Abstract
BACKGROUND:
Newborn hearing screening has a high participation rate of ∼97% of infants nationally, but a high lost to follow-up of ∼32% limits the effectiveness of the program. This study tested an intervention of targeted outpatient rescreening of infants through collaboration with the Women, Infants, and Children (WIC) program to improve follow-up rates for newborn hearing screen referrals.
METHODS:
Controlled intervention study of WIC-eligible infants who referred on newborn hearing screens at target hospitals. Hearing rescreens were performed by using screening auditory brainstem response testing by trained research assistants, coordinated with the infant’s WIC appointment. Loss to follow-up rates and age at follow-up were compared with non-WIC infants tracked via the Ohio Department of Health during the same time periods at the same hospitals and at nonintervention hospitals.
RESULTS:
During a 2-year period, there were 1493 hearing screen referrals at 6 hospitals in the Cincinnati region recorded by the Ohio Department of Health. Of these, 260 WIC-eligible infants were referred to the study. Among WIC-eligible intervention infants, the lost to follow-up rate over 2 years was 9.6%, compared with 28.7% for nonintervention infants in the same hospitals and 18.1% for nonintervention hospitals. The average age of hearing confirmation for the WIC intervention group was 34.8 days, compared with 63.6 days in non-WIC infants. One-third of mothers reported barriers to follow-up.
CONCLUSIONS:
Collaborating with WIC to provide targeted follow-up for newborn hearing screening improved loss to follow-up rates, decreased the age at hearing confirmation by 1 month, and addressed reported care barriers.
What’s Known on This Subject:
Nearly one-third of newborns do not receive timely follow-up for non-pass newborn hearing screen, limiting access to appropriate diagnosis and intervention for congenital hearing loss. Low-income mothers are at risk for loss to follow-up because of multiple barriers.
What This Study Adds:
Collaborating with the Women, Infants, and Children program to provide targeted follow-up for newborn hearing screening significantly improves timely follow-up. Care coordination and colocation of services significantly improved effectiveness of newborn hearing screening programs for low-income mothers and their newborns.
Congenital hearing loss is the most common preventable cause of developmental delay in infants, with an incidence of 2 to 3 infants per 1000 live births.1 Through Early Hearing Detection and Intervention (EHDI) state systems, ∼97% of infants are screened for hearing loss in the United States.2 The Joint Committee on Infant Hearing (JCIH) guidelines3 recommend timely follow-up to newborn hearing screening because early intervention has been found to be successful in preventing speech and language delay in infants who are diagnosed and receive early intervention.4–7 Unfortunately, of the 1.5% of US newborns who did not pass newborn hearing screening in 2012, >1 in 3 (36%) failed to receive timely follow-up necessary to diagnose whether a hearing loss was present.2 Nationally, lost to follow-up rates for universal newborn hearing screening (UNHS) varies from <5% in some states to >75% in others, reflecting highly variable and unacceptable systems-based differences in outcomes across the United States.2,8 Children are at higher risk of becoming lost to follow-up if their mothers are nonwhite, <30 years old, covered by public insurance, have lower levels of maternal education, smoked during pregnancy, or live in nonurban locations.8–11 Two program characteristics that have been reported by 2 studies to be important in improving follow-up are prenatal education about newborn screening and timely reporting of screening results from hospitals to medical homes and state public health agencies.12,13
Many UNHS screening protocols and the JCIH recommend an outpatient rescreening for nonpass infants within 1 month of hospital discharge to minimize the number of infants referred on for diagnostic audiologic and medical evaluation.3 However, effectiveness of rescreening has not been studied. We designed this intervention study of follow-up rescreening by collaborating with the Women, Infants, and Children (WIC) program in greater Cincinnati, Ohio. This WIC program was designed to address barriers to follow-up in low-income populations, as well as to improve functional integration between birth hospitals, the medical home, diagnostic services, and public health systems. The federally funded WIC organization was selected for this intervention because of its high success in supporting lower-income women and their children <5 years of age.14 WIC staff members offer mothers professional nutrition guidance, lactation counseling, and supplemental nutrition to procure healthy foods for their families. The WIC program has shown a significant positive impact on immunization rates.14 Nationally, WIC serves about half of the 2 million infants born annually in the United States and thus has great potential to improve loss to follow-up for newborn screening. The primary aim of this study was to determine whether outpatient rescreening intervention at WIC locations for infants who receive a nonpass result on the newborn hearing screen reduces the rate of loss to follow-up for diagnosis. The secondary aim was to evaluate whether infants who do not pass on the WIC rescreening received follow-up at an earlier age than comparison infants who were not enrolled in the rescreening study. Barriers to care were assessed in one to one interviews with mothers.
Methods
The study design was a controlled intervention, with loss to follow-up and age of hearing confirmation for infants in the WIC program compared with non-WIC infants at 4 targeted hospitals in Butler and Hamilton Counties and 2 control hospitals in Hamilton County. Together, Butler and Hamilton County have 18 300 annual total births, and ∼700 (3.8%) of those infants do not pass hearing screening. The intervention hospitals were selected because they had larger proportions of WIC participants and higher loss to follow-up rates, thus greater need for intervention. The control hospitals were located within the same counties and had similar percentages of infants referred from newborn screening. Human subject ethics approval was obtained from the institutional review board of Cincinnati Children’s Hospital and the Ohio Department of Health (ODH). All hospitals were informed of the study in a series of community stakeholder meetings.
All 6 newborn nurseries in these counties are managed by Newborn Care Associates, a 60-physician pediatric group within the division of neonatology at Cincinnati Children’s Hospital Medical Center. This group maintains a clinical database of all infants who did not pass newborn hearing and metabolic screening that was used for study referrals, screening eligibility, and tracking purposes. The ODH EHDI program also maintains a screening and tracking database and provided comparisons to control infants at the same hospitals and at control hospitals. Loss to follow-up was defined in each infant as reaching the age of 6 months with no success in completing rescreening or diagnostic assessment. This is consistent with the definition used by the ODH.
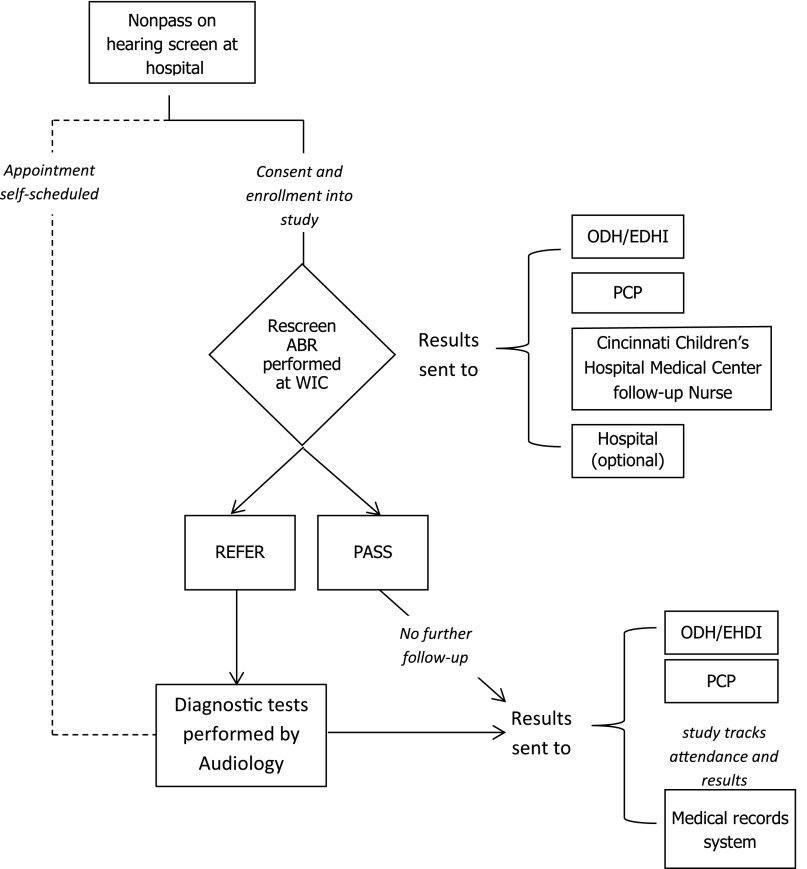
Figure 1 illustrates the enrollment and follow-up process for the intervention study. Infants who did not pass their hearing screen were referred to the study by birth hospitals or by WIC. If infants were enrolled or intended to enroll in WIC services, they were eligible for the rescreening intervention study. In Ohio, the current standard of care for infants who do not pass their hearing screening is to refer directly for a diagnostic evaluation, whereas some states include an intermediate rescreening step. Because the intervention was designed to supplement these existing follow-up services by targeting WIC follow-up to those at risk, infants who were already scheduled for diagnostic audiology appointments were not initially rescreened. All WIC-eligible infants, including those who attended diagnostic audiology, were tracked for follow-up purposes through the same neonatal care practice. This process assured that loss to documentation did not occur. Consultation with the EHDI network and Ohio chapter champion (Dr. Wiley) resulted in the recommendation that we not cancel self-scheduled appointments, but rather intervene with those infants who had not followed up by the time of their WIC appointment, because they were considered to be at higher risk for loss to follow-up. Infants scheduled for diagnostic evaluations were later offered rescreening at a WIC office if they failed to attend their scheduled visit.
FIGURE 1.
Intervention flow diagram for recruitment, enrollment, and follow-up stages of the study. ABR, auditory brain stem response; PCP, primary care provider.
Exclusion criteria were infants with stigmata or syndromes associated with congenital hearing loss (eg, Down, Waardenburg, cleft palate) or who were cared for in the NICU for ≥5 days. These infants were directed to diagnostic audiology, because they have higher risk for congenital and progressive hearing loss.3
Families were contacted by a research coordinator by letters and phone calls to screen for eligibility and explain the study and options for follow-up. If the parent indicated interest in the study, they were scheduled for rescreening, and informed consent was obtained in person before the rescreen at the mother’s WIC location. Enrollment and outpatient hearing rescreens were conducted by trained research coordinators. The rescreening test was automated auditory brainstem response using click stimuli at 30 dB nHL. Both ears were tested regardless of the screening result, in accordance with JCIH guidelines.3 After rescreening, parents were counseled by the research coordinator regarding the results and the need for diagnostic follow-up if they did not pass. Infants who did not pass rescreening were scheduled for a diagnostic evaluation at the audiology facility of their choice.
The study was initiated in November 2012 and developed in a phased rollout, initially at WIC locations serving infants born at 2 hospitals in Butler County. In February 2014, the study was expanded to Hamilton County for infants born at 2 target hospitals. Because other interventions were occurring across the state of Ohio to reduce loss to follow-up, we compared intervention infants at the same hospitals as well as to control hospitals to understand the impact of this intervention compared with the system as a whole. Infants were tracked until 6 months of age, with attempts to contact the family via phone and letter, for the intervention infants and for the general population according to ODH protocol.
To assess the primary aim, loss to follow-up rates for WIC-eligible infants born at intervention birth hospitals were compared with non-WIC infants at the same hospitals (control infants) and to those born in similar nonintervention birth hospitals (control hospitals). To assess the secondary aim, age at follow-up for enrolled infants was compared for WIC-eligible versus control infants. The age at follow-up was defined as the first follow-up visit for hearing services: either rescreening for those who were enrolled in the intervention or at diagnostic assessment for those infants who were eligible and tracked. As a balancing measure, the age of hearing loss diagnosis was also measured for both enrolled and nonenrolled infants to ensure that rescreening did not increase age at diagnosis.
Statistical Analysis
Data were entered into a customized REDCap database for analysis. The main outcome variables were (1) proportion of infants who followed through to desired outcomes of a passed screening test or a diagnostic evaluation; and (2) the time to rescreening and, if necessary, diagnostic assessment. All statistical analyses were conducted by using SAS version 9.3 (SAS Institute, Inc, Cary, NC). Descriptive statistics were calculated between the outcome variables. WIC infants born at the intervention hospitals were compared with non-WIC infants born at the same hospitals and similar infants born at nonintervention hospitals (control sites) with regard to the main outcome variables. Differences in the loss to follow-up rates at ages 1, 3, and 6 months between groups were tested with χ2 test statistic. Differences in the age at rescreening/assessment between groups were tested by using Student’s t test.
Results
During the 2-year period, there were 1493 hearing screen referrals at the 6 hospitals in the greater Cincinnati region recorded within the tracking database by the ODH. A total of 260 eligible referrals were made to the study, of which 128 (49.2%) were enrolled and rescreened at WIC locations and 107 (41.2%) were self-scheduled at diagnostic audiology evaluations. The eligible infants are detailed in Table 1 for the 2 counties for each year and for the duration of the study.
TABLE 1.
Eligible and Enrolled Infants, Detailed by County and Year
| Eligibility Status | Categories | Butler County 2013 | Butler County 2014 | Hamilton County 2014 | Total 2013–2014 |
|---|---|---|---|---|---|
| Ineligible for study | Not in WIC program | 19 | 17 | 7 | 43 |
| Deceased | 0 | 0 | 2 | 2 | |
| Miscellaneousa | 4 | 3 | 16 | 23 | |
| Refused | 3 | 0 | 3 | 6 | |
| Eligible for study | Enrolled | 25 | 49 | 54 | 128 |
| Rescreening scheduled | 0 | 0 | 1 | 1 | |
| Diagnostic completed | 18 | 35 | 51 | 104 | |
| Diagnostic scheduled | 0 | 0 | 2 | 2 | |
| Lost to follow-up | Could not contact (n) | 6 | 7 | 12 | 25 |
| Eligible referrals | Total eligible (t) | 49 | 91 | 120 | 260 |
| Total loss to follow-up = n/t, % | 12.2 | 7.7 | 10 | 9.6 |
Infants not eligible for the study for reasons including: NICU stay, birth at nontarget hospital, lives outside counties with participating WIC program.
Demographics are detailed in Table 2 for infants enrolled and rescreened within the intervention study, compared with nonstudy infants in the same hospitals and infants in control hospitals. The WIC study sample was comprised of higher proportions of Black or African-American (36.7%) infants compared with the control hospitals, and more Hispanic or Latino (21.1%) infants relative to nonintervention infants in the same and control hospitals. Public insurance was reported by 86% of families, which was significantly higher than nonstudy and control families. Maternal education was significantly lower in the study participants; 64% of mothers had completed high school or less, whereas only 6% were college graduates. About one-third of study families reported at least 1 barrier to obtaining follow-up services, with the most common being transportation or distance from the diagnostic facility, non-English speaking, lack of child care, work or school schedule, and insurance coverage.
TABLE 2.
Demographic Characteristics of the Enrolled Intervention Group Compared With Nonstudy Infants Referred Within the Same Hospitals and Infants Referred in Control Hospitals
| WIC Study, n = 128 (%) | Nonstudy Control, Same Hospitals, n = 917 (%) | Nonintervention Hospitals, n = 448 (%) | P | |
|---|---|---|---|---|
| Gender – boy | 68 (53.1) | 484 (52.8) | 277(61.8) | .006 |
| Race | <.0001 | |||
| White | 58 (45.3) | 402 (43.8) | 320 (71.4) | |
| African American | 47 (36.7) | 317 (34.6) | 74 (16.5) | |
| Other/unknown | 23 (18) | 198 (21.6) | 54 (12.1) | |
| Hispanic | 27 (21.1) | 113 (12.3) | 11 (2.5) | <.0001 |
| Maternal education | <.0001 | |||
| <HS | 33 (25.8) | 194 (21.2) | 36 (8.0) | |
| HS/GED | 49 (38.3) | 212 (23.1) | 68 (15.2) | |
| Some college | 37 (28.9) | 280 (30.5) | 133 (29.7) | |
| College/postgraduate | 8 (6.3) | 167(18.2) | 185 (41.3) | |
| Unknown | 1 (0.8) | 64 (7) | 26 (5.8) | |
| Payer of delivery | <.0001 | |||
| Medicaid | 110 (86) | 423 (46.1) | 123 (27.5) | |
| Private | 4 (3.1) | 295 (32.2) | 275 (61.4) | |
| Other/unknown | 14 (10.9) | 199 (21.7) | 50 (11.1) |
GED, general equivalency diploma; HS, high school.
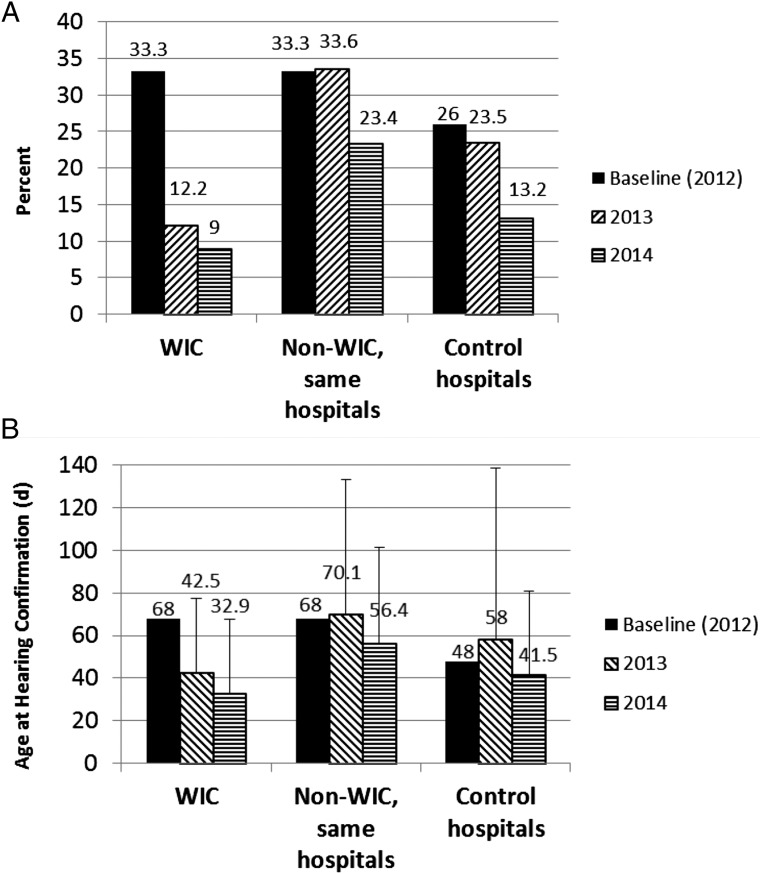
Lost to follow-up rates for the WIC intervention, WIC nonintervention in the same hospitals, and control hospitals are shown in Fig 2A. WIC intervention infants had a decrease in lost to follow-up rates from 33% in 2012 at baseline to 9.6% in 2013 to 2014 (P < .0001). The WIC intervention lost to follow-up rate was lower than the rate for nonintervention infants born in the same hospitals (28.7% for 2013–2014, P < .0001). It was also lower than the lost to follow-up rate in control hospitals (18.1% in 2013–2014, P = .002). The study lost to follow-up rate declined from the first to the second year as referrals to the study and effectiveness in contacting families improved. Rates of lost to follow-up were similar in the second year of the study for the rural county (Butler, 7.7%) compared with the urban county (Hamilton, 10%).
FIGURE 2.
A, Lost to follow-up rates for infants enrolled in the WIC intervention study, non-WIC infants in the same hospitals, and control hospitals. B, Age at hearing confirmation, including rescreening and diagnostic testing, for infants enrolled in the WIC intervention study, non-WIC infants in the same hospitals, and control hospitals. Error bars are SD.
The age of infants at the time of hearing confirmation for intervention and nonintervention groups is shown in Fig 2B. Average age at hearing confirmation across the 2 years combined for the WIC rescreening group was 34.8 days (median, 27.5 days; range, 2–283 days), which was significantly lower than non-WIC infants in the same hospitals (average, 63.6 days; median, 45 days; range, 1–411 days; P < .0001) and was also significantly lower than in control hospitals (average, 49.4 days; median, 29 days, range, 1–495 days; P = .0007). Timely follow-up (rescreening by 1 month and diagnosis by 3 months of age) was examined by comparing the proportion of infants who had hearing confirmation by these target ages. By 1 month of age, 61% of study infants were confirmed by screening or diagnostic tests compared with only 24% of nonstudy infants in the same hospitals (P < .0001). By 3 months of age, 96% of study infants had received hearing confirmation, compared with 82% of nonstudy infants in the same hospitals (P < .0001).
The average age at WIC rescreening was within the recommended guidelines set forth by the JCIH, whereby infants should be rescreened by 1 month of age and receive diagnostic testing by 3 months3. However, 3 study infants had late follow-up after 3 months of age. These infants were originally scheduled for diagnostic follow-up, but did not attend their appointments. All 3 families were contacted, enrolled, and successfully rescreened at WIC. The parents of these infants reported barriers for not attending their diagnostic visits, including transportation problems, work, and school schedules.
Of the 128 infants enrolled, 113 (88.3%) passed and 15 (11.7%) did not pass the rescreening in 1 or both ears. Of the 15 nonpass infants, 5 had normal hearing after audiologic assessment, 4 had fluctuating conductive loss, 4 had permanent hearing loss, and 2 were still in the diagnostic process. All children with permanent hearing loss were referred for early intervention services. Of the 15 infants who did not pass rescreening, all but 1 (93%) attended their first scheduled diagnostic hearing evaluation. In comparison, the average show rate of newborn hearing rescreens for diagnostic evaluation at the tertiary diagnostic center was 67% according to hospital statistics over the same time period.
Discussion
Overall, this WIC–EHDI collaboration met the goal of shifting the focus from searching for infants who were lost to follow-up to preventing loss to follow-up from occurring and decreasing the age of hearing confirmation. Rates of lost to follow-up and age at follow-up were each significantly improved with the WIC rescreening intervention compared with control infants and control hospitals, with no added delay in hearing confirmation at the diagnostic follow-up. Significant barriers to care were identified in this population in 34% of mothers. These included transportation or distance from a diagnostic facility, non-English speaking, and lack of child care, work hours, and insurance coverage. Because the study participants were more likely to be African-American or Hispanic, to have lower maternal education, and to be enrolled in Medicaid than the control infants and hospitals, the improvement demonstrated with this simple intervention is of great importance because previous studies have identified these factors to be associated with higher loss to follow-up rates.8–10
Collaboration with WIC to offer onsite rescreening allowed the research team to address barriers effectively because interpreters were on site, services were free and close to home, and work and school schedules could be accommodated. Individualized care coordination provided education at the time of rescreening and scheduling support that appeared to improve follow-up rates for diagnosis and intervention. Assistance with securing transportation and vouchers was provided as needed.
An important factor that appeared to reduce loss to follow-up within this study was contacting families quickly to schedule follow-up. We found it necessary to use multiple methods to contact families. Many families had transient phone numbers and addresses, making it more difficult to reach them with increasing time after birth. For this reason, it is best practice for follow-up to be proactively scheduled at the birth hospital, rather than waiting for families to initiate follow-up. In fact, states that report the lowest lost to follow-up rates use this practice.2,13 A strength of this study was careful tracking and documentation within the electronic medical record, which assured that loss to documentation did not occur.
A second important principle supporting the success of this intervention was collaboration among agencies, which was vital to impacting a problem as multifaceted as loss to follow-up. The study received support from WIC directors and staff, the DOH and Part C newborn hearing program, audiologists, neonatologists, otolaryngologists, and hearing screen coordinators at the birth hospitals. To reduce loss to follow-up of newborn hearing screening, it was important for study staff to identify the root causes of loss to follow-up. The WIC program provided an important means to reach families at high risk for loss to follow-up by implementing Health Insurance Portability and Accountability Act–compliant intake forms that asked mothers about UNHS results and obtained permission to provide contact information to the study coordinator. Introducing the extra step of rescreening at WIC did not interfere with the age of confirmation for diagnosis of hearing loss, and in fact improved on it–an important balancing measure.
A third principle was consistent counseling and education about the importance of a nonpass screening and the need to follow-up as soon as possible. Despite a relatively low likelihood of a child with a nonpass screen having a hearing loss diagnosed (4.7% in this study), primary care providers should not encourage watchful waiting, as delayed identification is detrimental for developmental outcomes. Although the study did not directly compare counseling to no counseling, the importance of the follow-up appointment was emphasized by the coordinator, and 93% of infants referred on for diagnostic testing attended their appointments.
Future research needs include investigating sustainable methods of reducing loss to follow-up by providing care coordination between birth hospitals and diagnostic centers. A major limitation for sustained improvement is funding and acceptance of the model on a broader scale. The WIC program is under constant pressure to provide a wide range of ancillary services and does not have funding to support rescreening within their system, so funds and personnel would need to be provided to implement this on a system-wide scale. It is possible that a rescreening protocol may be acceptable for all well infants who do not pass UNHS rather than starting with the more costly, time-consuming diagnostic evaluation. A limitation of this study was the lack of randomization for intervention. It is possible that the improvement could be due to the co-location with WIC, care coordination and education, or a combination of these factors. Randomizing to rescreening compared with diagnostic testing would be needed to determine patient preference, efficiency, and effectiveness of the 2 approaches.
A significant challenge in this study was to differentiate the intervention impact on loss to follow-up while other statewide initiatives were occurring at both the target and comparison hospitals. The simultaneous statewide efforts reduced the loss to follow-up rate from 33% in 2012, as our study was starting, to 23% in the same hospitals and 13% in control hospitals in 2014. The dual comparison with nonintervention infants at the same hospital and control hospitals showed that the improvement was not solely due to the study because overall system improvement occurred over the same time period in control hospitals. It is likely that similar improvement could be realized for all infants through systems improvement models because high rates of follow-up in a few states suggest that this is possible.
Conclusions
Collaborating with WIC to provide targeted follow-up for newborn hearing screening significantly improved lost to follow-up rates from 33.3% at baseline to 9.6% in year 1 and 2 (a reduction of 71%) and significantly improved the age at hearing diagnosis from 68 days at baseline to 34.8 days across the 2 years of the study (a reduction of 48.8%). Care coordination and colocation of services with WIC was effective in improving the system of follow-up from newborn hearing screening for low income mothers and their newborns.
Acknowledgments
We thank Laura Rolfes and Karen Davies as well as Cindy Meale and Betsy Buchanan, Directors of the Butler County and Hamilton County Women, Infant and Children Programs, for assistance with critical aspects of the study.
Glossary
- EHDI
Early Hearing Detection and Intervention
- JCIH
Joint Committee on Infant Hearing
- ODH
Ohio Department of Health
- WIC
Women, Infants, and Children
- UNHS
universal newborn hearing screen
Footnotes
Dr Hunter conceptualized and designed the study, analyzed and interpreted the data, and drafted and approved the final manuscript; Dr Meinzen-Derr designed and performed the analyses and critically reviewed, revised, and approved the final manuscript; Dr Wiley assisted in analysis and interpretation of the data and critically reviewed and approved the final manuscript; Ms Horvath and Dr Kothari provided statewide data, assisted in analysis and interpretation of the data, and critically reviewed and approved the final manuscript; Dr. Wexelblatt conceptualized and designed the study and drafted, critically reviewed and approved the final manuscript; and all authors reviewed and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control or the National Institutes of Health.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Disability Research and Dissemination Center (DRDC) through its grant number 5U01DD001007, FAIN No. U01DD001007 from the Centers for Disease Control and Prevention (CDC), from a Place Award from Cincinnati Children’s Hospital Medical Center and by Grant 8 UL1 TR000077 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the CDC or the NIH. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Centers for Disease Control and Prevention . Identifying infants with hearing loss - United States, 1999-2007. MMWR Morb Mortal Wkly Rep. 2010;59(8):220–223 [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Summary of 2012 National CDC EHDI data. Available at: www.cdc.gov/ncbddd/hearingloss/2012-data/2012_ehdi_hsfs_summary_b.pdf. Accessed May 28, 2015
- 3.American Academy of Pediatrics, Joint Committee on Infant Hearing . Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120(4):898–921 [DOI] [PubMed] [Google Scholar]
- 4.Kennedy CR, McCann DC, Campbell MJ, et al. Language ability after early detection of permanent childhood hearing impairment. N Engl J Med. 2006;354(20):2131–2141 [DOI] [PubMed] [Google Scholar]
- 5.Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early- and later-identified children with hearing loss. Pediatrics. 1998;102(5):1161–1171 [DOI] [PubMed] [Google Scholar]
- 6.Thomas TN, Kolasa MS, Zhang F, Shefer AM. Assessing immunization interventions in the Women, Infants, and Children (WIC) program. Am J Prev Med. 2014;47(5):624–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moeller MP. Early intervention and language development in children who are deaf and hard of hearing. Pediatrics. 2000;106(3). Available at: http://pediatrics.aappublications.org/content/106/3/e43 [DOI] [PubMed] [Google Scholar]
- 8.Liu CL, Farrell J, MacNeil JR, Stone S, Barfield W. Evaluating loss to follow-up in newborn hearing screening in Massachusetts. Pediatrics. 2008;121(2). Available at: http://pediatrics.aappublications.org/content/121/2/e335 [DOI] [PubMed] [Google Scholar]
- 9.Todd NW. Universal newborn hearing screening follow-up in two Georgia populations: newborn, mother and system correlates. Int J Pediatr Otorhinolaryngol. 2006;70(5):807–815 [DOI] [PubMed] [Google Scholar]
- 10.Holte L, Walker E, Oleson J, et al. Factors influencing follow-up to newborn hearing screening for infants who are hard of hearing. Am J Audiol. 2012;21(2):163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lloyd-Puryear MA, Brower A. Long-term follow-up in newborn screening: A systems approach for improving health outcomes. Genet Med. 2010;12(12 Suppl):S256–S260 [DOI] [PubMed] [Google Scholar]
- 12.Bush ML, Bianchi K, Lester C, et al. Delays in diagnosis of congenital hearing loss in rural children. J Pediatr. 2014;164(2):393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russ SA, Hanna D, DesGeorges J, Forsman I. Improving follow-up to newborn hearing screening: a learning-collaborative experience. Pediatrics. 2010;126(suppl 1):S59–S69 [DOI] [PubMed] [Google Scholar]
- 14.Black MM, Cutts DB, Frank DA, et al. ; Children’s Sentinel Nutritional Assessment Program Study Group . Special Supplemental Nutrition Program for Women, Infants, and Children participation and infants’ growth and health: a multisite surveillance study. Pediatrics. 2004;114(1):169–176 [DOI] [PubMed] [Google Scholar]