Goodnow et al. show that in vaccinated humans, anergic autoreactive B cells can hypermutate the B cell receptor during the immune response and remove binding to self while enhancing binding to the vaccine.
Abstract
Clonal anergy is an enigmatic self-tolerance mechanism because no apparent purpose is served by retaining functionally silenced B cells bearing autoantibodies. Human autoantibodies with IGHV4-34*01 heavy chains bind to poly-N-acetyllactosamine carbohydrates (I/i antigen) on erythrocytes and B lymphocytes, cause cold agglutinin disease, and are carried by 5% of naive B cells that are anergic. We analyzed the specificity of three IGHV4-34*01 IgG antibodies isolated from healthy donors immunized against foreign rhesus D alloantigen or vaccinia virus. Each IgG was expressed and analyzed either in a hypermutated immune state or after reverting each antibody to its unmutated preimmune ancestor. In each case, the preimmune ancestor IgG bound intensely to normal human B cells bearing I/i antigen. Self-reactivity was removed by a single somatic mutation that paradoxically decreased binding to the foreign immunogen, whereas other mutations conferred increased foreign binding. These data demonstrate the existence of a mechanism for mutation away from self-reactivity in humans. Because 2.5% of switched memory B cells use IGHV4-34*01 and >43% of these have mutations that remove I/i binding, clonal redemption of anergic cells appears efficient during physiological human antibody responses.
Horror autotoxicus—avoidance of antibody formation against our own blood cell surface antigens (Ehrlich and Morgenroth, 1957)—is mostly viewed as arising from three conceptual mechanisms for actively acquired tolerance, for which there is now extensive experimental evidence (Goodnow, 2007; Goodnow and Ohashi, 2013). The first mechanism conceived is clonal deletion of immature B cells bearing autoantibodies during a tolerance-susceptible developmental window in the primary lymphoid organs (Burnet, 1959; Lederberg, 1959). This mechanism potentially creates holes in the repertoire by removing antibodies before they can be tested for binding to foreign antigens. An alternative two-signal concept articulated by Talmage and Pearlman (1963) and Claman (1963) proposed clonal deletion of self-antigen–stimulated B cells in primary or secondary lymphoid organs through terminal differentiation without cell division, in the absence of a second, proliferative signal from bacterial products such as lipopolysaccharide. Bretscher and Cohn (1970) extended the two-signal concept to the requirement for T cell help, raising the possibility that tolerance is not acquired by self-reactive B cells themselves but that autoantibodies are avoided because helper T cells do not recognize self-antigens. Nossal and Pike (1980) conceived a third mechanism, clonal anergy, wherein self-reactive B cells persist in an unresponsive state. Despite experimental evidence that a large fraction of the mouse and human naive B cell repertoires comprises anergic cells with self-reactive antibodies (Goodnow et al., 1989; Duty et al., 2009; Quách et al., 2011; Zikherman et al., 2012), it remains to be shown that autoantibodies carried by anergic cells contribute to antibody responses against foreign immunogens in healthy, nonautoimmune people.
A fourth theoretical mechanism for actively acquired self-tolerance (hypermutation away from self-reactivity) was hypothesized by Jerne (1971) 45 years ago but has received little serious attention. Jerne proposed that the primary lymphoid organs (thymus and Bursa of Fabricius–equivalent) served as mutant-breeding sites seeded with proliferating lymphocytes bearing antibody variable (V) segments that bind self-antigens, particularly against cell surface histocompatibility antigens. Hypermutation of the V-segments and active suppression of the cells that retain binding to self would select mutant progeny with V regions that no longer bind to self but comprise a diverse repertoire of potential antibodies against foreign antigens, including many that bind allogeneic histocompatibility antigens (thus explaining the unusually strong immune response to alloantigens). Jerne’s concept has parallels with the well established mechanism of receptor editing, whereby immature bone marrow B cells replace one self-binding V-segment with another to evade clonal deletion (Gay et al., 1993; Tiegs et al., 1993; Casellas et al., 2001). Upon finding that anergic B cells regain the capacity to secrete antibody when transplanted into animals lacking the relevant self-antigen and provide a source of T cell help, we previously hypothesized that a physiological rationale for reversal of anergy might be following V-segment hypermutation and mutation away from self-reactivity in germinal centers (Goodnow et al., 1991; Goodnow, 1996, 1997). Diaz and Klinman (2000) considered a similar scenario as one theoretical explanation for their evidence that a CD24low subset of B cells contributed little antibody in a primary response but much of the secondary response antibody. Recently, we provided direct experimental evidence in transgenic mice for clonal redemption: anergic B cells with high affinity for self-lysozyme were induced to hypermutate their V-segments in germinal centers upon immunization with lysozyme coupled to foreign red cells, and mutant progeny were efficiently selected for loss of binding to self-lysozyme (Sabouri et al., 2014). It nevertheless remains an open question whether hypermutation away from self actually occurs, particularly during physiological human antibody responses.
The goal of the current study was to determine whether clonal redemption occurs in normal human immune responses. To address this question, we required a source of antibody from B cells that were (a) anergic, (b) reactive with a well defined, pathologically significant blood cell surface autoantigen, and (c) frequent in healthy humans. B cells carrying antibodies with the heavy chain (H chain) V-segment IGHV4-34*01 meet these criteria, accounting for 5–10% of circulating naive B cells in healthy individuals and displaying anergic characteristics of down-regulated surface IgM, but not surface IgD, and functional unresponsiveness (Cappione et al., 2005). Most unmutated IGHV4-34 IgM antibodies agglutinate human erythrocytes at low temperatures, causing cold agglutinin hemolytic disease if secreted at sufficient titer because they bind poly-N-acetyllactosamine chains comprising the I/i blood group antigen or attached as O-linked carbohydrates to proteins, notably the B220 isoform of CD45 (Childs et al., 1983; Pascual et al., 1991; Silberstein et al., 1991; Thompson et al., 1991; Grillot-Courvalin et al., 1992; Cappione et al., 2004). The IGHV4-34 sequence contains a unique group of amino acids, Q6, W7, A24, V25, and Y26 (International Immunogenetics [IMGT] numbering), that form a hydrophobic patch and are essential for poly-N-acetyllactosamine binding and red cell agglutination (Li et al., 1996; Potter et al., 2002). Although B cells expressing IGHV4-34 with an intact hydrophobic patch are counter selected in the germinal center, memory, and secreted repertoire, IgG and IgA IGHV4-34 antibodies with hydrophobic patch mutations are observed at a considerable frequency (Chapman et al., 1996; Zheng et al., 2004; Cappione et al., 2005; Sabouri et al., 2014). However, it is not known whether these antibodies have mutated away from initial self-reactivity or were simply not I/i self-reactive in their preimmune state because of differences in their light chain (L chain) or CDR3 of the H chain. Here, we address this question for three IGHV4-34 IgG antibodies elicited against well defined foreign antigens in healthy vaccinated donors. By reverting somatic mutations to the preimmune sequence, we found that each was strongly self-reactive on the ancestral naive B cell. During the immune response, each has acquired mutations that remove binding to self-blood cells that are distinct from the mutations that enhance binding to the vaccine and come at a cost of decreased avidity for the vaccine antigen, demonstrating that humans have a clonal redemption mechanism for acquiring self–nonself discrimination by mutation away from self.
RESULTS
A previous Blastn search of deposited nucleotide sequences in the NCBI database identified foreign antigen-specific IgG antibodies with hypermutated IGHV4-34 H chains that had been elicited by immunization of healthy individuals (Sabouri et al., 2014). Here, we experimentally evaluated the self- and foreign reactivity of three of these antibodies, one against the erythrocyte alloantigen rhesus D (RhD) and two against vaccinia virus, for which the entire Ig H and L chain variable sequences were available and the foreign antigen readily obtained for binding studies. Each immune antibody was expressed as human IgG1. In parallel, the somatic mutations in the H and L chain of each antibody were reverted to the IMGT closest matched germline V, D, and J sequences to express IgG1 corresponding to each antibody’s unmutated, preimmune ancestor (designated with the prefix “p”). IgG1 antibodies containing single forward or reversion mutations in the hydrophobic patch were also expressed and purified.
Fog-1 RhD antibody
Anti-RhD antibodies are routinely elicited by repeated alloimmunization of healthy RhD-negative men with foreign RhD-positive erythrocytes to provide Rho immunoglobulin for prevention of erythroblastosis fetalis in primiparous RhD-negative mothers and to isolate large panels of monoclonal anti-RhD antibodies for typing and a potential substitute to polyclonal gamma globulin fractions. A high-affinity anti-RhD IgG antibody isolated in this manner, Fog-1 (Bye et al., 1992), is highly somatically mutated using the IGHV4-34*01 V, IGHD2-21*02, and IGHJ6*02 H chain elements with 19 amino acid substitutions and the IGKV1-17*01 and IGKJ2*01 L chain elements with 6 substitutions (Fig. 1, A and B). To test self-reactivity of Fog-1 and preimmune Fog-1 (pFog-1), we used flow cytometry to measure binding of the different expressed IgG antibodies to CD19+ IgD+ CD27− mature naive human B cells from normal blood donors, which display high densities of poly-N-acetyllactosamine I/i antigen O-linked carbohydrates on the B220 isoform of CD45 (Childs et al., 1983; Grillot-Courvalin et al., 1992). pFog-1 specifically and homogeneously bound CD19+ B cells compared with CD19− lymphocytes in a dose-dependent fashion, whereas the hypermutated Fog-1 antibody had no measurable binding at any concentration tested (Fig. 1 C). pFog-1 binding to self-blood B cells occurred at 37°C or 4°C, although over a range of antibody concentrations, binding was slightly reduced when performed at 37°C compared with 4°C (12% decreased; Fig. 1 E). One of the 19 H chain somatic mutations in Fog-1, Y26H (IMGT numbering), alters the hydrophobic patch alanine, valine, tyrosine (AVY) sequence (Fig. 1, A and F). When this single somatic mutation was introduced into the pFog-1 antibody, it was sufficient to completely abolish self-reactivity, comparable with Fog-1 with 18 additional H chain and 6 L chain mutations (pFog-1 Y26H; Fig. 1 C). Conversely, self-reactivity was restored when only this somatic mutation was reverted in Fog-1 (Fog-1 H26Y), albeit not to the level of pFog-1. Thus, a single Y26H somatic mutation acquired by pFog-1 was sufficient to remove all detectable self-reactivity, whereas all the other acquired mutations in Fog-1 were not sufficient to abolish binding to self-blood cells (Fig. 1 C).
Figure 1.
Somatic hypermutation of IGHV4-34 autoantibody Fog-1 removes self-reactivity while increasing binding to RhD alloantigen. (A and B) Variable domain amino acid sequence of the IMGT-predicted pFog-1 H chain (A) and L chain (B) and sequence substitutions acquired in the immune Fog-1 antibody (shown below in red). (C) Gating strategy for measuring self-reactivity of IgG by binding to mature naive B cells (CD19+, IgD+ CD27−, CD14−, and CD3−), flow cytometric histograms representative of four independent experiments, and dose-dependent binding measured as relative MFI. Data points are the mean and standard deviation of four separate experiments using PBMCs from four individual donors. Statistical significance was assessed using two-way ANOVA (*, P ≤ 0.05 for Fog-1 H26Y vs. Fog-1 and pFog-1 Y26H; ****, P ≤ 0.0001 for pFog-1 vs. Fog-1, pFog-1 Y26H, and Fog-1 H26Y). The key demonstrates the number of amino acid substitutions (#aa subs) in each antibody. Numbering follows IMGT. SHM, somatic hypermutation. (D) Binding of Fog-1 and pFog-1 IgG to RhD+ and RhD− erythrocytes. Data in D are representative of three separate experiments using erythrocytes from three individual donors. Statistical significance was determined by two-way ANOVA (****, P ≤ 0.0001 for Fog-1 and Fog-1 H26Y vs. pFog-1 and pFog Y26H). (E) Binding of pFog-1 to mature naive B cells at 4°C (continuous line) and 37°C (dashed line) by flow cytometry. Data are representative of two independent experiments, and statistical significance was determined by Student’s paired t test (P = 0.10). (F) Structural models depicting the hydrophobic patch region of pFog-1 (left) and Fog-1 (right). Preimmune residues are depicted in blue with immune substitutions in dark red and oxygen in light red.
Flow cytometry was also used to measure binding of Fog-1 and pFog-1 to foreign RhD+ and self-RhD− erythrocytes from healthy donors. Fog-1 bound homogeneously and specifically to RhD+ erythrocytes compared with erythrocytes from an RhD− donor (Fig. 1 D). The pFog-1 antibody showed minimal binding to RhD+ erythrocytes compared with RhD− erythrocytes, consistent with the very low affinity of the preimmune antibody for the immunizing foreign blood cells. Note that there is too little binding of pFog-1 IgG to the low density of I/i antigens on RhD− erythrocytes, even at 4°C, in contrast to the strong binding of pFog-1 to B cells displaying a high density of I/i antigens on B220. Interestingly, the Y26H somatic mutation that completely removed self-reactivity conferred no detectable increase in binding of pFog-1 Y26H to foreign RhD+ erythrocytes, nor did its reversion cause any measurable decrease in RhD− binding of Fog-1 H26Y (Fig. 1 D). Although we cannot determine at which point Y26H was acquired in the evolution of Fog-1, whether it was acquired as the first or as the last mutation, it conferred no measurable benefit for binding foreign RhD but completely removed binding to self.
166 vaccinia antibody
Having shown evidence for autoantibody redemption in alloimmunization, we next evaluated this hypothesis in the context of a procedure that led to global eradication of smallpox: immunization of healthy people with vaccinia virus. The vaccinia-specific IgG antibodies 166 and 589 were isolated by single-cell PCR from peripheral blood plasmablasts 9–20 d after standard immunization with vaccinia virus Lister strain in healthy, previously vaccinated volunteers (Lantto et al., 2011). The 166 antibody employs an IGHV4-34*01, IGHD3-3*01, and IGHJ4*02 H chain with 13 nonsynonymous somatic mutations paired with an IGKV3-11*01 and IGKJ1*01 L chain carrying five somatic substitutions (Fig. 2, A and B). The hypermutated 166 antibody exhibited no detectable binding to normal naive B cells, whereas when all the somatic mutations were reverted, the preimmune 166 (p166) antibody demonstrated homogenous, dose-dependent self-reactivity (Fig. 2 C). p166 antibody binding to normal human B cells occurred at 37° or 4°C (Fig. 2 E). Acquisition of a single mutation within the hydrophobic patch Y26S was sufficient to completely remove self-reactivity from the p166 antibody (p166 Y26S; Fig. 2, C and F). However, in the context of the other somatic mutations in 166, the Y26S mutation was redundant for reducing autoreactivity because when the S26 substitution was reverted to Y in the immune 166 antibody, this single back mutation alone did not restore self-binding (166 S26Y; Fig. 2, C and F).
Figure 2.
Discrete somatic mutations acquired by antivaccinia antibody 166 independently remove self-reactivity and increase virus reactivity. (A and B) Variable domain amino acid sequence of the p166 H chain (A) and L chain (B) and sequence substitutions acquired in the immune 166 antibody (shown below in red). (C) Number of amino acid substitutions (#aa subs) in each antibody and binding to mature naive B cells. Data points are the mean and standard deviation of four independent experiments using PBMCs from four individual donors. Statistical significance was assessed using two-way ANOVA (****, P ≤ 0.0001 for p166 vs. 166, p166 Y26S, and 166 S26Y). SHM, somatic hypermutation. (D) Binding of each antibody to vaccinia virus measured by ELISA. The data are representative of three independent experiments with each data point showing the mean of duplicates. Statistical significance was determined by two-way ANOVA (*, P ≤ 0.05 for p166 vs. p166 Y26S; ***, P ≤ 0.001 for 166 and 166 S26Y vs. p166 and p166 Y26S). (E) Binding of p166 to mature naive B cells at 4°C (continuous line) and 37°C (dashed line). Data are representative of two independent experiments, and statistical significance was determined by Student’s paired t test (P = 0.05). (F) Three-dimensional structure of a hydrophobic patch for p166 (left) and 166 (right). Preimmune residues are depicted in blue with immune substitutions in dark red and oxygen in light red.
Binding to vaccinia virus by the 166 antibody and its reverted preimmune ancestor was measured by ELISA (Fig. 2 D). Binding of p166 to the virus was less than the fully mutated 166, consistent with affinity maturation against the virus. However, acquisition of the Y26S mutation in the hydrophobic patch did not appear to contribute to affinity maturation because it decreased p166 binding to the virus (Fig. 2 D), providing further evidence for a mechanism that allows selection of mutations that reduce antibody affinity for self, independent of mutations that increase binding to foreign antigens.
589 vaccinia antibody
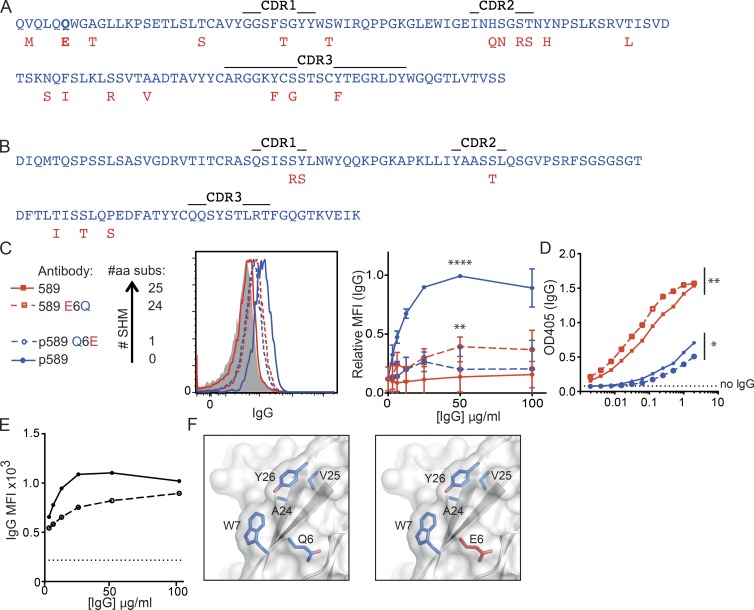
Both Fog-1 and 166 antibodies escaped self-reactivity by acquiring mutations in the Y26 residue, whereas the 589 vaccinia antibody maintained Y26 but acquired a mutation in another hydrophobic patch residue, Q6E (Fig. 3 F). The 589 antibody binds to a major membrane protein of the mature vaccinia virion A14 (Lantto et al., 2011) and employs an IGHV4-34*01, IGHD2-2*02, and IGHJ4*02 H chain with 19 somatic substitutions paired with an IGKV1-39*01 and IGKJ1*01 L chain with 6 substitutions (Fig. 3, A and B). The hypermutated antibody lacked binding to self–B cells, but the preimmune p589 derivative was self-reactive, binding B cells at concentrations <4 µg/ml at 37 or 4°C (Fig. 3 E). B cell binding was reduced but not eliminated by acquisition of the Q6E somatic mutation on its own (p589 Q6E; Fig. 3 C), and reversion of this somatic mutation alone was sufficient to restore an intermediate level of self-binding in the otherwise fully mutated antibody (589 E6Q). Although the single point mutation Q6E was necessary and sufficient to decrease self-reactivity, it also decreased binding to the virus: acquisition of this somatic mutation alone markedly decreased binding of p589 Q6E to vaccinia, and reversion of just this mutation enhanced binding of the otherwise fully hypermutated 589 E6Q (Fig. 3 D). The Q6E mutation provides a clear example of antibody mutation away from self-reactivity at the cost of decreased binding to the eliciting foreign antigen.
Figure 3.
A single somatic mutation acquired by antivaccinia antibody 589 reduces self- and foreign reactivity, whereas other mutations increase binding to foreign antigens. (A and B) Variable region amino acid sequence of the p589 H chain (A) and L chain (B) and sequence substitutions acquired in the immune 589 antibody (shown below in red). (C) Key depicting the number of amino acids substitutions (#aa subs) in each antibody and its binding to mature naive B cells. Data points are the mean and standard deviation of three separate experiments using PBMCs from three individual donors. Statistical significance was assessed by two-way ANOVA (**, P ≤ 0.01 for 589 vs. 589 E6Q; ****, P ≤ 0.0001 for p589 vs. 589, p589 Q6E, and 589 E6Q). SHM, somatic hypermutation. (D) Binding of each antibody to vaccinia virus measured by ELISA. Data are representative of two separate experiments with each point the mean of duplicates. Statistical significance was determined by two-way ANOVA (*, P ≤ 0.05 for p589 vs. p589 Q6E; **, P ≤ 0.01 for 589 vs. 589 E6Q). (E) Binding of p589 at 4°C (continuous line) and 37°C (dashed line). Data are representative of two independent experiments with differences evaluated by Student’s paired t test (P = 0.02). (F) Structural modeling of a hydrophobic patch of p589 (left) and 589 (right). Preimmune residues are depicted in blue with immune substitutions in dark red and oxygen in light red.
Acquisition of very low polyreactivity
Mutation away from self-reactivity with cell surface poly-N-acetyllactosamine, as observed for the IGHV4-34 antibodies studied here, contrasts to previous data demonstrating acquisition of polyreactivity with self-antigens through hypermutation in antibodies in the circulating IgG–memory B cell pool (Tiller et al., 2007). To determine whether Fog-1, 166, or 589 IGHV4-34 antibodies become polyreactive after somatic mutation, we evaluated the binding of preimmune and mutated antibodies to HEK293 cells, double-stranded DNA (dsDNA), and LPS compared with established polyreactive antibody controls. The positive control used was the weakly polyreactive eiJB40 antibody cloned from an early immature bone marrow B cell with down-regulated surface IgM, and the negative control was the nonpolyreactive mGO53 antibody cloned from a mature naive B cell (Wardemann et al., 2003). None of the IGHV4-34 antibodies or mGO53 bound to surface or intracellular self-antigens expressed by HEK293 cells, whereas eiJB40 showed dose-dependent binding to both intact and permeabilized HEK293 (Fig. 4 A). ELISAs evaluating LPS and dsDNA reactivity showed dose-dependent binding of eiJB40. The reactivity of IGHV4-34 antibodies was equivalent to the negative control mGO53 with the exception of Fog-1 and 589, which showed low binding to dsDNA and LPS that was much less than eiJB40 and would be classed as negative on this basis (Tiller et al., 2007) but was, nevertheless, greater than mGO53 or the pFog-1 and p589 antibodies (Fig. 4 B). The rise in very low binding to these antigens that accompanies affinity maturation to foreign RhD and vaccinia in Fog-1 and 589 is consistent with the increased polyreactivity observed in randomly sampled switched memory B cells (Tiller et al., 2007), indicating that clonal selection to mutate away from specific binding to an abundant cell surface autoantigen, poly-N-acetyllactosamine, differs from selection acting upon polyreactive binding to DNA. The latter property may be ignored because of low affinity and limited B cell exposure to DNA and arise simply as an accidental byproduct of mutations that enhance binding to the foreign antigens. Alternatively, weak polyreactivity could be positively selected to enhance persistence of memory B cells when insufficient foreign antigen remains (Mouquet et al., 2010; Wrammert et al., 2011).
Figure 4.
Testing polyreactivity of IGHV4-34 antibodies. (A) Flow cytometric evaluation of binding preimmune and mutated IGHV4-34 and negative and low-positive control antibodies mGO53 and eiJB40, respectively, to intact (extracellular) and permeabilized (intracellular) HEK293 cells. Data are representative of two independent experiments. (B) Binding of IGHV4-34 IgG and control antibodies to LPS and dsDNA by ELISA. Data points are the mean and standard deviation of triplicate data points, representative of two independent experiments.
DISCUSSION
All three of the human IgG antibodies, Fog-1, 166, and 589, are shown to have started out before somatic hypermutation as potentially pathogenic autoantibodies that bind under physiological conditions to an abundant human blood cell surface autoantigen, poly-N-acetyllactosamine. Rather than triggering clonal deletion or editing in the bone marrow, secretion of these potentially damaging autoantibodies was likely controlled by B cell clonal anergy because B cells expressing the IGHV4-34 segment without hydrophobic patch mutations exhibit the phenotypic and functional characteristics of anergic B cells in the naive B cell repertoire and are much less frequent in the germinal center and memory B cell subsets in healthy people (Cappione et al., 2005). B cells carrying each autoantibody were, nevertheless, recruited into physiological immunization responses and acquired numerous somatic mutations that had two effects: removal of self-reactivity and increased binding to the foreign antigen. These results are consistent with the Jerne (1971) concept of mutant-breeding organs seeded with self-reactive precursors and with experimental evidence in transgenic mice that anergic B cells can be reactivated by foreign antigens and suitable T cell help to proliferate in germinal centers and undergo hypermutation away from self (Sabouri et al., 2014).
In each of the human antibodies analyzed here, we identified a single, acquired somatic mutation that was sufficient to decrease or eliminate self-reactivity. In the two vaccinia-elicited antibodies, this single mutation also decreased binding to the foreign antigen. In the case of the RhD antibody, binding of the unmutated preimmune antibody to foreign RhD+ erythrocytes was too weak to test whether the mutation that removed self-reactivity also decreased binding to the foreign antigen. Diminished binding to RhD is nevertheless likely because experimental mutagenesis of an IGHV4-34 IgM antibody to RhD revealed that substitution of Y26 or other hydrophobic patch residues diminished or abolished binding to RhD+ erythrocytes in parallel with abrogating I/i erythrocyte self-reactivity (Thorpe et al., 2008). Acquisition of these affinity-lowering hydrophobic patch mutations is difficult to explain by the conventional view of germinal center selection for increased affinity against the foreign antigen. These mutations can be explained, however, by positing existence in humans of a germinal center mechanism for selecting decreased binding to self, even at the expense of decreased affinity for foreign antigen (Sabouri et al., 2014). Two mechanisms could both account for these observations. First, lowered affinity for self-antigen would decrease occupancy and down-regulation of antigen receptors by poly-N-acetyllactosamine, increasing receptor availability to bind RhD or vaccinia and compensating for lower affinity to foreign antigens. Second, lowered affinity for self may decrease antigen receptor–induced apoptosis and migration away from the germinal center light zone (Shokat and Goodnow, 1995).
A recent study evaluating vaccination responses in patients with systemic lupus erythematosus reverted four influenza-specific antibodies (not IGHV4-34) to their predicted preimmune configuration and noted anti-dsDNA reactivity in two ancestral preimmune antibodies (Kaur et al., 2015). However, it cannot be determined whether the loss of DNA recognition was a passive consequence of affinity maturation toward influenza or an active selection for mutations that reduced self-reactivity. Our study provides clear evidence for the latter in healthy immunized individuals because mutations that increased affinity to foreign antigens could be shown to be separate from mutations within the hydrophobic patch that reduced binding to both self- and foreign antigens.
Unlike the three IgG antibodies studied here, some antibodies may be trapped in an evolutionary cul-de-sac where it is difficult or impossible to mutate away from self without also decreasing binding to foreign antigens. This situation appears to exist for the majority of IgM antibodies initially elicited by RhD alloimmunization, which use the same IGHV4-34 segment as Fog-1 but paired with an IGLV3-3 lambda L chain (Thompson et al., 1991; Bye et al., 1992; Siegel et al., 2002). These do acquire somatic mutations but in regions other than the hydrophobic patch residues, presumably because mutating the latter also abrogates RhD binding (Thorpe et al., 2008), apparently trapping the IGHV4-34/IGLV3-3 combination to explain why it is not found among the high-affinity RhD IgGs (Bye et al., 1992; Siegel et al., 2002). Fog-1 is an interesting exception: pairing of IGHV4-34 with IGKV1-17 kappa appears to open up a structure-evolving pathway to lose self-reactivity by acquiring a hydrophobic patch mutation without loss of RhD binding.
Does mutation of IGHV4-34 antibodies away from self-reactivity, as established in the three examples studied here, represent a rare salvage pathway or an efficient process for specificity maturation? Previous studies showed that in nonautoimmune humans, the frequency of B cells binding an IGHV4-34 antiidiotype antibody, 9G4, was 5–7% in naive B cells but only 0.4–1.2% of germinal center B cells and 0.6–1.6% of IgG memory B cells (Pugh-Bernard et al., 2001; Cappione et al., 2005). However, only a modest decrease in the use of the IGHV4-34*01 element was measured by deep sequencing IGH repertoires of normal human B cell subsets: from 5% of naive B cells to 2.5% of switched memory B cells (Wu et al., 2010, 2011). Of the switched memory IGHV4-34 sequences, 43% had acquired AVY somatic mutations (Sabouri et al., 2014), which typically inactivate 9G4 binding as well as I/i self-reactivity because 9G4 binding overlaps the hydrophobic patch and competes with I/i binding (Potter et al., 1993; Zheng et al., 2004). Similarly, Chapman et al. (1996) found AVY mutations in 4/14 IgG and 4/6 IgA sequences from tonsillectomy samples, and Zheng et al. (2004) found hydrophobic patch mutations in 38% of IGHV4-34 IgG-switched B cells in human tonsils. Scoring for mutations in the AVY residues alone will underestimate the frequency of mutations that remove self-reactivity, as illustrated here by the 589 antibody. Collectively, these data indicate that IGHV4-34 antibodies, despite being self-reactive and inducing anergy in naive B cells, efficiently mutate away from self so that their contribution to the switched memory antibody repertoire is 50% of their contribution to the naive repertoire. The loss of the 9G4 idiotype from most of the germinal center and memory B cells of healthy people (Pugh-Bernard et al., 2001; Cappione et al., 2005) may primarily reflect efficient selection in the germinal center for mutation away from self.
In contrast to healthy donors, patients with systemic lupus erythematosus have little decrease in the frequency of 9G4-reactive B cells in the germinal center, switched memory, and plasma cell compartments (Cappione et al., 2005; Richardson et al., 2013). In light of the data presented here, these people may not have a deficit in clonal anergy per se but instead a failure to select germinal center progeny for mutations that remove self-reactivity, e.g., because of overabundant T follicular helper cells (Vinuesa et al., 2005; Simpson et al., 2010) or intrinsic abnormalities in the B cells (Butt et al., 2015). Cold agglutinin IGHV4-34 IgM antibodies are often produced during chronic EBV or mycoplasma infection, although rarely in sufficient titer to cause hemolytic anemia, and six EBV-induced antibodies that were cloned and sequenced all lacked somatic mutations (Chapman et al., 1993). In Wiskott-Aldrich syndrome, there is consistent secretion of IGHV4-34 IgM antibodies that bind poly-N-acetyllactosamine on mature naive B cells, with little or no somatic mutation in the cloned antibodies (Grillot-Courvalin et al., 1992). Thus, certain infections and inherited defects allow anergic B cells to be reactivated and proceed into antibody secretion without hypermutation or selection for loss of self-reactivity.
Pathological formation of IGHV4-34 autoantibodies in infectious and autoimmune diseases raises the question: what might be the advantage of clonal redemption of anergic B cells as a tolerance mechanism when it has the inherent risk of autoimmunity? For T cells, holes in the naive repertoire produced by clonal deletion are difficult for microbes to exploit because MHC polymorphism ensures the holes are different in each individual of a species. For the naive B cell repertoire, however, holes produced by deletion or editing will be the same in all individuals and indeed in most species because the self-antigen repertoire evolves slowly, providing steady targets for microbes to mimic and evade antibody formation. One evolutionary solution may lie in the high degree of inherited polymorphism in the IGHV repertoire (Kidd et al., 2012; Collins et al., 2015). The complementary solution is to retain autoreactive B cells in an anergic state within the naive repertoire, enabling somatic mutation away from self-reactivity when activated and receiving help from T follicular helper cells upon binding to a microbial antigen, as shown here for antibodies against vaccinia virus.
MATERIALS AND METHODS
IGHV4-34 antibodies
Deposited H and L chain sequences for each antibody were obtained from the NCBI database, and somatic mutations in the closest matching V, D and J elements were manually annotated using the IMGT V-quest junction analysis tool (Brochet et al., 2008; Giudicelli et al., 2011). Numbering follows the IMGT schema. Mutated and preimmune-reverted H and L chain cDNA sequences were synthesized by Biomatik and cloned into pCDNA3.1+ expression vectors. IgG1 antibodies were transiently expressed in HEK293 cells using standard plate transfection and the Expi system (Thermo Fisher Scientific) and purified with protein G Sepharose (GE Healthcare) according to the manufacturers’ recommendations. After dialyzing against PBS and concentrating, antibodies were quantified by Nanodrop and ELISA.
Assay for self-reactivity
Whole blood from random healthy donors was obtained from the Australian Red Cross Blood Service. PBMCs were prepared by Ficoll Histopaque and frozen. Thawed PBMCs were washed twice in PBS/1% BSA and incubated with Fc Block (BD) for 30 min at 4°C. After washing, cells were incubated with IGHV4-34 antibodies diluted in PBS and incubated for 30 min at 4°C or 37°C. Cells were washed twice in PBS and stained with antibodies against IgG, CD19, IgD, CD27, Live Dead, CD3, and CD14. Mature naive B cells (CD19+, IgD+, and CD27−) were evaluated for IGHV4-34 antibody binding on an LSRII or CANTO II flow cytometer (BD). Each group of preimmune, hypermutated, and intermediate IGHV4-34 antibodies (e.g., pFog-1, Fog-1, pFog-1 Y26H, and Fog-1 H26Y) were always assessed within the same experiment for three to four independent experiments. To normalize data from the three to four experiments performed on different days and with different flow cytometers, antibody reactivity was expressed as relative mean fluorescence intensity (MFI) by dividing the MFI value by the MFI of the highest binding antibody within the experiment.
Assay for RhD reactivity
Erythrocytes were isolated from the whole blood of RhD+ and RhD− donors from the Red Cross Blood Service. Approximately 10 ml of whole blood was diluted 1:1 in PBS and centrifuged at 400 g for 15 min. Plasma and buffy coat layers were removed, and red cell pellets were washed twice in PBS before incubating with IGHV4-34 antibodies at room temperature for 30 min. After washing twice in PBS, erythrocytes were stained with anti–human IgG antibody for 30 min at room temperature and analyzed by flow cytometry.
Assay for vaccinia virus reactivity
IGHV4-34 antibody binding to vaccinia virus was evaluated by ELISA. MaxiSorp plates (Thermo Fisher Scientific) were coated with sucrose cushion–purified stocks of Western Reserve vaccinia virus (provided by D. Tscharke, Australian National University, Canberra, ACT, Australia), inactivated with 1 µg/ml 4,5,8-trimethylpsoralen, and irradiated with UV-A for 20 min. ELISA plates were blocked with 2% BSA/PBS and then incubated with IGHV4-34 antibodies diluted in 1% BSA/PBS. After washing with 0.1% Tween 20/PBS, anti–human IgG alkaline phosphatase was applied, followed by para-nitrophenylphosphate substrate. All incubations were done for 1 h at room temperature. Binding of IGHV4-34 antibodies to vaccinia virus was quantified by measuring optical density at 405 nm.
Assay for polyreactivity
Polyreactivity was evaluated by flow cytometry and ELISA. For flow cytometry experiments, HEK293 cells were incubated with preimmune and mutated IGHV4-34 antibodies for 30 min at 4°C. After washing, anti–human IgG–BV605 was added for 30 min at 4°C and analyzed on a CANTO II flow cytometer. HEK293 cells were also permeabilized with Cytofix reagent (BD) to assess binding of antibodies to intracellular self-antigens. Polyreactivity ELISAs were performed as described previously using dsDNA and LPS (Tiller et al., 2008). For both flow cytometry and ELISA assays, established polyreactivity control antibodies were used: eiJB40, a low-positive control, and mGO53, a negative control antibody (Wardemann et al., 2003). These antibodies were produced as IgG1 as described in the IGHV4-34 antibodies section.
Structural modeling
Structural models depicting hydrophobic patch regions of IGHV4-34 were based on Protein Data Bank accession no. 1DNO (Cauerhff et al., 2000). All illustrations were generated using the PyMOL molecular graphics system (Schrödinger, LLC).
Statistical analysis
Dose-dependent binding of IGHV4-34 antibodies to either self- or foreign antigens was compared by two-way ANOVA with Tukey’s multiple comparison tests. Calculations were performed using Prism software (version 6; GraphPad Software). P-values <0.05 were considered significant.
ACKNOWLEDGMENTS
We thank Dr. Mary Christie for the generation of structural models, Prof. David Tscharke for the gift of vaccinia virus, and Dr. Peter Schofield and Prof. Robert Brink for experimental advice and feedback on the manuscript.
This work was supported by a National Health and Medical Research Council (NHMRC) Early Career fellowship (595989) to J.H. Reed and an NHMRC program grant (1016953), an Australia fellowship (585490), and a Senior Principal Research fellowship (1081858) to C.C. Goodnow.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- dsDNA
- double-stranded DNA
- H chain
- heavy chain
- L chain
- light chain
- MFI
- mean fluorescence intensity
- RhD
- rhesus D
References
- Bretscher P., and Cohn M.. 1970. A Theory of self-nonself discrimination: Paralysis and induction involve the recognition of one and two determinants on an antigen, respectively. Science. 169:1042–1049. 10.1126/science.169.3950.1042 [DOI] [PubMed] [Google Scholar]
- Brochet X., Lefranc M.P., and Giudicelli V.. 2008. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 36:W503–W508. 10.1093/nar/gkn316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236 pp.Burnet F.M. 1959. The clonal selection theory of acquired immunity. Cambridge University Press, Cambridge, MA: 10.5962/bhl.title.8281 [DOI] [Google Scholar]
- Butt D., Chan T.D., Bourne K., Hermes J.R., Nguyen A., Statham A., O’Reilly L.A., Strasser A., Price S., Schofield P., et al. 2015. FAS inactivation releases unconventional germinal center B cells that escape antigen control and drive IgE and autoantibody production. Immunity. 42:890–902. 10.1016/j.immuni.2015.04.010 [DOI] [PubMed] [Google Scholar]
- Bye J.M., Carter C., Cui Y., Gorick B.D., Songsivilai S., Winter G., Hughes-Jones N.C., and Marks J.D.. 1992. Germline variable region gene segment derivation of human monoclonal anti-Rh(D) antibodies. Evidence for affinity maturation by somatic hypermutation and repertoire shift. J. Clin. Invest. 90:2481–2490. 10.1172/JCI116140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappione A.J., Pugh-Bernard A.E., Anolik J.H., and Sanz I.. 2004. Lupus IgG VH4.34 antibodies bind to a 220-kDa glycoform of CD45/B220 on the surface of human B lymphocytes. J. Immunol. 172:4298–4307. 10.4049/jimmunol.172.7.4298 [DOI] [PubMed] [Google Scholar]
- Cappione A. III, Anolik J.H., Pugh-Bernard A., Barnard J., Dutcher P., Silverman G., and Sanz I.. 2005. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J. Clin. Invest. 115:3205–3216. 10.1172/JCI24179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casellas R., Shih T.A., Kleinewietfeld M., Rakonjac J., Nemazee D., Rajewsky K., and Nussenzweig M.C.. 2001. Contribution of receptor editing to the antibody repertoire. Science. 291:1541–1544. 10.1126/science.1056600 [DOI] [PubMed] [Google Scholar]
- Cauerhff A., Braden B.C., Carvalho J.G., Aparicio R., Polikarpov I., Leoni J., and Goldbaum F.A.. 2000. Three-dimensional structure of the Fab from a human IgM cold agglutinin. J. Immunol. 165:6422–6428. 10.4049/jimmunol.165.11.6422 [DOI] [PubMed] [Google Scholar]
- Chapman C.J., Spellerberg M.B., Smith G.A., Carter S.J., Hamblin T.J., and Stevenson F.K.. 1993. Autoanti-red cell antibodies synthesized by patients with infectious mononucleosis utilize the VH4-21 gene segment. J. Immunol. 151:1051–1061. [PubMed] [Google Scholar]
- Chapman C.J., Mockridge C.I., Hamblin T.J., and Stevenson F.K.. 1996. Tracking of the V4-34 (VH4-21) gene in human tonsil reveals clonal isotype switch events and a highly variable degree of somatic hypermutation. Clin. Exp. Immunol. 105:360–368. 10.1046/j.1365-2249.1996.d01-769.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs R.A., Dalchau R., Scudder P., Hounsell E.F., Fabre J.W., and Feizi T.. 1983. Evidence for the occurrence of O-glycosidically linked oligosaccharides of poly-N-acetyllactosamine type on the human leucocyte common antigen. Biochem. Biophys. Res. Commun. 110:424–431. 10.1016/0006-291X(83)91166-X [DOI] [PubMed] [Google Scholar]
- Claman H.N. 1963. Tolerance to a protein antigen in adult mice and the effect of nonspecific factors. J. Immunol. 91:833–839. [PubMed] [Google Scholar]
- Collins A.M., Wang Y., Roskin K.M., Marquis C.P., and Jackson K.J.. 2015. The mouse antibody heavy chain repertoire is germline-focused and highly variable between inbred strains. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370:20140236 10.1098/rstb.2014.0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M., and Klinman N.R.. 2000. Relative roles of somatic and Darwinian evolution in shaping the antibody response. Immunol. Res. 21:89–102. 10.1385/IR:21:2-3:89 [DOI] [PubMed] [Google Scholar]
- Duty J.A., Szodoray P., Zheng N.Y., Koelsch K.A., Zhang Q., Swiatkowski M., Mathias M., Garman L., Helms C., Nakken B., et al. 2009. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J. Exp. Med. 206:139–151. 10.1084/jem.20080611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich P., and Morgenroth J.. 1957. On Haemolysins. Pergamon Press, London. 246 pp. [Google Scholar]
- Gay D., Saunders T., Camper S., and Weigert M.. 1993. Receptor editing: an approach by autoreactive B cells to escape tolerance. J. Exp. Med. 177:999–1008. 10.1084/jem.177.4.999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudicelli V., Brochet X., and Lefranc M.P.. 2011. IMGT/V-QUEST: IMGT standardized analysis of the immunoglobulin (IG) and T cell receptor (TR) nucleotide sequences. Cold Spring Harb. Protoc. 2011:695–715. 10.1101/pdb.prot5633 [DOI] [PubMed] [Google Scholar]
- Goodnow C.C. 1996. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc. Natl. Acad. Sci. USA. 93:2264–2271. 10.1073/pnas.93.6.2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C.C. 1997. Glimpses into the balance between immunity and self-tolerance. Ciba Found. Symp. 204:190–207. [DOI] [PubMed] [Google Scholar]
- Goodnow C.C. 2007. Multistep pathogenesis of autoimmune disease. Cell. 130:25–35. 10.1016/j.cell.2007.06.033 [DOI] [PubMed] [Google Scholar]
- Goodnow C.C., and Ohashi P.S.. 2013. Immunological tolerance. In Fundamental Immunology. Paul W.E., editor. Wolters Kluwer Health/Lippincott Williams and Wilkins, Philadelphia: 765–794. [Google Scholar]
- Goodnow C.C., Crosbie J., Jorgensen H., Brink R.A., and Basten A.. 1989. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 342:385–391. 10.1038/342385a0 [DOI] [PubMed] [Google Scholar]
- Goodnow C.C., Brink R., and Adams E.. 1991. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 352:532–536. 10.1038/352532a0 [DOI] [PubMed] [Google Scholar]
- Grillot-Courvalin C., Brouet J.C., Piller F., Rassenti L.Z., Labaume S., Silverman G.J., Silberstein L., and Kipps T.J.. 1992. An anti-B cell autoantibody from Wiskott-Aldrich syndrome which recognizes i blood group specificity on normal human B cells. Eur. J. Immunol. 22:1781–1788. 10.1002/eji.1830220717 [DOI] [PubMed] [Google Scholar]
- Jerne N.K. 1971. The somatic generation of immune recognition. Eur. J. Immunol. 1:1–9. 10.1002/eji.1830010102 [DOI] [PubMed] [Google Scholar]
- Kaur K., Zheng N.Y., Smith K., Huang M., Li L., Pauli N.T., Henry Dunand C.J., Lee J.H., Morrissey M., Wu Y., et al. 2015. High affinity antibodies against influenza characterize the plasmablast response in SLE patients after vaccination. PLoS One. 10:e0125618 10.1371/journal.pone.0125618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd M.J., Chen Z., Wang Y., Jackson K.J., Zhang L., Boyd S.D., Fire A.Z., Tanaka M.M., Gaëta B.A., and Collins A.M.. 2012. The inference of phased haplotypes for the immunoglobulin H chain V region gene loci by analysis of VDJ gene rearrangements. J. Immunol. 188:1333–1340. 10.4049/jimmunol.1102097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantto J., Haahr Hansen M., Rasmussen S.K., Steinaa L., Poulsen T.R., Duggan J., Dennis M., Naylor I., Easterbrook L., Bregenholt S., et al. 2011. Capturing the natural diversity of the human antibody response against vaccinia virus. J. Virol. 85:1820–1833. 10.1128/JVI.02127-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J. 1959. Genes and antibodies: Do antigens bear instructions for antibody specificity or do they select cell lines that arise by mutation? Science. 129:1649–1653. 10.1126/science.129.3364.1649 [DOI] [PubMed] [Google Scholar]
- Li Y., Spellerberg M.B., Stevenson F.K., Capra J.D., and Potter K.N.. 1996. The I binding specificity of human VH 4-34 (VH 4-21) encoded antibodies is determined by both VH framework region 1 and complementarity determining region 3. J. Mol. Biol. 256:577–589. 10.1006/jmbi.1996.0110 [DOI] [PubMed] [Google Scholar]
- Mouquet H., Scheid J.F., Zoller M.J., Krogsgaard M., Ott R.G., Shukair S., Artyomov M.N., Pietzsch J., Connors M., Pereyra F., et al. 2010. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 467:591–595. 10.1038/nature09385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G.J., and Pike B.L.. 1980. Clonal anergy: persistence in tolerant mice of antigen-binding B lymphocytes incapable of responding to antigen or mitogen. Proc. Natl. Acad. Sci. USA. 77:1602–1606. 10.1073/pnas.77.3.1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual V., Victor K., Lelsz D., Spellerberg M.B., Hamblin T.J., Thompson K.M., Randen I., Natvig J., Capra J.D., and Stevenson F.K.. 1991. Nucleotide sequence analysis of the V regions of two IgM cold agglutinins. Evidence that the VH4-21 gene segment is responsible for the major cross-reactive idiotype. J. Immunol. 146:4385–4391. [PubMed] [Google Scholar]
- Potter K.N., Li Y., Pascual V., Williams R.C. Jr., Byres L.C., Spellerberg M., Stevenson F.K., and Capra J.D.. 1993. Molecular characterization of a cross-reactive idiotope on human immunoglobulins utilizing the VH4-21 gene segment. J. Exp. Med. 178:1419–1428. 10.1084/jem.178.4.1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter K.N., Hobby P., Klijn S., Stevenson F.K., and Sutton B.J.. 2002. Evidence for involvement of a hydrophobic patch in framework region 1 of human V4-34-encoded Igs in recognition of the red blood cell I antigen. J. Immunol. 169:3777–3782. 10.4049/jimmunol.169.7.3777 [DOI] [PubMed] [Google Scholar]
- Pugh-Bernard A.E., Silverman G.J., Cappione A.J., Villano M.E., Ryan D.H., Insel R.A., and Sanz I.. 2001. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J. Clin. Invest. 108:1061–1070. 10.1172/JCI200112462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quách T.D., Manjarrez-Orduño N., Adlowitz D.G., Silver L., Yang H., Wei C., Milner E.C., and Sanz I.. 2011. Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. J. Immunol. 186:4640–4648. 10.4049/jimmunol.1001946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C., Chida A.S., Adlowitz D., Silver L., Fox E., Jenks S.A., Palmer E., Wang Y., Heimburg-Molinaro J., Li Q.Z., et al. 2013. Molecular basis of 9G4 B cell autoreactivity in human systemic lupus erythematosus. J. Immunol. 191:4926–4939. 10.4049/jimmunol.1202263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabouri Z., Schofield P., Horikawa K., Spierings E., Kipling D., Randall K.L., Langley D., Roome B., Vazquez-Lombardi R., Rouet R., et al. 2014. Redemption of autoantibodies on anergic B cells by variable-region glycosylation and mutation away from self-reactivity. Proc. Natl. Acad. Sci. USA. 111:E2567–E2575. 10.1073/pnas.1406974111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokat K.M., and Goodnow C.C.. 1995. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 375:334–338. 10.1038/375334a0 [DOI] [PubMed] [Google Scholar]
- Siegel D.L., Czerwinski M., and Spitalnik S.L.. 2002. Section 5: Structural/genetic analysis of mAbs to blood group antigens. Coordinator’s report. Transfus. Clin. Biol. 9:83–97. 10.1016/S1246-7820(01)00224-5 [DOI] [PubMed] [Google Scholar]
- Silberstein L.E., Jefferies L.C., Goldman J., Friedman D., Moore J.S., Nowell P.C., Roelcke D., Pruzanski W., Roudier J., and Silverman G.J.. 1991. Variable region gene analysis of pathologic human autoantibodies to the related i and I red blood cell antigens. Blood. 78:2372–2386. [PubMed] [Google Scholar]
- Simpson N., Gatenby P.A., Wilson A., Malik S., Fulcher D.A., Tangye S.G., Manku H., Vyse T.J., Roncador G., Huttley G.A., et al. 2010. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 62:234–244. 10.1002/art.25032 [DOI] [PubMed] [Google Scholar]
- Talmage D.W., and Pearlman D.S.. 1963. The antibody response: a model based on antagonistic actions of antigen. J. Theor. Biol. 5:321–339. 10.1016/0022-5193(63)90067-5 [DOI] [PubMed] [Google Scholar]
- Thompson K.M., Sutherland J., Barden G., Melamed M.D., Randen I., Natvig J.B., Pascual V., Capra J.D., and Stevenson F.K.. 1991. Human monoclonal antibodies against blood group antigens preferentially express a VH4-21 variable region gene-associated epitope. Scand. J. Immunol. 34:509–518. 10.1111/j.1365-3083.1991.tb01574.x [DOI] [PubMed] [Google Scholar]
- Thorpe S.J., Ball C., Fox B., Thompson K.M., Thorpe R., and Bristow A.. 2008. Anti-D and anti-i activities are inseparable in V4-34-encoded monoclonal anti-D: the same framework 1 residues are required for both reactivities. Transfusion. 48:930–940. 10.1111/j.1537-2995.2007.01624.x [DOI] [PubMed] [Google Scholar]
- Tiegs S.L., Russell D.M., and Nemazee D.. 1993. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 177:1009–1020. 10.1084/jem.177.4.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T., Tsuiji M., Yurasov S., Velinzon K., Nussenzweig M.C., and Wardemann H.. 2007. Autoreactivity in human IgG+ memory B cells. Immunity. 26:205–213. 10.1016/j.immuni.2007.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T., Meffre E., Yurasov S., Tsuiji M., Nussenzweig M.C., and Wardemann H.. 2008. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods. 329:112–124. 10.1016/j.jim.2007.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa C.G., Cook M.C., Angelucci C., Athanasopoulos V., Rui L., Hill K.M., Yu D., Domaschenz H., Whittle B., Lambe T., et al. 2005. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 435:452–458. 10.1038/nature03555 [DOI] [PubMed] [Google Scholar]
- Wardemann H., Yurasov S., Schaefer A., Young J.W., Meffre E., and Nussenzweig M.C.. 2003. Predominant autoantibody production by early human B cell precursors. Science. 301:1374–1377. 10.1126/science.1086907 [DOI] [PubMed] [Google Scholar]
- Wrammert J., Koutsonanos D., Li G.M., Edupuganti S., Sui J., Morrissey M., McCausland M., Skountzou I., Hornig M., Lipkin W.I., et al. 2011. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208:181–193. 10.1084/jem.20101352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.C., Kipling D., Leong H.S., Martin V., Ademokun A.A., and Dunn-Walters D.K.. 2010. High-throughput immunoglobulin repertoire analysis distinguishes between human IgM memory and switched memory B-cell populations. Blood. 116:1070–1078. 10.1182/blood-2010-03-275859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.C., Kipling D., and Dunn-Walters D.K.. 2011. The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front. Immunol. 2:81 10.3389/fimmu.2011.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N.Y., Wilson K., Wang X., Boston A., Kolar G., Jackson S.M., Liu Y.J., Pascual V., Capra J.D., and Wilson P.C.. 2004. Human immunoglobulin selection associated with class switch and possible tolerogenic origins for Cδ class-switched B cells. J. Clin. Invest. 113:1188–1201. 10.1172/JCI20255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikherman J., Parameswaran R., and Weiss A.. 2012. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 489:160–164. 10.1038/nature11311 [DOI] [PMC free article] [PubMed] [Google Scholar]