Homeostatic levels of IL-4 are necessary for normal development of memory phenotype CD8+ T cells and naive CD8+ T cells and for a robust CD8+ T cell response to LCMV infection.
Abstract
Previous studies have revealed that a population of innate memory CD8+ T cells is generated in response to IL-4, first appearing in the thymus and bearing high expression levels of Eomesodermin (Eomes) but not T-bet. However, the antigen specificity and functional properties of these cells is poorly defined. In this study, we show that IL-4 regulates not only the frequency and function of innate memory CD8+ T cells, but also regulates Eomes expression levels and functional reactivity of naive CD8+ T cells. Lack of IL-4 responsiveness attenuates the capacity of CD8+ T cells to mount a robust response to lymphocytic choriomeningitis virus infection, with both quantitative and qualitative effects on effector and memory antigen-specific CD8+ T cells. Unexpectedly, we found that, although numerically rare, memory phenotype CD8+ T cells in IL-4Rα–deficient mice exhibited enhanced reactivity after in vitro and in vivo stimulation. Importantly, our data revealed that these effects of IL-4 exposure occur before, not during, infection. Together, these data show that IL-4 influences the entire peripheral CD8+ T cell pool, influencing expression of T-box transcription factors, functional reactivity, and the capacity to respond to infection. These findings indicate that IL-4, a canonical Th2 cell cytokine, can sometimes promote rather than impair Th1 cell–type immune responses.
Memory CD8+ T cells are generated after an immune response dependent on suitable TCR, costimulatory, and cytokine signals (Kaech and Cui, 2012). However, naive CD8+ T cells can also acquire the phenotypic and functional traits of memory cells in the absence of stimulation by foreign antigens through responses to homeostatic cues (Lee et al., 2011; Sprent and Surh, 2011; Jameson et al., 2015). This pathway was initially observed in the context of the proliferative response made by naive CD8+ T cells in lymphopenic conditions, but such cells are also generated under normal homeostatic conditions (Sprent and Surh, 2011; Jameson et al., 2015). The homeostatic cytokines IL-7 and IL-15 play an important role in inducing and perpetuating these innate or homeostatic memory CD8+ T cells, but recent studies indicated an unexpected role for IL-4. Specifically, mice that develop a prominent population of IL-4–producing NK T cells show the generation of abundant memory-like CD8+ T cells (Lee et al., 2011; Jameson et al., 2015). The generation of these memory-like cells (which have been termed innate or bystander memory CD8+ T cells) requires that CD8+ T cells be intrinsically responsive to IL-4 (Weinreich et al., 2009; Lee et al., 2011; Jameson et al., 2015). Although IL-4 is best known as a prototypical feature of the Th2 responses, the innate memory CD8+ T cells produced in response to IL-4 were found to exhibit Tc1 properties, including the ability to rapidly produce IFN-γ (Weinreich et al., 2009, 2010; Lai et al., 2011). Although originally identified in genetically manipulated C57BL/6 mice, this pathway was also observed in normal mouse strains, most prominently the BALB/c strain (Weinreich et al., 2010; Lee et al., 2013b).
Two unique features of IL-4–induced innate memory CD8+ T cells have been reported: The first is that IL-4–induced memory phenotype CD8+ T cells are first detected within the thymus and appear to arise soon after CD8+ thymocyte maturation (Weinreich et al., 2009; Gordon et al., 2011; Lai et al., 2011; Lee et al., 2011). In contrast, innate memory CD8+ T cells produced in C57BL/6 mice, which have low steady-state IL-4 levels, are rare in the thymus, and this population appears first in peripheral lymphoid tissues (Akue et al., 2012). Second, IL-4–induced memory CD8+ T cells show striking up-regulation of the transcription factor Eomesodermin (Eomes) but not the related T-box factor, T-bet (Weinreich et al., 2009; Gordon et al., 2011; Lai et al., 2011; Lee et al., 2011). In contrast, memory-like CD8+ T cells generated in C57BL/6 mice express both Eomes and T-bet, similarly to antigen-driven memory CD8+ T cells (Lee et al., 2013a). How these differences influence the functional response of antigen-specific CD8+ T cells remains unclear.
The relative expression of T-bet and Eomes is thought to play an important role in activated CD8+ T cell differentiation (Kaech and Cui, 2012). Soon after CD8+ T cell activation, T-bet and Eomes are thought to cooperate in inducing the effector program, and in established memory CD8+ T cells, T-bet and Eomes cooperate to promote IL-2Rβ (CD122) expression, which is required for memory cell homeostasis (Kaech and Cui, 2012). However, the two transcription factors also have nonredundant roles and different expression profiles in short-lived effector cells (SLECs) versus memory precursor effector cells (MPECs): High expression levels of T-bet promotes terminal effector cell differentiation, whereas Eomes expression levels peak in memory CD8+ T cells, and Eomes is required for efficient production of central memory CD8+ T cells (Joshi et al., 2007; Banerjee et al., 2010; Rao et al., 2010), as well as innate memory CD8+ T cells (Weinreich et al., 2009; Sosinowski et al., 2013).
Although the role of IL-4 in inducing innate memory CD8+ T cells in the thymus has been well studied, little is known about how the response to IL-4 shapes homeostasis and function of the peripheral CD8+ T cell pool. This is important to assess because some studies have shown that memory phenotype CD8+ T cells generated through homeostatic mechanisms act by inhibiting rather than enhancing the T cell immune response (Rifa’i et al., 2004; Lin et al., 2007; Dai et al., 2010; Kim et al., 2010, 2011; Sakuraba et al., 2013). Here, we show that responsiveness to IL-4 during CD8+ T cell development and homeostasis leads to diverse changes in functional response and gene expression not only of memory phenotype CD8+ T cells, but also of naive CD8+ T cells. Although IL-4Rα–deficient memory phenotype CD8+ T cells exhibited low Eomes expression, our data unexpectedly revealed that these cells had enhanced capacity to produce inflammatory cytokines in vitro and expand to lymphocytic choriomeningitis virus (LCMV) infection in vivo. These data suggest that a lack of IL-4 sensitivity chiefly compromises the numbers rather than intrinsic function of memory-like cells. Through adoptive transfer studies, we could also show that sensitivity to IL-4 encounter during the response to LCMV was not necessary to support an effective CD8+ T cell response, suggesting that the impact of IL-4 is primarily through its effects on CD8+ T cell development and homeostasis. Collectively, our results indicate that IL-4 has diverse effects on the phenotype and function of the whole peripheral CD8+ T cell population and that IL-4 responsiveness enhances the CD8+ T cell response to pathogens.
RESULTS
IL-4 responsiveness dictates the frequency of innate memory CD8+ T cells in secondary lymphoid tissues
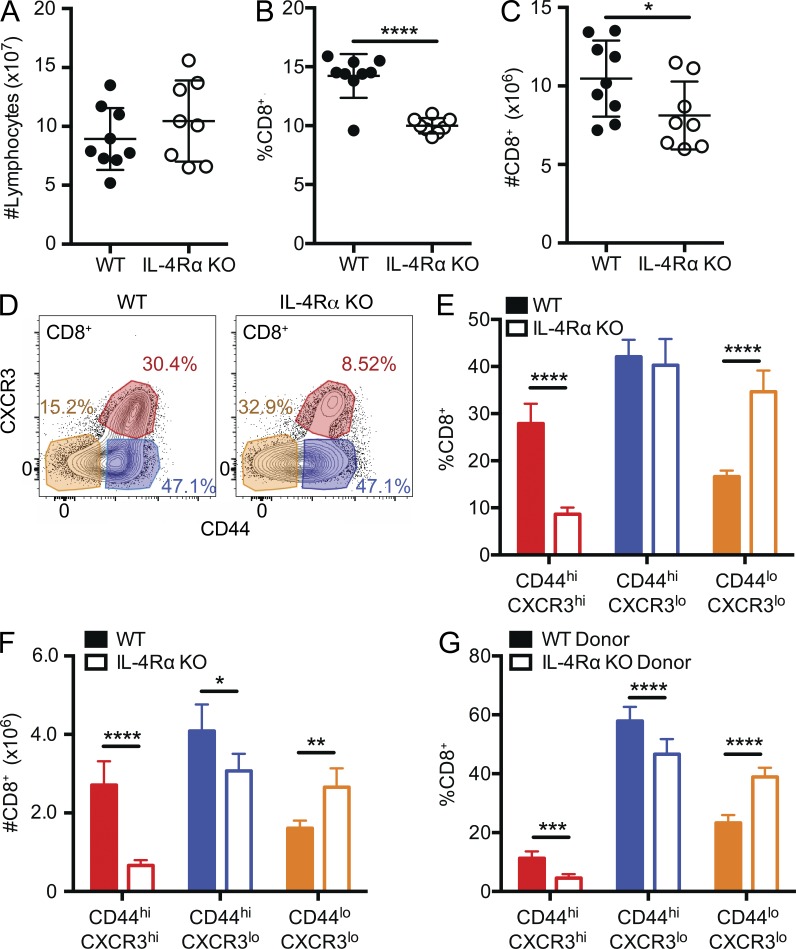
Innate (bystander) memory CD8+ T cells are generated in the thymus of certain mouse strains in a process dependent on IL-4 produced by NK T cells and IL-4 sensitivity by CD8+ T cells themselves (Weinreich et al., 2010; Lee et al., 2013b). However, it is less clear whether and how IL-4 reactivity affects the peripheral pool of memory phenotype CD8+ T cells. We initially compared the CD8+ T cell pools from spleen and lymph nodes of naive WT and IL-4Rα KO BALB/cByJ mice. Although the total numbers of lymphocytes were similar (Fig. 1 A), IL-4Rα KO mice exhibited a decreased frequency of CD8+ T cells (Fig. 1 B), leading to reduced numbers of CD8+ T cells (Fig. 1 C). Preliminary studies indicated total lymphocyte numbers in IL-4Rα KO mice were normalized by an increased frequency of B cells (not depicted). Using CD44 and CXCR3 surface expression as markers (Ventre et al., 2012), we differentiated the CD8+ T cell pool into three populations: naive phenotype (CD44lo CXR3lo), memory phenotype (CD44hi CXCR3hi), and an intermediate phenotype (CD44hi CXCR3lo) population (gating shown in Fig. 1 D). IL-4Rα KO mice had decreased frequencies of memory phenotype CD8+ T cells in the periphery, with reciprocal increased frequencies of naive phenotype cells, compared with WT (Fig. 1 E). These differences resulted in a substantial decrease in memory phenotype CD8+ T cell numbers (and slightly fewer intermediate cells), whereas the number of naive CD8+ T cells increased in IL-4Rα KO mice (Fig. 1 F). To determine whether the impact of IL-4 responsiveness was cell extrinsic or intrinsic, we generated mixed BM chimeras, in which congenically distinct IL-4Rα KO and WT BM cells were coinjected to reconstitute lethally irradiated hosts. As in the intact mice, the frequency of memory phenotype CD8+ T cells was significantly reduced in the IL-4Rα KO donor population, whereas naive phenotype CD8+ T cells frequencies were increased (Fig. 1 G), demonstrating that these effects reflect a cell-intrinsic role for IL-4Rα on CD8+ T cells (as was found for thymic IL-4–induced memory phenotype CD8+ T cells [Weinreich et al., 2009]).
Figure 1.
IL-4Rα expression is required for generation/maintenance of bystander memory CD8+ T cells in the periphery. (A–C) Combined spleen and lymph node lymphocytes from age-matched WT BALB/cByJ and IL-4Rα KO BALB/cByJ were analyzed for total lymphocyte count (A), frequency (B), and total number of CD8+ T cells (C). (D–F) As shown in D, CD8+ T cells were segregated into three populations based on CD44 and CXCR3 expression. The frequency (E) and number (F) of CD44lo CXCR3lo, CD44hi CXCR3lo, and CD44hi CXCR3hi CD8+ T cells was determined in WT and IL-4Rα KO BALB/cByJ mice. (G) Mixed BM chimeras were generated by introducing WT and IL-4Rα KO BALB/cByJ donor BM into lethally irradiated WT BALB/cByJ hosts. Chimeric mice were bled 8 wk later, and the frequency of CD8+ T cells with the indicated CD44/CXCR3 phenotype was determined for both donor populations. Error bars indicate mean ± SD. P-values are represented as *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 by unpaired (A–C, E, and F) or paired (G) Student’s t test for BM chimeras. (A–C) Data are combined from two separate experiments (n = 9 for WT, n = 8 for IL-4Rα KO) and are representative of five experiments. (D–F) n = 4 for WT and n = 4 for IL-4Rα KO, and data are representative of five experiments. (G) n = 7 for mixed BM chimeras, and data are representative of three experiments.
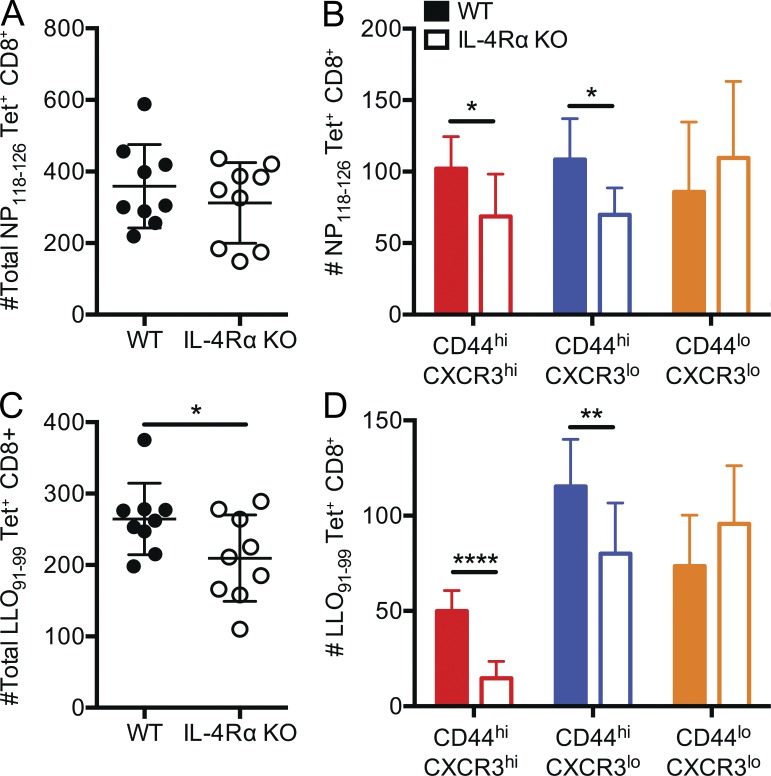
It was possible that IL-4Rα deficiency caused a reduction in conventional rather than innate memory phenotype CD8+ T cells. We and others have shown that a significant fraction of C57BL/6 CD8+ T cells specific for varied foreign antigens exhibit a memory phenotype despite no previous exposure to those antigens; this population of innate memory cells has also been called virtual memory CD8+ T cells (Haluszczak et al., 2009; La Gruta et al., 2010). This population was only modestly reduced in IL-4– or IL-4Rα–deficient C57BL/6 mice (Akue et al., 2012; Sosinowski et al., 2013), but it was unclear whether the much larger population of IL-4–dependent memory-like cells in BALB/c mice included virtual memory cells. To address this, we performed tetramer enrichment assays to isolate antigen-specific CD8+ T cell populations in unimmunized WT and IL-4Rα KO BALB/c mice. Total numbers of CD8+ T cells specific for the Ld-restricted LCMV nucleoprotein 118–126 (NP118–126) epitope and the Kd-restricted Listeria monocytogenes listeriolysin O 91–99 (LLO91–99) epitope were modestly reduced in IL-4Rα–deficient BALB/c mice (Fig. 2, A and C). On resolution of phenotypic subsets, it became apparent that the numbers of foreign antigen–specific naive CD8+ T cells were not significantly changed in unimmunized WT and IL-4Rα–deficient mice but that numbers of both memory and intermediate phenotype cells were reduced in the IL-4Rα KO animals (Fig. 2, B and D). Similar findings were observed when Ld/NP118–126-specific CD8+ T cells were characterized from the spleens of WT, IL-4Rα KO, and IL-4 KO animals (not depicted). These results indicate that our findings for the bulk CD8+ T cell pool also apply to precursors specific for unencountered antigens. Together, these results indicate that IL-4Rα expression by CD8+ T cells is important for the generation and/or maintenance of innate memory phenotype CD8+ T cells in peripheral tissues.
Figure 2.
IL-4Rα expression is required for normal numbers of antigen-specific memory phenotype CD8+ T cell precursors. (A–D) Tetramer enrichment was performed on spleen and lymph nodes from individual age-matched WT BALB/cByJ and IL-4Rα KO BALB/cByJ using tetramers containing LCMV NP118–126/Ld (labeled NP118–126 Tet+) and L. monocytogenes LLO91–99/Kd (labeled LLO91–99 Tet+). The total numbers of tetramer-binding cells (A and C) and the numbers of cells in the indicated phenotypic subsets (B and D) are indicated. Each data point represents cells recovered from an individual mouse. Error bars indicate mean ± SD. P-values are represented as *, P < 0.05; **, P < 0.01; ****, P < 0.0001 by unpaired Student’s t test. Data are combined from two separate experiments (experiment 1: n = 5 for WT, n = 4 for IL-4Rα KO; experiment 2: n = 4 for WT, n = 4 for IL-4Rα KO) and are representative of four experiments (n = 15 for WT, n = 15 IL-4Rα KO in total).
IL-4 receptor regulates Eomes expression in peripheral memory phenotype CD8+ T cells
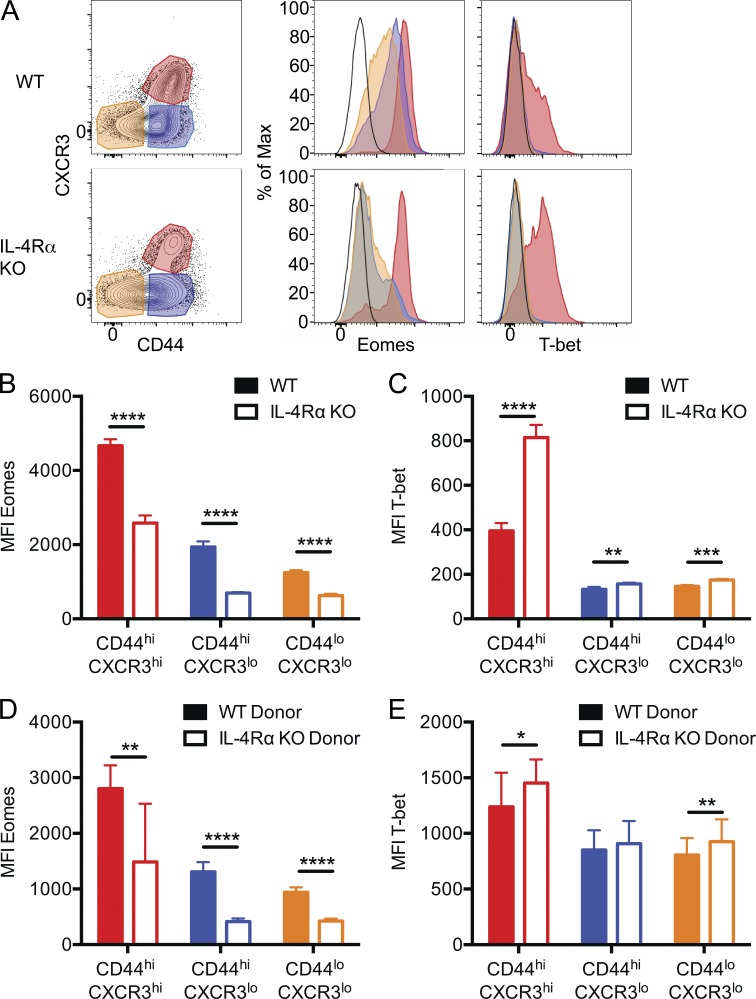
The T-box transcription factors T-bet and Eomes play a crucial role in activated CD8+ T cell fate and function. Antigen-induced memory CD8+ T cells and innate memory CD8+ T cells in C57BL/6 mice express both Eomes and T-bet (Lee et al., 2013a). In contrast, the thymic innate memory population in BALB/c mice is characterized by high Eomes expression and low T-bet expression (Weinreich et al., 2010). It is unclear, however, whether this Eomeshi, T-betlo phenotype is a unique feature of thymic innate memory phenotype CD8+ T cells and how IL-4 responsiveness impacts T-box factor expression by naive and memory phenotype peripheral CD8+ T cells in BALB/c mice. Hence, we measured expression of Eomes and T-bet by CD8+ T cells from the spleen and lymph nodes of WT and IL-4Rα KO BALB/cByJ mice (Fig. 3 A). As we reported for thymic innate memory CD8+ T cells (Weinreich et al., 2010), BALB/c peripheral memory phenotype CD8+ T cells were Eomeshi and T-betlo. In contrast, IL-4Rα deficiency resulted in reduced Eomes and elevated T-bet expression by memory phenotype cells. Unexpectedly, analysis of IL-4Rα KO mice also revealed reduced Eomes expression (and moderately increased T-bet expression) in the naive and intermediate phenotype CD8+ T cell populations (Fig. 3, B and C). This conclusion was supported when Eomeshi and Eomeslo peaks were analyzed separately; Eomes expression levels were higher in WT versus IL-4Rα KO CD8+ T cells within these populations (not depicted). It was possible that IL-4Rα–dependent Eomes expression by CD8+ T cell subsets was not caused by IL-4 sensitivity by CD8+ T cells themselves. Hence, we again examined mixed BM chimeras (as in Fig. 1) and found that Eomes expression was highly dependent on IL-4Rα expression by CD8+ T cells (Fig. 3 D), indicating a CD8+ T cell–intrinsic role of IL-4 sensitivity. Again, measuring the Eomes expression level after gating on Eomeshi and Eomeslo populations confirmed this conclusion (not depicted). In contrast, the elevated T-bet expression observed in CD8+ T cell subsets of intact IL-4Rα KO mice was not reliably observed in IL-4Rα–deficient cells from the mixed BM chimeras (Fig. 3 E), suggesting this effect was not cell autonomous.
Figure 3.
IL-4Rα regulates Eomes and T-bet expression by all CD8+ T cell subsets. (A) Cells were isolated from naive WT and IL-4Rα KO BALB/cByJ spleen and lymph nodes, and CD8+ T cells with the indicated CXCR3/CD44 phenotype were analyzed for Eomes and T-bet expression after intracellular staining. The black line (unfilled) represents naive CD4 T cells as a control. (B–E) The mean fluorescence intensity (MFI) for expression of Eomes (B and D) and T-bet (C and E) was determined in CD8+ T cells from intact WT BALB/cByJ and IL-4Rα KO BALB/cByJ mice (B and C) and from mixed BM chimeras, generated as in Fig. 1 (D and E). Error bars indicate mean ± SD. P-values are represented as *, P < 0.05; **, P < 0.001; ***, P < 0.001; ****, P < 0.0001 by unpaired (B and C) or paired (D and E) Student’s t test. (B and C) n = 4 for WT and n = 4 for IL-4Rα KO, and data are representative of four experiments. (D and E) n = 7 for mixed BM chimeras, and data are representative of three experiments.
Together, these data show that IL-4 sensitivity is required for maximal Eomes expression not only by memory phenotype BALB/c CD8+ T cells, but also in naive (and intermediate) phenotype CD8+ T cells. These findings indicate that IL-4 influences gene expression in the entire peripheral CD8+ T cell pool.
IL-4 responsiveness influences cytokine and effector molecule production by stimulated CD8+ T cells
CD8+ T cells with high expression of Eomes and low expression of T-bet have been associated with not only differentiation of functionally potent memory CD8+ T cells in acute infection models (Banerjee et al., 2010), but also with terminal differentiation of exhausted CD8+ T cells in chronic infection studies (Paley et al., 2012). Hence, the functional impact of altered Eomes and T-bet ratios observed in WT and IL-4Rα–deficient BALB/c CD8+ T cells is difficult to predict.
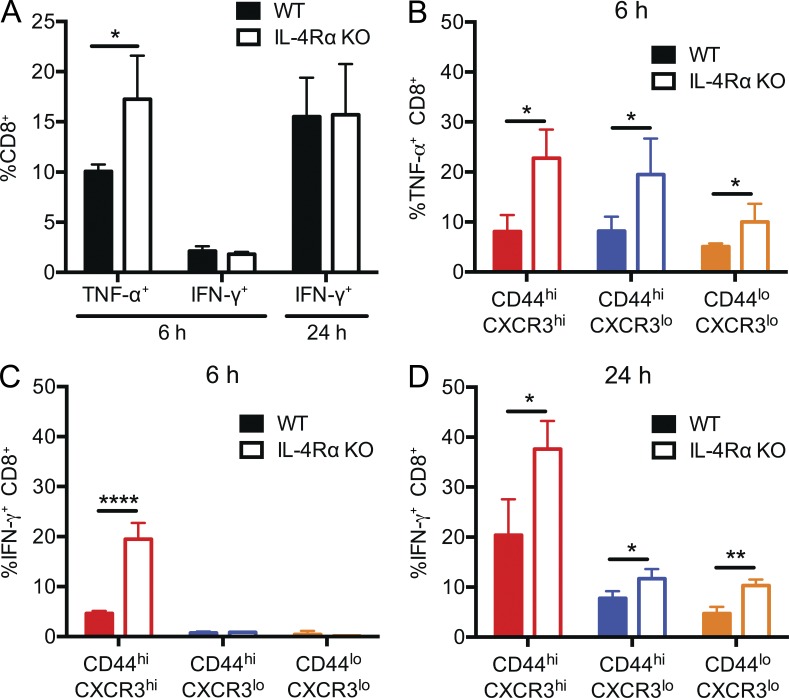
To address this, we first measured cytokine production after T cell activation in vitro. We and others previously showed that IL-4–induced memory phenotype cells in the thymus were capable of rapid IFN-γ production upon TCR stimulation; however, these studies entailed comparison with the response of IL-4 KO or IL-4Rα KO CD4− CD8+ thymocytes, which are dominated by naive phenotype cells (Weinreich et al., 2010; Lai et al., 2011). A direct comparison between the responses of naive and memory CD8+ subsets in WT and IL-4Rα–deficient BALB/c mice has not been carefully investigated. First, we stimulated bulk CD8+ T cells from peripheral lymphoid tissues of WT and IL-4Rα KO mice with anti-CD3 and anti-CD28 and assayed production of the inflammatory cytokines TNF and IFN-γ. Unexpectedly, despite the increased frequency of memory phenotype CD8+ T cells in WT animals, similar frequencies of WT and IL-4Rα KO CD8+ T cells produced IFN-γ at 6- and 24-h time points, and a greater proportion of IL-4Rα KO CD8+ T cells expressed TNF at 6 h (a time point chosen because this cytokine is produced early after stimulation; Fig. 4 A; Badovinac et al., 2000). These findings prompted us to study the responses of WT and IL-4Rα KO CD8+ T cell subpopulations. Hence, we next sorted the three CD8+ T cell subsets (see Fig. 1) and tested their individual responses to T cell activation (Fig. 4, B–D). Similar to the bulk populations (Fig. 4 A), IL-4Rα KO CD8+ T cells from all three subsets showed an increased frequency of TNF-producing cells compared with their WT counterparts (Fig. 4 B). Interestingly, at 24 h of stimulation, we observed increased percentages of IFN-γ–producing cells from all three subsets of CD8+ T cells derived from IL-4Rα KO animals relative to WT (Fig. 4 D). This result was not caused by altered kinetics of IFN-γ expression by WT versus IL-4Rα–deficient populations because a significantly higher fraction of IL-4Rα KO CD8+ memory phenotype T cells produced IFN-γ even at 6 h of stimulation (a time point at which very few CXCR3lo/CD44hi/lo populations expressed IFN-γ; Fig. 4 C).
Figure 4.
Enhanced cytokine production by IL-4Rα KO CD8+ T cells. (A) Bulk CD8+ T cells from WT and IL-4Rα KO were stimulated with plate-immobilized anti-CD3 and anti-CD28 for 6 or 24 h and stained for intracellular TNF or IFN-γ. (B–D) WT and IL-4Rα KO CD8+ T cells were sorted into the subsets indicated and then stimulated with anti-CD3 and anti-CD28 for 6 (B and C) or 24 h (D) before intracellular staining for TNF (B) or IFN-γ (C and D). Error bars indicate mean ± SD. P-values are represented as *, P < 0.05; **, P < 0.01; ****, P < 0.0001 by unpaired Student’s t test. n = 4 for WT and n = 4 for IL-4Rα KO for each graph, and data are representative of three (A) or two (B–D) experiments.
These data suggest that the reduced frequency of memory phenotype and Eomeshi CD8+ T cell populations in IL-4Rα–deficient mice may be partially offset by increased functional reactivity, at least in terms of inflammatory cytokine production. Hence, we next sought to investigate the consequences of these differences by tracking the in vivo response of WT and IL-4Rα KO CD8+ T cells to infection.
IL-4 sensitivity regulates the CD8+ T cell response to acute viral infection
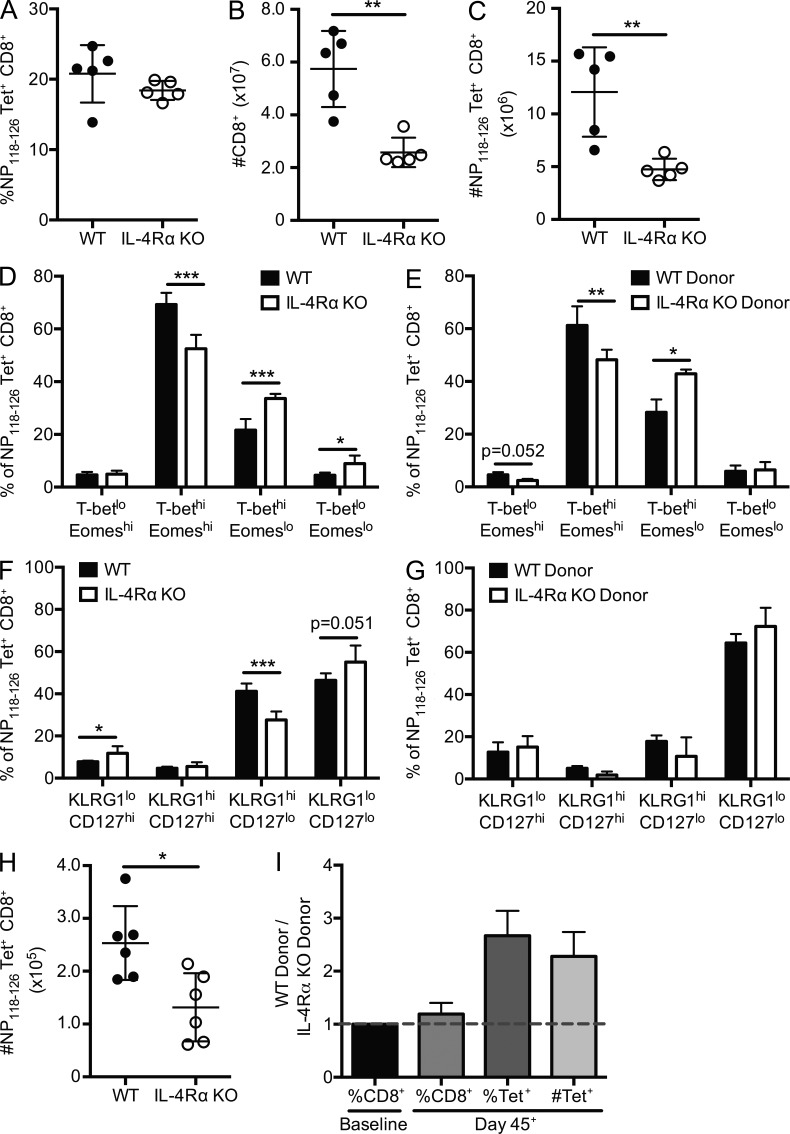
The role for IL-4 in regulating the CD8+ T cell response to infection is unclear. IL-4 reactivity was proposed to be important for optimal effector (Carvalho et al., 2002) and memory (Morrot et al., 2005) CD8+ T cell responses to a mouse model of malaria. However, IL-4 signaling may inhibit polyfunctional responses to viral infection (Wijesundara et al., 2013) and may reduce activation of memory CD8+ T cells after in vitro restimulation (Ventre et al., 2012). To investigate whether IL-4 sensitivity impacted the CD8+ T cell response to infection, we infected WT and IL-4Rα KO mice with an acute viral pathogen, LCMV Armstrong, and tracked the response of Ld/NP118–126-specific CD8+ T cells, as well as the bulk CD8+ T cell pool. In part, we chose to follow the response to Ld/NP118–126 because, in our hands, the total numbers of antigen-specific precursors were not substantially different between WT and IL-4Rα KO mice, whereas the numbers of nonnaive phenotype antigen-specific cells was significantly reduced (Fig. 2). WT and IL-4Rα KO mice had similar frequencies of NP118–126-specific CD8+ T cells, as measured by tetramer binding (Fig. 5 A). However, we found significantly decreased numbers of total CD8+ T cells in the spleens of IL-4Rα KO mice 7 d after LCMV infection (Fig. 5 B), and accordingly, the absolute numbers of antigen-specific CD8+ T cells were reduced in IL-4Rα animals (Fig. 5 C). It is worth noting that the fold difference in bulk and antigen-specific CD8+ T cell numbers between WT and IL-4Rα–deficient mice was more substantial after LCMV infection (Fig. 5, B and C) than in unimmunized mice (Figs. 1 C and 2 A), e.g., the difference in bulk CD8+ T cells before infection was 1.3-fold, whereas it was 2.2-fold after infection (Figs. 1 C and 5 B), indicating that the response to infection exacerbates the deficit in IL-4Rα KO CD8+ T cells. Because we found that IL-4 sensitivity influenced Eomes and T-bet expression in unimmunized CD8+ T cell populations (Fig. 3), we next measured intracellular expression of these factors in the antigen-specific CD8+ T cells at the effector stage of the response to LCMV. We observed decreased frequency and numbers of Eomes-expressing CD8+ T cells in IL-4Rα KO mice (Fig. 5 D and not depicted). Although an increased frequency of IL-4Rα KO antigen-specific CD8+ T cells was either T-bet single positive or expressed low levels of both transcription factors (Fig. 5 D), this did not translate into increased absolute numbers of these populations (not depicted).
Figure 5.
IL-4Rα deficiency compromises the antigen-specific CD8+ T cell response to LCMV. (A–G) Intact WT and IL-4Rα KO BALB/cByJ mice (A–D and F) or mixed BM chimeras containing WT and IL-4Rα KO donor cells (E and G) were infected with LCMV Armstrong, and spleens were analyzed on day 7 after infection. (A–C) The frequency (A) and number (C) of NP118–126/Ld tetramer–positive (Tet+) CD8+ T cells were measured, as was the number of total CD8+ T cells (B). (D–G) The frequency of NP118–126/Ld tetramer–positive CD8+ T cells expressing the indicated pattern of T-bet/Eomes expression (D and E) or KLRG-1/CD127 expression (F and G) was determined in LCMV-infected WT and IL-4Rα KO mice (D and F) or mixed BM chimeras (E and G). (H and I) NP118–126/Ld tetramer enrichment was used to enumerate the antigen-specific memory CD8+ T cells in WT and IL-4Rα KO mice (H) and mixed BM chimeras (I) at least 45 d after infection with LCMV. Error bars indicate mean ± SD. P-values are represented as *, P < 0.05; **, P < 0.01; ***, P < 0.001 by unpaired (A–D, F, and H) or paired (E and G) Student’s t test. (A–D and F) Data (n = 5 for WT, n = 5 for IL-4Rα KO) are representative of two individual experiments. (E and G) Data (n = 5 for mixed BM chimeras) are representative of two individual experiments. (H) Data are combined from two separate experiments (n = 6 for WT, n = 6 for IL-4Rα KO). (I) Data are combined from two separate experiments (n = 6 for mixed BM chimeras) and are representative of three individual experiments.
It was possible that the differences we observed in the nature of the CD8+ T cell response to LCMV in WT versus IL-4Rα KO animals reflected alterations in the response of other IL-4–sensitive cells and/or aspects of viral control (e.g., viral clearance kinetics). To control for this, we conducted similar experiments in mixed BM chimeras, in which we could determine the intrinsic impact of IL-4Rα deficiency among the CD8+ T cell population. Those studies revealed that the differences in T-bet and Eomes expression held for WT and IL-4Rα KO cells responding to LCMV in the same host animals, arguing for a cell-autonomous role for IL-4Rα expression in this system (Fig. 5 E). Thus, the impact of IL-4 sensitivity on T-bet/Eomes expression is carried into the effector phase of the antiviral response.
Elevated T-bet expression levels are required for effective generation of SLECs, identified in LCMV infections by a CD127lo KLRG1hi phenotype, whereas MPECs identified as CD127hi KLRG1lo are more likely to survive contraction and develop into long-lived memory cells (Kaech et al., 2003). Hence, we also investigated whether the frequency of SLEC and MPEC populations (and other CD127/KLRG1-defined subsets) differed in LCMV-infected WT and IL-4Rα KO mice. At an effector stage time point, we observed reduced frequencies of SLEC phenotype cells and a modest increase in the frequency of MPEC phenotype cells in IL-4Rα KO animals (Fig. 5 F), although all antigen-specific IL-4Rα KO subpopulations were decreased in absolute numbers compared with WT (not depicted). The changes in KLRG1/CD127 expression were, however, not well recapitulated in the BM chimeras (Fig. 5 G), suggesting effects of IL-4Rα expression on cells other than CD8+ T cells may influence these phenotypic differences.
We next extended these data to a memory time point. Antigen-specific CD8+ T cells were isolated by peptide–MHC tetramer enrichment from spleen and lymph nodes of WT, IL-4Rα KO, and mixed BM chimeras infected with LCMV at least 45 d previously. Fewer antigen-specific cells were recovered from the IL-4Rα KO CD8+ T cell population relative to WT cells (Fig. 5, H and I). Note that the BM chimera approach allowed us to normalize the ratio of IL-4Rα KO and WT cells in the preimmune population (Fig. 5 I, first column); hence, the dominance of WT over IL-4Rα KO antigen-specific memory CD8+ T cells in this case suggests a qualitative rather than quantitative difference in the capacity of LCMV-induced IL-4Rα CD8+ T cells to reach memory phase.
These data suggest that differences in IL-4 sensitivity affect both the magnitude and quality of the CD8+ T cell immune response toward acute viral infection.
The impact of CD8+ T cell responsiveness to IL-4 occurs before LCMV infection
Although our data suggested that CD8+ T cell sensitivity to IL-4 was important for an optimal response to LCMV, it was not possible to determine whether this chiefly reflected a requirement for IL-4 during the infection itself or whether the effects of IL-4 occurred before infection, by programming the preimmune CD8+ T cell population.
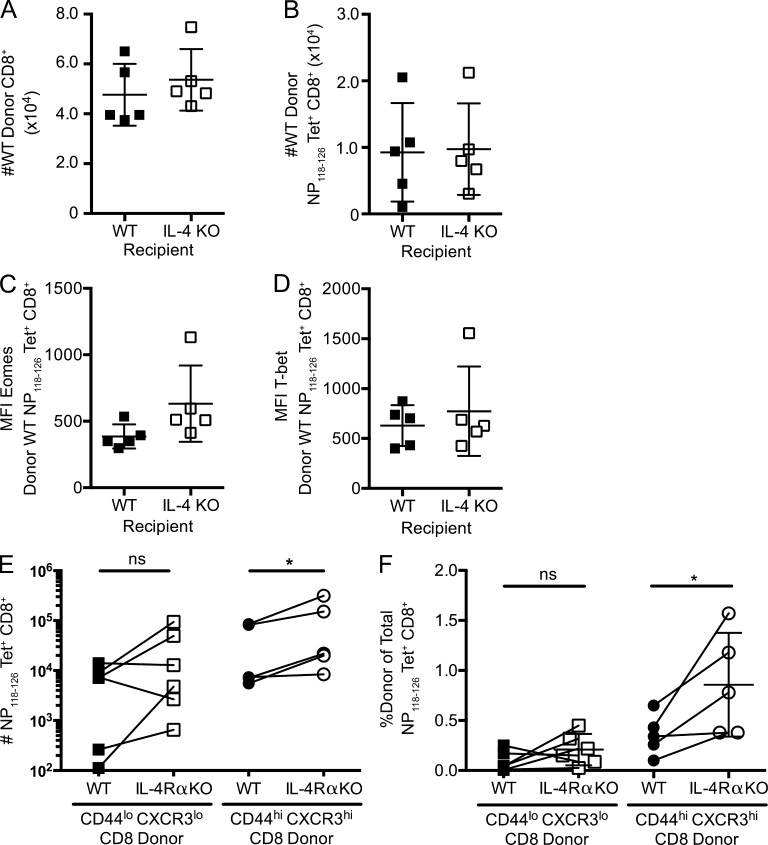
To distinguish between these possibilities, we performed adoptive transfer experiments. Congenically distinct WT CD8+ T cells were transferred into either WT (IL-4 sufficient) or IL-4 KO (IL-4 deficient) recipients. Engraftment was determined 1 d after adoptive transfer in some mice (n = 3 for WT, n = 4 for IL-4 KO) and found to be equivalent in both recipients (not depicted). 7 d after LCMV infection, we observed similar numbers of total and antigen-specific donor CD8+ T cells in both the WT and IL-4 KO recipient groups (Fig. 6, A and B). Additionally, no statistical differences in Eomes (Fig. 6 C) and T-bet (Fig. 6 D) were observed in WT antigen-specific CD8+ T cells transferred to WT or IL-4 KO recipients. These findings suggested that exposure to IL-4 during the immune response to LCMV is not required for an effective CD8+ T cell response.
Figure 6.
IL-4 exposure before but not during LCMV infection regulates the CD8+ T cell response. (A–D) WT BALB/cByJ (CD45.1) CD8+ T cells were transferred into WT BALB/cByJ (CD45.2) or IL-4 KO BALB/cJ recipients (CD45.2), which were then infected with LCMV and analyzed 7 d after infection. The numbers of total splenic donor CD8+ T cells (A) and donor NP118–126/Ld tetramer–binding CD8+ T cells (B) were calculated for each recipient group. (C and D) Expression levels of Eomes (C) and T-bet (D) on antigen-specific CD8+ T cells in the indicated recipient animals. MFI, mean fluorescence intensity. (E and F) Naive (CD44lo CXCR3lo) and memory phenotype (CD44hi CXCR3hi) CD8+ T cells were sorted from WT BALB/cByJ (CD45.1) and IL-4Rα KO BALB/cByJ (CD45.2) mice, and equal numbers of phenotype-matched WT and IL-4Rα KO cells were cotransferred into WT BALB/cByJ recipients (CD45.1/2). 1 d after adoptive transfer, recipients were infected with LCMV. Total numbers of splenic NP118–126/Ld tetramer–binding CD8+ T cells (E) or the frequency of donor NP118–126/Ld tetramer–positive (Tet+) cells (F) within the total tetramer-positive population was calculated for each recipient. Error bars indicate mean ± SD. (A–D) P > 0.05 (not significant [ns]) for all comparisons, using unpaired Student’s t test. (E and F) P-values are represented as *, P < 0.05 by paired Student’s t test. (A–D) Data (n = 5 for WT, n = 5 for IL-4 KO recipients) are representative of two experiments. (E and F) Data are combined from two separate experiments (n = 6 for naive phenotype recipients, n = 5 for memory phenotype recipients total).
We used a complementary approach to determine the impact of IL-4Rα deficiency on the response of memory phenotype CD8+ T cells. Because frequencies of naive and memory phenotype CD8+ T cells differ in WT and IL-4Rα KO mice (Fig. 1), we sorted congenically distinct WT and IL-4Rα KO CD8+ T cells into naive (CD44lo CXCR3lo) or memory phenotype (CD44hi CXCR3hi) subsets before coadoptive transfer into WT host BALB/c mice that were subsequently infected with LCMV. The magnitude of the LCMV-specific response by both donor populations was determined 7 d after infection. Fig. 6 E shows the numbers of donor Ld/NP118–128 tetramer–stained cells, whereas Fig. 6 F shows the response by each donor population as a percentage of the host response, which helps normalize interexperiment variability. These experiments revealed no significant difference in the magnitude of the response of antigen-specific WT and IL-4Rα KO naive CD8+ T cells, in contrast to what might be expected were reactivity to IL-4 critical for driving expansion of LCMV-specific CD8+ T cells. Interestingly, the expansion of memory phenotype IL-4Rα KO donor cells was at least as great as that of their WT counterparts, and in many cases, this IL-4Rα KO population responded more vigorously than the WT population (Fig. 6, E and F). These findings reinforce the conclusion that IL-4Rα engagement during the response to LCMV is not required to induce a strong proliferative response. Furthermore, these results suggest that memory phenotype IL-4Rα KO CD8+ T cells are not intrinsically defective in their in vivo response to LCMV but that their scarcity limits the overall magnitude of the response in IL-4Rα–deficient mice.
Combined, these results strongly suggest that it is IL-4 exposure during development and homeostasis that shapes the subsequent CD8+ T cell response to LCMV infection and that IL-4 conditioning primarily increases the quantity but not the quality of memory-like CD8+ T cells that participate in the immune response to LCMV.
DISCUSSION
Our data indicate that, in BALB/c mice, the response to steady-state levels of IL-4 substantially alters the CD8+ T cell pool. IL-4Rα–deficient mice had reduced total CD8+ T cell numbers, chiefly arising because of a severe reduction in the frequency of memory phenotype cells (including virtual memory cells specific for unencountered foreign antigens). However, beyond these quantitative effects, qualitative changes in the CD8+ T cell pool were evident. Our previous study indicates that IL-4 exposure in the thymus resulted in high expression levels of the Eomes transcription factor (but not the related factor T-bet) in thymic memory phenotype CD8+ T cells (Weinreich et al., 2010). Peripheral memory phenotype CD8+ T cells in IL-4Rα KO mice were reduced in frequency and showed reduced Eomes expression. Surprisingly, we also observed that IL-4 responsiveness correlated with heightened Eomes expression in all CD8+ T cells, including phenotypically naive CD8+ T cells. A study by Carty et al. (2014) using C57BL/6 mice suggested that naive CD8+ T cells do not up-regulate Eomes in response to IL-4 alone in vitro, but rather, Eomes could be induced by a combination of IL-4 together with weak TCR stimulation. In contrast, in vivo treatment of mice with IL-4 complexes (which induce a heightened response to IL-4) could provoke naive CD8+ T cell proliferation (Morris et al., 2009) and induce their acquisition of innate memory phenotype, including elevated Eomes expression (Ventre et al., 2012). Hence, the elevated Eomes levels we observe in WT naive CD8+ T cells in BALB/c mice might arise because of sustained IL-4Rα signals in the periphery or potentially be carried over from IL-4Rα stimulation during thymic differentiation; whether, in either case, basal TCR stimulation through self-peptide–MHC interactions is required to preserve Eomes expression levels is unclear.
Our experiments investigating the impact of IL-4Rα signals on functional responses of peripheral CD8+ T cell populations also led to unexpected findings. Although numerically reduced, IL-4Rα–deficient memory phenotype CD8+ T cells showed significantly enhanced capacity to make IFN-γ and TNF compared with their WT counterparts. Furthermore, this functional superiority extended to the IL-4Rα–deficient naive and intermediate phenotype populations. On a per cell basis then, acute inflammatory cytokine production is compromised rather than enhanced by developmental exposure to IL-4. This finding resonates with the long-established principle that IL-4Rα signals promote Th2 and impair Th1 cell differentiation. Likewise, we found that IL-4Rα–deficient memory phenotype CD8+ T cells exhibited enhanced not diminished expansion in response to LCMV infection when their numbers were normalized with WT cells through adoptive transfer. Hence, IL-4 appears to act to both increase the number of memory-like CD8+ T cells while also reducing their individual capacity to elicit inflammatory cytokines in vitro and mount a robust response to viral infection in vivo.
Because these data suggest that IL-4 sensitivity has both positive and negative effects on the quantity/quality of the CD8+ T cell pool, they raise the question of whether developmental exposure to IL-4 would improve or diminish the CD8+ T cell response to pathogens. Previous studies have indicated that some homeostatic memory-like CD8+ T cell populations are regulatory cells, capable of restraining CD4+ and CD8+ T cell responses (Rifa’i et al., 2004; Lin et al., 2007; Dai et al., 2010; Kim et al., 2010, 2011; Sakuraba et al., 2013). Published studies suggest loss of IL-4 sensitivity compromises the CD8+ T cell response to liver stage malaria (Carvalho et al., 2002; Morrot et al., 2005). In our experiments, we found that loss of IL-4 sensitivity by the CD8+ T cell population led to diverse changes in the response to acute LCMV infection. The magnitude of the antigen-specific response was compromised in IL-4Rα–deficient mice, as was the production of effector cells expressing high levels of Eomes. Interpreting the basis for these changes in the intact IL-4Rα KO mice is complicated by the fact that these mice exhibit both slightly reduced numbers of preimmune CD8+ T cells and changes in gene expression and functional traits within the CD8+ population. However, similar conclusions were reached from studies using mixed BM chimeras, in which the numbers of preimmune WT and IL-4Rα KO CD8+ T cells were normalized. Collectively with the data discussed in the previous paragraph on the robust reactivity of memory phenotype IL-4Rα KO CD8+ T cells, our data suggest that the impaired LCMV-specific response observed in IL-4Rα KO mice is chiefly caused by a deficit in the numbers rather than the intrinsic quality of memory phenotype CD8+ T cells.
The chimera and adoptive transfer approaches also indicated that IL-4–dependent changes in the pre- and postimmune CD8+ T cell pool were dependent on IL-4 sensitivity by the CD8+ T cell population itself. Furthermore, these data suggested that the effects of IL-4 reactivity occurred during T cell development or homeostasis, rather than during the response to pathogen infection, because we found no evidence for a role of IL-4 in supporting the response to LCMV. Hence, our data are compatible with a model in which IL-4 induces altered thymic differentiation of CD8+ T cells, and these effects are maintained into the peripheral antigen-responsive pool.
Collectively, our experiments suggest diverse effects of steady-state IL-4 exposure on CD8+ T cell subsets, gene expression, functional reactivity, and response to pathogens. Our findings indicate that IL-4 exposure may impair the intrinsic capacity of CD8+ T cells to rapidly produce IFN-γ and to mount a robust proliferative response to LCMV; yet, at the same time, IL-4 drives differentiation of significantly greater numbers of memory phenotype CD8+ T cells that participate effectively in Th1 responses. In this regard, our findings suggest a need to reevaluate ways in which IL-4, the prototypical Th2 cell cytokine, may ironically improve rather than diminish proinflammatory immune responses.
In this regard, a recent study (Lee et al., 2015) used distinct approaches to manipulate the frequency of IL-4–induced memory-like cells in BALB/c mice and found that the presence of this population correlated with improved control of LCMV cl13 (which can establish a chronic infection in mice).
An important question is whether similar effects are mediated by IL-4 in humans. Populations of Eomeshi memory-like CD8+ T cells have been described in fetal humans (Min et al., 2011), and related subsets have been observed in adult humans (Jacomet et al., 2015). How variability in IL-4 expression levels alters the frequency or characteristics of these CD8+ T cells is unclear. In a set of mouse strains, there was a correlation between the frequencies of IL-4–producing invariant NK T cells and thymic innate memory CD8+ T cells (Lee et al., 2013b). It is possible that humans with sustained elevated levels of IL-4 (e.g., individuals with atopic diseases or infection with pathogens inducing chronic Th2 responses) may undergo similar changes in the developing CD8+ T cell pool, which would promote balance in the immune system by increasing the numbers of cells competent to participate in type-1 immune responses.
MATERIALS AND METHODS
Mice and virus
BALB/cByJ and IL-4 KO BALB/cJ mice were purchased from The Jackson Laboratory. IL-4Rα KO BALB/cByJ mice were generated by backcrossing IL-4Rα KO BALB/cJ (The Jackson Laboratory) with BALB/cByJ mice for at least five generations. All animal experiments were done with approved Institutional Animal Care and Use Committee protocols at the University of Minnesota. LCMV Armstrong was provided by D. Masopust (University of Minnesota, Minneapolis, MN). Mice were infected with 2 × 105 plaque forming units i.p.
Mixed BM chimeras
BM was harvested from congenically distinct WT and IL-4Rα KO BALB/c mice. After T cell depletion, BM was mixed at ∼45% WT and ∼55% IL-4Rα KO, as preliminary data showed disadvantaged engraftment of IL-4Rα KO BM. Recipient congenic WT BALB/c mice were given 500 cGy (in a split dose) and reconstituted with 10 × 106 BM cells i.v. Chimeras were used >8 wk after transplantation.
Peptide–MHC class I tetramer enrichment
Enrichment of antigen-specific CD8+ T cell precursors or LCMV-specific CD8+ T cells at memory time points was performed as previously described (Haluszczak et al., 2009). Combined spleen and lymph nodes or spleen only, as indicated, was digested with collagenase D. Cells were labeled with PE- or APC-conjugated tetramers, followed by magnetic enrichment over columns using anti-PE or anti-APC magnetic beads (Miltenyi Biotec). Enriched samples were stained with surface antibodies, and AccuCheck counting beads (Invitrogen) were used for calculating cell number.
Surface and intracellular flow cytometry
Single-cell suspensions were generated from spleen and lymph nodes as designated. Cells were stained with the following extracellular antibodies: anti-CD4 (RM4-5), anti-CD8 (53-6.7), anti-CD44 (IM7), anti-CXCR3 (CXCR3-173), anti-CD3ε (145-2C11), anti-CD45.1 (A20), anti-CD45.2 (104), anti-KLRG1 (2F1), and anti-CD127 (A7R34). Antibodies were purchased from eBioscience, BD, or Tonbo. LCMV NP118–126 and L. monocytogenes LLO91–99 tetramers were provided by the National Institutes of Health Tetramer Facility. For intracellular staining, cells were fixed with the FoxP3 Fix/Perm kit (eBioscience) and stained with the following antibodies: anti-Eomes (Dan11mag), anti–T-bet (4B10), anti–IFN-γ (XMG1.2), and anti-TNF (MP6-XT22).
In vitro stimulation
CD8+ T cells were enriched using negative magnetic isolation (Miltenyi Biotec) and cultured for bulk CD8+ T cell analysis or FACS sorted based on CD44 and CXCR3 expression (Fig. 1 D). Cells were cultured at 5.0 × 104 cells per 200 µl in a flat-bottom 96-well plate with immobilized anti-CD3 (145-2C11) and anti-CD28 (37.51) for 6–24 h. GolgiPlug (BD) was added for the final 4 h, before antibody staining. Cells were prepared for flow cytometry as described in the previous section, with the addition of fixable Live/Dead (Thermo Fisher Scientific) staining to exclude dead cells from analysis.
Adoptive transfers
CD8+ T cells were magnetically isolated as described in the previous section from congenic donors and either transferred as bulk CD8+ populations or first subjected to FACS sorting to isolate CD44hi CXCR3hi and CD44lo CXCR3lo populations. In some experiments, 1.5–2 × 106 WT BALB/c CD8+ T cells were adoptively transferred into congenic WT and IL-4 KO BALB/c mice. In the competitive adoptive transfer experiments, equal numbers of WT and IL-4 KO BALB/c CD8+ T cells (1.8–2.8 × 106 cells total, depending on the population and experiment) were adoptively transferred i.v. into WT BALB/c congenic recipients.
Data analysis and statistics
Flow cytometry data were analyzed using FlowJo (Tree Star). A two-tailed, unpaired or paired Student’s t test was performed on log-transformed data using Prism (GraphPad Software). P-values are represented as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
ACKNOWLEDGMENTS
We thank the members of the Jamequist Laboratory and the University of Minnesota Center for Immunology for critical input.
This work was supported by the National Institutes of Health grants R21 AI100088 and R01 AI075168 to S.C. Jameson and grant K99-AI114884 to Y.J. Lee and a postdoctoral fellowship from the University of Minnesota Cancer Biology Training Grant (T32 CA009138) to K.R. Renkema.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- LCMV
- lymphocytic choriomeningitis virus
- MPEC
- memory precursor effector cell
- SLEC
- short-lived effector cell
References
- Akue A.D., Lee J.Y., and Jameson S.C.. 2012. Derivation and maintenance of virtual memory CD8 T cells. J. Immunol. 188:2516–2523. 10.4049/jimmunol.1102213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac V.P., Corbin G.A., and Harty J.T.. 2000. Cutting edge: OFF cycling of TNF production by antigen-specific CD8+ T cells is antigen independent. J. Immunol. 165:5387–5391. 10.4049/jimmunol.165.10.5387 [DOI] [PubMed] [Google Scholar]
- Banerjee A., Gordon S.M., Intlekofer A.M., Paley M.A., Mooney E.C., Lindsten T., Wherry E.J., and Reiner S.L.. 2010. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 185:4988–4992. 10.4049/jimmunol.1002042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty S.A., Koretzky G.A., and Jordan M.S.. 2014. Interleukin-4 regulates eomesodermin in CD8+ T cell development and differentiation. PLoS One. 9:e106659 10.1371/journal.pone.0106659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho L.H., Sano G., Hafalla J.C., Morrot A., Curotto de Lafaille M.A., and Zavala F.. 2002. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat. Med. 8:166–170. 10.1038/nm0202-166 [DOI] [PubMed] [Google Scholar]
- Dai H., Wan N., Zhang S., Moore Y., Wan F., and Dai Z.. 2010. Cutting edge: programmed death-1 defines CD8+CD122+ T cells as regulatory versus memory T cells. J. Immunol. 185:803–807. 10.4049/jimmunol.1000661 [DOI] [PubMed] [Google Scholar]
- Gordon S.M., Carty S.A., Kim J.S., Zou T., Smith-Garvin J., Alonzo E.S., Haimm E., Sant’Angelo D.B., Koretzky G.A., Reiner S.L., and Jordan M.S.. 2011. Requirements for eomesodermin and promyelocytic leukemia zinc finger in the development of innate-like CD8+ T cells. J. Immunol. 186:4573–4578. 10.4049/jimmunol.1100037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluszczak C., Akue A.D., Hamilton S.E., Johnson L.D., Pujanauski L., Teodorovic L., Jameson S.C., and Kedl R.M.. 2009. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J. Exp. Med. 206:435–448. 10.1084/jem.20081829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacomet F., Cayssials E., Basbous S., Levescot A., Piccirilli N., Desmier D., Robin A., Barra A., Giraud C., Guilhot F., et al. 2015. Evidence for eomesodermin-expressing innate-like CD8+ KIR/NKG2A+ T cells in human adults and cord blood samples. Eur. J. Immunol. 45:1926–1933. 10.1002/eji.201545539 [DOI] [PubMed] [Google Scholar]
- Jameson S.C., Lee Y.J., and Hogquist K.A.. 2015. Innate memory T cells. Adv. Immunol. 126:173–213. 10.1016/bs.ai.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi N.S., Cui W., Chandele A., Lee H.K., Urso D.R., Hagman J., Gapin L., and Kaech S.M.. 2007. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 27:281–295. 10.1016/j.immuni.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S.M., and Cui W.. 2012. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12:749–761. 10.1038/nri3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S.M., Tan J.T., Wherry E.J., Konieczny B.T., Surh C.D., and Ahmed R.. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191–1198. 10.1038/ni1009 [DOI] [PubMed] [Google Scholar]
- Kim H.J., Verbinnen B., Tang X., Lu L., and Cantor H.. 2010. Inhibition of follicular T-helper cells by CD8+ regulatory T cells is essential for self tolerance. Nature. 467:328–332. 10.1038/nature09370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Wang X., Radfar S., Sproule T.J., Roopenian D.C., and Cantor H.. 2011. CD8+ T regulatory cells express the Ly49 class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc. Natl. Acad. Sci. USA. 108:2010–2015. 10.1073/pnas.1018974108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Gruta N.L., Rothwell W.T., Cukalac T., Swan N.G., Valkenburg S.A., Kedzierska K., Thomas P.G., Doherty P.C., and Turner S.J.. 2010. Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. J. Clin. Invest. 120:1885–1894. 10.1172/JCI41538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai D., Zhu J., Wang T., Hu-Li J., Terabe M., Berzofsky J.A., Clayberger C., and Krensky A.M.. 2011. KLF13 sustains thymic memory-like CD8+ T cells in BALB/c mice by regulating IL-4–generating invariant natural killer T cells. J. Exp. Med. 208:1093–1103. 10.1084/jem.20101527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Park S.P., Park C.H., Kang B.H., Park S.H., Ha S.J., and Jung K.C.. 2015. IL-4 induced innate CD8+ T cells control persistent viral infection. PLoS Pathog. 11:e1005193 10.1371/journal.ppat.1005193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y., Hamilton S.E., Akue A.D., Hogquist K.A., and Jameson S.C.. 2013a Virtual memory CD8 T cells display unique functional properties. Proc. Natl. Acad. Sci. USA. 110:13498–13503. 10.1073/pnas.1307572110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Jameson S.C., and Hogquist K.A.. 2011. Alternative memory in the CD8 T cell lineage. Trends Immunol. 32:50–56. 10.1016/j.it.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Holzapfel K.L., Zhu J., Jameson S.C., and Hogquist K.A.. 2013b Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 14:1146–1154. 10.1038/ni.2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.J., Peacock C.D., Bahl K., and Welsh R.M.. 2007. Programmed death-1 (PD-1) defines a transient and dysfunctional oligoclonal T cell population in acute homeostatic proliferation. J. Exp. Med. 204:2321–2333. 10.1084/jem.20062150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H.S., Lee Y.J., Jeon Y.K., Kim E.J., Kang B.H., Jung K.C., Chang C.H., and Park S.H.. 2011. MHC class II-restricted interaction between thymocytes plays an essential role in the production of innate CD8+ T cells. J. Immunol. 186:5749–5757. 10.4049/jimmunol.1002825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S.C., Heidorn S.M., Herbert D.R., Perkins C., Hildeman D.A., Khodoun M.V., and Finkelman F.D.. 2009. Endogenously produced IL-4 nonredundantly stimulates CD8+ T cell proliferation. J. Immunol. 182:1429–1438. 10.4049/jimmunol.182.3.1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrot A., Hafalla J.C., Cockburn I.A., Carvalho L.H., and Zavala F.. 2005. IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites. J. Exp. Med. 202:551–560. 10.1084/jem.20042463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley M.A., Kroy D.C., Odorizzi P.M., Johnnidis J.B., Dolfi D.V., Barnett B.E., Bikoff E.K., Robertson E.J., Lauer G.M., Reiner S.L., and Wherry E.J.. 2012. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 338:1220–1225. 10.1126/science.1229620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R.R., Li Q., Odunsi K., and Shrikant P.A.. 2010. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 32:67–78. 10.1016/j.immuni.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifa’i M., Kawamoto Y., Nakashima I., and Suzuki H.. 2004. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J. Exp. Med. 200:1123–1134. 10.1084/jem.20040395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba K., Shibata K., Iwamoto Y., Yoshikai Y., and Yamada H.. 2013. Naturally occurring PD-1+ memory phenotype CD8 T cells belong to nonconventional CD8 T cells and are cyclophosphamide-sensitive regulatory T cells. J. Immunol. 190:1560–1566. 10.4049/jimmunol.1202464 [DOI] [PubMed] [Google Scholar]
- Sosinowski T., White J.T., Cross E.W., Haluszczak C., Marrack P., Gapin L., and Kedl R.M.. 2013. CD8α+ dendritic cell trans presentation of IL-15 to naive CD8+ T cells produces antigen-inexperienced T cells in the periphery with memory phenotype and function. J. Immunol. 190:1936–1947. 10.4049/jimmunol.1203149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., and Surh C.D.. 2011. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat. Immunol. 12:478–484. 10.1038/ni.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventre E., Brinza L., Schicklin S., Mafille J., Coupet C.A., Marçais A., Djebali S., Jubin V., Walzer T., and Marvel J.. 2012. Negative regulation of NKG2D expression by IL-4 in memory CD8 T cells. J. Immunol. 189:3480–3489. 10.4049/jimmunol.1102954 [DOI] [PubMed] [Google Scholar]
- Weinreich M.A., Takada K., Skon C., Reiner S.L., Jameson S.C., and Hogquist K.A.. 2009. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 31:122–130. 10.1016/j.immuni.2009.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich M.A., Odumade O.A., Jameson S.C., and Hogquist K.A.. 2010. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat. Immunol. 11:709–716. 10.1038/ni.1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesundara D.K., Tscharke D.C., Jackson R.J., and Ranasinghe C.. 2013. Reduced interleukin-4 receptor α expression on CD8+ T cells correlates with higher quality anti-viral immunity. PLoS One. 8:e55788 10.1371/journal.pone.0055788 [DOI] [PMC free article] [PubMed] [Google Scholar]