Nguyen and Ehrenstein reveal that anti-TNF antibodies paradoxically enhance membrane TNF–TNF-RII interactions to increase Foxp3 expression and confer upon T reg cells the ability to suppress Th17 cells in rheumatoid arthritis patients.
Abstract
The interplay between inflammatory and regulatory pathways orchestrates an effective immune response that provides protection from pathogens while limiting injury to host tissue. Tumor necrosis factor (TNF) is a pivotal inflammatory cytokine, but there is conflicting evidence as to whether it boosts or inhibits regulatory T cells (T reg cells). In this study, we show that the therapeutic anti-TNF antibody adalimumab, but not the soluble TNF receptor etanercept, paradoxically promoted the interaction between monocytes and T reg cells isolated from patients with rheumatoid arthritis (RA). Adalimumab bound to monocyte membrane TNF from RA patients and unexpectedly enhanced its expression and its binding to TNF-RII expressed on T reg cells. As a consequence, adalimumab expanded functional Foxp3+ T reg cells equipped to suppress Th17 cells through an IL-2/STAT5-dependent mechanism. Our data not only highlight the beneficial effect of membrane TNF on T reg cell numbers during chronic inflammation, but in addition reveal how a therapeutic antibody that is thought to act by simply blocking its target can enhance the regulatory properties of this proinflammatory cytokine.
Effective resolution of inflammation is orchestrated through a complex array of mediators and cellular mechanisms. Increasing evidence indicates that the seeds of this resolution phase exist even at the height of inflammation. Regulatory T cells (T reg cells) are potent suppressors of immune responses and are considered pivotal in resolving inflammation and autoimmunity (Miyara et al., 2011). T reg cells occur in increased numbers in a wide variety of inflammatory diseases such as the synovium of patients with rheumatoid arthritis (RA; Cao et al., 2004; van Amelsfort et al., 2004), although one group found no difference in the frequency of T reg cells between the inflamed synovial fluid and peripheral blood (Nie et al., 2013). There is substantial controversy as to whether these T reg cells are fully suppressive, and the precise mechanisms that modulate T reg cell number and function during inflammation remain unclear. We and others have shown that T reg cells from RA patients are defective in their ability to suppress proinflammatory cytokines (Ehrenstein et al., 2004; Valencia et al., 2006; Flores-Borja et al., 2008; Zanin-Zhorov et al., 2010; Cribbs et al., 2014).
To understand the interrelationship between inflammation and T reg cell number and function, significant attention has been paid to the actions of TNF, which is known to play a pivotal role in several inflammatory disorders including RA. However, recent evidence studying this cytokine’s impact on T reg cells has led to contradictory and controversial results. Although some investigators have shown that TNF can impair T reg cell function (Valencia et al., 2006; Nagar et al., 2010; Nie et al., 2013), others have found that TNF enhances their capacity to suppress via its interaction with TNF-RII expressed by T reg cells (Grinberg-Bleyer et al., 2010; Kleijwegt et al., 2010; Chen et al., 2013; Chopra et al., 2013; Zaragoza et al., 2016).
Anti-TNF therapy has revolutionized the therapy of a variety of inflammatory diseases including RA. We have previously shown that adalimumab, an anti-TNF antibody, but not etanercept, a soluble TNF receptor, increased T reg cell numbers in patients with RA and that these T reg cells were capable of suppressing the highly inflammatory cytokine IL-17 (McGovern et al., 2012). Our data implied that TNF compromised the potency of T reg cell suppression in RA, which was reversed by therapeutic TNF blockade. However, it was unclear why etanercept, which is as equally effective as adalimumab in the treatment of RA, lacked T reg cell modulatory properties. Here, we reveal that adalimumab, but not etanercept, binds to membrane TNF expressed by RA monocytes and promotes T reg cell expansion through enhanced TNF-RII–mediated IL-2/STAT5 signaling.
RESULTS
Adalimumab increased functionally suppressive T reg cells in PBMCs from RA patients but not healthy controls
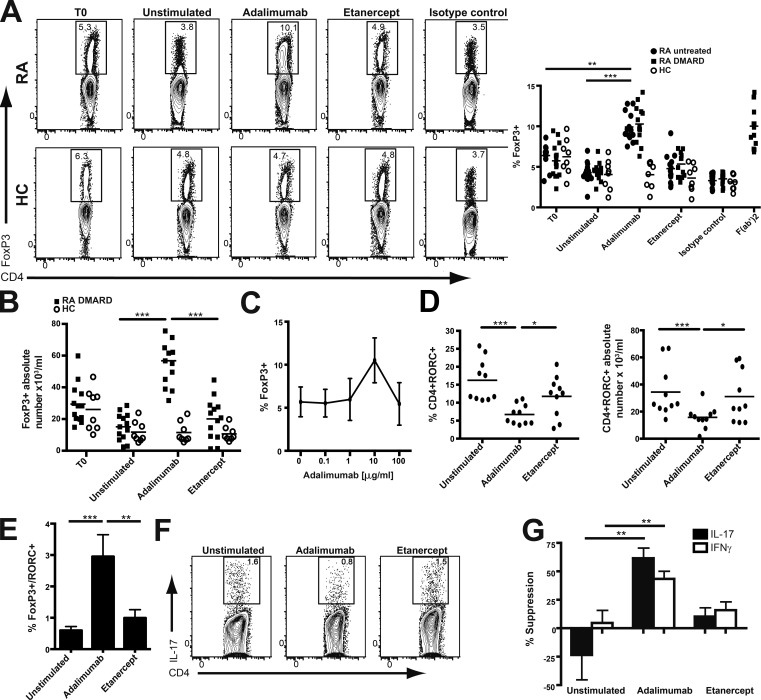
We have previously shown that RA patients receiving adalimumab but not etanercept therapy have increased peripheral CD4+ T reg cells (McGovern et al., 2012). To elucidate the underlying mechanisms and explain the differing effects of these two anti-TNF agents, we established an in vitro model avoiding the use of anti-CD3 that can artificially modulate Foxp3 expression (Tran et al., 2007; Sakaguchi et al., 2010). PBMCs from RA patients or healthy controls were cultured for 3 d with either adalimumab or etanercept. Adalimumab (or its Fab′2 fragment) but not etanercept (or an isotype control) increased the percentage and the absolute number of CD4+Foxp3+ T reg cells in PBMCs from RA patients (Fig. 1, A and B). Of note, adalimumab had the same effect on T reg cell enrichment in PBMCs from RA patients treated with disease-modifying antirheumatic drugs compared with PBMCs from untreated patients. In contrast, the number of T reg cells in PBMCs from healthy individuals was unaffected by adalimumab or etanercept (Fig. 1, A and B). Without the addition of adalimumab, the number of T reg cells was reduced over the course of the culture. We noted that the effect of adalimumab was dose dependent, with the optimal concentration at 10 µg/ml (Fig. 1 C) corresponding to serum concentrations found in patients responding to adalimumab (Bartelds et al., 2007). Lower concentrations in the range of 1 µg/ml were associated with clinical nonresponsiveness (Bartelds et al., 2007) and did not increase T reg cells (Fig. 1 C), whereas higher concentrations abrogated this effect of adalimumab.
Figure 1.
Adalimumab increased the number of functionally suppressive T reg cells in PBMCs from RA patients but not healthy controls. (A, B, D, and E) 2 × 105 PBMCs from untreated or disease-modifying antirheumatic drug (DMARD)–treated RA patients and healthy controls (HC) were cultured with 10 µg/ml adalimumab, adalimumab F(ab’)2 (n = 11), etanercept, or isotype control for 3 d. (A and B) Representative flow cytometry and cumulative data indicating the percentage and absolute number of CD4+FoxP3+ from RA patients divided into untreated (n = 13), on disease-modifying antirheumatic drug (n = 13), and healthy controls (n = 8) at time zero (T0) and at day 3 of the culture. (C) Adalimumab dose–response curve from RA PBMCs exposed to increasing concentrations of adalimumab for 3 d. Cumulative data depict percentage of CD4+FoxP3+ T reg cells (n = 7). (D) Percentage and absolute number of CD4+RORC+ cells in the cultures at day 3 (n = 10). (E) The ratio of CD4+FoxP3+/CD4+RORC+ in the PBMC cultures from RA patients at day 3 (n = 10). (F) After exposure to either anti-TNF agent for 3 d, CD4+ T cells were stained for IL-17 cytokine production. Representative flow cytometry shows IL-17 production from CD4+ in RA (n = 18). (G) Classical T reg cell suppression assay: adalimumab- or etanercept-treated RA T reg cells were purified (CD4+CD25+CD127−) and co-cultured for 5 d with monocytes and untreated responder T cells (CD4+CD25−CD127+) at a 1:3 ratio in the presence of 1 µg/ml anti-CD3/CD28. Cumulative data indicate percentage of suppression of IL-17 (n = 6) and IFN-γ (n = 6) production. Data in A and B were obtained from five experimental repeats. C was obtained from two experimental repeats. D, E, and G were obtained from three experimental repeats. F is representative of six independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 using Kruskal-Wallis with Dunn’s post-hoc analysis. Error bars represent mean ± SE.
We have demonstrated that adalimumab therapy led to the generation of functional T reg cells equipped to suppress Th17 cells in patients treated with this anti-TNF therapy in vivo (McGovern et al., 2012). Therefore, we examined RORC expression in CD4+ T cells from RA PBMCs exposed to adalimumab. The number of CD4+RORC+ T cells was significantly reduced in RA PBMCs after culture with adalimumab (Fig. 1 D), matching our in vivo observations (McGovern et al., 2012). This decrease in the CD4+RORC+ subset together with the rise in T reg cell number led to a fivefold increase in the ratio of T reg cells to CD4+RORC+ T cells (Fig. 1 E). The reduction in CD4+RORC+ T cells driven by adalimumab was reflected by a decrease in Th17 cells (Fig. 1 F). To determine whether these T reg cells generated by adalimumab in vitro were functionally suppressive, we established classical suppression assays using untreated responder T cells and monocytes together with T reg cells (CD4+CD25+CD127−) isolated from the cultures described in the previous paragraph. T reg cells purified from RA PBMCs exposed to adalimumab, but not etanercept, significantly suppressed IL-17 and IFN-γ production (Fig. 1 G). Consistent with previous findings (Ehrenstein et al., 2004; Valencia et al., 2006; Flores-Borja et al., 2008; Zanin-Zhorov et al., 2010; Cribbs et al., 2014), unmanipulated RA T reg cells were defective with respect to regulating IFN-γ production (Fig. 1 G).
T reg cells and monocytes were required for the adalimumab-induced T reg cell expansion
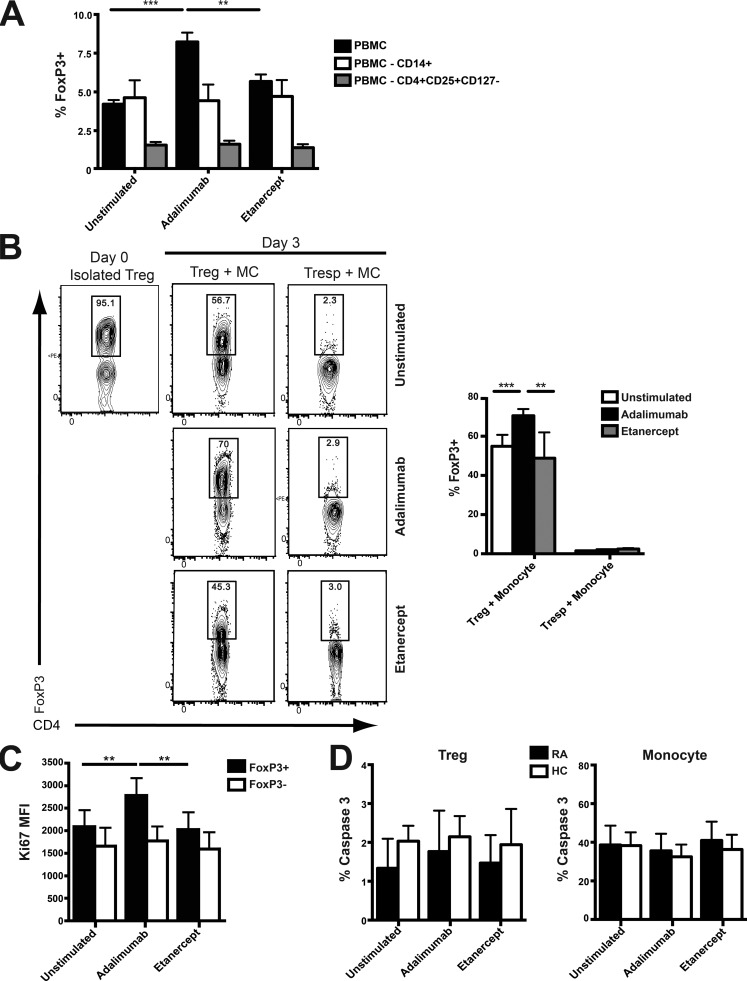
To examine which cell subsets were targeted by adalimumab to mediate T reg cell expansion, we first depleted T reg cells or monocytes from PBMCs. Removal of either monocytes or T reg cells from PBMCs from RA patients abolished the increase in T reg cells upon addition of adalimumab (Fig. 2 A). When responder T cells or T reg cells from RA patients were stimulated with adalimumab in the presence of monocytes, only the latter responded to adalimumab with respect to increased T reg cells compared with unstimulated cultures at day 3 (Fig. 2 B). In keeping with the data in Fig. 1, etanercept had no effect on Foxp3 expression (Fig. 2 B). There was some reduction in Foxp3 expression over the course of the culture, though this was least when adalimumab was present. Adalimumab but not etanercept significantly promoted the proliferation of T reg cells but had no effect on CD4+Foxp3− responder T cells (Fig. 2 C). There was no difference in T reg or monocyte cell death between the different treatment groups (Fig. 2 D).
Figure 2.
T reg cells and monocytes were required for the adalimumab-driven T reg cell expansion. (A) PBMCs from RA patients were sorted into three populations: whole PBMCs, PBMCs depleted of CD14+ monocytes, and PBMCs depleted of CD4+CD25+CD127− T reg cells and stimulated with adalimumab, etanercept, or an isotype control for 3 d. The percentage of CD4+Foxp3+ T cells is shown (n = 4). (B) T reg cells or responder T cells (Tresp) and CD14+ monocytes (MC) from RA patients were isolated by FACS before being co-cultured in the presence of adalimumab or etanercept for 3 d. The cumulative data and representative flow cytometry plot indicate the percentage of CD4+FoxP3+ on day 0 and in each set of co-cultures on day 3 (n = 4). (C) Ki67 staining gated on CD4+FoxP3+ and CD4+FoxP3− in PBMCs from RA patients (n = 8) exposed for 3 d to either adalimumab or etanercept. MFI, mean fluorescence intensity. (D) The cumulative data of active Caspase 3 expression on CD4+FoxP3+ T reg cells or monocytes from RA patients (n = 14) and healthy controls (HC; n = 8) after culturing PBMCs for 3 d with either adalimumab or etanercept. The data in A and B were obtained from two experimental repeats, and data in C and D were obtained from four experimental repeats. **, P < 0.01; ***, P < 0.001 using Kruskal-Wallis tests with Dunn’s post-hoc analysis. Error bars represent mean ± SE.
Adalimumab bound to membrane TNF expressed by RA monocytes
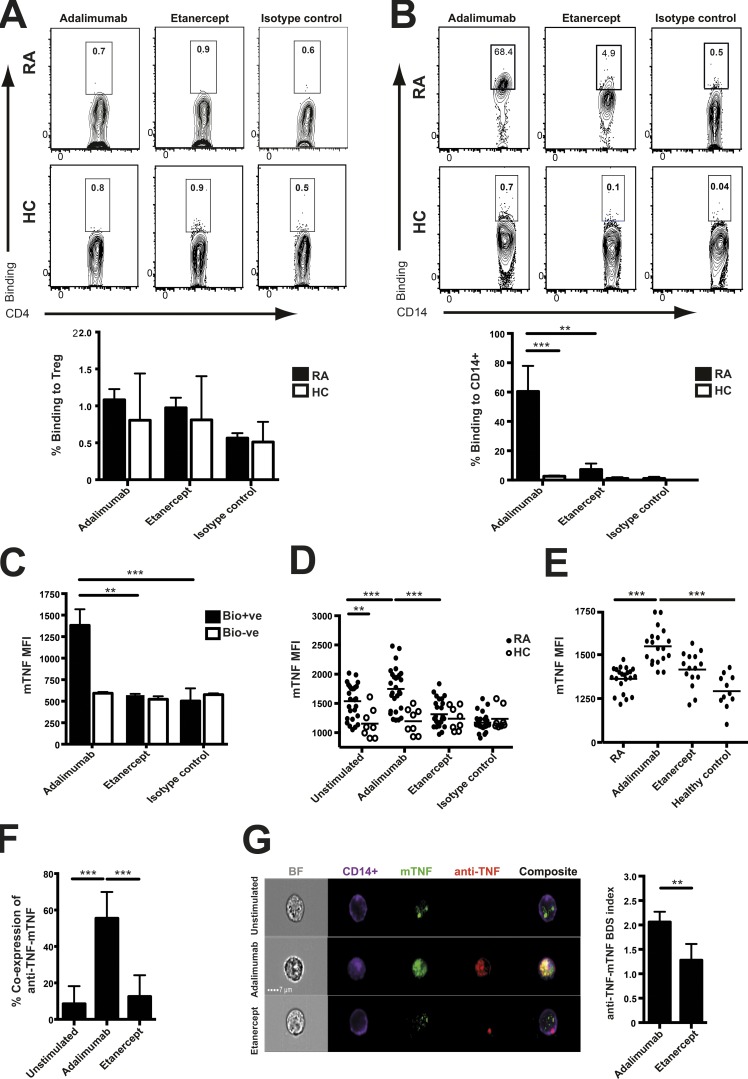
These results led us to explore the molecular basis for the interaction between monocytes and T reg cells. Having identified the two cell types required for adalimumab to expand T reg cells, we examined the binding of adalimumab to purified T reg cells and monocytes. Neither biotinylated adalimumab nor etanercept bound to T reg cells from RA patients or healthy controls (Fig. 3 A). In contrast, adalimumab but not etanercept bound to monocytes from RA patients but not healthy controls (Fig. 3 B). Unexpectedly, we noted that the monocytes that had bound the biotinylated adalimumab increased their membrane TNF expression (Fig. 3 C). Repeating this experiment with adalimumab that had not been biotinylated, we confirmed that membrane TNF expression was elevated after addition of adalimumab to purified RA monocytes. Adalimumab did not have any effect on monocyte TNF expression in healthy controls (Fig. 3 D). These findings paralleled in vivo observations from patients responding to adalimumab therapy, where monocytes from adalimumab responders had increased TNF expression compared with RA patients treated with methotrexate or etanercept (Fig. 3 E). Quantitative ImageStream technology confirmed the coexpression of membrane TNF and adalimumab (Fig. 3 F). Moreover, there was significant colocalization between adalimumab and membrane TNF on RA monocytes as assessed by the bright detailed similarity (BDS) index (Fig. 3 G).
Figure 3.
Adalimumab bound to membrane TNF on RA monocytes. (A and B) Adalimumab and etanercept were biotinylated and added to purified CD4+CD25+CD127− T reg cells (A) or purified CD14+ monocytes (B) from RA patients and healthy controls (HC) for 30 min. Bound adalimumab and etanercept were detected using an APC-streptavidin secondary antibody. Representative plots and cumulative data depict percentage of binding of each anti-TNF agent to T reg cells from RA patients (n = 12) and healthy individuals (n = 10; A) and to monocytes (number of RA = 8; number of healthy controls = 10; B). (C) The bars depict membrane TNF (mTNF) mean fluorescence intensity (MFI) on RA monocytes either bound (Bio+ve) or unbound (Bio−ve) by biotinylated adalimumab for 30 min (n = 11). (D) Membrane TNF expression on purified CD14+ monocytes from RA patients (n = 28) and healthy controls (n = 8) exposed for 24 h to adalimumab, etanercept, or isotype control. (E) Ex vivo expression of monocyte membrane TNF from RA patients with active disease (n = 23), adalimumab responders (n = 19), etanercept responders (n = 15), and healthy individuals (n = 11). (F) Purified monocytes were stained for CD14+ and membrane TNF after co-culture with biotinylated adalimumab or etanercept for 30 min. ImageStream cumulative data indicate coexpression of membrane TNF and either adalimumab or etanercept on monocytes. Unstimulated are shown as background (n = 5). (G) The ImageStream gallery includes brightfield (BF), CD14, membrane TNF, biotinylated adalimumab, and etanercept (anti-TNF), composite merged images. The bars depict the BDS index (colocalization) of adalimumab and etanercept with membrane TNF occurring at the surface of CD14+ monocytes (n = 5). Data in A–C were obtained from three experimental repeats. Data in D and E were from six and F and G were from two experimental repeats. **, P < 0.01; ***, P < 0.001 using Kruskal-Wallis (A–D, F, and G) or Friedman tests (E) with Dunn’s post-hoc analysis. Error bars represent mean ± SE.
Adalimumab promoted binding between monocyte membrane TNF and TNF-RII expressed by T reg cells
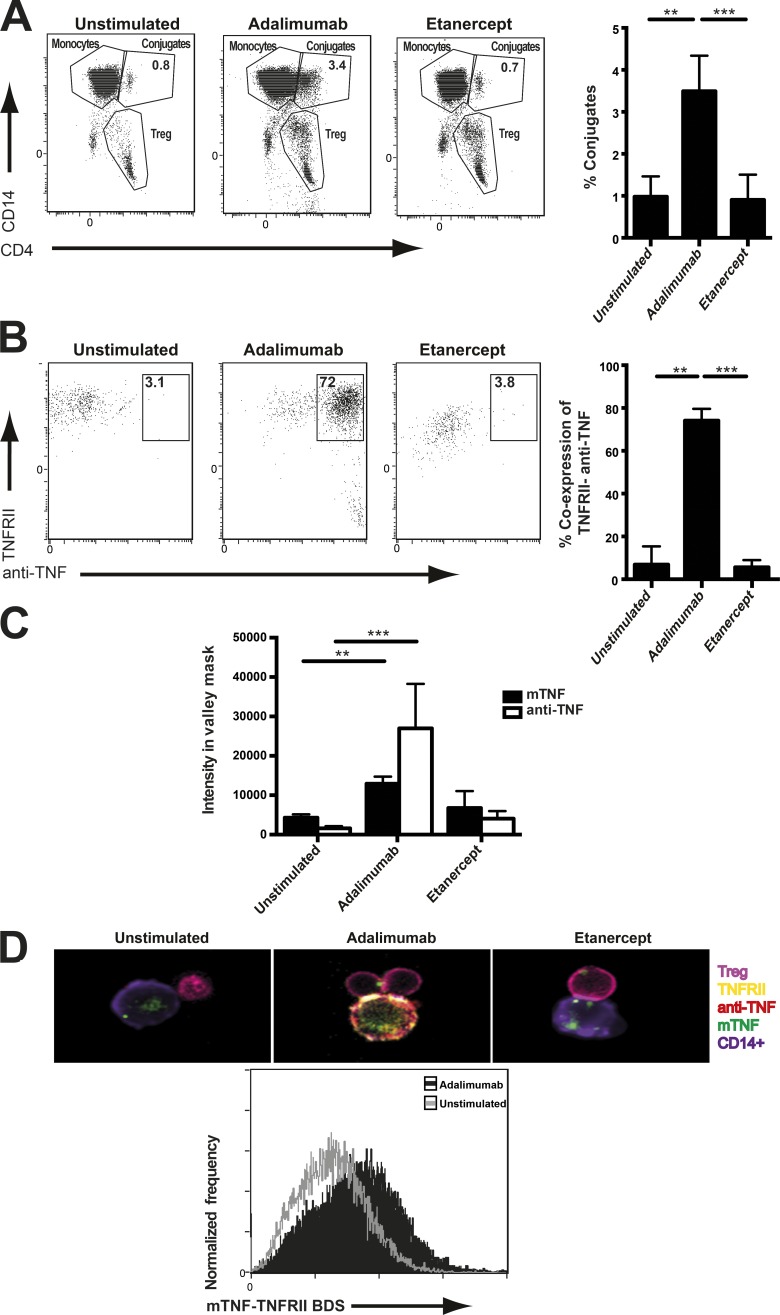
We next investigated whether adalimumab expands T reg cells through the interaction between monocyte membrane TNF and T reg cell TNF-RII. ImageStream technology has the potential to both image and quantify many thousands of interactions between monocytes and T reg cells. Monocytes and T reg cells were stained for TNF-RII and membrane TNF after culture with biotinylated adalimumab or etanercept. Adalimumab but not etanercept promoted T reg cell–monocyte conjugate formation after a 30-min incubation (Fig. 4 A). The majority of these conjugates coexpressed adalimumab and TNF-RII (Fig. 4 B). Both membrane TNF and adalimumab were present in the synapse between monocytes and T reg cells as defined by the valley mask (Fig. 4 C; Ahmed et al., 2009). Colocalization of membrane TNF and TNF-RII in the T reg cell–monocyte conjugates was demonstrated by analysis of the BDS index using ImageStream (Fig. 4 D).
Figure 4.
Adalimumab promoted the interaction between monocyte membrane TNF and TNF-RII expressed by T reg cells. (A) Purified T reg cells and monocytes from RA patients were treated with biotinylated adalimumab or etanercept for 30 min. Representative imaging flow cytometry (ImageStream) and cumulative data of T reg cell (Treg)–monocyte conjugate formation in the presence of either anti-TNF agent. (B) Representative ImageStream plots and cumulative data reveal the percentage of conjugates coexpressing either anti-TNF agent or TNF-RII. (C) Cumulative data depicting membrane TNF (mTNF) and either adalimumab or etanercept intensity in the valley mask of the conjugate synapse. (A–C) n = 5. Data were obtained from three experimental repeats. **, P < 0.01; ***, P < 0.001 using Kruskal-Wallis tests with Dunn’s post-hoc analysis. Error bars represent mean ± SE. (D) Representative merged images of CD4+ T reg cell, CD14+ monocyte, membrane TNF, TNF-RII, and anti-TNF. The BDS index (colocalization) of membrane TNF and TNF-RII at the conjugate interface in monocyte–T reg cell cultures treated with or without adalimumab is shown. Data are representative of three experimental repeats.
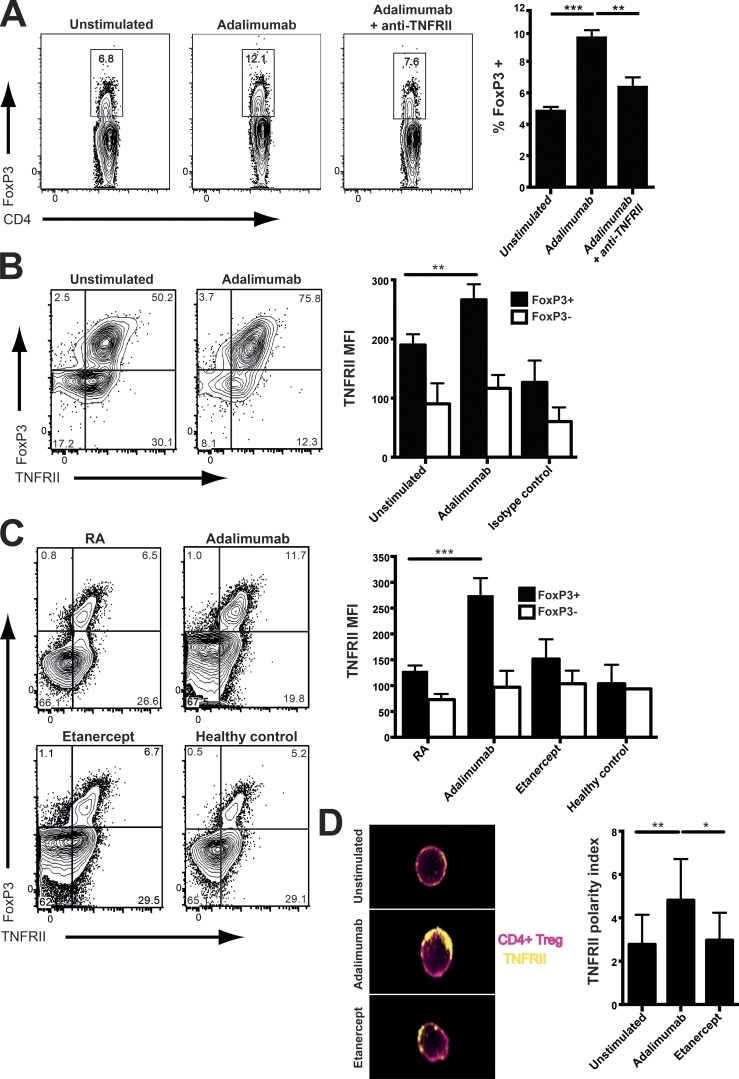
TNF-RII stimulation triggered expansion of the T reg cell subset
Blockade of TNF-RII abolished the increase in T reg cells driven by adalimumab, confirming the pivotal role of the TNF–TNF-RII interaction between monocytes and T reg cells (Fig. 5 A). TNF-RII expression on RA T reg cells was enhanced in the presence of adalimumab in vitro (Fig. 5 B). This effect was mirrored in vivo in RA patients responding to adalimumab (Fig. 5 C). No increase in TNF-RII expression occurred in responder T cells from patients treated with and responding to adalimumab (Fig. 5 C). An increase in the polarization of TNF-RII distribution was detected on the T reg cell surface in the presence of adalimumab (Fig. 5 D).
Figure 5.
Adalimumab-enhanced TNF-RII expression and polarization on the surface of T reg cells. (A) 10 µg/ml anti–TNF-RII antagonist antibody was added to PBMCs from RA patients treated with adalimumab for 3 d. Representative flow cytometry and cumulative data indicate percentage of CD4+FoxP3+ T reg cells (n = 10). (B) Purified CD4+CD25+CD127− T reg cells from RA patients were cultured with purified monocytes and treated with either adalimumab or isotype control for 24 h. The representative flow cytometry plots and cumulative data show expression of TNF-RII on FoxP3+ T reg cells and those purified T reg cells that had low Foxp3 expression (n = 21). MFI, mean fluorescence intensity. (C) Ex vivo TNF-RII expression on CD4+FoxP3+ T reg cells and CD4+FoxP3− responder T cells from RA patients with active disease (n = 29), patients responding to adalimumab (n = 27) or etanercept therapy (n = 13), and healthy individuals (n = 8). (D) Adalimumab or etanercept was added to purified RA T reg cells cultured for 24 h in the presence of monocytes. Samples were analyzed using ImageStream after staining for CD4+ T reg cells (red) and TNF-RII (yellow). The representative composite image shows polarized TNF-RII distribution on the surface of T reg cells. The cumulative data depict the polarity index of TNF-RII on T reg cells (n = 6). Data in A was obtained from three experimental repeats, B and C were obtained from six, and D was obtained from two experimental repeats. *, P < 0.05; **, P < 0.01; ***, P < 0.001 using Kruskal-Wallis (A, B, and D) or Friedman tests (C) with Dunn’s post-hoc analysis. Error bars represent mean ± SE.
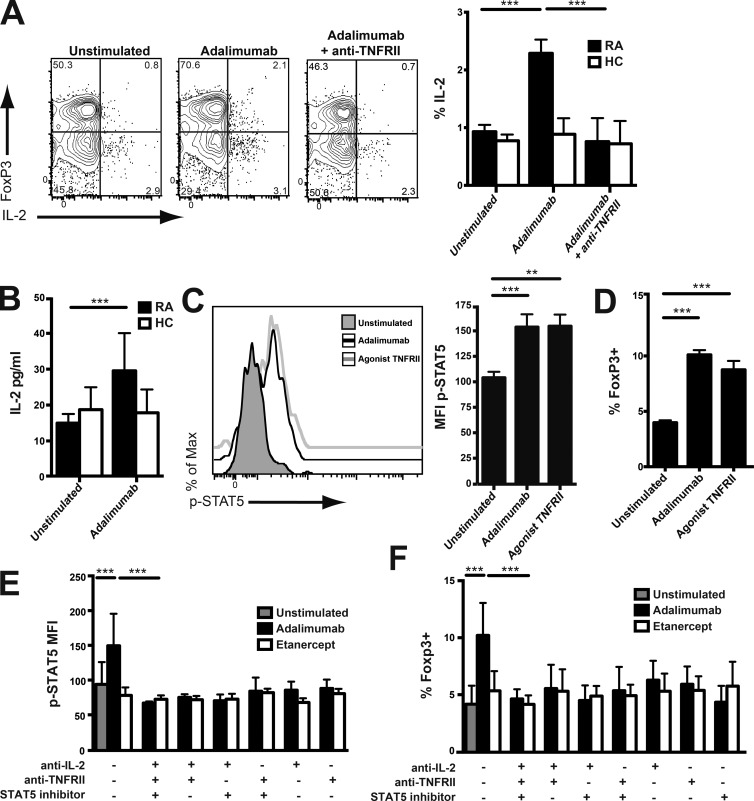
IL-2/STAT5 signaling was required for adalimumab-driven T reg cell expansion
TNF-RII promotes the production of IL-2 (McKarns and Schwartz, 2008; Miller et al., 2015), which has a pivotal role in the generation, homeostasis, and in vivo proliferation of T reg cells (Cheng et al., 2011). Moreover, T reg cells from patients with autoimmune disease can produce increased amounts of IL-2 compared with healthy T reg cells (Carbone et al., 2014). Therefore, we examined IL-2 secretion by purified T reg cells from RA patients cultured in the presence of monocytes. Adalimumab increased the production of IL-2 by purified T reg cells from RA patients but not healthy controls (Fig. 6, A and B). Moreover, TNF-RII blockade abrogated the enhanced IL-2 production triggered by adalimumab (Fig. 6 A). IL-2 production was increased in those purified T reg cells that maintained high expression of Foxp3, though low Foxp3 cells also produced this cytokine.
Figure 6.
Adalimumab T reg cell expansion was driven by IL-2/STAT5 signaling. (A) Purified CD4+CD25+CD127− T reg cells from patients with RA or healthy controls (HC) were co-cultured for 72 h with their autologous CD14+ monocytes and adalimumab with or without an anti–TNF-RII antagonist. Representative FACS plots (RA only) and cumulative data depicting CD4+FoxP3+ T reg cell IL-2 production in RA (n = 11) and healthy individuals (n = 5) are shown. (B) IL-2 production assayed by ELISA from purified T reg cells and monocytes from RA patients after 3-d exposure to adalimumab (n = 22) and healthy controls (n = 13). (C and D) RA PBMCs were exposed for 3 d either to adalimumab or an anti–TNF-RII agonist. Representative histograms and bars show phosphor-STAT5 (p-STAT5) expression on CD4+FoxP3+ T reg cells (n = 9; C) and percentage of CD4+FoxP3+ T reg cells (n = 9; D). MFI, mean fluorescence intensity. (E) T reg cell phosphor-STAT5 expression in RA PBMCs after blockade of IL-2, TNF-RII, and STAT5 inhibition, either alone or in combination, in the presence of adalimumab (n = 9) or etanercept (n = 5). (F) Frequency of FoxP3 T reg cells in RA PBMCs using the same conditions as in E. Data were obtained from three experimental repeats. **, P < 0.01; ***, P < 0.001 using Kruskal-Wallis test with Dunn’s post-hoc analysis.
IL-2 signaling in human T reg cells results in the phosphorylation of STAT5 (Zorn et al., 2006). TNF-RII drives STAT5 phosphorylation in synergy with IL-2 in mouse T reg cells (Chen et al., 2007). Therefore, we examined whether adalimumab affected T reg cell STAT5 signaling. Adalimumab increased STAT5 phosphorylation in RA T reg cells (Fig. 6 C) and Foxp3 expression (Fig. 6 D) to a similar degree as direct stimulation of TNF-RII. Blockade of IL-2 and TNF-RII alone or together abrogated the increase of STAT5 phosphorylation (Fig. 6 E) and T reg cell expansion (Fig. 6 F) stimulated by adalimumab. Addition of a STAT5 inhibitor alone or together with anti–IL-2 and anti–TNF-RII antagonist prevented the T reg cell enrichment by adalimumab (Fig. 6 F), indicating that T reg cell expansion was dependent on the STAT5 pathway. Incubation of PBMCs with etanercept did not increase STAT5 phosphorylation (Fig. 6 E).
DISCUSSION
The importance of TNF has been clearly demonstrated through therapeutic blockade in patients with a variety of inflammatory disorders. However, its role in modulating the function of T reg cells has remained controversial because of several apparently contradictory findings in mice and humans (Valencia et al., 2006; Grinberg-Bleyer et al., 2010; Kleijwegt et al., 2010; Nagar et al., 2010; Chen et al., 2013; Chopra et al., 2013; Nie et al., 2013; Zaragoza et al., 2016). Here, we reveal how anti-TNF antibodies can not only preserve, but also enhance the regulatory properties of this proinflammatory cytokine in patients with chronic inflammation.
Through a series of linked observations, we have demonstrated that adalimumab binds to membrane TNF on monocytes, enhancing both the expression of membrane TNF and its binding to TNF-RII on T reg cells. We show that TNF-RII expressed by T reg cells from RA patients drives the expansion of T reg cells able to suppress Th17 cells via STAT5, which is consistent with previous data from mouse and human studies implicating TNF-RII as a potent enhancer of T reg cell function (Grinberg-Bleyer et al., 2010; Chen et al., 2013; Beilhack et al., 2014; McCann et al., 2014). The fact that T reg cell suppression was restored in RA patients after anti-TNF therapy (Ehrenstein et al., 2004) appeared to support the notion that TNF was detrimental to T reg cell function. However, the data presented here implicate an alternative interpretation, specifically that therapeutic anti-TNF antibodies paradoxically promote T reg cell TNF-RII signaling to drive their expansion. These results highlight how a treatment that targets a pivotal inflammatory cytokine not only preserves, but actually boosts the proresolution forces driven by that pathway, thereby highlighting a novel therapeutic paradigm.
We have previously published that T reg cells endowed with the ability to suppress Th17 cell responses are increased in patients responding to adalimumab therapy (McGovern et al., 2012). We have now established an in vitro model of these in vivo observations, thereby enabling the dissection of the underlying mechanisms. Adalimumab bound to monocytes rather than T reg cells and increased the expression of monocyte membrane TNF. Monocytes are known to be activated in RA patients (Evans et al., 2009; McGovern et al., 2012) and express more membrane TNF compared with their healthy counterparts (Meusch et al., 2009). Membrane-bound TNF on monocytes has been shown to be superior to soluble TNF in activating TNF-RII (Kleijwegt et al., 2010; Rauert et al., 2010). It has been reported that infliximab, a chimeric anti-TNF antibody, bound strongly to monocytes but not CD4+ T cells (Coulthard et al., 2012), which is consistent with our results using the purely human anti-TNF antibody adalimumab. However, our data indicate that adalimumab not only passively detects membrane TNF, but actually increases its expression in RA monocytes. The mechanism that may underlie this surprising result is unclear, but one possibility is that adalimumab stabilizes membrane TNF at the cell surface and prevents recycling or cleavage to soluble TNF.
The increase in monocyte membrane TNF driven by adalimumab, which occurred both in vitro and in vivo, led not only to enhancement of T reg cell TNF-RII expression, but also its binding to membrane TNF. Recent structural comparisons of TNF-adalimumab Fab and TNF–TNF-RII reveal that only 7 of 21 TNF residues involved in the TNF-RII binding also participate in contacting the adalimumab Fab molecule (Hu et al., 2013). These data raise the possibility that adalimumab and TNF-RII present at the conjugate synapse may not block each other’s binding by interacting with different parts of the same or another membrane TNF molecule in close proximity. Notwithstanding these observations, crystallographic studies of TNF–anti-TNF complexes are derived from the interaction between soluble molecules, making it difficult to draw precise conclusions about the binding between membrane-bound molecules. Our data indicate that the T reg cell expansion driven by adalimumab occurs in a relatively narrow concentration range, which closely relates to therapeutic efficacy (Bartelds et al., 2007). It is tempting to speculate that the spatiotemporal geometry that permits adalimumab to enhance TNF–TNF-RII interactions is particularly sensitive to changes in adalimumab concentration. An intermediate adalimumab concentration may block some but not all membrane TNF domains, allowing for not only cross-linking of TNF trimers, but also enough unoccupied TNF domains to bind TNF-RII. The polarization of TNF-RII on T reg cells induced by adalimumab is consistent with the formation of TNF–TNF-RII aggregates, which are required for the initiation of TNF-RII intracellular signaling (Mukai et al., 2010). TNF-RII expression on T reg cells has been linked with enhanced suppressive capacity (Chen et al., 2007), which agrees with our observations that adalimumab-expanded T reg cells can control IL-17 production unlike their healthy counterparts (McGovern et al., 2012).
Etanercept bound weakly to RA monocytes and did not result in the cascade of events including TNF-RII polarization at the surface of T reg cells and STAT5 signaling that led to Foxp3 expansion. Several studies have examined the distinct actions of anti-TNF antibodies and etanercept, initially prompted by the divergent efficacy of these two classes of TNF antagonists in the treatment of Crohn’s disease (Patil et al., 2013; Peake et al., 2013). Differences in their half-life and effects on membrane TNF have been suggested, but no definitive explanation has been obtained (Horiuchi et al., 2010). Our data favor the interpretation that the key distinction between these two types of TNF inhibitors resides in their differing avidity for monocyte membrane TNF expressed by monocytes from RA patients. A further advantage of establishing the in vitro model is that it allows for a comparison of the effects of adalimumab on healthy controls who would not receive anti-TNF therapy. Monocytes from healthy controls expressed minimal levels of membrane TNF (Meusch et al., 2009), which could account for the lack of binding of adalimumab. These findings reinforce not only the notion that the inflammatory milieu is important in promoting T reg cell expansion, but also that understanding how inflammation is resolved requires the analysis of patient as well as healthy samples.
The up-regulation of Foxp3 by adalimumab was reliant upon low levels of IL-2 production and subsequently STAT5 activation. The dependence of T reg cells on IL-2 is well established (Cheng et al., 2011; Zaragoza et al., 2016). Human T reg cells are known to be highly sensitive to low doses of IL-2, some 100-fold less than responder T cells (Yu et al., 2015). Moreover, similarly low concentrations of IL-2 have been recently shown to boost the effects of TNF and expand human T reg cells (Zaragoza et al., 2016). TNF-RII is able to increase the sensitivity of IL-2 signaling, thereby amplifying the impact of small changes in IL-2 production (Chen et al., 2007; Mahmud et al., 2014). We found that adalimumab stimulated IL-2 production consistent with a recent study indicating that IL-2 transcription was directly triggered by TNF-RII (Miller et al., 2015). The defective regulation of IL-2 production reported in autoimmune disease (Carbone et al., 2014) may enhance susceptibility to T reg cell expansion driven by adalimumab. Upon addition of adalimumab, IL-2 production was increased by those purified T reg cells that maintained a high expression of Foxp3, but low Foxp3 T cells also increased their production of IL-2. The provenance of these IL-2–secreting Foxp3 low T cells is unclear but could derive either from T reg cells that had lost Foxp3 expression over the course of the culture or from an expansion of responder (conventional) T cells contaminating the FACS-purified T reg cells. Indeed, in vivo, it is possible that responder T cells are the main source of the IL-2 driving T reg cell expansion in response to adalimumab. TNF-RII expression tended to be highest on T reg cells, and its expression was increased by adalimumab in contrast to the unaltered TNF-RII expression on responder T cells. However, a proportion of responder T cells express TNF-RII at levels comparable to T reg cells, and these could also be triggered by monocyte membrane TNF to produce IL-2 in the same manner as T reg cells.
Anti-TNF antibodies appear to be the first example of a therapeutic cytokine inhibitor that paradoxically promotes the regulatory functions of its target. Anti-TNF antibodies block the proinflammatory soluble TNF while appearing to augment the immunosuppressive properties of membrane TNF. Future studies will need to examine how these two opposing effects of TNF can be individually targeted to resolve inflammation and promote tolerance.
MATERIALS AND METHODS
Patient population
We recruited patients with active RA, whose diagnosis fulfilled the American College of Rheumatology 1987 revised classification criteria for RA (Arnett et al., 1988), and healthy volunteers. The University College London Hospital Ethics Committee approved the study. Patients were untreated or receiving methotrexate, sulfalsalazine, hydroxychloroquine, or leflunomide and not receiving prednisolone doses >7.5 mg/d, with their Disease Activity Score (DAS28; Prevoo et al., 1995) >5.1 and their C-reactive protein levels >5 mg/liter. Patients were designated as responding to anti-TNF therapy if they had a reduction in their DAS28 score ≥1.2 and a C-reactive protein <5 mg/liter.
Antibodies and inhibitors
The following antibodies were used: Alexa Fluor 700–conjugated CD4 (RPA-TA; BD), allophycocyanin (APC)-Cy7–conjugated CD25 (M-A251; BD), PE-Cy5–conjugated CD127 (eBioRDR5; eBioscience), Pacific blue–conjugated CD14 (M5E2; BD), FITC-conjugated Ki67 (35 Ki-67; BD), PE-conjugated RORγτ (AFKJS-9; eBioscience), Alexa Fluor 647–conjugated FoxP3 (PCH101; eBioscience), PE-conjugated FoxP3 (236A/E; eBioscience), FITC-conjugated membrane TNF (FAB210F; R&D Systems), PE-conjugated TNF-RII (22235; R&D Systems), PE-conjugated STAT5 (PY694; BD), FITC-conjugated active Caspase 3 (C92-605; BD), FITC-conjugated IL-2 (MQ1-17H12; eBioscience), Alexa Fluor 647–conjugated Il-17A (BL168; BioLegend), PE-Cy7–conjugated IFN-γ (B27; BD), soluble anti-CD3 (HIT-3a; eBioscience), anti-CD28 (CD28.2; eBioscience), anti–TNF-RII antagonist (22221; R&D Systems), anti–TNF-RII agonist (MR2-1; Hycult Biotech), and anti–IL-2 neutralizing mAb (MQ1-17H12; BD). STAT5 inhibitor N′([4-oxo-4H-chromen-3-yl]methylene) nicotinohydrazide (EMD Millipore) was also used. Of relevance, FITC-conjugated membrane TNF (FAB210F; R&D Systems) does not interfere with membrane TNF’s binding to TNF-RII (Gerspach et al., 2000).
Anti-TNF agents
Adalimumab or etanercept was added at a final concentration of 10 µg/ml (except for Fig. 1 C, where a range of concentrations was used) to PBMCs or co-cultures of purified T reg cell and monocyte cultures for 3 d.
Cell purification
Ficoll-Paque (GE Healthcare) was used to isolate PBMCs. Specific cell populations were purified by flow cytometry (Aria; BD). The gating strategy for T reg cell isolation and depletion and the resulting purities obtained are shown in Fig. S1.
Depletion assay
Whole PBMCs or PBMCs depleted of CD4+CD25+CD127− (T reg cell) or CD14+ (monocyte) were purified using flow cytometry. These populations were cultured at 3 × 105 cells per well in 96-well U-bottomed plates (Thermo Fisher Scientific) with adalimumab, etanercept, or isotype control at a concentration of 10 µg/ml for 3 d.
Flow cytometric analysis and soluble cytokine detection
Cell surface and intracellular staining were performed in accordance with the manufacturers’ instructions. A FoxP3 staining buffer set (eBioscience) was used for all intracellular staining. The secretion of IL-17A, IFN-γ, and IL-2 was determined by flow cytometry after 4-h stimulation with 4 µg/ml phorbol myristate acetate, 1 µg/ml ionomycin, and 2 µg/ml Golgi Stop (BD).
Preparation of Fab′2 fragment
Fab′2 fragments of adalimumab were prepared with the Pierce Fab′2 Micro kit (Thermo Fisher Scientific).
IL-2 ELISA
IL-2 production by purified monocytes and T reg cell cultures was determined according to human IL-2 ELISA (Ready-SET-Go; eBioscience).
Suppression assay
CD4+CD25+CD127− (T reg cells) were isolated by FACS (Aria; BD) from PBMCs cultured with adalimumab or etanercept for 3 d. A classical co-culture suppression assay was then performed by co-culturing these anti-TNF–treated T reg cells with freshly purified untreated autologous responder T cells at a 1:3 ratio in the presence of monocytes and 1 µg/ml soluble anti-CD3/CD28 for 5 d. IL-17 and IFN-γ production were measured by flow cytometry after a 4-h stimulation with 4 µg/ml phorbol myristate acetate, 1 µg/ml ionomycin, and 2 µg/ml Golgi Stop. The percentage of suppression of IL-17 and IFN-γ was calculated using the following formula: percentage of suppression = (Tresp + MC) − (Tresp + MC+ Treg) × 100 (Tresp + MC), where Tresp is responder T cells, MC is monocytes, and Treg is T reg cells.
Biotinylation binding assay
Biotinylation of adalimumab and etanercept was performed in accordance with Thermo Fisher Scientific’s instructions for the EZ-Link NHS-PEO Solid-Phase Biotinylation kit. Biotinylated adalimumab, etanercept, or human IgG1 isotype control was added to purified T reg cells or monocytes from RA patients for 30 min before staining for lineage markers CD4, CD25, CD14, TNF-RII, and membrane TNF. Cells were then resuspended in APC-conjugated streptavidin for 30 min before acquisition on FACS.
ImageStream analysis
Sorted T reg cells and monocytes were cultured with biotinylated adalimumab or etanercept at 37°C for the time specified. The cells were then stained with CD4, CD14, membrane TNF, TNF-RII, and streptavidin, all labeled with their respective fluorochromes, fixed, permeabilized, and run on a cytometer (ImageStream X Mark II; Amnis). 50–100,000 event image files were automatically acquired using the ImageStream X (Amnis). Single-color controls were used to create a compensation matrix that was applied to all files to correct for spectral overlap. The resulting compensated image files were analyzed using image-based algorithms available in the IDEAS statistical analysis software (version 6.2). Parameters were set to prevent the collection of small debris and dead cells.
Colocalization
Colocalization was assessed by comparing the small bright image detail of two fluorescent channels and thus calculating the BDS index of anti-TNF agent (red) and membrane TNF (green) in CD14+ cell subsets or anti-TNF (red), TNF-RII (yellow), and membrane TNF (green) in the CD4+ T reg cell (pink)–CD14+(purple) conjugates. Colocalization was considered as a BDS of ≥1.75.
Intensity at the synapse valley mask
IDEAS identified a valley mask, a region of contact between T reg cell–monocyte conjugates, before quantifying the expression intensity of anti-TNF, membrane TNF, or TNF-RII located within the mask.
Polarization
Delta Centroid values were calculated by IDEAS to determine the polarization index of TNF-RII distribution on the T reg cell surface.
STAT5 Phosflow
PBMCs were fixed with an equal volume of warm Phosflow Fix buffer I (BD) for 10 min at 37°C and then permeabilized for 10 min by cold Phosflow Perm/wash buffer III (BD) at 4°C, followed by surface CD4 and CD14, intracellular Foxp3, and STAT5 staining. For some experiments, STAT5 inhibitor (N′[(4-oxo-4H-chromen-3-yl)methylene] nicotinohydrazide) was added to PBMCs for 1 h before adalimumab treatment.
Statistical analysis
Statistically significant differences were determined using Kruskal-Wallis or Friedman tests with Dunn’s post-hoc analysis using Prism software (GraphPad Software). P-values <0.05 were considered as statistically significant. P-values are denoted in figures as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Online supplemental material
Fig. S1 describes the gating strategy for T reg cell purification and T reg cell depletion. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20151255/DC1.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank P.J. Chana (King's College London) for his technical assistance with the ImageStream experiments. We thank Alice Cotton for her help with obtaining blood samples from patients.
This work was supported by the University College London Hospitals Biomedical Research Centre and Arthritis Research UK (20687).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- APC
- allophycocyanin
- BDS
- bright detailed similarity
- RA
- rheumatoid arthritis
References
- Ahmed F., Friend S., George T.C., Barteneva N., and Lieberman J.. 2009. Numbers matter: quantitative and dynamic analysis of the formation of an immunological synapse using imaging flow cytometry. J. Immunol. Methods. 347:79–86. 10.1016/j.jim.2009.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F.C., Edworthy S.M., Bloch D.A., McShane D.J., Fries J.F., Cooper N.S., Healey L.A., Kaplan S.R., Liang M.H., Luthra H.S., et al. 1988. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31:315–324. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- Bartelds G.M., Wijbrandts C.A., Nurmohamed M.T., Stapel S., Lems W.F., Aarden L., Dijkmans B.A., Tak P.P., and Wolbink G.J.. 2007. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann. Rheum. Dis. 66:921–926. 10.1136/ard.2006.065615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilhack A., Chopra M., Biehl M., Vaeth M., Brandl A., Amich J., Findeis J., Garrote A.-L.J., Brede C., Bäuerlein C.A., et al. 2014. A selective TNFR2 agonist expands host Treg cells in vivo to protect from acute graft-versus-host disease. Blood. 124:1099.24986687 [Google Scholar]
- Cao D., van Vollenhoven R., Klareskog L., Trollmo C., and Malmström V.. 2004. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res. Ther. 6:R335–R346. 10.1186/ar1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone F., De Rosa V., Carrieri P.B., Montella S., Bruzzese D., Porcellini A., Procaccini C., La Cava A., and Matarese G.. 2014. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat. Med. 20:69–74. 10.1038/nm.3411 [DOI] [PubMed] [Google Scholar]
- Chen X., Bäumel M., Männel D.N., Howard O.M., and Oppenheim J.J.. 2007. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J. Immunol. 179:154–161. 10.4049/jimmunol.179.1.154 [DOI] [PubMed] [Google Scholar]
- Chen X., Wu X., Zhou Q., Howard O.M., Netea M.G., and Oppenhein J.J.. 2013. TNFR2 is critical for the stabilization of CD4+FoxP3+ regulatory T cell phenotype in the inflammatory environment. J. Immunol. 190:1076–1084. 10.4049/jimmunol.1202659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Yu A., and Malek T.R.. 2011. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol. Rev. 241:63–76. 10.1111/j.1600-065X.2011.01004.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra M., Riedel S.S., Biehl M., Krieger S., von Krosigk V., Bäuerlein C.A., Brede C., Jordan Garrote A.L., Kraus S., Schäfer V., et al. 2013. Tumor necrosis factor receptor 2-dependent homeostasis of regulatory T cells as a player in TNF-induced experimental metastasis. Carcinogenesis. 34:1296–1303. 10.1093/carcin/bgt038 [DOI] [PubMed] [Google Scholar]
- Coulthard L.R., Geiler J., Mathews R.J., Church L.D., Dickie L.J., Cooper D.L., Wong C., Savic S., Bryer D., Buch M.H., et al. 2012. Differential effects of infliximab on absolute circulating blood leucocyte counts of innate immune cells in early and late rheumatoid arthritis patients. Clin. Exp. Immunol. 170:36–46. 10.1111/j.1365-2249.2012.04626.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs A.P., Kennedy A., Penn H., Read J.E., Amjadi P., Green P., Syed K., Manka S.W., Brennan F.M., Gregory B., and Williams R.O.. 2014. Treg cell function in rheumatoid arthritis is compromised by CTLA-4 promoter methylation resulting in a failure to activate the indoleamine 2,3-dioxygenase pathway. Arthritis Rheumatol. 66:2344–2354. 10.1002/art.38715 [DOI] [PubMed] [Google Scholar]
- Ehrenstein M.R., Evans J.G., Singh A., Moore S., Warnes G., Isenberg D.A., and Mauri C.. 2004. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 200:277–285. 10.1084/jem.20040165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H.G., Gullick N.J., Kelly S., Pitzalis C., Lord G.M., Kirkham B.W., and Taams L.S.. 2009. In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc. Natl. Acad. Sci. USA. 106:6232–6237. 10.1073/pnas.0808144106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Borja F., Jury E.C., Mauri C., and Ehrenstein M.R.. 2008. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 105:19396–19401. 10.1073/pnas.0806855105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerspach J., Götz A., Zimmermann G., Kolle C., Böttinger H., and Grell M.. 2000. Detection of membrane-bound tumor necrosis factor (TNF): An analysis of TNF-specific reagents. Microsc. Res. Tech. 50:243–250. [DOI] [PubMed] [Google Scholar]
- Grinberg-Bleyer Y., Saadoun D., Baeyens A., Billiard F., Goldstein J.D., Grégoire S., Martin G.H., Elhage R., Derian N., Carpentier W., et al. 2010. Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs. J. Clin. Invest. 120:4558–4568. 10.1172/JCI42945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi T., Mitoma H., Harashima S., Tsukamoto H., and Shimoda T.. 2010. Transmembrane TNF-α: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford). 49:1215–1228. 10.1093/rheumatology/keq031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Liang S., Guo H., Zhang D., Li H., Wang X., Yang W., Qian W., Hou S., Wang H., et al. 2013. Comparison of the inhibition mechanisms of adalimumab and infliximab in treating tumor necrosis factor α-associated diseases from a molecular view. J. Biol. Chem. 288:27059–27067. 10.1074/jbc.M113.491530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijwegt F.S., Laban S., Duinkerken G., Joosten A.M., Zaldumbide A., Nikolic T., and Roep B.O.. 2010. Critical role for TNF in the induction of human antigen-specific regulatory T cells by tolerogenic dendritic cells. J. Immunol. 185:1412–1418. 10.4049/jimmunol.1000560 [DOI] [PubMed] [Google Scholar]
- Mahmud S.A., Manlove L.S., Schmitz H.M., Xing Y., Wang Y., Owen D.L., Schenkel J.M., Boomer J.S., Green J.M., Yagita H., et al. 2014. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat. Immunol. 15:473–481. 10.1038/ni.2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann F.E., Perocheau D.P., Ruspi G., Blazek K., Davies M.L., Feldmann M., Dean J.L., Stoop A.A., and Williams R.O.. 2014. Selective tumor necrosis factor receptor I blockade is antiinflammatory and reveals immunoregulatory role of tumor necrosis factor receptor II in collagen-induced arthritis. Arthritis Rheumatol. 66:2728–2738. 10.1002/art.38755 [DOI] [PubMed] [Google Scholar]
- McGovern J.L., Nguyen D.X., Notley C.A., Mauri C., Isenberg D.A., and Ehrenstein M.R.. 2012. Th17 cells are restrained by Treg cells via the inhibition of interleukin-6 in patients with rheumatoid arthritis responding to anti-tumor necrosis factor antibody therapy. Arthritis Rheum. 64:3129–3138. 10.1002/art.34565 [DOI] [PubMed] [Google Scholar]
- McKarns S.C., and Schwartz R.H.. 2008. Biphasic regulation of Il2 transcription in CD4+ T cells: roles for TNF-α receptor signaling and chromatin structure. J. Immunol. 181:1272–1281. 10.4049/jimmunol.181.2.1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusch U., Rossol M., Baerwald C., Hauschildt S., and Wagner U.. 2009. Outside-to-inside signaling through transmembrane tumor necrosis factor reverses pathologic interleukin-1β production and deficient apoptosis of rheumatoid arthritis monocytes. Arthritis Rheum. 60:2612–2621. 10.1002/art.24778 [DOI] [PubMed] [Google Scholar]
- Miller P.G., Bonn M.B., and McKarns S.C.. 2015. Transmembrane TNF-TNFR2 impairs Th17 differentiation by promoting Il2 expression. J. Immunol. 195:2633–2647. 10.4049/jimmunol.1500286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyara M., Gorochov G., Ehrenstein M., Musset L., Sakaguchi S., and Amoura Z.. 2011. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun. Rev. 10:744–755. 10.1016/j.autrev.2011.05.004 [DOI] [PubMed] [Google Scholar]
- Mukai Y., Nakamura T., Yoshikawa M., Yoshioka Y., Tsunoda S., Nakagawa S., Yamagata Y., and Tsutsumi Y.. 2010. Solution of the structure of the TNF-TNFR2 complex. Sci. Signal. 3:ra83 10.1126/scisignal.2000954 [DOI] [PubMed] [Google Scholar]
- Nagar M., Jacob-Hirsch J., Vernitsky H., Berkun Y., Ben-Horin S., Amariglio N., Bank I., Kloog Y., Rechavi G., and Goldstein I.. 2010. TNF activates a NF-κB–regulated cellular program in human CD45RA− regulatory T cells that modulates their suppressive function. J. Immunol. 184:3570–3581. 10.4049/jimmunol.0902070 [DOI] [PubMed] [Google Scholar]
- Nie H., Zheng Y., Li R., Guo T.B., He D., Fang L., Liu X., Xiao L., Chen X., Wan B., et al. 2013. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat. Med. 19:322–328. 10.1038/nm.3085 [DOI] [PubMed] [Google Scholar]
- Patil S.A., Rustgi A., Langenberg P., and Cross R.K.. 2013. Comparative effectiveness of anti-TNF agents for Crohn’s disease in a tertiary referral IBD practice. Dig. Dis. Sci. 58:209–215. 10.1007/s10620-012-2323-0 [DOI] [PubMed] [Google Scholar]
- Peake S.T., Bernardo D., Mann E.R., Al-Hassi H.O., Knight S.C., and Hart A.L.. 2013. Mechanisms of action of anti-tumor necrosis factor α agents in Crohn’s disease. Inflamm. Bowel Dis. 19:1546–1555. 10.1097/MIB.0b013e318281333b [DOI] [PubMed] [Google Scholar]
- Prevoo M.L., Van’T Hof M.A., Kuper H.H., van Leeuwen M.A., van de Putte L.B., and van Riel P.L.. 1995. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 38:44–48. 10.1002/art.1780380107 [DOI] [PubMed] [Google Scholar]
- Rauert H., Wicovsky A., Müller N., Siegmund D., Spindler V., Waschke J., Kneitz C., and Wajant H.. 2010. Membrane tumor necrosis factor (TNF) induces p100 processing via TNF receptor-2 (TNFR2). J. Biol. Chem. 285:7394–7404. 10.1074/jbc.M109.037341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Miyara M., Costantino C.M., and Hafler D.A.. 2010. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 10:490–500. 10.1038/nri2785 [DOI] [PubMed] [Google Scholar]
- Tran D.Q., Ramsey H., and Shevach E.M.. 2007. Induction of FOXP3 expression in naive human CD4+FOXP3− T cells by T-cell receptor stimulation is transforming growth factor-β–dependent but does not confer a regulatory phenotype. Blood. 110:2983–2990. 10.1182/blood-2007-06-094656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia X., Stephens G., Goldbach-Mansky R., Wilson M., Shevach E.M., and Lipsky P.E.. 2006. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 108:253–261. 10.1182/blood-2005-11-4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amelsfort J.M., Jacobs K.M., Bijlsma J.W., Lafeber F.P., and Taams L.S.. 2004. CD4+CD25+ regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 50:2775–2785. 10.1002/art.20499 [DOI] [PubMed] [Google Scholar]
- Yu A., Snowhite I., Vendrame F., Rosenzwajg M., Klatzmann D., Pugliese A., and Malek T.R.. 2015. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in type 1 diabetes. Diabetes. 64:2172–2183. 10.2337/db14-1322 [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A., Ding Y., Kumari S., Attur M., Hippen K.L., Brown M., Blazar B.R., Abramson S.B., Lafaille J.J., and Dustin M.L.. 2010. Protein kinase C-θ mediates negative feedback on regulatory T cell function. Science. 328:372–376. 10.1126/science.1186068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza B., Chen X., Oppenheim J.J., Baeyens A., Gregoire S., Chader D., Gorochov G., Miyara M., and Salomon B.L.. 2016. Suppressive activity of human regulatory T cells is maintained in the presence of TNF. Nat. Med. 22:16–17. 10.1038/nm.4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn E., Nelson E.A., Mohseni M., Porcheray F., Kim H., Litsa D., Bellucci R., Raderschall E., Canning C., Soiffer R.J., et al. 2006. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 108:1571–1579. 10.1182/blood-2006-02-004747 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.