Abstract
Background
Ulcerative colitis (UC) can be treated with surgery or medications. Patients often must choose between long-term immunosuppressive therapy or total colectomy. It is uncertain if there is a mortality benefit to one of these treatment approaches.
Objective
To determine whether patients with advanced UC treated with elective colectomy have an improved survival compared to patients treated with medical therapy.
Design
Retrospective matched cohort study
Setting
50-state Medicaid and beneficiaries (2000–2005), Medicare-beneficiaries (2006–2011) and dual-eligible individuals (2000–2011)
Patients
830 UC patients pursuing elective colectomy surgery and 7,541 matched UC patients pursuing medical therapy.
Measurements
The primary outcome was time to death. Cox proportional hazard models were used to compare the survival of advanced UC patients treated with elective colectomy or medical therapy. The models controlled for significant comorbidities through matched and adjusted analysis.
Results
The mortality rates associated with elective surgery and medical therapy were 34 and 54 per 1,000 person-years, respectively. Elective colectomy was associated with improved survival compared to pursuing chronic medical therapy (adjusted HR 0.67, 95% CI 0.52–0.87) although not all results remained statistically significant in the sensitivity analyses. Post-hoc analysis by age group showed improved survival with surgery in patients 50 years and older with advanced UC (HR 0.60, 95% CI 0.45–0.79, age by treatment interaction p=0.032).
Limitation
Retrospective non-randomized analysis can be subject to residual confounding. The source cohort was derived from different databases across the study period. Sensitivity and secondary analyses had reduced statistical power.
Conclusion
Elective colectomy surgery appeared to be associated with an improved survival rate relative to medical therapy among patients 50 years and older with advanced UC.
Introduction
Current medical therapies for ulcerative colitis (UC), a type of inflammatory bowel disease (IBD) with inflammation confined to the colon, are inadequate to achieve remission in all patients. Mesalamine fails to induce a remission in more than 50% of patients and relapse rates are high even for those who do achieve remission (1). These patients often must choose between surgery or escalation of medical therapy with corticosteroids and/or chronic immunosuppressant therapy. Corticosteroids have been associated with increased infection and mortality risks (2–4). Immunosuppressant therapy (e.g. thiopurine or anti-TNF medications) is associated with increased infection and cancer risks (3, 5–13), and a significant portion of patients will still fail to achieve or maintain remission (5, 14, 15). These patients are exposed to additional courses of corticosteroids and, in some, emergent colectomy which carries higher morbidity and mortality than elective surgery (16–19).
Alternatively, UC patients can pursue elective colectomy which involves a total proctocolectomy with ileostomy and often restorative ileal pouch anal anastomosis. While quality of life following surgery is altered, and up to 40% experience pouchitis (20), when pursued electively, these surgeries carry a low morbidity and mortality (16, 21–23). Two prior studies have suggested there may be a survival benefit with elective colectomy in UC, and that this benefit may vary by patient age; however, both studies were subject to confounding by indication due to an inability to adjust for disease severity (19, 24).
Quality of life, morbidity and mortality are each important factors that drive patients’ and physicians’ treatment decisions (25). Therefore, clarifying whether elective surgery provides a survival advantage relative to medical therapy for UC is important. In this study, we utilized national U.S. Medicare and Medicaid data to conduct a retrospective cohort study examining whether patients with advanced UC pursuing elective colectomy had improved survival compared to similar patients pursuing chronic medical therapy.
Methods
Data source
Medicare and Medicaid data from the Centers for Medicare and Medicaid Services (CMS) and have been widely used for epidemiologic research (see Appendix Methods for further details) (26–30). This study utilized data from all 50 states for Medicaid beneficiaries (2000–2005), Medicare beneficiaries (2006–2011) and dual-eligible individuals (2000–2011).
Study Sample
We identified 182,235 UC patients (ICD-9 code 556.0–556.6, 556.8–556.9) ≥ 18 years and with ≥ 6 months of Medicare/Medicaid eligibility to allow comorbidity measurement and assure capturing the start of medical therapies (Figure 1, Appendix Methods and Appendix Figure 1). We defined advanced UC as having at least one of the following: a hospitalization with a primary diagnosis of UC; 2 or more oral corticosteroid prescriptions within a 90 day period; or any prescription for immunosuppressant therapy (cyclosporine, tacrolimus, azathioprine, 6-mercaptopurine and/or infliximab, n = 50,105. Advanced disease time began when the patient first met any of these criteria. We focused on this group of UC patients who often face decisions regarding escalation of medical therapy versus surgery.
Figure 1.
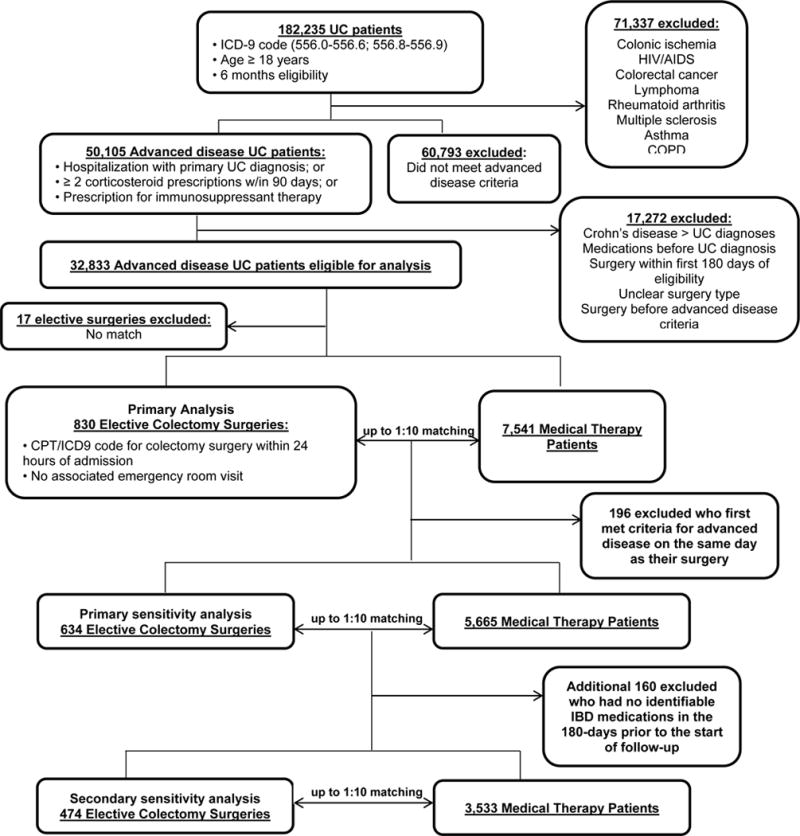
Identification of elective colectomy surgery patients
Exclusion criteria are described in detail in Appendix Methods and Appendix Table 1. Within the remaining 32,833 advanced UC patients, we identified patients pursuing elective colectomy using CPT and ICD-9 codes (Appendix Table 1) (31). To ensure capturing elective surgeries, surgical codes were required to occur within 24 hours of the admission associated with that surgery and could have no emergency department visit on that day or the day prior. Patients having surgery greater than 24 hours after the associated admission or having an emergency department visit on the same day or the day prior to that admission were defined as having an emergent colectomy.
Appendix Table 1.
Diagnostic codes used
| Exclusion Criteria | |
|---|---|
| ICD9 or CPT codes | |
| Colonic ischemia | 557 |
| HIV/AIDS | 079.53, V08, 795.71, 042, 795.8 |
| Colorectal cancer | 153.0–153.9, 154.0, 230.3 |
| Lymphoma | 200.0–200.9, 202.0–202.1, 202.7–202.8, 785.6 |
| Rheumatoid arthritis | 714.0, 714.2, 714.30–714.33, 714.4 |
| Multiple sclerosis | 340 |
| Chronic obstructive pulmonary disease | 490–496 |
| Asthma | 493.00–493.02, 493.10–492.12, 493.20–493.22, 493.8, 493.81–493.82, 493.90–493.92 |
| Colectomy codes | |||
|---|---|---|---|
| ICD9 or CPT codes | Number of patients age < 50 | Number of patients age ≥ 50 | |
| Colectomy, partial, with anastomosis | 44140 | 14 | 61 |
| Colectomy, partial, with skin-level cecostomy or colostomy | 44141 | 1 | 9 |
| Colectomy, partial, with end colostomy and closure of distal segement (Hartmann-type procedure) | 44143 | 10 | 24 |
| Laparoscopy, surgical; colectomy, partial with end colostomy and closure of distal segment (Hartmann type procedure) | 44206 | 1 | 3 |
| Colectomy, partial, with resection, with colostomy or ileostomy and creation of mucofistula | 44144 | 2 | 5 |
| Colectomy, partial, with coloproctostomy | 44145 | 7 | 32 |
| Laparoscopy, surgical; colectomy, partial, with anastomosis, with coloproctostomy | 44207 | 1 | 15 |
| Colectomy, partial; with coloproctostomy, with colostomy | 44146 | 0 | 9 |
| Laparoscopy, surgical; colectomy, partial, with anastomosis, with coloproctostomy | 44208 | 0 | 4 |
| Colectomy, partial; abdominal and transanal approach | 44147 | 2 | 3 |
| Colectomy, total; abdominal, without proctectomy; with ileostomy or ileoproctostomy | 44150 | 19 | 67 |
| Laparoscopy, surgical; colectomy, total, abdominal, without proctectomy, with ileostomy or ileoproctostomy | 44210 | 8 | 39 |
| Colectomy, total; abdominal, without proctectomy; with continent ileostomy | 44151 | 0 | 3 |
| Total proctocolectomy with ileo pouch-anal anastomosis | 44152 | 1 | 1 |
| Laparoscopy, surgical; colectomy, total, abdominal, with proctectomy, with ileoanal anastomosis, creation of ileal reservoir, with loop ileostomy, with or without rectal mucosectomy | 44211 | 6 | 33 |
| Colectomy | 44153 | 17 | 8 |
| Colectomy, total; abdominal, with proctectomy; with ileostomy | 44155 | 21 | 139 |
| Laparoscopy, surgical; colectomy, total, abdominal, with proctectomy, with ileostomy | 44212 | 4 | 81 |
| Colectomy, total; abdominal, with proctectomy; with continent ileostomy | 44156 | 0 | 2 |
| Total intra-abdominal colectomy | 45.8, 45.80, 45.81, 45.82, 45.83 | 66 | 15 |
| Formation of endorectal ileal pouch with anastomosis of small intestine to anus | 45.95 | 6 | 0 |
| Bowel excision resection ostomy | 44209 | 0 | 0 |
| “with ileoanal anastamosis, includes loop ileostomy, andrectal mucosectomy, when performed” | 44157 | 1 | 4 |
| “with ileoanal anastomosis, creation of ileal reservoir, includes loop ileostomy, and rectal mucosectomy, when performed” | 44158 | 11 | 26 |
| laparoscopic colectomy, partial, with anastomosis | 44204 | 1 | 23 |
| Laparoscopy, surgical; colectomy, partial, with removal of terminal ileum with ileocolostomy | 44205 | 3 | 6 |
| Proctocolectomy with ileal anastomosis | 45113 | 10 | 6 |
Cohort Selection
The goal was to select a cohort of medically-treated patients that were as similar as possible to our surgery patients, yet did not have elective surgery. Each elective surgery patient was matched to up to 10 medically-treated patients with advanced UC on the time from advanced disease to having surgery as described below (see Appendix Methods). In addition, the medical therapy patient had to match the surgical patient at the start of follow-up on: age (categories shown in Table 1); sex; specific immunosuppressant therapy used in the 6-months prior to start of follow-up (including corticosteroids); reason for inclusion in the advanced disease cohort; and Medicaid eligibility (as a proxy for low-income) (Appendix Methods).
Table 1.
Baseline demographics
| VARIABLE | ELECTIVE SURGERY N = 830 |
MEDICAL THERAPY† N = 7,541 |
|---|---|---|
| Age, n (%)* | ||
| 18 ≤ age < 30 | 52 (6) | 442 (6) |
| 30 ≤ age < 40 | 81 (10) | 703 (9) |
| 40 ≤ age < 45 | 36 (4) | 308 (4) |
| 45 ≤ age < 50 | 38 (5) | 331 (4) |
| 50 ≤ age < 55 | 38 (5) | 328 (4) |
| 55 ≤ age < 60 | 40 (5) | 336 (5) |
| 60 ≤ age < 65 | 31 (4) | 242 (3) |
| 65 ≤ age < 70 | 169 (20) | 1,575 (21) |
| 70 ≤ age < 75 | 171 (21) | 1,629 (22) |
| 75 ≤ age < 80 | 114 (14) | 1,063 (14) |
| ≥ 80 | 60 (7) | 584 (8) |
| Gender, n (%)* | ||
| Female | 478 (58) | 4,410 (59) |
| Male | 352 (42) | 3,131 (42) |
| Medication use in prior 180 days, n (%)* | ||
| Corticosteroids | 388 (47) | 3,282 (44) |
| Cyclosporine/Tacrolimus | < 11 (<1)‡ | < 11 (<1)‡ |
| Azathioprine/6-mercaptopurine | 161 (19) | 1,125 (15) |
| Infliximab | 65 (8) | 414 (6) |
| Hospitalized for UC, n (%)* | 518 (62) | 4,739 (63) |
| Number of criteria for advanced disease met between cohort entry to start of follow-up** | ||
| One criteria met | 354 (43) | 4,268 (57) |
| Two criteria met | 251 (30) | 2,286 (30) |
| Three criteria met | 225 (27) | 987 (13) |
| Days from cohort entry to start of follow-up* | ||
| Mean (SD) | 302 (424) | 283 (403) |
| Median (IQR) | 120 (1–425) | 104 (1–405) |
| Medicaid beneficiary, n (%)* | 324 (39) | 2,862 (38) |
| Comorbidity score (preceding 180 days) | ||
| Score > 1 | 391 (47) | 3,278 (44) |
| Mean (SD) | 2.0 (2.5) | 2.0 (2.6) |
| Median (IQR) | 1 (0–3) | 1 (0–3) |
| Non-IBD medications in prior 180 days | ||
| mean (SD) | 9.7 (4.8) | 9.5 (5.1) |
| median (IQR) | 9.0 (6.0–13.0) | 9.0 (6.0–12.0) |
| Emergency Department visits in prior 180 days | ||
| mean (SD) | 0.8 (1.5) | 1.0 (1.8) |
| median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
Not all medical therapy patients had exposure to immunosuppressants or corticosteroids in the 180-days prior to follow-up; some had exposure to mesalamine therapy or had no medication exposure (and were removed in sensitivity analysis)
Medical therapy patients were weighted in the analysis according to the number of patients in each matched group. The numbers reported here are not weighted and do not reflect the balance achieved with the matching.
Use of the data was governed by a Data Use Agreement with CMS, which prohibits displaying any cell size < 11
criteria for advanced disease include: a hospitalization with a primary diagnosis of UC; 2 or more oral corticosteroid prescriptions within a 90 day period; or any prescription for immunosuppressant therapy including cyclosporine, tacrolimus, azathioprine, 6-mercaptopurine and/or infliximab therapy. The p-value for the difference between cohorts was <0.001.
Follow-up time for the surgery cohort started on the date of their elective surgery. The time from advanced disease to surgery was used to match up to 10 medically-treated patients (see Appendix Figure 1). Follow-up for the medically-treated group began the same number of days after they met the definition of advanced disease as the surgery patient underwent colectomy. Any advanced UC patient that had not had a surgery prior to this date was eligible to be a match.
Matched medical therapy patients who underwent an emergent colectomy after starting follow-up continued to contribute follow-up time to the medical therapy cohort in an intention-to-treat manner. If medical therapy patients had an elective surgery after the start of follow-up, they were re-matched at the time of their elective surgery with an appropriate medical therapy patient and contributed follow-up to both the medical treatment and surgery cohorts from that point forward.
Statistical Analysis
The primary outcome of interest was mortality which is identifiable in the Medicare-Medicaid records by the date of death. Cox regression was used to compute unadjusted and adjusted hazard ratios and 95% confidence intervals. To account for the variable number of matches, we used a weighted Cox regression (see Appendix Methods). Our primary exposure of interest was treatment strategy (surgery vs. medical therapy). Additional potential confounders were also evaluated in the 180 days prior to start of follow-up (Table 1). Comoribidites were categorized (≤ 1 or 2+) using the combined Romano/Charlson-Elixhauser comorbidity index (32). Polypharmacy was assessed by counting the number of unique non-IBD medications prescribed based on National Drug Category (NDC) codes. Potential confounders were added separately; variables with a greater than 10% change in the effect estimate were retained in the final model. The test of the proportional hazards assumption evaluated the interaction of treatment and the natural log of follow-up time. Comorbidity standardized survival proportion and cumulative incidence were calculated using inverse probability weighting (33) (Appendix Methods).
A sensitivity analysis was conducted excluding 196 elective colectomy patients who first met the criteria for advanced disease on the same day as their surgery as well as their matched medically-treated patients. In a second sensitivity analysis we excluded these patients along with an additional 160 UC elective colectomy patients and their medically-treated matches who had no identifiable IBD medications in the 180-days prior to the start of follow-up in the Medicare-Medicaid data (Appendix Table 2). Additional sensitivity analyses were stratified by treatment prior to start of follow-up.
Appendix Table 2.
Demographics for Primary and Secondary Sensitivity Analysis
| PRIMARY SENSITIVITY ANALYSIS | SECONDARY SENSITIVITY ANALYSIS | |||
|---|---|---|---|---|
| VARIABLE | ELECTIVE SURGERY N = 634 |
MEDICAL THERAPY† N = 5,665 |
ELECTIVE SURGERY N = 474 |
MEDICAL THERAPY† N = 3,533 |
| Age, n (%)* | ||||
| 18 ≤ age < 30 | 38 (6) | 320 (6) | 35 (7) | 286 (8) |
| 30 ≤ age < 40 | 60 (10) | 495 (9) | 43 (9) | 298 (8) |
| 40 ≤ age < 45 | 28 (4) | 236 (4) | 17 (4) | 109 (3) |
| 45 ≤ age < 50 | 27 (4) | 234 (4) | 19 (4) | 134 (4) |
| 50 ≤ age < 55 | 27 (4) | 224 (4) | 18 (4) | 121 (3) |
| 55 ≤ age < 60 | 28 (4) | 224 (4) | 19 (4) | 111 (3) |
| 60 ≤ age < 65 | 24 (4) | 172 (3) | 17 (4) | 98 (3) |
| 65 ≤ age < 70 | 127 (20) | 1,174 (21) | 101 (21) | 821 (23) |
| 70 ≤ age < 75 | 128 (20) | 1,209 (21) | 96 (20) | 747 (21) |
| 75 ≤ age < 80 | 91 (14) | 833 (15) | 69 (15) | 519 (15) |
| ≥ 80 | 56 (9) | 544 (10) | 40 (8) | 289 (8) |
| Gender, n (%)* | ||||
| Female | 372 (59) | 3,377 (60) | 267 (56) | 2,025 (57) |
| Male | 262 (41) | 2,288 (40) | 207 (44) | 1,508 (43) |
| Medication use in prior 180 days, n (%)* | ||||
| Corticosteroids | 334 (53) | 2,808 (50) | 334 (71) | 2,766 (78) |
| Cyclosporine/Tacrolimus | <11 (<1)** | <11 (<1)** | <11 (<1)** | <11 (<1)** |
| Azathioprine/6-MP | 157 (25) | 1,119 (20) | 157 (33) | 1,112 (32) |
| Infliximab | 63 (10) | 411 (7) | 63 (13) | 408 (12) |
| Hospitalized for UC, n (%)* | 322 (51) | 2,863 (51) | 207 (44) | 1,339 (40) |
| Days from cohort entry to start of follow-up* | ||||
| Mean (SD) | 396 (445) | 376 (426) | 372 (398) | 328 (348) |
| Median (IQR) | 231 (68–619) | 212 (62–572) | 217 (88–549) | 184 (77–478) |
| Medicaid beneficiary, n (%)* | 250 (39) | 2,175 (38) | 184 (39) | 1,337 (38) |
| Comorbidity score (preceding 180 days) | ||||
| Score > 1 | 266 (42) | 1,691 (30) | 184 (39) | 972 (28) |
| Mean (SD) | 1.7 (2) | 1.2 (2) | 1.5 (2) | 1.1 (2) |
| Median (IQR) | 1.0 (0–3.0) | 1.0 (0–2.0) | 1.0 (0–3.0) | 0 (0–2.0) |
| Non-IBD medications in prior 180 days | ||||
| mean (SD) | 10 (5) | 10 (5) | 11 (5) | 10 (5) |
| median (IQR) | 10 (6–13) | 9 (6–12) | 10 (7–14) | 9 (6–13) |
| Emergency Department visits in prior 180 days | ||||
| mean (SD) | 0.9 (1.6) | 0.8 (1.7) | 0.9 (1.7) | 0.7 (1.6) |
| median (IQR) | 0 (0–1.0) | 0 (0–1.0) | 0 (0–1.0) | 0 (0–1.0) |
Not all medical therapy patients had exposure to immunosuppressants or corticosteroids in the 180-days prior to follow-up; some had exposure to mesalamine therapy or had no medication exposure (and were removed in the secondary sensitivity analysis)
Medical therapy patients were weighted in the analysis according to the number of patients in each matched group. The numbers reported here are not weighted and do not reflect the balance achieved with the matching.
Use of the data was governed by a Data Use Agreement with CMS, which prohibits displaying any cell size < 11.
To assess the robustness of our findings, we re-ran the main and primary sensitivity analysis 20 times with randomly resampled matched medical-therapy patients. All statistical analyses were performed using SAS v.9.3 (SAS Institute, Cary, NC).
Although the study sample size was limited by available data, a priori power calculations (assuming α = 0.05) estimated that with a 1:1 matching of medical therapy to elective surgery patients, and assuming a relative survival of 0.5 in the medical therapy group, we would need approximately 350 surgery and 350 medical therapy patients to reject the null hypothesis of equal survival with 80% power.
Ethical Considerations
The study was approved by the Institution Review Boards at the participating institutions.
Role of Funding Source
The funding agencies played no role in the design or conduct of the study; collection, management, analysis or interpretation of data; or preparation, review or approval of manuscript.
Results
We identified 32,833 patients with advanced UC as defined by our inclusion criteria (Figure 1). Of these patients, 847 underwent elective colectomy. Seventeen of these patients could not be matched for reasons of prior medication use and duration of advanced disease; and were excluded from our analysis. Thus 830 elective surgery patients were in our primary analysis. Table 1 and Appendix Table 2 describe the demographics for the overall and sensitivity analyses, respectively.
The mortality rates associated with elective colectomy and medical therapy in the overall cohort were 34 and 54 per 1,000 person-years, respectively (Table 2). Elective colectomy was associated with an improved survival compared to medical therapy in unadjusted analyses (HR 0.70, 95% CI 0.54–0.90) (Table 2), although neither of the sensitivity analyses showed significantly greater survival in those pursuing elective surgery. When the analysis was limited to the patients who were removed in the primary sensitivity analysis (i.e. the 196 surgical patients who entered the advanced cohort on the day of elective surgery and their matches), patients pursuing elective colectomy had a longer survival (HR 0.30, 95% CI 0.16–0.56).
Table 2.
Mortality Rates and Hazard Ratios for Relative Survival of Elective Colectomy Compared to Medical Therapy Among Patients with Advanced Ulcerative Colitis*
| Main Analysis | Primary Sensitivity Analysis** | Secondary Sensitivity Analysisπ | Patients Excluded in Primary Sensitivity Analysis | Patients with only immunosuppressant exposure† pre-follow-up | Patients with only corticosteroid exposure pre-follow-up | |
|---|---|---|---|---|---|---|
| Elective colectomy patients (n) | 830 | 634 | 474 | 196 | 139 | 195 |
| Matched medical therapy patients (n) | 7541 | 5665 | 3533 | 1876 | 919 | 1889 |
| Entire cohort | ||||||
| unadjusted | 0.70 (0.54–0.90) test of proportional hazard assumption p = 0.094 |
0.93 (0.70–1.24) test of proportional hazard assumption p = 0.075 |
0.89 (0.61–1.30) test of proportional hazard assumption p = 0.073 |
0.30 (0.16–0.56) test of proportional hazard assumption p = 0.182 |
1.04 (0.53–2.01) |
0.95 (0.58–1.56) |
| adjusted for comorbidities | 0.67 (0.52–0.87) |
0.81 (0.61–1.08) |
0.79 (0.53–1.16) |
0.38 (0.20–0.72) |
1.09 (0.56–2.12) |
0.84 (0.51–1.38) |
| Age < 50 | ||||||
| unadjusted | 1.38 (0.70–2.70) |
1.84 (0.87–3.88) |
1.93 (0.75–4.98) |
0.42 (0.06–2.95) |
0.70 (0.08–6.10) |
3.57 (1.16–11.00) |
| adjusted for comorbidities | 1.35 (0.69–2.66) |
1.71 (0.79–3.69) |
1.78 (0.67–4.71) |
0.51 (0.08–3.14) |
0.71 (0.09–5.86) |
2.82 (0.87–9.15) |
| Age ≥ 50 | ||||||
| unadjusted | 0.62 (0.47–0.82) |
0.83 (0.61–1.13) |
0.78 (0.52–1.18) |
0.28 (0.14–0.54) |
1.10 (0.55–2.20) |
0.76 (0.43–1.33) |
| adjusted for comorbidities | 0.60 (0.45–0.79) |
0.72 (0.52–0.98) |
0.69 (0.45–1.05) |
0.35 (0.18–0.68) |
1.15 (0.57–2.33) |
0.69 (0.39–1.21) |
| p-value for interaction of age and treatment‡ | 0.032 | 0.047 | 0.085 | 0.72 | 0.66 | 0.027 |
| Deaths, (IR, 95% CI per 1,000 person-years)†† | ||||||
| Elective surgery entire cohort | 63 (IR 34, 26–44) | 53 (IR 39, 29–51) | 32 (IR 31, 21–43) | 10 (IR 21, 10–39) | 10 (IR 32, 15–58) | 17 (IR 42, 24–67) |
| Medical therapy entire cohort | 783 (IR 54, 50–57) | 479 (IR 46, 42–50) | 251 (IR 37, 33–42) | 304 (IR 73, 65–82) | 59 (IR 31, 23–40) | 154 (IR 45, 38–52) |
| Elective surgery age < 50 | 10 (IR 25, 12–46) | 9 (IR 30, 14–56) | 6 (IR 26, 9–56) | 1 (IR 11, 0.27–59) | 1 (IR 11, 0.29–63) | 4 (IR 50, 14–127) |
| Medical therapy age < 50 | 66 (IR 19, 14–24) | 40 (IR 16, 12–22) | 21 (IR 14, 9–22) | 26 (IR 24, 15–35) | 7 (IR 15, 6–32) | 10 (IR 13, 6–23) |
| Elective surgery age ≥ 50 | 53 (IR 37, 28–48) | 44 (IR 42, 30–56) | 26 (IR 32, 21–47) | 9 (IR 24, 11–45) | 9 (IR 40, 18–76) | 13 (IR 40, 21–68) |
| Medical therapy age ≥ 50 | 717 (IR 65, 60–70) | 439 (IR 55, 50–60) | 230 (IR 43, 38–49) | 278 (IR 91, 80–102) | 52 (IR 36, 27–47) | 144 (IR 54, 46–64) |
Data are presented as hazard ratio and 95% confidence intervals unless otherwise specified
Primary sensitivity analysis excluding 196 UC elective colectomy patients who first met the criteria for advanced disease on the same day as their surgery as well as their matched medically-treated patients
Secondary sensitivity analysis excluded those patients excluded in the primary sensitivity analysis as well as an additional 160 UC elective colectomy patients and their medically-treated matched patients who had no identifiable IBD medications in the 180-days prior to the start of follow-up
Immunosuppressant exposure defined as azathioprine/6-mercaptopurine, infliximab and/or cyclosporine/tacrolimus
adjusted for comorbidities
IR incidence rate; CI confidence interval
The number of non-IBD medications, emergency department visits in the prior 180 days and calendar year of cohort entry did not meet our definition of confounders. Adjustment for comorbidities increased the strength of association between treatment and mortality in the primary (HR 0.67 (95% CI 0.52–0.87) and sensitivity analysis (Table 2). Divergence in the survival proportion started at 180 days post-operatively and continued into follow-up (Table 3, Figure 2).
Table 3.
Follow-up Time and Cumulative Incidence of Death in Elective Surgery versus Medical therapy†
| Time from start of follow-up | Number Died | Number Remaining | Survival Proportion* | Cumulative Incidence per 1,000 persons* | ||||
|---|---|---|---|---|---|---|---|---|
| Surgical Therapy | Medical Therapy | Surgical Therapy | Medical Therapy | Surgical Therapy | Medical Therapy | Surgical Therapy | Medical Therapy | |
| 30 days | 12 | 91 | 799 | 7210 | 0.99 (0.98–0.99) |
0.99 (0.98–1.00) |
14.5 (6.3–22.7) |
12.0 (4.5–19.4) |
| 60 days | 20 | 172 | 771 | 6901 | 0.98 (0.96–0.99) |
0.98 (0.97–0.99) |
24.5 (13.8–35) |
24.2 (13.5–34.8) |
| 90 days | 22 | 227 | 744 | 6593 | 0.97 (0.96–0.98) |
0.97 (0.96–0.98) |
27.1 (15.8–39.1) |
32.0 (19.7–44.1) |
| 180 days | 26 | 337 | 689 | 5868 | 0.97 (0.96–0.98) |
0.95 (0.94–0.97) |
32.5 (20.1–44.7) |
48.8 (33.5–64) |
| 365 days | 37 | 476 | 566 | 4724 | 0.95 (0.94–0.97) |
0.93 (0.91–0.95) |
48.9 (33.3–64.2) |
73.5 (54.1–92.5) |
| 2 years | 48 | 615 | 392 | 2994 | 0.93 (0.91–0.95) |
0.89 (0.87–0.92) |
68.7 (49.4–87.6) |
106.8 (81.8–131.1) |
| 3 years | 55 | 704 | 234 | 1743 | 0.91 (0.89–0.94) |
0.86 (0.83–0.89) |
89.1 (64.8–112.8) |
141.9 (109.8–172.8) |
| 4 years | 60 | 745 | 124 | 964 | 0.89 (0.85–0.92) |
0.83 (0.79–0.87) |
113.9 (81.5–145) |
168.7 (129.2–206.3) |
| 5 years | 62 | 770 | 55 | 393 | 0.86 (0.81–0.91) |
0.79 (0.74–0.85) |
141.4 (91.3–188.7) |
205.9 (149.3–258.7) |
| 6 years | 62 | 777 | 28 | 166 | 0.86 (0.81–0.91) |
0.77 (0.71–0.85) |
141.4 (91.3–188.7) |
225.5 (151.9–292.7) |
| 7 years | 62 | 780 | 17 | 110 | 0.86 (0.81–0.91) |
0.76 (0.68–0.85) |
141.4 (91.3–188.7) |
238.7 (151.3–317.1) |
| 8 years | 63 | 781 | 12 | 79 | 0.80 (0.69–0.93) |
0.75 (0.67–0.85) |
200.6 (70.2–312.6) |
245.8 (148.6–331.9) |
| 9 years | 63 | 783 | <11** | 48 | 0.80 (0.69–0.93) |
0.73 (0.62–0.87) |
200.6 (70.2–312.6) |
266.8 (132.5–380.4) |
Survival proportion and cumulative incidence estimates for medical therapy patients are standardized to the surgery group using the Romano/Charlson-Elixhauser comorbidity index
Use of the data was governed by a Data Use Agreement with CMS, which prohibits displaying any cell size < 11.
Figure 2.
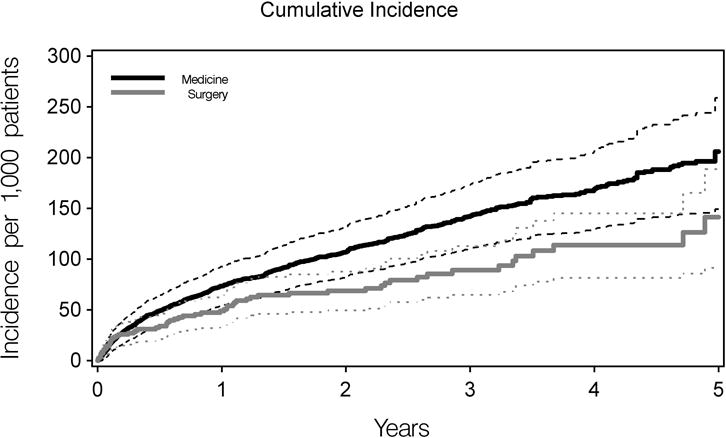
Cumulative incidence of death. Cumulative incidence is shown in bold lines; and 95% CI’s are indicated with thin lines. Medical therapy patients are illustrated in black and elective surgery in grey. Data utilized to generate Figure 2 are provided in Table 3.
To assure the robustness of our analysis, we re-ran the models in 20 different randomly-selected matched cohorts. The mean hazard ratio of these was less than 2% different from the reported results in each analysis evaluated, and the maximum difference of any individual analysis was 7.24%.
Based on prior studies showing an age-based survival benefit of elective colectomy,(24) we also examined age as an effect modifier. The test for interaction of age ≥ 65 with treatment was not significant (p=0.27) despite a survival benefit for surgery in UC patients age 65 and older (HR adjusted for comorbidities 0.6, 95% CI 0.45–0.80). However, the test for interaction of age ≥ 50 was significant (p=0.032). Therefore, post-hoc analysis was performed examining age < 50 and age ≥ 50, adjusting for comorbidities. In patients < 50 years, survival with elective colectomy and medical therapy was not significantly different. However, in patients ≥ 50 years, elective colectomy was associated with a survival benefit compared to medical therapy in both the overall main analysis (HR 0.60, 95% CI 0.45–0.79) and primary sensitivity analysis (HR 0.72, 95% CI 0.52–0.98); and the strength of association was comparable but with wider confidence intervals in the secondary sensitivity analysis (HR 0.69, 95% CI 0.45–1.05) (Table 2).
An analysis was performed that was limited to patients treated with only immunosuppressant therapy (azathioprine/6-mercaptopurine, infliximab, and/or cyclosporine/tacrolimus) or only corticosteroids in the 6 months prior to the start of follow-up. Survival did not differ between the medical and surgically treated patients in these subgroups, although these analyses were underpowered (Table 2). Analysis was also performed examining potential causes of increased mortality in medical therapy patients. During follow-up, those pursuing colectomy were more likely to have infections and use narcotics, but less likely to use corticosteroids after the first several months (Appendix Table 3).
Appendix Table 3.
Descriptive analysis of patients in surgery and medical therapy cohorts during follow-up†
| VARIABLE | ELECTIVE SURGERY N = 830 |
MEDICAL THERAPY N = 7541 |
p-value |
|---|---|---|---|
| Subsequent elective surgery, n (%) | N/A | 198 (3) | |
| Days from start of follow-up to elective surgery | |||
| Mean (SD) | 362 (395) | ||
| Median (IQR) | 223 (63–533) | ||
| Subsequent emergent surgery | 0 | 226 (3) | |
| Days from start of follow-up to emergent surgery | |||
| Mean (SD) | 362 (395) | ||
| Median (IQR) | 223 (63–533) | ||
| New drug therapy initiated during follow-up‡ | 160 (19) | 1965 (26) | <0.001 |
| Azathioprine/6-MP use during: | |||
| follow-up, n (%) | 37 (5) | 1300 (17) | <0.001 |
| first year of follow-up, n (%) | 20 (2) | 1192 (16) | <0.001 |
| Cyclosporine/tacrolimus use during: | |||
| follow-up, n (%) | 3 (0.4) | 26 (0.3) | 0.94 |
| first year of follow-up, n (%) | 3 (0.4) | 18 (0.2) | 0.50 |
| Corticosteroid use during: | |||
| follow-up, n (%) | 316 (38) | 3,090 (41) | 0.11 |
| first year of follow-up, n (%) | 249 (30) | 2,511 (33) | 0.06 |
| follow-up, excluding first month, n (%) | 244 (29) | 2,801 (37) | <0.001 |
| follow-up, excluding first three months, n (%) | 208 (25) | 2,450 (33) | <0.001 |
| 2 or more corticosteroid prescriptions within 90 days during follow-up | 150 (18) | 1,729 (23) | 0.002 |
| Infliximab use during: | |||
| follow-up, n (%) | 76 (9) | 924 (12) | 0.009 |
| first year of follow-up, n (%) | 41 (5) | 646 (9) | <0.001 |
| Mesalamine use during: | |||
| follow-up, n (%) | 148 (18) | 3131 (42) | <0.001 |
| first year of follow-up, n (%) | 119 (14) | 2918 (39) | <0.001 |
| Narcotic use in first year of follow-up*, n (%) | 459 (55) | 3,102 (41) | <0.001 |
| Infections in first year of follow-up*, n (%) | |||
| Any infection | 91 (11) | 654 (9) | 0.030 |
| Serious bacterial infections | 87 (11) | 606 (8) | 0.020 |
| Opportunistic infections | <11 (1)** | 58 (1) | 0.36 |
| Tuberculosis | <11 (0)** | <11 (0)** | 0.64 |
| Zoster infections | < 11 (1)** | 38 (1) | 0.93 |
p-values are based on simple χ2 test and do not account for follow-up time.
Excludes first month of follow-up
Inclusive of any prescription of mesalamine, corticosteroids, thiopurines, calciurein inhibitor therapy, or infliximab therapy.
Use of the data was governed by a Data Use Agreement with CMS, which prohibits displaying any cell size < 11.
Discussion
We observed that among Medicare and Medicaid beneficiaries with advanced UC, and specifically among those ≥ 50 years, elective colectomy is associated with a significantly improved relative survival compared to those who pursue medical therapy. To our knowledge, this is the first population-based study to examine relative survival in elective colectomy versus medical therapy for UC in the United States; and the first such study to adjust for medication use prior to surgery. Two prior European population-based studies observed similar improved relative survival in UC patients pursuing elective colectomy compared to those admitted to the hospital for their UC (19, 24). However, neither study was able to adjust for medication use prior to surgery, allowing for confounding by indication, if the choice to not pursue surgery was linked to risk of death through some factor other than the treatment decision. To address this, we matched our medical and surgical cohorts on key factors including age, definition of advanced disease, duration with advanced disease and recent medical therapy; and further adjusted for concomitant comorbidities. We also performed additional sensitivity analyses to address this particular concern. Specifically, in our primary sensitivity analysis, we removed a group of elective colectomy patients who were (by our definition) relatively well and only were labeled as advanced due to hospitalization for their surgery; their matched medical-therapy patients consisted of UC patients with severe enough disease to also warrant a hospitalization for their disease but not elective surgery (Table 2). Not surprisingly, there was a very large survival benefit associated with surgery in this excluded cohort. However, even after excluding these patients, surgical therapy was associated with a survival benefit in older patients.
Prior work has indicated that there is a survival benefit of elective colectomy in UC starting at age 50 (24). In our data, we performed a post-hoc analysis that also confirmed a survival benefit in those 50 years and older. Notably, we also found a survival benefit with surgery in UC patients age 65 and older, although the interaction of age (< 65, ≥ 65) with treatment (adjusted for comorbidities) was non-significant.
We did not find a survival benefit associated with elective colectomy in patients aged < 50. These results should be interpreted with caution, given that these analyses were performed post-hoc and involved a smaller sample size and low absolute number of deaths (78 total deaths). We found the proportion of medical-therapy patients receiving immunosuppressants was similar in those < 50 and ≥ 50 years (data not shown). Nonetheless, because complications from medical therapy are less common in young patients (34) and death from any cause is relatively rare under the age of 50, even the small absolute risk of perioperative mortality represents a larger relative risk in this population. However, in younger UC patients with medication-refractory disease, elective surgery can certainly be of benefit in reducing morbidity and mortality, and thus our findings should not be interpreted as indicating that surgery should be universally avoided in this younger population.
When examining only those patients on immunosuppressant therapy or corticosteroids prior to follow-up, survival was not statistically significantly different between medical and surgical therapy. These analyses were underpowered, but suggest some important concepts. In patients treated only with corticosteroids, particularly those over age 50, the mortality hazard ratio was similar to prior studies finding increased mortality associated with corticosteroids (4, 35). Our data also point to a comparable survival with medical or surgical therapy in those patients in a stable remission on immunosuppressant therapy. Alternatively, the finding of a survival benefit for elective colectomy among patients over age 50, but not in the subgroup treated with immunosuppressant therapy, could possibly have been due to an unmeasured comorbidity that precluded use of these immunosuppressant therapies or surgery and increased the risk of death. The proportion of patients with advanced disease who were receiving chronic immunosuppressant therapies was relatively small, which may be due to patients having disease refractory to these therapies, misunderstanding regarding the risks of continued active disease, or patients’ preferences to avoid chronic immunosuppression. The comparative effectiveness of the different medical treatment strategies, particularly in the elderly, should be a focus of further research.
The findings of this study have important implications for both patients and providers with regards to informed decision making in UC. Traditionally, providers have assumed that UC patients desire to avoid colectomy surgery given resulting changes to quality of life. Discussion of surgical options in UC are therefore often not initiated, or initiated only when all other medical therapies have failed, despite a literature suggesting that post-operative UC patients report improved quality of life with surgery (21, 36–39). Recent work has shown that UC patients are willing to accept surgery in order to avoid potential lethal serious adverse effects of chronic immunosuppressant therapies, especially if these therapies are incompletely effective in maintaining a durable remission (25). Our findings of a survival advantage associated with elective colectomy in patients 50 years or older underline the need for earlier and more informed discussions regarding surgical options in UC to improve shared decision-making, and to specifically emphasize that a strategy of intermittent corticosteroid use or incompletely controlled disease in advanced UC may be associated with an increased mortality risk.
There are potential limitations to our study. The Medicare and Medicaid data are collected for administrative purposes and the diagnosis of UC has not been validated. In our study, UC patients had, on average, 13.6 encounters (median 6) with an ICD9 coded diagnosis for UC. Our inclusion criteria also required patients to additionally have concomitant corticosteroids and/or immunosuppressant prescriptions, or have a primary hospitalization for UC, thus limiting the potential misclassification. The Medicare and Medicaid data do not explicitly label surgery as emergent or elective. However, our definition of elective surgery produced post-operative mortality rates comparable to other national datasets examining mortality in Medicare and Medicaid beneficiaries in which surgery was defined as elective (16), thus lending support to our methodology and findings.
Our source populations differed by time frames. To account for this, we matched on age and Medicaid status and tested calendar year of cohort entry as a potential confounder and effect modifier, although there was no evidence of confounding or modification by time period.
Medicare and Medicaid are the primary insurance for 18% of the U.S. population, making it the coverage for an overall large population of UC patients. However, findings among Medicare and Medicaid beneficiaries may not be generalizable to the general population. For example, our patients < 65 years qualified for Medicare because of disability, although we could not determine if this was directly a result of their UC. Importantly, however, our analysis was limited to comparison within the Medicare-Medicaid population itself, thus while our absolute mortality rates may differ from other populations, our relative mortality rates should not. The similarity of our results to two population-based studies from Europe (19, 24) suggests that these results are likely to be generalizable to the broader U.S. population.
We did not examine cause of death as this is subject to significant misclassification in administrative databases (40, 41). We did not find any differences in infections or narcotic use that would explain the difference in mortality rates. Corticosteroid use after the first few months was more common in the medically treated patients. We were unable to adjust for smoking as it is not a coded diagnosis within the dataset. There is some evidence that tobacco use may improve UC symptoms and therefore one could speculate that smokers may be less likely to pursue surgery, but more likely to die from smoking-related diseases (42). We did exclude patients with concomitant diagnoses for asthma or COPD, both of which are associated with tobacco use. We also adjusted for the Romano/Charlson-Elixhauser comorbidity index which contains several smoking-related comorbidities (32).
In conclusion, we have found that in Medicare and Medicaid beneficiaries with UC requiring hospitalization, multiple corticosteroid prescriptions or immunosuppressant use, there is greater long-term survival following elective surgery when compared to pursuing long-term medical therapy. Post-hoc analysis suggests that this survival benefit was most evident in those patients 50 years or older and, although this was not evident in the subgroup of older patients treated with immunosuppressant drugs for their advanced UC. These findings warrant discussion with patients when weighing the risks and benefits of different medical therapies and total colectomy.
Supplementary Material
Acknowledgments
We would like to acknowledge Dr. Samy Suissa, PhD, for technical input regarding study design
Grant Support: NIH K08 grant K08 DK084347-01, K24 DK078228 and Agency for Healthcare Research and Quality (AHRQ) (R01HS018517)
Footnotes
Protocol: available to interested readers by contacting Dr. Bewtra at mbewtra@upenn.edu
Statistical Code: available to interested readers by contacting Craig Newcomb at cnewcomb@mail.med.upenn.edu
Data: patient-level data not available; research-identifiable data files available to all approved investigators through Centers for Medicare and Medicaid Services (CMS).
References
- 1.Harrell LE, Hanauer SB. Mesalamine derivatives in the treatment of Crohn’s disease. Gastroenterol Clin North Am. 2004;33(2):303. doi: 10.1016/j.gtc.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Chen DM, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4(5):621–30. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Toruner M, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134(4):929–36. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JD, Gelfand JM, Troxel AB, Forde KA, Newcomb C, Kim H, et al. Immunosuppressant medications and mortality in inflammatory bowel disease. Am J Gastroenterol. 2008;103(6):1428. doi: 10.1111/j.1572-0241.2008.01836.x. [DOI] [PubMed] [Google Scholar]
- 5.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 6.Colombel JF, Loftus EV, Jr, Tremaine WJ, Egan LJ, Harmsen WS, Schleck CD, et al. The safety profile of infliximab in patients with Crohn’s disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004;126(1):19–31. doi: 10.1053/j.gastro.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 7.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 8.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 9.Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7(8):874–81. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54(8):1121–5. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beigel F, Jurgens M, Tillack C, Subklewe M, Mayr D, Goke B, et al. Hepatosplenic T-cell lymphoma in a patient with Crohn’s disease. Nat Rev Gastroenterol Hepatol. 2009;6(7):433–6. doi: 10.1038/nrgastro.2009.87. [DOI] [PubMed] [Google Scholar]
- 12.Mackey AC, Green L, Leptak C, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease: update. J Pediatr Gastroenterol Nutr. 2009;48(3):386–8. doi: 10.1097/mpg.0b013e3181957a11. [DOI] [PubMed] [Google Scholar]
- 13.Ochenrider MG, Patterson DJ, Aboulafia DM. Hepatosplenic T-cell lymphoma in a young man with Crohn’s disease: case report and literature review. Clin Lymphoma Myeloma Leuk. 2010;10(2):144–8. doi: 10.3816/CLML.2010.n.021. [DOI] [PubMed] [Google Scholar]
- 14.Su C, Lewis JD, Goldberg B, Brensinger C, Lichtenstein GR. A meta-analysis of the placebo rates of remission and response in clinical trials of active ulcerative colitis. Gastroenterology. 2007;132(2):516–26. doi: 10.1053/j.gastro.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 15.Timmer A, McDonald JW, Tsoulis DJ, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;9:CD000478. doi: 10.1002/14651858.CD000478.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan GG, McCarthy EP, Ayanian JZ, Korzenik J, Hodin R, Sands BE. Impact of hospital volume on postoperative morbidity and mortality following a colectomy for ulcerative colitis. Gastroenterology. 2008;134(3):680–7. doi: 10.1053/j.gastro.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Alves A, Panis Y, Bouhnik Y, Maylin V, Lavergne-Slove A, Valleur P. Subtotal colectomy for severe acute colitis: a 20-year experience of a tertiary care center with an aggressive and early surgical policy. J Am Coll Surg. 2003;197(3):379–85. doi: 10.1016/S1072-7515(03)00434-4. [DOI] [PubMed] [Google Scholar]
- 18.Pal S, Sahni P, Pande GK, Acharya SK, Chattopadhyay TK. Outcome following emergency surgery for refractory severe ulcerative colitis in a tertiary care centre in India. BMC Gastroenterol. 2005;5:39. doi: 10.1186/1471-230X-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts SE, Williams JG, Yeates D, Goldacre MJ. Mortality in patients with and without colectomy admitted to hospital for ulcerative colitis and Crohn’s disease: record linkage studies. BMJ. 2007;335(7628):1033. doi: 10.1136/bmj.39345.714039.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sagar PM, Pemberton JH. Intraoperative, postoperative and reoperative problems with ileoanal pouches. Br J Surg. 2012;99(4):454–68. doi: 10.1002/bjs.8697. [DOI] [PubMed] [Google Scholar]
- 21.Michelassi F, Lee J, Rubin M, Fichera A, Kasza K, Karrison T, et al. Long-term functional results after ileal pouch anal restorative proctocolectomy for ulcerative colitis: a prospective observational study. Ann Surg. 2003;238(3):433. doi: 10.1097/01.sla.0000086658.60555.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fazio VW, Ziv Y, Church JM, Oakley JR, Lavery IC, Milsom JW, et al. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg. 1995;222(2):120–7. doi: 10.1097/00000658-199508000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bach SP, Mortensen NJ. Ileal pouch surgery for ulcerative colitis. World J Gastroenterol. 2007;13(24):3288–300. doi: 10.3748/wjg.v13.i24.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholls RJ, Clark DN, Kelso L, Crowe AM, Knight AD, Hodgkins P, et al. Nationwide linkage analysis in Scotland implicates age as the critical overall determinant of mortality in ulcerative colitis. Aliment Pharmacol Ther. 2010;31(12):1310–21. doi: 10.1111/j.1365-2036.2010.04302.x. [DOI] [PubMed] [Google Scholar]
- 25.Bewtra M, Kilambi V, Fairchild AO, Siegel CA, Lewis JD, Johnson FR. Patient preferences for surgical versus medical therapy for ulcerative colitis. Inflamm Bowel Dis. 2014;20(1):103–14. doi: 10.1097/01.MIB.0000437498.14804.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arora G, Singh G, Vadhavkar S, Shah SB, Mannalithara A, Mithal A, et al. Incidence and risk of intestinal and extra-intestinal complications in Medicaid patients with inflammatory bowel disease: a 5-year population-based study. Dig Dis Sci. 2010;55(6):1689–95. doi: 10.1007/s10620-010-1236-z. [DOI] [PubMed] [Google Scholar]
- 27.Osterman MT, Haynes K, Delzell E, Zhang J, Bewtra M, Brensinger C, et al. Comparative Effectiveness of Infliximab and Adalimumab for Crohn’s Disease. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winthrop KL, Baddley JW, Chen L, Liu L, Grijalva CG, Delzell E, et al. Association between the initiation of anti-tumor necrosis factor therapy and the risk of herpes zoster. JAMA. 2013;309(9):887–95. doi: 10.1001/jama.2013.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gross R, Bilker WB, Strom BL, Hennessy S. Validity and comparison of two measures of days supply in Medicaid claims data. Pharmacoepidemiol Drug Saf. 2008;17(10):1029–32. doi: 10.1002/pds.1606. [DOI] [PubMed] [Google Scholar]
- 30.Hennessy S, Leonard CE, Palumbo CM, Newcomb C, Bilker WB. Quality of Medicaid and Medicare data obtained through Centers for Medicare and Medicaid Services (CMS) Med Care. 2007;45(12):1216–20. doi: 10.1097/MLR.0b013e318148435a. [DOI] [PubMed] [Google Scholar]
- 31.Bewtra M, Su C, Lewis JD. Trends in hospitalization rates for inflammatory bowel disease in the United States. Clin Gastroenterol Hepatol. 2007;5(5):597–601. doi: 10.1016/j.cgh.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–59. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14(6):680–6. doi: 10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- 34.Scott FI, Vajravelu R, Bewtra M, Mamtani R, Lee D, Goldberg D, et al. The Benefit to Risk Balance of Combining Infliximab with Azathioprine Varies With Age: A Markov Model. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lichtenstein GR, Cohen R, Feagan BG, Sandborn WJ, Salzberg B, Chen D, et al. Safety of Infliximab and Other Crohn’s Disease Therapies: TreatTM Registry Data with 24,575 Patient-Years of Follow-up. American Journal of Gastroenterology. 2008;103(s1) [Google Scholar]
- 36.Bewtra M, Siegel C, Lewis J. Ulcerative Colitis Patients Taking Immunosuppressive Medications Report Inadequate Understanding of Surgical Options. Gastroenterology. 2012;142(5):S-794. [Google Scholar]
- 37.Waljee AK, Higgins PD, Waljee JF, Tujios SR, Saxena A, Brown LK, et al. Perceived and actual quality of life with ulcerative colitis: a comparison of medically and surgically treated patients. Am J Gastroenterol. 2011;106(4):794–9. doi: 10.1038/ajg.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLeod RS, Baxter NN. Quality of life of patients with inflammatory bowel disease after surgery. World J Surg. 1998;22(4):375–81. doi: 10.1007/s002689900400. [DOI] [PubMed] [Google Scholar]
- 39.Davies RJ, O’Connor BI, Victor C, MacRae HM, Cohen Z, McLeod RS. A prospective evaluation of sexual function and quality of life after ileal pouch-anal anastomosis. Dis Colon Rectum. 2008;51(7):1032–5. doi: 10.1007/s10350-008-9248-x. [DOI] [PubMed] [Google Scholar]
- 40.Wexelman B, Eden E, Rose K. Survey of New York City Resident Physicians on Cause-of-Death Reporting, 2010. Prev Chronic Dis. 2013;10 doi: 10.5888/pcd10.120288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravakhah K. Death certificates are not reliable: revivification of the autopsy. South Med J. 2006;99(7):728–33. doi: 10.1097/01.smj.0000224337.77074.57. [DOI] [PubMed] [Google Scholar]
- 42.Ananthakrishnan AN. Environmental triggers for inflammatory bowel disease. Curr Gastroenterol Rep. 2013;15(1):302. doi: 10.1007/s11894-012-0302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


