Abstract
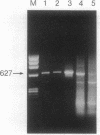
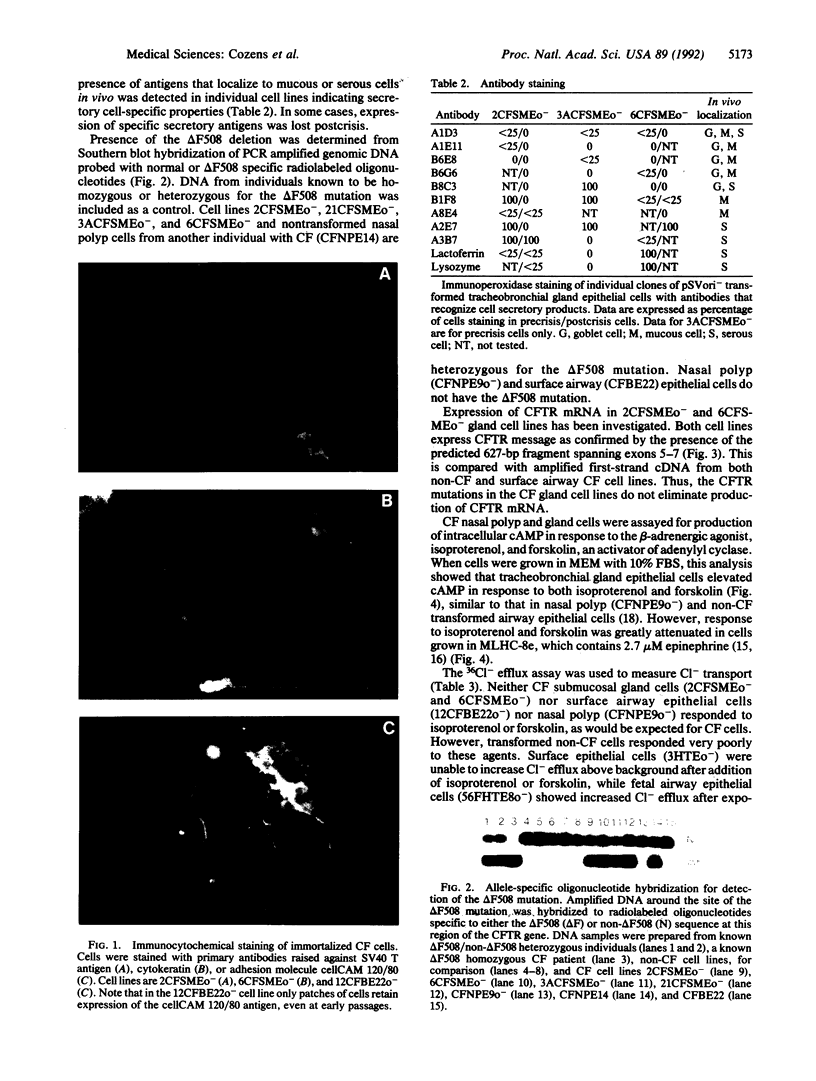
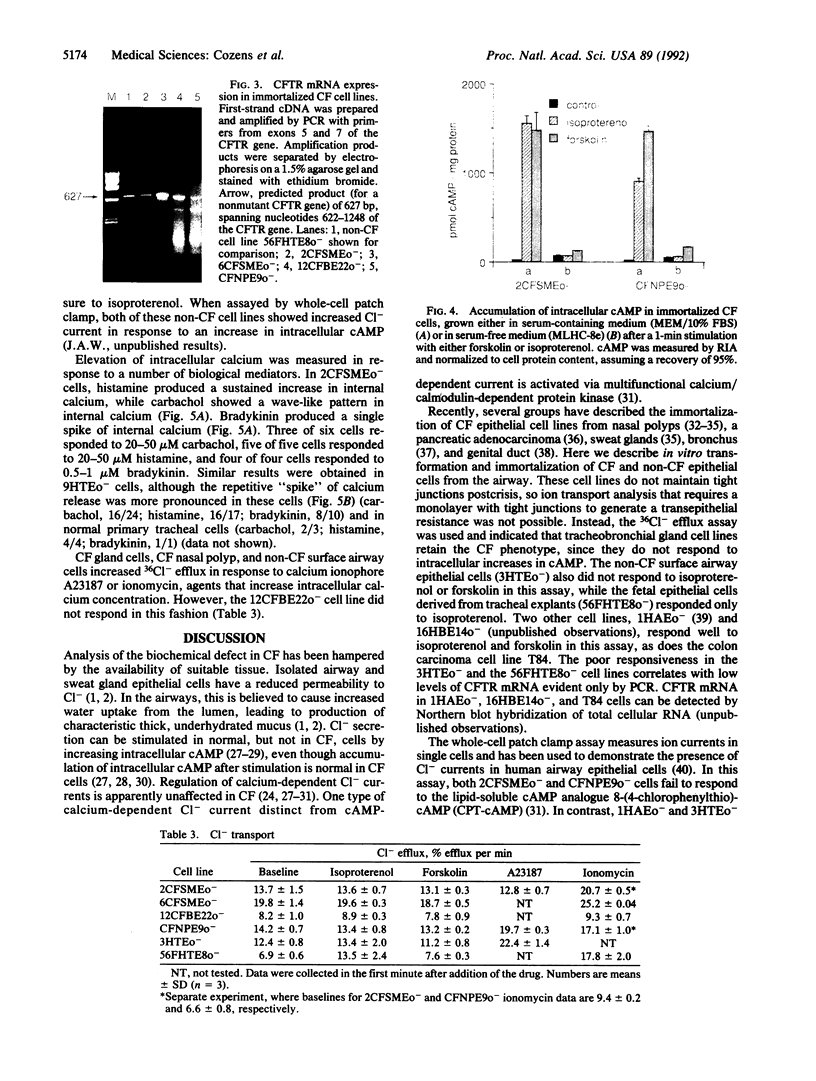
Tracheobronchial glands were isolated and cultured from a patient with cystic fibrosis (CF). Cultured epithelial cells were transformed with pSVori-. All transformed cell lines express cytokeratin filaments and at early passages express the junctional complex molecule cell CAM 120/80, indicating their epithelial origin. Several gland cell lines express antigens that localize to secretory cells in vivo. Cl- transport measured by 36Cl efflux shows that CF gland epithelial cells, like CF surface airway and nasal polyp epithelial cells, are unable to respond to increases in intracellular cAMP. However, they do produce an increase in intracellular cAMP after treatment with isoproterenol or forskolin. One CF gland cell line shows increased intracellular calcium in response to a number of agents and increased Cl- efflux comparable to that observed in a non-CF airway surface epithelial cell line after addition of calcium ionophore. All cell lines express CF transmembrane conductance regulator mRNA, as measured by PCR amplification of first-strand cDNA. The CF tracheobronchial gland cell lines described here are compound heterozygotes, having a single copy of the delta F508 mutation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991 Jul 12;253(5016):202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Barasch J., Kiss B., Prince A., Saiman L., Gruenert D., al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991 Jul 4;352(6330):70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- Beckstead J. H. Optimal antigen localization in human tissues using aldehyde-fixed plastic-embedded sections. J Histochem Cytochem. 1985 Sep;33(9):954–958. doi: 10.1177/33.9.4020104. [DOI] [PubMed] [Google Scholar]
- Boat T. F., Cheng P. W. Epithelial cell dysfunction in cystic fibrosis: implications for airways disease. Acta Paediatr Scand Suppl. 1989;363:25–30. doi: 10.1111/apa.1989.78.s363.25. [DOI] [PubMed] [Google Scholar]
- Boucher R. C., Cheng E. H., Paradiso A. M., Stutts M. J., Knowles M. R., Earp H. S. Chloride secretory response of cystic fibrosis human airway epithelia. Preservation of calcium but not protein kinase C- and A-dependent mechanisms. J Clin Invest. 1989 Nov;84(5):1424–1431. doi: 10.1172/JCI114316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan J. A., Yeger H., Tabcharani J. A., Jensen T. J., Auerbach W., Hanrahan J. W., Riodan J. R., Buchwald M. Transformed sweat gland and nasal epithelial cell lines from control and cystic fibrosis individuals. J Cell Sci. 1990 Jan;95(Pt 1):109–123. doi: 10.1242/jcs.95.1.109. [DOI] [PubMed] [Google Scholar]
- Clancy J. P., McCann J. D., Li M., Welsh M. J. Calcium-dependent regulation of airway epithelial chloride channels. Am J Physiol. 1990 Feb;258(2 Pt 1):L25–L32. doi: 10.1152/ajplung.1990.258.2.L25. [DOI] [PubMed] [Google Scholar]
- Coleman L., Harris A. Immortalization of male genital duct epithelium: an assay system for the cystic fibrosis gene. J Cell Sci. 1991 Jan;98(Pt 1):85–89. doi: 10.1242/jcs.98.1.85. [DOI] [PubMed] [Google Scholar]
- Cozens A. L., Yezzi M. J., Chin L., Simon E. M., Friend D. S., Gruenert D. C. Chloride ion transport in transformed normal and cystic fibrosis epithelial cells. Adv Exp Med Biol. 1991;290:187–196. doi: 10.1007/978-1-4684-5934-0_19. [DOI] [PubMed] [Google Scholar]
- Drumm M. L., Pope H. A., Cliff W. H., Rommens J. M., Marvin S. A., Tsui L. C., Collins F. S., Frizzell R. A., Wilson J. M. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990 Sep 21;62(6):1227–1233. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- Finkbeiner W. E., Basbaum C. B. Monoclonal antibodies directed against human airway secretions. Localization and characterization of antigens. Am J Pathol. 1988 May;131(2):290–297. [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A., Rechkemmer G., Shoemaker R. L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986 Aug 1;233(4763):558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- Gruenert D. C., Basbaum C. B., Welsh M. J., Li M., Finkbeiner W. E., Nadel J. A. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenert D. C., Basbaum C. B., Widdicombe J. H. Long-term culture of normal and cystic fibrosis epithelial cells grown under serum-free conditions. In Vitro Cell Dev Biol. 1990 Apr;26(4):411–418. doi: 10.1007/BF02623833. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde S. C., Emsley P., Hartshorn M. J., Mimmack M. M., Gileadi U., Pearce S. R., Gallagher M. P., Gill D. R., Hubbard R. E., Higgins C. F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990 Jul 26;346(6282):362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Jefferson D. M., Valentich J. D., Marini F. C., Grubman S. A., Iannuzzi M. C., Dorkin H. L., Li M., Klinger K. W., Welsh M. J. Expression of normal and cystic fibrosis phenotypes by continuous airway epithelial cell lines. Am J Physiol. 1990 Dec;259(6 Pt 1):L496–L505. doi: 10.1152/ajplung.1990.259.6.L496. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Yankaskas J. R., Stutts M. J., Willumsen N. J., Boucher R. C. Persistence of abnormal chloride conductance regulation in transformed cystic fibrosis epithelia. Science. 1989 Jun 23;244(4911):1472–1475. doi: 10.1126/science.2472008. [DOI] [PubMed] [Google Scholar]
- Kartner N., Hanrahan J. W., Jensen T. J., Naismith A. L., Sun S. Z., Ackerley C. A., Reyes E. F., Tsui L. C., Rommens J. M., Bear C. E. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell. 1991 Feb 22;64(4):681–691. doi: 10.1016/0092-8674(91)90498-n. [DOI] [PubMed] [Google Scholar]
- Kerem B. S., Buchanan J. A., Durie P., Corey M. L., Levison H., Rommens J. M., Buchwald M., Tsui L. C. DNA marker haplotype association with pancreatic sufficiency in cystic fibrosis. Am J Hum Genet. 1989 Jun;44(6):827–834. [PMC free article] [PubMed] [Google Scholar]
- Laski F. A., Alzner-DeWeerd B., RajBhandary U. L., Sharp P. A. Expression of a X. laevis tRNATyr gene in mammalian cells. Nucleic Acids Res. 1982 Aug 11;10(15):4609–4626. doi: 10.1093/nar/10.15.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J. D., Li M., Welsh M. J. Identification and regulation of whole-cell chloride currents in airway epithelium. J Gen Physiol. 1989 Dec;94(6):1015–1036. doi: 10.1085/jgp.94.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton P. M. Cystic fibrosis: a disease in electrolyte transport. FASEB J. 1990 Jul;4(10):2709–2717. doi: 10.1096/fasebj.4.10.2197151. [DOI] [PubMed] [Google Scholar]
- Rich D. P., Anderson M. P., Gregory R. J., Cheng S. H., Paul S., Jefferson D. M., McCann J. D., Klinger K. W., Smith A. E., Welsh M. J. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990 Sep 27;347(6291):358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rugolo M., Romeo G., Lenaz G. Kinetic analysis of chloride efflux from normal and cystic fibrosis fibroblasts. Biochem Biophys Res Commun. 1986 Jan 14;134(1):233–239. doi: 10.1016/0006-291x(86)90552-8. [DOI] [PubMed] [Google Scholar]
- Scholte B. J., Kansen M., Hoogeveen A. T., Willemse R., Rhim J. S., van der Kamp A. W., Bijman J. Immortalization of nasal polyp epithelial cells from cystic fibrosis patients. Exp Cell Res. 1989 Jun;182(2):559–571. doi: 10.1016/0014-4827(89)90259-0. [DOI] [PubMed] [Google Scholar]
- Schoumacher R. A., Ram J., Iannuzzi M. C., Bradbury N. A., Wallace R. W., Hon C. T., Kelly D. R., Schmid S. M., Gelder F. B., Rado T. A. A cystic fibrosis pancreatic adenocarcinoma cell line. Proc Natl Acad Sci U S A. 1990 May;87(10):4012–4016. doi: 10.1073/pnas.87.10.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small M. B., Gluzman Y., Ozer H. L. Enhanced transformation of human fibroblasts by origin-defective simian virus 40. Nature. 1982 Apr 15;296(5858):671–672. doi: 10.1038/296671a0. [DOI] [PubMed] [Google Scholar]
- Sommerhoff C. P., Finkbeiner W. E. Human tracheobronchial submucosal gland cells in culture. Am J Respir Cell Mol Biol. 1990 Jan;2(1):41–50. doi: 10.1165/ajrcmb/2.1.41. [DOI] [PubMed] [Google Scholar]
- Venglarik C. J., Bridges R. J., Frizzell R. A. A simple assay for agonist-regulated Cl and K conductances in salt-secreting epithelial cells. Am J Physiol. 1990 Aug;259(2 Pt 1):C358–C364. doi: 10.1152/ajpcell.1990.259.2.C358. [DOI] [PubMed] [Google Scholar]
- Wagner J. A., Cozens A. L., Schulman H., Gruenert D. C., Stryer L., Gardner P. Activation of chloride channels in normal and cystic fibrosis airway epithelial cells by multifunctional calcium/calmodulin-dependent protein kinase. Nature. 1991 Feb 28;349(6312):793–796. doi: 10.1038/349793a0. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Liedtke C. M. Chloride and potassium channels in cystic fibrosis airway epithelia. 1986 Jul 31-Aug 6Nature. 322(6078):467–470. doi: 10.1038/322467a0. [DOI] [PubMed] [Google Scholar]
- Wheelock M. J., Buck C. A., Bechtol K. B., Damsky C. H. Soluble 80-kd fragment of cell-CAM 120/80 disrupts cell-cell adhesion. J Cell Biochem. 1987 Jul;34(3):187–202. doi: 10.1002/jcb.240340305. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H. Cystic fibrosis and beta-adrenergic response of airway epithelial cell cultures. Am J Physiol. 1986 Oct;251(4 Pt 2):R818–R822. doi: 10.1152/ajpregu.1986.251.4.R818. [DOI] [PubMed] [Google Scholar]
- Yamaya M., Finkbeiner W. E., Widdicombe J. H. Altered ion transport by tracheal glands in cystic fibrosis. Am J Physiol. 1991 Dec;261(6 Pt 1):L491–L494. doi: 10.1152/ajplung.1991.261.6.L491. [DOI] [PubMed] [Google Scholar]
- Yamaya M., Finkbeiner W. E., Widdicombe J. H. Ion transport by cultures of human tracheobronchial submucosal glands. Am J Physiol. 1991 Dec;261(6 Pt 1):L485–L490. doi: 10.1152/ajplung.1991.261.6.L485. [DOI] [PubMed] [Google Scholar]
- Zeitlin P. L., Lu L., Rhim J., Cutting G., Stetten G., Kieffer K. A., Craig R., Guggino W. B. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am J Respir Cell Mol Biol. 1991 Apr;4(4):313–319. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]