Abstract
BACKGROUND:
Little is known about the association of gut microbiota, a potentially modifiable factor, with bronchiolitis in infants. We aimed to determine the association of fecal microbiota with bronchiolitis in infants.
METHODS:
We conducted a case–control study. As a part of multicenter prospective study, we collected stool samples from 40 infants hospitalized with bronchiolitis. We concurrently enrolled 115 age-matched healthy controls. By applying 16S rRNA gene sequencing and an unbiased clustering approach to these 155 fecal samples, we identified microbiota profiles and determined the association of microbiota profiles with likelihood of bronchiolitis.
RESULTS:
Overall, the median age was 3 months, 55% were male, and 54% were non-Hispanic white. Unbiased clustering of fecal microbiota identified 4 distinct profiles: Escherichia-dominant profile (30%), Bifidobacterium-dominant profile (21%), Enterobacter/Veillonella-dominant profile (22%), and Bacteroides-dominant profile (28%). The proportion of bronchiolitis was lowest in infants with the Enterobacter/Veillonella-dominant profile (15%) and highest in the Bacteroides-dominant profile (44%), corresponding to an odds ratio of 4.59 (95% confidence interval, 1.58–15.5; P = .008). In the multivariable model, the significant association between the Bacteroides-dominant profile and a greater likelihood of bronchiolitis persisted (odds ratio for comparison with the Enterobacter/Veillonella-dominant profile, 4.24; 95% confidence interval, 1.56–12.0; P = .005). In contrast, the likelihood of bronchiolitis in infants with the Escherichia-dominant or Bifidobacterium-dominant profile was not significantly different compared with those with the Enterobacter/Veillonella-dominant profile.
CONCLUSIONS:
In this case–control study, we identified 4 distinct fecal microbiota profiles in infants. The Bacteroides-dominant profile was associated with a higher likelihood of bronchiolitis.
What’s Known on This Subject:
Recent studies have demonstrated a link between gut microbiota and respiratory diseases, such as asthma. However, little is known about the association of gut microbiota, a potentially modifiable factor, with bronchiolitis in infants.
What This Study Adds:
In this case–control study of infants hospitalized with bronchiolitis and healthy age-matched controls, we identified 4 distinct fecal microbiota profiles in their fecal samples. We found that the Bacteroides-dominant microbiota profile was associated with a higher likelihood of bronchiolitis.
Bronchiolitis is a major public health problem for children in the United States and worldwide.1–3 Indeed, bronchiolitis is the leading cause of hospitalizations in US infants, accounting for 18% of all infant hospitalizations.3 Although causative viral pathogens (eg, respiratory syncytial virus [RSV]) are ubiquitous, not all infants develop bronchiolitis.4 Likewise, severity of infection ranges from a minor nuisance to fatal bronchiolitis. The reasons for these differences remain largely unclear.5
The recent advent of culture-independent techniques revealed the presence of highly functional communities of microbes inhabiting the human body (ie, the human microbiota) that contribute to host immune development and homeostasis.6 Within the human body, the intestinal tract is the most densely colonized surface, with bacterial loads of ∼1014 bacteria.7 Disruption of balance in the gut microbiota and microbiota-derived regulatory T cell response are linked with inflammatory diseases in the local environment (eg, inflammatory bowel disease).8,9 Recent studies also demonstrate that the gut microbiota modulates the immune function in distant mucosal locations, such as the respiratory tract,7 and thereby remotely contributes to pathogenesis of asthma and cystic fibrosis.10–12 Despite the evidence suggesting the existence of a “common mucosal response” in host immune development,13,14 to the best of our knowledge no studies have investigated the relationship of gut microbiota, a potentially modifiable factor, with bronchiolitis in infants.
In this context, we conducted a case–control study of a multicenter prospective cohort of infants hospitalized with bronchiolitis and healthy matched controls to determine the association of fecal microbiota and bronchiolitis in infants.
Methods
Study Design, Setting, and Participants
We conducted a case–control study to examine the fecal microbiota of infants hospitalized with bronchiolitis (cases) and that of healthy infants (controls). As part of a multicenter prospective cohort study, the 35th Multicenter Airway Research Collaboration,15 we enrolled 40 infants age <12 months hospitalized with an attending physician diagnosis of bronchiolitis at 1 of 3 US hospitals (Alfred I. duPont Hospital for Children, Wilmington, DE; Boston Children’s Hospital, Boston, MA; and Kosair Children’s Hospital, Louisville, KY) during a bronchiolitis season from November 2013 through April 2014. Bronchiolitis was defined by American Academy of Pediatrics guidelines as an acute respiratory illness with some combination of rhinitis, cough, tachypnea, wheezing, crackles, and retractions.4 We excluded infants with previous enrollment into the 35th Multicenter Airway Research Collaboration, those who were transferred to a participating hospital >48 hours after the original hospitalization, those whose parents gave consent >24 hours after hospitalization, or those with known cardiopulmonary disease, immunodeficiency, immunosuppression, or gestational age ≤32 weeks.
Healthy infants (n = 115) were enrolled as the controls in this case–control study. The setting and participants have been reported previously.16 Briefly, using a standardized protocol, we enrolled healthy infants (age-matched within 1.5 months of cases) from a primary care group practice at Massachusetts General Hospital (Boston, MA) from November 2013 through May 2014. We excluded infants with current fever, respiratory illness, or gastrointestinal illness,17 or antibiotic treatment in the preceding 7 days. The institutional review board at each of the participating hospitals approved the study. Written informed consent was obtained from the parent or guardian.
Data and Sample Collection
Site investigators conducted a structured interview and chart review that assessed patients’ demographic characteristics, family history, prenatal and past medical history, home environmental characteristics, and hospital course (in the cases only). All data were reviewed at the Study Coordinating Center, and site investigators were queried about missing data and discrepancies identified by manual data checks.
Fecal samples were collected via a standardized protocol at the time of hospitalization (in the cases) or at home before the clinic visit (in the controls). First, diapers containing feces were refrigerated or stored in a cooler by hospital staff or parents immediately after collection. The fecal samples were then placed in sterile Sarstedt feces collection containers (Sarstedt, Nümbrecht, Germany) and immediately stored at −80°C. Frozen samples were shipped on dry ice to Baylor College of Medicine, where we characterized the microbiota via 16S rRNA gene sequencing.
16s rRNA Gene Sequencing
We adapted 16S rRNA gene sequencing methods from the methods developed for the National Institutes of Health Human Microbiome Project.18,19 Briefly, bacterial genomic DNA was extracted with a Mo BIO PowerMag DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA). The 16S rDNA V4 region was amplified by polymerase chain reaction (PCR) and sequenced in the MiSeq platform (Illumina, San Diego, CA) via the 2- × 250-bp paired-end protocol, yielding pair-end reads that overlap almost completely. The primers used for amplification contain adapters for MiSeq sequencing and single-end barcodes, allowing pooling and direct sequencing of PCR products.20
Sequencing read pairs were demultiplexed based on the unique molecular barcodes, and reads were merged in USEARCH v7.0.1090,21 allowing 0 mismatches and a minimum overlap of 50 bases. Merged reads were trimmed at the first base with a Q5 quality score. In addition, a quality filter was applied to the resulting merged reads, and reads containing >0.05 expected errors were discarded. Rarefaction curves of bacterial operational taxonomic units (OTUs) were constructed with sequence data for each sample to ensure coverage of the bacterial diversity present. Samples with suboptimal amounts of sequencings reads (<80% of the taxa are represented) were resequenced to ensure that the majority of bacterial taxa were encompassed in our analyses. Details of the quality control may be found in the Supplemental Information.
The 16S rRNA gene sequences were clustered into OTUs at a similarity cutoff value of 97% via the UPARSE algorithm.22 OTUs were mapped to the SILVA Database23 containing only the 16S V4 region to determine taxonomies. We recovered abundances by mapping the demultiplexed reads to the UPARSE OTUs. A custom script constructed a rarefied OTU table from the output files generated in the previous 2 steps for downstream analyses of α-diversity (eg, Shannon index) and β-diversity (eg, Bray–Curtis distance).
Statistical Analyses
We calculated the relative abundance of each OTU for each fecal sample. We conducted analyses at the genus level; because our sequences were dominated by 1 OTU per genus, we collapsed all OTUs assigned to the same genus into a single group for reporting.24 To identify fecal microbiota profiles, we performed unbiased clustering by partitioning around medoids25 by using Bray–Curtis distances. Each cluster is defined by a point designated as the center, the “medoid,” and minimizes the distance between samples in a cluster. We determined the number of clusters to choose for the data by using the gap statistic.26
To examine the association of microbiota profiles with the likelihood of being a bronchiolitis case, we constructed 2 logistic regression models. First, we fitted an unadjusted model that included only microbiota profiles as the independent variable. Second, we constructed an adjusted model controlling for ≤5 potential confounders (ie, age, gender, prematurity, mode of birth, and history of systemic antibiotic use before enrollment), given the small number of bronchiolitis cases. These variables were chosen based on clinical plausibility and a priori knowledge.4,5,27 We did not control for breastfeeding status because it was considered an ancestor variable of the association of interest (ie, the relationship between breastfeeding and likelihood of bronchiolitis may be mediated by gut microbiota), and adjustment of an ancestor variable would bias the inference toward the null.
Next, to compare the abundances of bacteria within fecal microbiota between bronchiolitis cases and healthy controls, we used the linear discriminant analysis effect size method.28 In this method, vectors resulting from the comparison of abundances (eg, Bacteroides relative abundance) between the groups are used as inputs to the linear discriminant analysis. This method has the advantage over traditional statistical tests (eg, pairwise tests) that an effect size is produced in addition to a P value. This advantage enables us to sort the results of multiple testing by the magnitude of the between-group difference, not only by P values, because the 2 are not necessarily correlated.28 Analyses used R version 3.2 with the phyloseq package.29
Results
Study Population
At the 4 participating hospitals, we enrolled a total of 40 infants hospitalized with bronchiolitis (cases) and 115 age-matched healthy infants (controls). Overall, the median age was 3 months (IQR, 2–5 months), 55% were male, and 54% were non-Hispanic white. Of cases of bronchiolitis, RSV was detected in 65% and rhinovirus in 23%. Subject characteristics differed between cases and controls (Table 1). For example, compared with healthy controls, infants with bronchiolitis were more likely to have a parental history of asthma, maternal antibiotic use during pregnancy, a history of premature birth, a sibling at home, and corticosteroid use before the enrollment, but they were less likely to be breastfed (all Ps < .05).
TABLE 1.
Characteristics of Infants With Bronchiolitis (Cases) and Healthy Infants (Controls)
| Characteristics | Infants With Bronchiolitis (Cases), n = 40 | Healthy Infants (Controls), n = 115 | P |
|---|---|---|---|
| Demographics | |||
| Age, mo, mean (SD) | 4 (3) | 4 (2) | .76 |
| Male gender | 18 (55) | 64 (56) | .99 |
| Race or ethnicity | .04 | ||
| Non-Hispanic white | 23 (57) | 61 (53) | |
| Non-Hispanic black | 6 (15) | 11 (10) | |
| Hispanic | 10 (25) | 19 (17) | |
| Other | 1 (2) | 24 (21) | |
| Parental history of asthma | 16 (40) | 21 (18) | .01 |
| Prenatal history | |||
| Maternal smoking during pregnancy | 8 (20) | 3 (3) | .001 |
| Maternal antibiotic use during pregnancy | 11 (29) | 13 (11) | .02 |
| Maternal antibiotic use during labor | 12 (34) | 35 (30) | .82 |
| Past medical history and home environmental characteristics | |||
| Mode of birth | .13 | ||
| Vaginal birth | 31 (78) | 72 (63) | |
| Cesarean delivery | 9 (22) | 43 (37) | |
| Prematurity (32–37 wk) | 12 (30) | 11 (10) | .004 |
| Previous breathing problems before enrollmenta | 8 (21) | 0 (0) | <.001 |
| History of eczema | 8 (21) | 17 (15) | .56 |
| Ever attended day care | 9 (23) | 14 (12) | .16 |
| Sibling at home | 34 (87) | 47 (41) | <.001 |
| Smoking exposure at home | 8 (21) | 4 (3) | .002 |
| Mostly breastfed for the first 3 mo of age | 16 (52) | 89 (77) | .009 |
| Systemic antibiotic use before enrollment | 8 (21) | 13 (11) | .24 |
| Systemic corticosteroid use before enrollment | 9 (23) | 0 (0) | <.001 |
| Hospitalization course | |||
| Hospital length-of-stay, d, median (IQR) | 3 (2–4) | — | — |
| Admission to ICU | 8 (20) | — | — |
| Use of mechanical ventilationb | 5 (16) | — | — |
Data are no. (%) of infants unless otherwise indicated. Percentages may not equal 100 because of missingness or rounding. —, not computed.
Defined as an infant having a cough that wakes him or her at night or causes emesis, or when the child has wheezing or shortness of breath without cough.
Defined as use of continuous positive airway pressure or intubation during inpatient stay, regardless of location at any time during the index hospitalization.
Fecal Microbiota Sequence and Profiles
We analyzed fecal samples from all enrolled infants by 16S rRNA gene sequencing and obtained 484 669 high-quality merged sequences, of which 456 888 (94%) were mapped to the database. All 155 samples had sufficient sequence depth to obtain a high degree of sequence coverage (rarefaction cutoff, 1470 reads per sample) and were used for the analysis. The fecal microbiota was composed primarily of 4 genera, Escherichia (22%), Bifidobacterium (19%), Enterobacter (15%), and Bacteroides (13%), followed by Veillonella (5%).
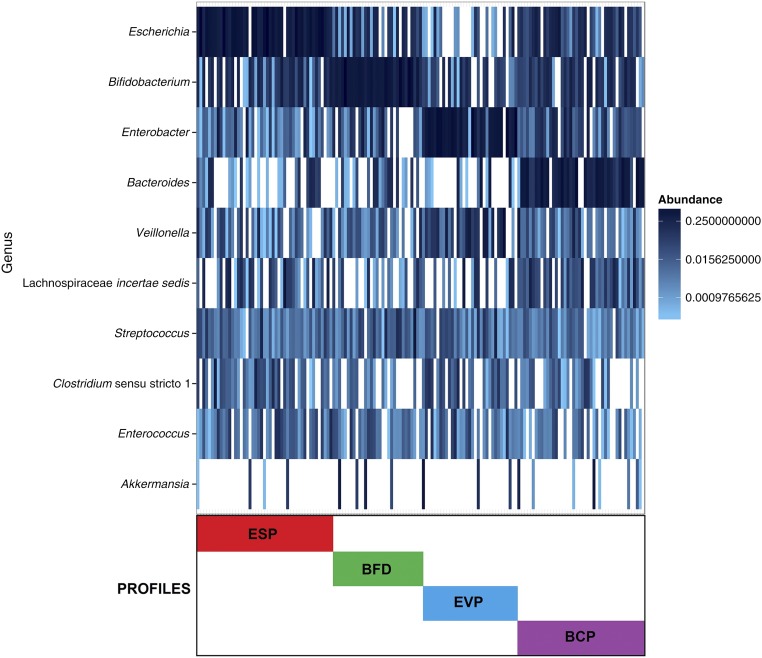
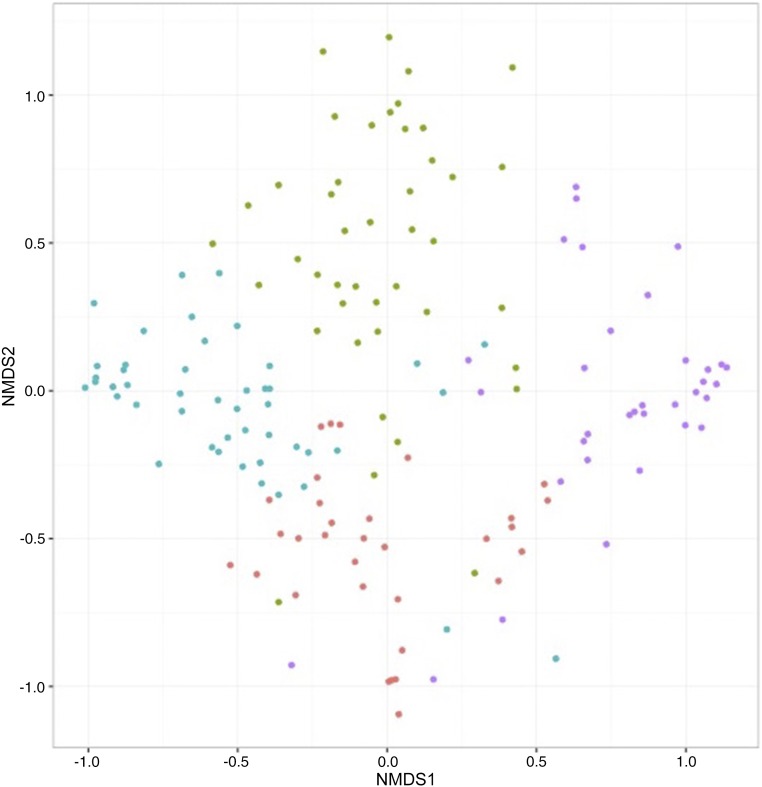
Partitioning around medoid clustering of fecal microbiota identified 4 distinct microbiota profiles (Fig 1): Escherichia-dominant profile (30%), Bifidobacterium-dominant profile (21%), Enterobacter/Veillonella-dominant profile (22%), and Bacteroides-dominant profile (28%). The first 2 profiles were dominated by either the Escherichia or Bifidobacterium genus, and the third profile was codominated by Enterobacter and Veillonella genera (Table 2). The fourth profile had the highest relative abundance of Bacteroides, with highest bacterial richness (P < .001) and α-diversity index (Shannon index, P < .001). The nonmetric multidimensional scaling of fecal microbiota also revealed that the subjects clustered together according to their microbiota profile (Fig 2). Some of the patient characteristics differed across the 4 microbiota profiles (Supplemental Table 4). For instance, compared with infants with an Enterobacter/Veillonella-dominant profile, those with a Bacteroides-dominant profile were older and more likely to have maternal smoking history during pregnancy and history of vaginal birth (both P < .05).
FIGURE 1.
Clustering and composition of fecal microbiota in 155 infants. All fecal microbiota profiles of cases and controls were clustered via partitioning around medoids clustering method with Bray–Curtis distance. Colored bars indicate 4 microbiota profiles: Escherichia-dominant profile (ESP; red), Bifidobacterium-dominant profile (BFD; green), Enterobacter/Veillonella-dominant profile (EVP; blue), and Bacteroides-dominant profile (BCP; purple). The optimal number of clusters was identified by use of the gap statistic. To obtain additional information about the bacterial composition of samples within microbiota profiles, the 10 most abundant genera present in an adjacent heatmap were displayed. The taxonomy depicted is on the genus level because our sequences were dominated by 1 OTU per genus.
TABLE 2.
Richness, α-Diversity, Relative Abundance, and Case–Control Status by Fecal Microbiota Profile
| Escherichia-Dominant Profile, n = 46 (30%) | Bifidobacterium-Dominant Profile, n = 32 (21%) | Enterobacter/Veillonella-Dominant Profile, n = 34 (22%) | Bacteroides-Dominant Profile, n = 43 (28%) | P | |
|---|---|---|---|---|---|
| Richness | |||||
| Number of genera, median (IQR) | 13 (10–17) | 15 (11–17) | 11 (9–14) | 20 (15–25) | <.001 |
| α-Diversity, median (IQR) | |||||
| Shannon index | 1.86 (1.20–2.46) | 1.96 (1.62–2.39) | 1.69 (1.35–2.20) | 2.51 (2.22–2.96) | <.001 |
| Relative abundance of 10 most abundant genera, mean (SD) | |||||
| Escherichia | 0.53 (0.22) | 0.12 (0.12) | 0.03 (0.08) | 0.13 (0.14) | .003a |
| Bifidobacterium | 0.12 (0.12) | 0.50 (0.19) | 0.07 (0.09) | 0.12 (0.12) | .003a |
| Enterobacter | 0.04 (0.10) | 0.06 (0.11) | 0.49 (0.26) | 0.08 (0.11) | .003a |
| Bacteroides | 0.03 (0.07) | 0.04 (0.07) | 0.04 (0.12) | 0.37 (0.23) | .003a |
| Veillonella | 0.03 (0.05) | 0.02 (0.05) | 0.15 (0.19) | 0.02 (0.05) | .003a |
| Lachnospiraceae incertae sedis | 0.07 (0.12) | 0.02 (0.04) | 0.04 (0.10) | 0.07 (0.09) | .85a |
| Streptococcus | 0.02 (0.04) | 0.05 (0.11) | 0.03 (0.06) | 0.01 (0.01) | .49a |
| Clostridium sensu stricto 1 | 0.04 (0.08) | 0.00 (0.01) | 0.03 (0.05) | 0.01 (0.02) | .20a |
| Enterococcus | 0.02 (0.04) | 0.02 (0.04) | 0.02 (0.05) | 0.01 (0.02) | .96a |
| Akkermansia | 0.00 (0.02) | 0.03 (0.09) | 0.03 (0.11) | 0.01 (0.08) | .99a |
| Case–control status | .01 | ||||
| Bronchiolitis | 10 (22%) | 6 (19%) | 5 (15%) | 19 (44%) | |
| Healthy control | 36 (78%) | 26 (81%) | 29 (85%) | 24 (56%) | |
Benjamini–Hochberg adjusted P value accounting for multiple comparisons.
FIGURE 2.
Nonmetric multidimensional scaling (NMDS) ordination of fecal microbiota. The Bray–Curtis distance between all cases and controls was calculated and used to generate nonmetric multidimensional scaling plots. Each dot in the figure represents the microbiota profile of a single subject in a low-dimensional space. Colored dots indicate 4 microbiota profiles: Escherichia-dominant profile (red), Bifidobacterium-dominant profile (green), Enterobacter/Veillonella-dominant profile (blue), and Bacteroides-dominant profile (purple). The subjects cluster together according to their microbiota profiles.
Microbiota Profiles and Bronchiolitis
The proportion of infants with severe bronchiolitis differed across the 4 microbiota profile groups: lowest in the Enterobacter/Veillonella-dominant profile (15%) and highest in the Bacteroides-dominant profile (44%; Table 2), corresponding to an odds ratio (OR) of 4.59 (95% confidence interval [CI], 1.58–15.5; P = .008). In the multivariable model adjusting for age, gender, prematurity, mode of birth, and history of systemic antibiotic use, the association between the Bacteroides-dominant profile and a greater likelihood of severe bronchiolitis case remained significant (OR for comparison with the Enterobacter/Veillonella-dominant profile, 3.89; 95% CI, 1.19–14.6; P = .03; Table 3). In a sensitivity analysis adjusting for a different set of covariates (age, gender, parental history of asthma, maternal antibiotic use during pregnancy, and systemic corticosteroid use before enrollment), the results did not change materially: Infants with a Bacteroides-dominant profile had a greater likelihood of bronchiolitis (OR, 4.12; 95% CI, 1.28–15.2; P = .02).
TABLE 3.
Unadjusted and Multivariable Associations Between Fecal Microbiota Profiles and Likelihood of Bronchiolitis
| Unadjusted Model | Adjusted Model | Sensitivity Analysis | ||||
|---|---|---|---|---|---|---|
| Variables | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Microbiome profile | ||||||
| Escherichia-dominant profile | 1.61 (0.51–5.66) | .43 | 1.63 (0.49–5.97) | .44 | 1.64 (0.49–6.07) | .44 |
| Bifidobacterium-dominant profile | 1.24 (0.36–5.14) | .66 | 1.28 (0.33–5.13) | .72 | 1.12 (0.26–4.79) | .88 |
| Enterobacter/Veillonella-dominant profile | Reference | Reference | Reference | |||
| Bacteroides-dominant profile | 4.59 (1.58–15.5) | .008 | 3.89 (1.19–14.6) | .03 | 4.12 (1.28–15.2) | .02 |
| Covariates | ||||||
| Age, mo (per incremental month) | — | — | 0.90 (0.75–1.07) | .24 | 0.89 (0.74–1.05) | .19 |
| Female gender | — | — | 1.22 (0.55–2.78) | .63 | 0.93 (0.40–2.19) | .87 |
| Prematurity | — | — | 4.24 (1.56–12.0) | .005 | — | — |
| Cesarean delivery | — | — | 0.63 (0.23–1.63) | .35 | — | — |
| Systemic antibiotic use before enrollment | — | — | 1.68 (0.55–4.95) | .35 | 2.12 (0.66–6.53) | .19 |
| Parental history of asthma | — | — | — | — | 3.27 (1.35–7.99) | .009 |
| Maternal antibiotic use during pregnancy | — | — | — | — | 3.11 (1.23–8.56) | .27 |
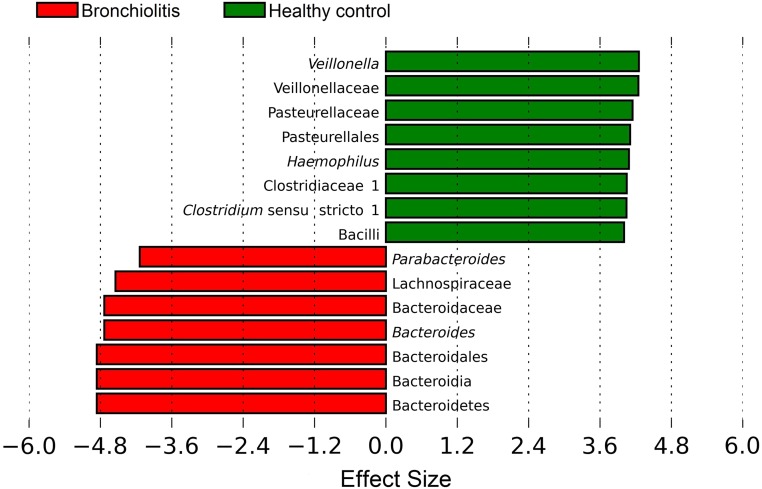
In contrast, the likelihood of bronchiolitis in infants with an Escherichia-dominant or Bifidobacterium-dominant profile was not significantly different compared with those with an Enterobacter/Veillonella-dominant profile in both unadjusted and adjusted analyses. Similarly, the use of linear discriminant effect size method demonstrated that Veillonella genus was negatively associated with likelihood of bronchiolitis, whereas Bacteroides genus was positively associated with likelihood (both Benjamini–Hochberg adjusted Ps < .05; Fig 3).
FIGURE 3.
Effect sizes of genera that were significantly associated with likelihood of being a case (bronchiolitis) or healthy control. The linear discriminant effect size method was used to compare the abundances of all detected bacteria between cases and controls, computing an effect size for each comparison. Results shown here are significant by Kruskal–Wallis test (Benjamini–Hochberg adjusted P < .05) and represent large differences between groups (absolute effect size >3.6). Positive values (right) correspond to the effect sizes representative of healthy infants (controls), and negative values (left) correspond to the effect sizes infants with bronchiolitis (cases). Veillonella genus was found to be overrepresented in healthy infants, whereas Bacteroides genus was overrepresented in infants with bronchiolitis.
Discussion
In this case–control study of 40 infants with bronchiolitis and 115 healthy age-matched controls, we identified 4 distinct fecal microbiota profiles. We found that, compared with infants with an Enterobacter/Veillonella-dominant profile, those with a Bacteroides-dominant profile had a higher likelihood of bronchiolitis. In contrast, the likelihood of bronchiolitis in infants with an Escherichia-dominant or Bifidobacterium-dominant profile was not significantly different. To our knowledge, this is the first study that has investigated the association of fecal microbiota with the risk of bronchiolitis in infants. Our study also highlights the importance of integrating discovery-driven (ie, the identification of microbiota profiles) and hypothesis-driven (ie, the determination of association between the microbiota profiles and bronchiolitis) approaches.
Studies of prebiotic and probiotic supplements, despite their heterogeneity in study populations, treatment regimens, and outcomes,30 have demonstrated the promise of modulating gut microbiota and potentially reducing the morbidity of viral acute respiratory infections (ARIs).31–33 For example, a randomized controlled trial of 94 preterm infants reported that supplementation of prebiotics and probiotics (Lactobacillus rhamnosus) led to a lower incidence of rhinovirus ARIs.31 Another clinical trial of 326 healthy children also reported that the use of Lactobacillus acidophilus and Bifidobacterium animalis reduced ARI symptoms.32 However, none of these trials has investigated the gut microbiota itself. Although data are scarce, murine studies have deciphered the relationship of gut microbiota with host response against viral ARIs (eg, influenza, RSV).34–36 For instance, Ichinohe et al,34 using an antibiotic-treated mouse model, reported that a disruption of gut microbiota (ie, dysbiosis) impairs the generation of virus-specific CD4 and CD8 T cells and antibody responses after influenza virus infection, suggesting the need for an intact commensal bacterial community in the establishment of immune response against viral ARIs. Our study corroborates these earlier findings and extends them by demonstrating the association of Bacteroides-dominant fecal microbiota profiles with bronchiolitis in infants.
Our observations, in conjunction with the earlier studies, suggest a causal pathway linking the gut microbiota in early infancy to the respiratory tract immune response against viral infection. That is, the Bacteroides-dominant gut microbiota in early infancy attenuates the development of immune function in the respiratory tract and thereby leads to an increased susceptibility to bronchiolitis. Indeed, Sjögren et al,37 examining a prospective cohort of 64 infants in Sweden, reported that a high abundance of Bacteroides fragilis in fecal samples during the first month of age was associated with lower levels of Toll-like receptor 4 expression and lipopolysaccharide-induced production of inflammatory cytokines in the peripheral blood mononuclear cells. These data fit into the larger concept of a “common mucosal response,”13,14 that is, antigen presentation at 1 mucosal site (eg, gut), via systemic immune responsiveness, shapes immune function at distant mucosal sites (eg, respiratory tract). Alternatively, the Bacteroides-dominant fecal microbiota might be simply a marker of infants who have a higher propensity for viral ARI, including bronchiolitis. It is also possible that viral ARI alters the gut microenvironment, leading to overgrowth of Bacteroides locally (ie, reverse causation38). Furthermore, any combinations of these mechanisms are also possible.
Interestingly, infants with a Bacteroides-dominant profile had the highest bacterial richness and diversity. Although it is generally considered that higher diversity is protective against morbidity, recent studies have demonstrated that higher bacterial diversity may be associated with higher disease morbidity. For example, Huang et al39 found that, compared with healthy controls, patients with asthma had a higher bacterial richness and diversity in their airway. This finding was concordant with an independent study of a European corticosteroid-using population of patients with asthma.40 Although the underlying mechanism remains to be elucidated, these data may suggest that a depletion of gut microbiota that protects against development of bronchiolitis (resilience microbiota6), rather than microbial richness or diversity, plays a role in the development of bronchiolitis. Despite the complexity, identification of the association between the Bacteroides-dominant microbiota profile and bronchiolitis is an important finding. Our data underscore the importance of understanding microbiome–host interactions by defining the responsible mechanisms, such as systemic dissemination of metabolites produced by the gut microbiota promoting the growth of certain bacteria or acting directly as immunomodulatory molecules in the respiratory tract.7
Several potential limitations of our study should be taken into account. First, the study cases consisted of infants hospitalized with bronchiolitis; therefore, our inference might not be extrapolated to those with milder illness (eg, bronchiolitis not necessitating hospitalization). However, our case selection approach, with its greater severity contrast, probably improved the efficiency of investigating the association of interest. Second, the study design precluded investigation of the dynamics and succession of the gut microbiota in relation to respiratory health in early childhood. To address this important question, we are following the study populations longitudinally up to age 6 years, with fecal sampling at multiple time points. Third, with the use of 16S rRNA gene sequence, we were unable to elucidate the differences in bacterial composition at the species level or their functional capacity. These important topics will be the focus of our future investigations. Fourth, as with any observational study, the association between fecal microbiota and bronchiolitis does not necessarily prove causality and might be explained, at least partly, by unmeasured confounders. Additionally, the small number of bronchiolitis cases prevented us from adjusting for all sets of potential confounders. However, the significant association persisted even after we controlled for clinically important covariates. Finally, participating sites were academic centers in the urban areas. Although these results may not be generalizable to infants in rural areas, our study participants consisted of racially and ethnically diverse samples.
Conclusions
In this case–control study of infants with bronchiolitis and healthy age-matched controls, we identified 4 distinct fecal microbiota profiles in their fecal samples. We also found that, compared with infants with the Enterobacter/Veillonella-dominant profile, those with the Bacteroides-dominant profile had a higher likelihood of bronchiolitis. Although causal inferences remain premature, the identification of a Bacteroides-dominant microbiota profile in early infancy as the primary culprit in the association between the gut microbiota and host immune response against viral ARIs is an important finding. Our data should facilitate epidemiologic, mechanistic, and interventional investigations to disentangle the complex web of the gut microbiome, respiratory viruses, host immune response, and bronchiolitis pathogenesis in children.
Glossary
- ARI
acute respiratory infection
- CI
confidence interval
- IQR
interquartile range
- OR
odds ratio
- OTU
operational taxonomic units
- PCR
polymerase chain reaction
- RSV
respiratory syncytial virus
Footnotes
Dr Hasegawa carried out the statistical analysis and drafted the initial manuscript; Dr Linnemann conceptualized and designed the study, enrolled the subjects, and reviewed and revised the manuscript; Drs Mansbach and Camargo conceptualized and designed the study, obtained the funding, and reviewed and revised the manuscript; Drs Ajami and Petrosino generated the microbiome data, carried out the initial statistical analysis, and reviewed and revised the manuscript; Ms Espinola carried out the initial analyses and reviewed and revised the manuscript; Dr Piedra was involved in the microbiome data generation and critically reviewed and revised the manuscript; Drs Stevenson and Thompson enrolled the subjects, coordinated and supervised data collection, and critically reviewed the manuscript; Ms Sullivan designed the data collection instruments, coordinated and supervised data collection, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: Dr Mansbach has provided bronchiolitis-related consultation for Regeneron; Drs Ajami and Petrosino own shares at Diversigen Inc, a microbiome research company; and the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grants U01 AI-087881, R01 AI-114552, R01 AI-108588, and R21 HL-129909 from the National Institutes of Health (Bethesda, MD). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Mansbach has provided bronchiolitis-related consultation for Regeneron; Drs Ajami and Petrosino own shares at Diversigen Inc, a microbiome research company; and the other authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2016-1377.
References
- 1.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA Jr. Temporal trends in emergency department visits for bronchiolitis in the United States, 2006 to 2010. Pediatr Infect Dis J. 2014;33(1):11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA Jr. Trends in bronchiolitis hospitalizations in the United States, 2000–2009. Pediatrics. 2013;132(1):28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ralston SL, Lieberthal AS, Meissner HC, et al. ; American Academy of Pediatrics . Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5). Available at: www.pediatrics.org/cgi/content/full/134/5/e1474 [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa K, Mansbach JM, Camargo CA Jr. Infectious pathogens and bronchiolitis outcomes. Expert Rev Anti Infect Ther. 2014;12(7):817–828 [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa K, Camargo CA Jr. Airway microbiota and acute respiratory infection in children. Expert Rev Clin Immunol. 2015;11(7):789–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsland BJ, Trompette A, Gollwitzer ES. The gut–lung axis in respiratory disease. Ann Am Thorac Soc. 2015;12(suppl 2):S150–S156 [DOI] [PubMed] [Google Scholar]
- 8.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host–bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920 [DOI] [PubMed] [Google Scholar]
- 10.Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44(6):842–850 [DOI] [PubMed] [Google Scholar]
- 11.Nakayama J, Kobayashi T, Tanaka S, et al. Aberrant structures of fecal bacterial community in allergic infants profiled by 16S rRNA gene pyrosequencing. FEMS Immunol Med Microbiol. 2011;63(3):397–406 [DOI] [PubMed] [Google Scholar]
- 12.Bruzzese E, Callegari ML, Raia V, et al. Disrupted intestinal microbiota and intestinal inflammation in children with cystic fibrosis and its restoration with Lactobacillus GG: a randomised clinical trial. PLoS One. 2014;9(2):e87796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czerkinsky C, Prince SJ, Michalek SM, et al. IgA antibody–producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc Natl Acad Sci USA. 1987;84(8):2449–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang YJ, Boushey HA. The microbiome in asthma. J Allergy Clin Immunol. 2015;135(1):25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansbach JM, Hasegawa K, Henke DM, et al. Respiratory syncytial virus and rhinovirus severe bronchiolitis are associated with distinct nasopharyngeal microbiota. J Allergy Clin Immunol. 10.1016/j.jaci.2016.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa K, Linnemann RW, Avadhanula V, et al. Detection of respiratory syncytial virus and rhinovirus in healthy infants. BMC Res Notes. 2015;8(1):718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee GM, Salomon JA, Friedman JF, et al. Illness transmission in the home: a possible role for alcohol-based hand gels. Pediatrics. 2005;115(4):852–860 [DOI] [PubMed] [Google Scholar]
- 18.Human Microbiome Project Consortium . A framework for human microbiome research. Nature. 2012;486(7402):215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461 [DOI] [PubMed] [Google Scholar]
- 22.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996–998 [DOI] [PubMed] [Google Scholar]
- 23.Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(Database issue):D590–D596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teo Shu M, Mok D, Pham K, et al. . The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17(5):704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman L, Rousseeuw PJ. Partitioning Around Medoids (Program PAM). In: Finding Groups in Data: An Introduction to Cluster Analysis. Hoboken, NJ: John Wiley and Sons, Inc.; 1990:68–125 [Google Scholar]
- 26.Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. JR Stat Soc. 2001;63(2):411–423 [Google Scholar]
- 27.Mansbach JM, Piedra PA, Stevenson MD, et al. ; MARC-30 Investigators . Prospective multicenter study of children with bronchiolitis requiring mechanical ventilation. Pediatrics. 2012;130(3). Available at: www.pediatrics.org/cgi/content/full/130/3/e492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4):e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azad MB, Coneys JG, Kozyrskyj AL, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. BMJ. 2013;347:f6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luoto R, Ruuskanen O, Waris M, Kalliomäki M, Salminen S, Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2014;133(2):405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leyer GJ, Li S, Mubasher ME, Reifer C, Ouwehand AC. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics. 2009;124(2). Available at: www.pediatrics.org/cgi/content/full/124/2/e172 [DOI] [PubMed] [Google Scholar]
- 33.Razi CH, Harmancı K, Abacı A, et al. The immunostimulant OM-85 BV prevents wheezing attacks in preschool children. J Allergy Clin Immunol. 2010;126(4):763–769 [DOI] [PubMed] [Google Scholar]
- 34.Ichinohe T, Pang IK, Kumamoto Y, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA. 2011;108(13):5354–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujimura KE, Demoor T, Rauch M, et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci USA. 2014;111(2):805–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomosada Y, Chiba E, Zelaya H, et al. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol. 2013;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sjögren YM, Tomicic S, Lundberg A, et al. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin Exp Allergy. 2009;39(12):1842–1851 [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211(12):2397–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, et al. . Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127(2):372–381, e371–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. [DOI] [PMC free article] [PubMed] [Google Scholar]