 49.4% | 9,010 | 49.5% | 2,727 | 50.6% | 0.0003* | 0.8702 | 0.1467 |
| Anti-inflammatory analgesics (non-NSAID) | 15 | 0.2% | 56 | 0.3% | 45 | 0.2% | 23 | 0.4% | 0.9173 | 0.6177 | 0.0306* |
| H2 blocker | 150 | 2.4% | 567 | 2.8% | 491 | 2.7% | 134 | 2.5% | 0.1992 | 0.6813 | 0.3996 |
| PPI | 1,304 | 20.8% | 4,280 | 20.9% | 3,872 | 21.3% | 1,108 | 20.6% | 0.4679 | 0.3410 | 0.2670 |
|
| |||||||||||
| Preindex healthcare costs (US $) | 10,732.4 | 11,117.9 | 10,747.3 | 11,356.2 | 0.9552 | 0.0438* | 0.0264* | ||||
Statistically significant at alpha level of 0.05.
Procedures of interest
The majority of patients in all four cohorts received a single course of HA treatment over the 3-year time period, ranging from 69.7% in the Synvisc cohort to 74.4% in the Supartz/Hyalgan cohort (Table 3). The number of injections patients received for the index course of HA treatment were 2.4 injections for the Synvisc cohort, 2.6 injections for the Euflexxa cohort, 2.8 injections for the Orthovisc cohort, and 3.7 injections for the Supartz/Hyalgan cohort. More than 50% of the patients in the Supartz/Hyalgan cohort received fewer than five injections for the index course, and 26.3% of the patients in the Supartz/Hyalgan cohort received 3 injections for the index course. The amount allowed for the index injections was greatest for the Synvisc cohort ($446) and smallest for the Supartz/Hyalgan cohort ($224). The paid amount of the index injections for the Synvisc cohort ($366) was nearly twice the paid amount of the index injections for the Supartz/Hyalgan cohort ($181).
Table 3.
Measures of procedural outcomes.
| Euflexxa only N=6,263 | Synvisc only N=20,518 | Supartz/Hyalgan N=18,217 | Orthovisc only N=5,391 | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N | % | N | % | N | % | N | % | |
| Injection courses | ||||||||
| 1 | 4,501 | 71.9% | 14,303 | 69.7% | 13,561 | 74.4% | 3,895 | 72.3% |
| 2 | 1,037 | 16.6% | 3,773 | 18.4% | 2,999 | 16.5% | 900 | 16.7% |
| 3 | 399 | 6.4% | 1,451 | 7.1% | 1,012 | 5.6% | 317 | 5.9% |
| 4 | 210 | 3.4% | 592 | 2.9% | 404 | 2.2% | 148 | 2.7% |
| 5 | 80 | 1.3% | 257 | 1.3% | 189 | 1.0% | 91 | 1.7% |
| 6+ | 36 | 0.6% | 142 | 0.7% | 52 | 0.3% | 40 | 0.7% |
|
| ||||||||
| Injections in index course | ||||||||
| 1 | 1,210 | 19.3% | 6,431 | 31.3% | 2,616 | 14.4% | 679 | 12.6% |
| 2 | 477 | 7.6% | 1,517 | 7.4% | 909 | 5.0% | 389 | 7.2% |
| 3 | 4,311 | 68.8% | 11,834 | 57.7% | 4,793 | 26.3% | 3,968 | 73.6% |
| 4 | 105 | 1.7% | 329 | 1.6% | 1,191 | 6.5% | 194 | 3.6% |
| 5+ | 160 | 2.6% | 407 | 2.0% | 8,708 | 47.8% | 161 | 3.0% |
|
| ||||||||
| Average number of injections in index course | 2.6 | 2.4 | 3.7 | 2.8 | ||||
|
| ||||||||
| Patients experiencing TKR (n, %) within 3-year postindex period | 1,553.0 | 24.8% | 5,484.0 | 26.7% | 4,566.0 | 25.1% | 1,355.0 | 25.1% |
|
| ||||||||
| Disease-specific cost (US $) | 13,160 | 14,959 | 13,947 | 14,224 | ||||
|
| ||||||||
| Charge amount of index injections (US $) | 346.2 | 575.7 | 296.8 | 512.1 | ||||
|
| ||||||||
| Allowed amount of index injections (US $) | 246.5 | 446.4 | 223.8 | 337.1 | ||||
|
| ||||||||
| Paid amount of index injections (US $) | 203.0 | 365.6 | 181.2 | 273.5 | ||||
Disease-specific healthcare costs
Comparison of the unadjusted disease-specific costs showed that the Euflexxa cohort incurred the lowest costs (mean=$13,160, median=$4,808) over the postindex period, whereas the Synvisc cohort incurred the highest costs (mean=$14,959, median=$6,388), the Supartz/Hyalgan cohort incurred mean cost of $13,947 (median=$5,720), and the Orthovisc cohort incurred mean cost of $14,224 (median=$6,188), respectively. Adjusting for confounders via a GLM showed that the Synvisc cohort (9.0%, p<0.0001) and the Orthovisc cohort (6.8%, p=0.0050) incurred greater patient-based changes in disease-specific costs than the Supartz/Hyalgan cohort with statistically significant difference (Table 4). After covariate adjustment, the patient-based change in disease-specific costs of the Euflexxa group was actually slightly greater than the Supartz/Hyalgan cohort but with no statistically significant difference (2.2%, p=0.3304). This implies that switching Synvisc and Orthovisc users with average disease-specific costs of $14,959 and $14,224, respectively to Supartz/Hyalgan could save $1,235 and $906 per patient over 3 years, but switching Euflexxa users to Supartz/Hyalgan would lead to little change in average disease-specific costs.
Table 4.
Generalized linear model: patient-based changes in disease-specific costs.
| Exponential of Wald 95% confidence limits | p value | |||||
|---|---|---|---|---|---|---|
| Parameters | Parameter estimate | Standard error | Exponential of parameter est. | Lower limit | Upper limit | |
| Euflexxa compared with Supartz/Hyalgan | 0.021 | 0.022 | 1.022 | 0.979 | 1.067 | 0.3304 |
| Synvisc compared with Supartz/Hyalgan | 0.086 | 0.015 | 1.090 | 1.058 | 1.123 | <0.0001* |
| Orthovisc compared with Supartz/Hyalgan | 0.065 | 0.023 | 1.068 | 1.020 | 1.117 | 0.0050* |
Statistically significant at alpha level of 0.05.
Time to TKR
The Synvisc cohort had a higher proportion of patients who received TKR (26.7%) than the other three cohorts (Table 3), which had similar rates of patients who received TKR (24.8–25.1%). The logistic regression model of incidence rates of TKR showed the Synvisc cohort had statistically significantly greater odds ratio (OR) of having a TKR than the Supartz/Hyalgan cohort (OR=1.077, p=0.0017), but there were no statistically significant differences in the ORs of having a TKR among the Euflexxa, Orthovisc, and Supartz/Hyalgan cohorts (Table 5).
Table 5.
Logistic regression model for odds of having a TKR.
| 95% confidence limits | ||||||
|---|---|---|---|---|---|---|
| Parameters | Parameter estimate | Standard error | Odds ratio | Lower limit | Upper limit | p value |
| Euflexxa compared with Supartz/Hyalgan | 0.000 | 0.035 | 1.000 | 0.935 | 1.071 | 0.9942 |
| Synvisc compared with Supartz/Hyalgan | 0.075 | 0.024 | 1.077 | 1.028 | 1.129 | 0.0017* |
| Orthovisc compared with Supartz/Hyalgan | 0.018 | 0.037 | 1.018 | 0.948 | 1.094 | 0.6199 |
Statistically significant at alpha level of 0.05.
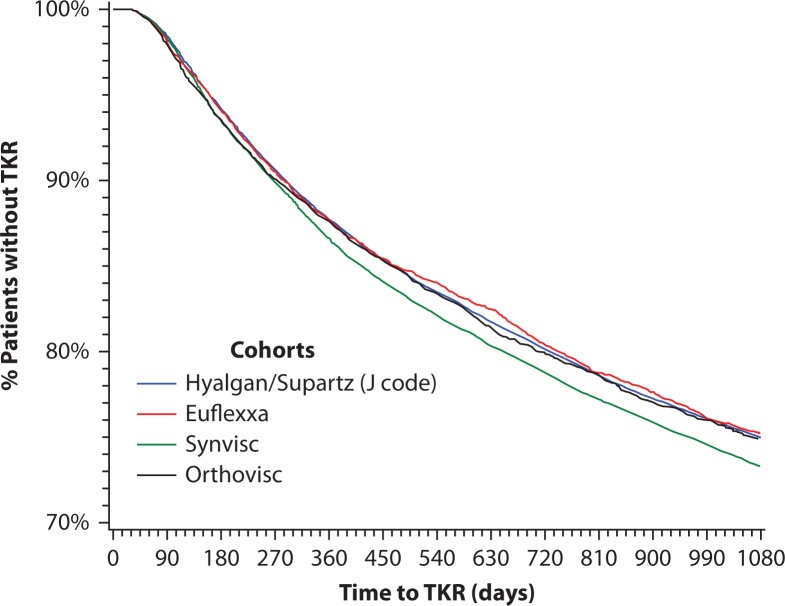
Kaplan–Meier survival curves representing proportions of subjects who had not experienced TKR across the 3-year time span were generated for the different HA cohorts (Figure 2). Survival curves for the Euflexxa, Orthovisc, and Supartz/Hyalgan cohorts were similar and close together. The results of a log-rank test of survival curves suggest that Synvisc patients tended to reach TKR earlier than patients receiving other HA injections; there was a statistically significant difference between survival times of the Synvisc cohort and the Supartz/Hyalgan cohort (p=0.0001) but no statistically significant differences among the survival times of the Euflexxa, Orthovisc, and Supartz/Hyalgan cohorts (Table 6). Likewise, the results of a Cox PHM for time-to-TKR (Table 7) adjusting for background covariates confirmed that the Synvisc cohort had statistically significantly greater hazard ratio (HR) of having a TKR than the Supartz/Hyalgan cohort (HR=1.069, p=0.0009), but there were no statistically significant differences in the HRs of having a TKR among the Euflexxa, Orthovisc, and Supartz/Hyalgan cohorts.
Figure 2.
Kaplan–Meier survival curves of time to TKR data for different HA cohorts.
The survival curve of the Synvisc cohort was statistically different from that of the Supartz/Hyalgan cohort (p=0.0001, log-rank test).
Table 6.
Log-rank tests of time to TKR among different HA cohorts.
| Log-rank test | ||
|---|---|---|
| Index therapy | Chi-square | p value |
| Euflexxa compared with Supartz/Hyalgan | 1.013 | 0.3142 |
| Synvisc compared with Supartz/Hyalgan | 14.914 | 0.0001* |
| Orthovisc compared with Supartz/Hyalgan | 2.274 | 0.1315 |
Statistically significant at alpha level of 0.05.
Table 7.
Cox proportional hazards model of the risk of having TKR.
| 95% CI of hazard ratio | ||||||
|---|---|---|---|---|---|---|
| Parameters | Coefficient | Standard error | Hazard ratio | Lower limit | Upper limit | p value |
| Euflexxa compared with Supartz/Hyalgan | 0.004 | 0.030 | 1.004 | 0.948 | 1.064 | 0.8806 |
| Synvisc compared with Supartz/Hyalgan | 0.067 | 0.020 | 1.069 | 1.027 | 1.112 | 0.0009* |
| Orthovisc compared with Supartz/Hyalgan | 0.021 | 0.031 | 1.021 | 0.961 | 1.085 | 0.5071 |
Statistically significant at alpha level of 0.05.
Discussion
Real-world evidence suggests that individual HA products have clinically detectable differences in clinical and cost outcomes. With regards to healthcare costs, in spite of the popular perception among payers that the HA products indicated for five injections per course are uneconomical, the Supartz/Hyalgan cohort demonstrated better cost outcomes than the Synvisc and Orthovisc cohorts and similar cost outcomes of the Euflexxa cohort. This confirms the finding from an earlier study which showed that though patients in the Supartz/Hyalgan received more injections per course than patients in the Synvisc cohort, the costs to the health plan were less for the Supartz/Hyalgan cohort than for the Synvisc cohort [29].
For the comparative effectiveness outcome of TKR delay, all HA cohorts were rather similar in their ability to delay TKR, except the Synvisc cohort which had a statistically significantly greater risk of having a TKR than the other cohorts. The statistical significance for the difference in time to TKR between the Synvisc cohort and the Supartz/Hyalgan cohort still persisted after adjustment of background covariates.This real-world finding sheds interesting light on another debate surrounding the use of IAHA regarding whether certain intrinsic properties of particular HA products such as molecular weights or production process can influence clinical outcomes [30]. Regarding molecular weight, one study suggested potential benefit of high molecular weight (HMW) HA through the CD44 receptor binding with greater affinity [31], but another study suggested better anti-inflammatory effects of HMW HA but better chondroprotective effects of low molecular weight (LMW) HA [32]. Another study concluded that low and high molecular weight HAs were similar in efficacy and safety [33]. The current study does not show any clear relationship between molecular weight of HA and its ability to delay TKR. Regarding production process, one study showed a HA product produced by biological fermentation (Bio-HA) has a significantly smaller incidence of injection site adverse reactions than a HA product derived from avian sources (AD-HA) [34]. However, in the current study, Supartz/Hyalgan, both of which are avian-derived hyaluronic acid (AD-HA) products, delayed TKR as effectively as other Bio-HA products, and delayed TKR longer than another AD-HA product (Synvisc). The current dataset and analyses do not provide a clear reason for this difference, but the unique chemical composition of Synvisc, which is an admixture of two distinctive chemically modified HAs (hylan A and hylan B) that are different from compositions of other HA products [35], may play a part. Our findings also suggest that one may need to go beyond simple categorizations of HAs by molecular weight or production sources to account for differences in real-world clinical outcomes among HA products.
By linking the clinical outcome of delay to a major surgery and the cost outcome, the current study shows that, among the HA products considered, Euflexxa, Orthovisc, and Supartz/Hyalgan may potentially offer more value than Synvisc for payers in the US healthcare system because those HA products are more effective in delaying TKR and less costly than Synvisc. Moreover, Euflexxa and Supartz/Hyalgan may be able to achieve better cost minimization than Orthovisc given similar clinical outcomes on time to TKR. This can be valuable information to healthcare payers that need to cope with the huge economic burden that the debilitating nature and high prevalence of OA imposes on the US healthcare system. In 2009, OA resulted in approximately 921,000 hospitalizations with a mean cost per stay of $45,443, and OA-related surgeries cost the US healthcare system $42.3 billion [36]. A recent meta-analysis showed evidence that US-approved HA viscosupplements are safe and efficacious through 26 weeks in patients with knee OA and had better efficacy than non–US approved HA viscosupplements [37]. The results of this study take one further step by yielding information on the clinical and cost differences amongst various HA products that may be of interest to payers who need to shape formulary and reimbursement policies in the presence of different intra-articular HA injection products in the US healthcare market.
Limitations
As the claims data for this study is representative of the US commercially insured population, results may not be generalizable to other non–commercially insured populations such as fee-for-service Medicare, who differ with regards to demographics such as age, resource utilization, and prescription usage patterns from the commercially insured patients in our study.
This retrospective study has limitations similar to other retrospective design studies that make use of claims data, such as coding errors or omissions. Because HA products were identified via J-codes, the products that share the same J-code (Supartz and Hyalgan, Synvisc, and Synvisc-One which is identical to Synvisc in chemical composition) were analyzed together as single groups. Because the index event was defined as the initiation of HA injection treatment, a non-HA cohort was not defined in this study due to the lack of a clearly identifiable index event equivalent to the initiation of HA treatment and its stage in knee OA treatment pathway. In addition, certain systemic factors that could affect care, such as utilization management policies or plan limits on medication use, are not available in this dataset.
Because knee OA progresses slowly and it may take a long time between OA diagnosis and TKR, a long follow-up period would be ideal. However, the follow-up period in this study had to be limited to 3 years post index date due to a lack of longer follow-up data at the time of study design. In addition, direct indicators of OA disease severity including Kellgren–Lawrence scores, complete medical records of past disease duration, or patient-reported outcomes such as pain and function questionnaire scores were not available in the claims database. In their place, other demographic or clinical characteristics at baseline available within the database were used in multivariate analyses to adjust for potential differences in disease state among patients in different cohorts.
Conclusion
Analysis of the retrospective cohort data in a US health plan claims database provides real-world evidence that some intra-articular HA products may offer more value in delaying TKR than other HA products. The current finding also suggests that explanation of real-world clinical outcomes for IAHA may require considerations that go beyond simple categorization of HA by molecular weight or production process. Additional research is needed to find out more about which aspects of intrinsic HA product properties and external factors may influence clinical outcomes associated with IAHA in the real-world clinical practice.
Acknowledgments
This research was sponsored by Seikagaku Corporation. We thank Ken Long of Bioventus LLC who helped with administration of this study.
Abbreviations:
- AD-HA
avian derived hyaluronic acid
- AAOS
American Academy of Orthopedic Surgeons
- ACR
American College of Rheumatology
- AMSSM
American Medical Society for Sport Medicine
- Bio-HA
biological fermentation hyaluronic acid
- BMI
body mass index
- GLM
generalized linear model
- HA
hyaluronic acid
- HMW
high molecular weight
- HR
hazard ratio
- IAHA
intra-articular hyaluronic acid
- LMW
low molecular weight
- OA
osteoarthritis
- OARSI
Osteoarthritis Research Society International
- OR
odds ratio
- PHM
proportional hazards model
- TKR
total knee replacement
Disclosure and potential conflicts of interest:
Funding for manuscript preparation and statistical analysis was provided by Seikagaku Corporation. VD is a consultant of Bioventus LLC; MD and KS are employees of IMS Health; AS is a former employee of Bioventus LLC; SL is an employee of Seikagaku Corporation and is listed as a patentee for a hyaluronic acid product. The International Committee of Medical Journal Editors’ (ICMJE) Potential Conflicts of Interests form for the author is available for download at: http://www.drugsincontext.com/wp-content/uploads/2016/06/dic.212296-COI.pdf.
Correct attribution:
Copyright © 2016 Dasa V, DeKoven M, Sun K, Scott A, Lim S. http://dx.doi.org/10.7573/dic.212296. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 3.0.
Article URL:
Provenance:
Submitted, externally peer reviewed
References
- 1.Bitton R. The economic burden of osteoarthrits. Am J Manag Care. 2009 Sep;15(8 Suppl):S230–5. [PubMed] [Google Scholar]
- 2.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, Fang F, Schwartz TA, Abbate LM, Callahan LF, Kalsbeek WD, Hochberg MC. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007 Jan;34(1):172–80. [PubMed] [Google Scholar]
- 3.Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005 Mar;17(2):195–200. doi: 10.1097/01.bor.0000151406.64393.00. [DOI] [PubMed] [Google Scholar]
- 4.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008 Sep;59(9):1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG. Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a US population-based survey. Arthritis Care Res. 2015 Aug; doi: 10.1002/acr.22721. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United States Bone and Joint Initiative: The Burden of Musculoskeletal Diseases in the United States (BMUS) Third Edition. Rosemont, IL: 2014. [cited 2016 May 31]. Available from: http://www.boneandjointburden.org. [Google Scholar]
- 7.Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi: 10.1186/s12891-015-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forsey R, Fisher J, Thompson J, Stone M, Bell C, Ingram E. The effect of hyaluronic acid and phospholipid based lubricants on friction within a human cartilage damage model. Biomaterials. 2006;27(26):4581–90. doi: 10.1016/j.biomaterials.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Mitsui Y, Gotoh M, Nakama K, Yamada T, Higuchi F, Nagata K. Hyaluronic acid inhibits mRNA expression of proinflammatory cytokines and cyclooxygenase-2/prostaglandin E2 production via CD44 in interleukin-1-stimulated subacromial synovial fibroblasts from patients with rotator cuff disease. J Orthop Res. 2008 July;26(7):1032–7. doi: 10.1002/jor.20558. [DOI] [PubMed] [Google Scholar]
- 10.Julovi SM, Ito H, Nishitani K, Jackson CJ, Nakamura T. Hyaluronan inhibits matrix metalloproteinase-13 in human arthritic chondrocytes via CD44 and P38. J Orthop Res. 2011 Feb;29(2):258–64. doi: 10.1002/jor.21216. [DOI] [PubMed] [Google Scholar]
- 11.Elmorsy S, Funakoshi T, Sasazawa F, Todoh M, Tadano S, Iwasaki N. Chondroprotective effects of high-molecular-weight cross-linked hyaluronic acid in a rabbit knee osteoarthritis model. Osteoarthritis Cartilage. 2014;22(1):121–7. doi: 10.1016/j.joca.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Yoshioka K, Yasuda Y, Kisukeda T, Nodera R, Tanaka Y, Miyamoto K. Pharmacological effects of novel cross-linked hyaluronate, Gel-200, in experimental animal models of osteoarthritis and human cell lines. Osteoarthritis Cartilage. 2014;22(6):879–87. doi: 10.1016/j.joca.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Reid MC. Viscosupplementation for osteoarthritis: a primer for primary care physicians. Adv Ther. 2013;30:967–86. doi: 10.1007/s12325-013-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strand V, Conaghan PG, Lohmander LS, Koutsoukos AD, Hurley FL, Bird H, Brooks P, Day R, Puhl W, Band PA. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006 Sep;14(9):859–66. doi: 10.1016/j.joca.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012 May;20(5):350–6. doi: 10.1016/j.joca.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Jevsevar DS, Brown GA, Jones DL, Matzkin EG, Manner PA, Mooar P, Schousboe JT, Stovitz S, Sanders JO, Bozic KJ, Goldberg MJ, Martin WR, 3rd, Cummins DS, Donnelly P, Woznica A, Gross L, American Academy of Orthopaedic Surgeons The American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition. J Bone Joint Surg Am. 2013 Oct;95(20):1885–6. doi: 10.2106/00004623-201310160-00010. [DOI] [PubMed] [Google Scholar]
- 17.Jevsevar D, Donnelly P, Brown GA, Cummins DS. Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. J Bone Joint Surg Am. 2015 Dec;97(24):2047–60. doi: 10.2106/JBJS.N.00743. [DOI] [PubMed] [Google Scholar]
- 18.Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015 Jan;162(1):46–54. doi: 10.7326/M14-1231. [DOI] [PubMed] [Google Scholar]
- 19.Mandl LA, Losina E. Relative efficacy of knee OA treatments: are all placebos born equal? Ann Intern Med. 2015 Jan;162(1):71–72. doi: 10.7326/M14-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P, American College of Rheumatology American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64:465–74. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 21. American College of Rheumatology Position Statement [cited 2016 May 31]. Available from: http://www.rheumatology.org/Practice-Quality/Administrative-Support/Position-Statements.
- 22.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H, Kwoh K, Lohmander S, Rannou F, Roos EM, Underwood M. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartil. 2014;22:363–88. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Trojian TH, Concoff AL, Joy SM, Hatzenbuehler JR, Saulsberry WJ, Coleman CI. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Br J Sports Med. 2016;50:84–92. doi: 10.1136/bjsports-2015-095683. [DOI] [PubMed] [Google Scholar]
- 24.Waddell DD, Joseph B. Delayed total knee replacement with hylan G-F 20. J Knee Surg. 2016 Feb;29(2):159–68. doi: 10.1055/s-0034-1395281. [DOI] [PubMed] [Google Scholar]
- 25.Dasa V, Lim S, Heeckt P. Real-world evidence for safety and effectiveness of repeated courses of hyaluronic acid injections on the time to knee replacement surgery. Am J Orthopedics. 2016. Under submission. [DOI] [PubMed]
- 26.Altman R, Lim S, Steen RG, Dasa V. Hyaluronic acid injections are associated with delay of total knee replacement surgery in patients with knee osteoarthritis: evidence from a large U.S. health claims database. PLoS One. 2015 Dec;10(12):e0145776. doi: 10.1371/journal.pone.0145776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altman R, Fredericson M, Bhattacharyya SK, Bisson B, Abbott T, Yadalam S, Kim M. Association between hyaluronic acid injections and time-to-total knee replacement surgery. J Knee Surg. 2015 Dec; doi: 10.1055/s-0035-1568992. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Hatoum HT, Fierlinger AL, Lin SJ, Altman RD. Cost-effectiveness analysis of intra-articular injections of a high molecular weight bioengineered hyaluronic acid for the treatment of osteoarthritis knee pain. J Med Econ. 2014 May;17(5):326–37. doi: 10.3111/13696998.2014.902843. [DOI] [PubMed] [Google Scholar]
- 29.Yeaw J, DeKoven M, Mozaffari E, Tierce J. Patterns of use, outcomes, and costs of selected viscosupplementation agents in health plan administrative data. J Manag Care Pharm. 2008;14(2):239. [Google Scholar]
- 30.Rosen J, Avram V, Fierlinger A, Niazi F, Sancheti P, Bedi A. Clinicians’ perspectives on the use of intra-articular hyaluronic acid as a treatment for knee osteoarthritis: a North American, multidisciplinary survey. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:21–27. doi: 10.4137/CMAMD.S34496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizrahy S, Raz SR, Hasgaard M, Liu H, Soffer-Tsur N, Cohen K, Dvash R, Landsman-Milo D, Bremer MG, Moghimi SM, Peer D. Hyaluronan-coated nanoparticles: the influence of the molecular weight on CD44-hyaluronan interactions and on the immune response. J Control Release. 2011;156(2):231–8. doi: 10.1016/j.jconrel.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 32.Huang TL, Hsu HC, Yang KC, Yao CH, Lin FH. Effect of different molecular weight hyaluronans on osteoarthritis-related protein production in fibroblast-like synoviocytes from patients with tibia plateau fracture. J Trauma. 2010;68(1):146–52. doi: 10.1097/TA.0b013e3181a92cf8. [DOI] [PubMed] [Google Scholar]
- 33.Lee PB, Kim YC, Lim YJ, Lee CJ, Sim WS, Ha CW, Bin SI, Lim KB, Choi SS, Lee SC. Comparison between high and low molecular weight hyaluronates in knee osteoarthritis patients: open-label, randomized, multicentre clinical trial. J Int Med Res. 2006;34(1):77–87. doi: 10.1177/147323000603400110. [DOI] [PubMed] [Google Scholar]
- 34.Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthr Cartil. 2006;14(2):154–62. doi: 10.1016/j.joca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki M, Miyazaki T, Nakamura T. Immunogenicity of hylan G-F 20 in guinea pigs and mice. J Rheumatol. 2004 May;31(5):943–50. [PubMed] [Google Scholar]
- 36.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective: a population-based review of the fourth most common cause of hospitalization in U.S. adults. Orthop Nurs. 2012 March–April;31(2):85–91. doi: 10.1097/NOR.0b013e31824fcd42. [DOI] [PubMed] [Google Scholar]
- 37.Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015 May;8:217–28. doi: 10.2147/JPR.S83076. [DOI] [PMC free article] [PubMed] [Google Scholar]