Abstract
We investigated the use of cucurbitacin B, a plant-derived tetracyclic triterpenoid, as a single agent or in combination with methotrexate (MTX) for human osteosarcoma (OS) treatment. Cucurbitacin B showed antiproliferative activity against seven human OS cell lines in vitro accompanying G2/M cell cycle arrest, apoptosis, and inhibition of ERK, Akt, and mTOR proteins. Cucurbitacin B in combination with MTX synergistically inhibited OS cell growth in vitro. Low-dose cucurbitacin B (LD-CuB, 0.5 mg/kg body weight) or low-dose MTX (LD-MTX, 150 mg/kg) failed to decrease the size of human OS xenografts in nude mice. However, combined therapy at identical concentrations inhibited tumor growth by 62% vs. LD-CuB and 81% vs. LD-MTX (p < 0.001). Strikingly, the effect persisted even when the dose of MTX was decreased by two thirds (VLD-MTX, 50 mg/kg). In conclusion, cucurbitacin B alone or in combination with MTX shows promising antiproliferative activity against human OS.
Keywords: Osteosarcoma, Cucurbitacin B, Methotrexate, Synergism, Xenograft
1. Introduction
Human osteosarcoma (OS) is the most common malignant bone tumor in the second decade of childhood. Well known for its metastasis and high local recurrence rate, OS is a type of cancer whose treatment requires an extensive multimodal approach including surgery, radiotherapy, and chemotherapy.
Currently, chemotherapeutic regimens for human OS treatment use the combination of multiple chemotherapeutic agents including high-dose methotrexate (HD-MTX) with leucovorin rescue, doxorubicin (adriamycin), cisplatin, and ifosfamide either with or without etoposide [1]. Although these regimens have remained the mainstay of OS chemotherapy for decades, none have provided any major advancement in survival compared to the original combination by Rosen et al. [2,3]. Furthermore, these regimens were only efficacious with localized OS and performed poorly with development of metastatic, recurrent OS [1].
Cucurbitacins are a group of plant-derived tetracyclic triterpenoids originally found in the plant family of Cucurbitaceae. Plants containing cucurbitacins have been known for their anti-pyretic, analgesic, anti-inflammatory, antimicrobial, and anti-tumor activities in folk medicine. They show strong antiproliferative activity against many human cancer cells, primarily as the inhibitor of the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway [4,5]. However, cucurbitacins can selectively inhibit different signaling pathways depending on the cancer cell type albeit the mechanisms are usually unknown [4,5].
Our preliminary study demonstrated that cucurbitacin B inhibited the growth of human OS cells, whose JAK–STAT pathway is known to be inactive [6]. This led us to study what other pathways are affected by cucurbitacin B. Here, we show that cucurbitacin B can inhibit the phosphorylation of extracellular signal-regulated kinase (ERK), Akt, and mammalian target of rapamycin (mTOR) proteins in human OS cells. This molecular insight led us to explore further the possible synergism of cucurbitacin B with MTX directed against human OS growing in a murine model system.
2. Materials and methods
2.1. Osteosarcoma cell culture
Seven human OS cell lines (U2OS, G292, MG-63, HT-161, HOS, SAOS-2, and SJSA) were used in the study. Each cell line except HT-161 was obtained from the American Type Culture Collection (ATCC, Rockville, MD). HT-161 was received from Bogenmann et al. [7]. OS cell lines were maintained in DMEM medium (Mediatech Inc., Herndon, VA) supplemented with 10% fetal bovine serum (FBS; Atlanta Biological, Lawrenceville, GA) in a humidified incubator at 37 °C supplied with 5% CO2. Only the cells in exponential growth phase were used in the study.
2.2. Chemical compounds
Cucurbitacin B (CKBP002, Fig. 1A) was generously provided by CK Life Sciences International (Holdings) Inc. (Hong Kong, China). Pure cucurbitacin B crystal was solubilized with 100% ethanol to 10 mM and diluted with PBS to a stock concentration of 0.1 mM. Methotrexate (MTX; Affymetrix–USB, Cleveland, OH) was dissolved in 0.1 M sodium carbonate buffer (pH = 9.6) to a stock concentration of 10 mM. All chemical compounds were freshly dissolved on the day of the experiment.
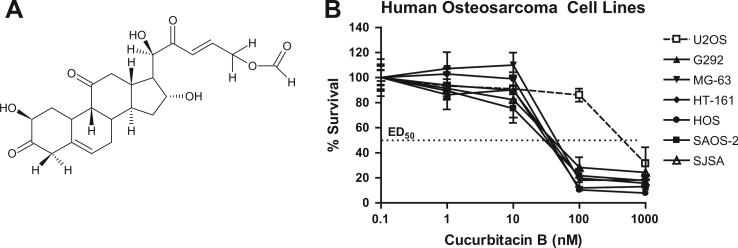
Fig. 1.
Growth inhibition of human osteosarcoma (OS) cell lines by cucurbitacin B. (A) Chemical structure of cucurbitacin B. (B) Dose-dependent antiproliferative activity of cucurbitacin B against seven human OS cell lines (U2OS, G292, MG-63, HT-161, HOS, SAOS-2, and SJSA) as measured by MTT assay after 48 h of exposure. Measurements repeated in triplicates. Data represent the mean ± standard deviation (SD; error bars).
2.3. Measurement of cell growth and survival by MTT assay
Cell growth and survival were measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. For dose–response tests, cells were exposed to media with increasing concentrations (1–1000 nM) of DMSO (negative control), cucurbitacin B, or MTX. After 48 h of incubation, 5 mg/ml MTT solution (Sigma–Aldrich, St. Louis, MO) was added, incubated for 2 h, and 20% SDS solution was added to stop the reaction. The absorbance was measured at 540 nm using an ELISA reader. For pulse-exposure experiments, MG-63 and SAOS-2 cells were exposed to cucurbitacin B at their ED50 (70 nM for MG-63 cells and 30 nM for SAOS-2 cells). After 2, 9, and 20 h of exposure, cells were washed extensively with PBS and returned to cucurbitacin B-free culture. Cell growth was checked by MTT assay at 24, 48, and 72 h.
2.4. Cell cycle analysis and apoptosis assay
For cell cycle analysis, MG-63 and SAOS-2 cells were exposed to either cucurbitacin B or MTX at their ED50. Cells were harvested and fixed with 70% ethanol at regular time intervals. Fixed cells were stained with propidium iodide (PI) for flow cytometry analysis using BD FACScan (BD Biosciences, San Jose, CA). For apoptosis assay, MG-63 and SAOS-2 cells were exposed to DMSO, 200 μM H2O2 (positive control), cucurbitacin B, or MTX at their ED50. After 72 h of exposure, cells were harvested and stained with PI and fluorescein isothiocyanate (FITC) using Annexin VFITC apoptosis detection kit (BD Biosciences) according to the manufacturer's protocol.
2.5. Western blotting
MG-63 and SAOS-2 cells were exposed to DMSO, cucurbitacin B, or MTX at their ED50. After 2, 9, and 20 h of exposure, cells were lysed with RIPA buffer (Millipore–Upstate, Temecula, CA) supplemented with protease inhibitor cocktail, 0.5 mM PMSF, and 0.2 mM Na3VO4. Samples were adjusted to the same protein concentration before loading. Proteins were then separated by SDS–PAGE, transferred to nitrocellulose membrane, and blotted with antibodies from Cell Signaling Technology (Danvers, MA), Santa Cruz Biotechnology (Santa Cruz, CA), or Assay Biotechnology (Sunnyvale, CA) (Supplementary Table S1). GAPDH protein was used as a loading control.
2.6. Drug affinity responsive target stability (DARTS) analysis
Direct interaction of cucurbitacin B and mTOR was assessed by DARTS as described in the original article with some modifications [8]. Briefly, confluent MG-63 cells were harvested and lysed with lysis buffer (1% Triton X-100, 150 mM NaCl. 100 mM Tris–HCl, pH 7.4, 1 mM EDTA, and 1 mM EGTA, pH 8.0) supplemented with protease inhibitor (Roche, San Francisco, CA), 0.2 mM PMSF (Roche), and 0.2 mM Na3VO4 (Sigma). Cell lysates were incubated with 0.1 volume of increasing concentrations (10–1000 nM) of either DMSO or cucurbitacin B at room temperature for 30 min. Lysates were then proteolyzed with thermolysin (Sigma) at room temperature for 3 min. Reactions were stopped by adding 0.5 M EDTA solution and 2 × Laemmli sample buffer (Bio-Rad, Hercules, CA) with 5% β-mercaptoethanol (Sigma). Western blotting was performed as described above.
2.7. Growth of MG-63 xenografts in athymic nude mice with in vivo treatment
All animal experiments strictly followed the guidelines of Cedars-Sinai Medical Center and the National Institute of Health (NIH). Female nu/nu athymic nude mice (5–6 weeks old; average weight 21 g; specific pathogen-free) from Harlan Laboratories (Indianapolis, IN) were maintained in a pathogen-free condition with sterilized chow and water. 5 × 106 cells of MG-63 cells were mixed with 200 μl of Matrigel solution (BD Biosciences) per injection, and the mixture was injected subcutaneously on the upper flanks of nude mice. After 24 h, tumor size was measured, and any outliers were ruled out by one-way analysis of variance (ANOVA) test. Five mice were randomly assigned to each experimental group: (1) PBS (diluent-specific control); (2) low-dose cucurbitacin B (LD-CuB, 0.5 mg/kg body weight); (3) high-dose cucurbitacin B (HD-CuB, 1.0 mg/kg); (4) low-dose methotrexate (LD-MTX, 150 mg/kg); (5) LD-CuB with LD-MTX; (6) HD-CuB with LD-MTX; and (7) LD-CuB with very low-dose MTX (VLD-MTX, 50 mg/kg). The dose of LD-MTX was the murine equivalent of the human dose of LD-MTX. The conversion was made using dose translation formula by Reagan-Shaw et al. [9]. Intraperitoneal (i.p.) injections of either PBS or cucurbitacin B were administered three times a week, and MTX was injected once every 2 weeks. Body weights were monitored every 2 days. Tumor size was measured every 2 days, and the tumor volume was calculated using the following formula: A (length) × B (width) × C (height) × 0.5236 [10]. The experiment was stopped at day 35, and all mice were sacrificed. The presence of metastatic spread was examined macroscopically at the time of autopsy followed by histological examination. At least two tumors from each group were snap-frozen in liquid nitrogen for Western blotting.
2.8. Immunohistochemistry (IHC)
At autopsy, tumors and internal organs including liver, spleen, kidneys were excised, weighed, and then fixed in 10% PBS-buffered formalin and maintained in 70% ethanol. For IHC, fixed tumors and organs were embedded in parap-last (Oxford Labware, St. Louis, MO), cut in 6 μm thick sections, and stained with hematoxylin and eosin (HE) for histopathological examination.
For Ki-67 proliferation assay, tumor sections were deparaffinized with xylene and rehydrated through graded ethanol. Endogenous peroxidase activity was blocked with 3% H2O2 in methanol. Heat-induced antigen retrieval (HIER) was carried out in 10 mM citrate buffer (pH = 6.0) using a vegetable steamer at 95 °C for 25 min. Tumor sections were incubated with a mouse monoclonal antibody for human Ki-67 (M7240, 1:100 dilution; Dako, Carpinteria, CA) followed by MACH2 Mouse HRP-Polymer mouse secondary antibody (MHRP520L, Biocare Medical, Concord, CA). After incubation, tumor sections were stained with diaminobenzidine (DAB) and counterstained with hematoxylin. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) apoptosis assay was performed using ApopTaq Plus Peroxidase In Situ Apoptosis Kit (Millipore) according to the manufacturer's protocol. Percent positive cells in representative microscopic fields were counted using ImageJ [11].
2.9. In vivo toxicity test
Whole-blood samples were obtained by submandibular bleeding and serum samples were harvested with serum separator tubes (BD Biosciences). Blood count and serum chemistry results were obtained by the Hemagen Analyst Benchtop Chemistry System (Hemagen Diagnostics, Inc. Columbia, MD). Colony-forming cell (CFC) assays were performed as previously described [12].
2.10. Drug interaction analysis
Synergism between cucurbitacin B and MTX in vitro was determined quantitatively by isobologram and combination index (CI) analysis adapted from the median-principle methods of Chou and Talalay [13,14]. Calcusyn 2.0 software (Biosoft, Ferguson, MO) was used for CI analysis [15].
2.11. Statistical analysis
All in vitro and in vivo experiments were repeated at least three times to ensure reproducibility. Two-tailed student t-test was used to compare differences between two groups. One-way ANOVA test was used to compare differences among three or more groups. P-values less than or equal to 0.05 were considered statistically significant in both tests.
3. Results
3.1. Effect of cucurbitacin B on human osteosarcoma (OS) cell lines in vitro
Seven human OS cell lines (U2OS, G292, MG-63, HT-161, HOS, SAOS-2, and SJSA) were exposed to increasing concentrations of cucurbitacin B, and dose–responses were determined (Fig. 1B). Cells showed similar ED50 values of approximately 50 nM, except U2OS (500 nM). When MG-63 and SAOS-2 cells were pulse-exposed at their corresponding ED50 (70 nM for MG-63 and 30 nM for SAOS-2 cells) for 2, 9, and 20 h, longer exposure to cucurbitacin B induced more cell death (Fig. 2A). Together, the cytotoxic activity of cucurbitacin B in human OS cells was dose- and time-dependent.
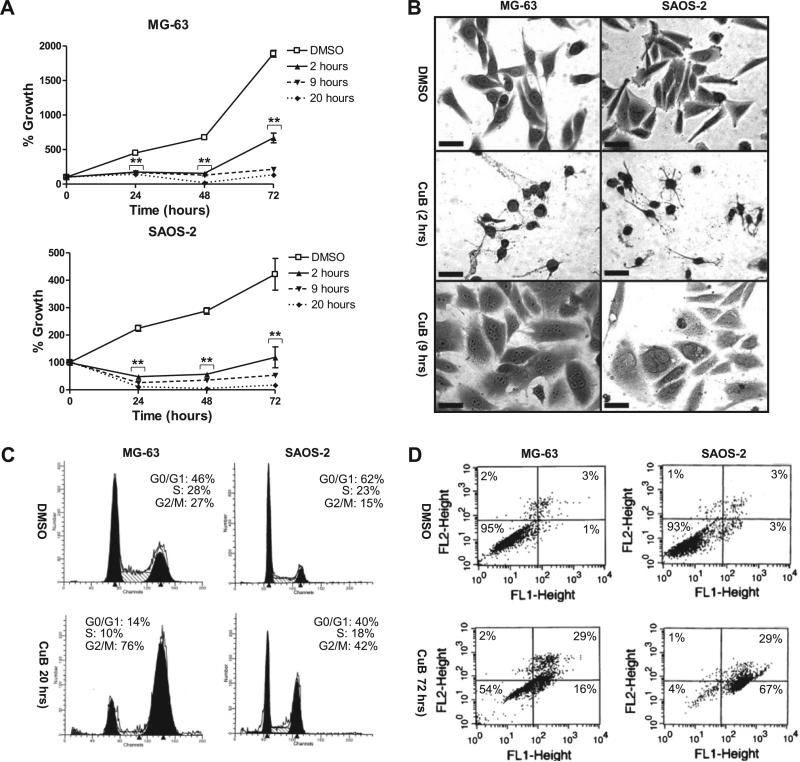
Fig. 2.
Effect of cucurbitacin B on MG-63 and SAOS-2 cells. Cells were exposed to cucurbitacin B at their ED50 (70 nM for MG-63 and 50 nM for SAOS-2 cells). CuB, cucurbitacin B. (A) Time-dependent antiproliferative activity of cucurbitacin B on MG-63 and SAOS-2 cells measured by pulse-exposure experiments. Cells were exposed to cucurbitacin B for 2, 9, or 20 h, washed extensively, and cultured in the absence of cucurbitacin B for an additional 24, 48, and 72 h. Cell growth was measured by MTT assay. Measurements were repeated in triplicates. Data represent mean ± SD. Asterisks (**) represent p < 0.001 vs. DMSO control by t-test. (B) Morphological changes of MG-63 and SAOS-2 cells after exposure to cucurbitacin B. Cells with normal morphology (top) were compared to rounded cells (middle) and multinucleated cells (bottom). Rounded cells were observed after 2 h exposure to cucurbitacin B. Multinucleated cells were observed at day 10 after longer exposure (9 h) to cucurbitacin B. Cells were visualized with crystal violet staining. Representative cells are shown. 400×; scale bar = 50 μm. (C) G2/M cell cycle arrest after 20 h of exposure to cucurbitacin B. Cells were stained with propidium iodide (PI) and analyzed by FACS. (D) Apoptosis after 72 h of exposure to cucurbitacin B. Cells were stained with PI and Annexin V-FITC, and analyzed by FACS.
Exposure to cucurbitacin B induced morphological changes in MG-63 and SAOS-2 cells. Rapid loss of pseudopodia and rounding was observed in both cells after 2 h exposure to cucurbitacin B (Fig. 2B, middle panels). 9 h-exposure to cucurbitacin B resulted in multinuclearity which was not reversed by removal of cucurbitacin B (Fig. 2B, bottom panels).
Other responses of MG-63 and SAOS-2 cells to cucurbitacin B exposure included G2/M cell cycle arrest and apoptosis. The G2/M phase increased around 3-fold for both MG-63 (27–76%) and SAOS-2 cells (15–42%) after 20 h of exposure to cucurbitacin B (Fig. 2C). The amount of apoptotic cells increased to 44% in MG-63 cells and 96% in SAOS-2 cells after 72 h of exposure (Fig. 2D).
3.2. Inhibition of ERK, Akt, and mTOR phosphorylation by cucurbitacin B
Western blot analysis demonstrated that cucurbitacin B inhibited the phosphorylation of some key signaling proteins in human OS cells. Cucurbitacin B inhibited ERK phosphorylation at T204 without changing the total levels of ERK in both MG-63 and SAOS-2 cells (Fig. 3A). Decreased level of phospho-c-Jun and subsequent decrease in c-Fos expression were associated with decreased levels of ERK. Other MAPK proteins such as p38 and JNK showed no significant changes (data not shown). Inhibition of Akt phosphorylation at S473 was observed in MG-63 cells, but not in SAOS-2 cells (Fig. 3B). Total amount of Akt remained unaffected in both cell lines.
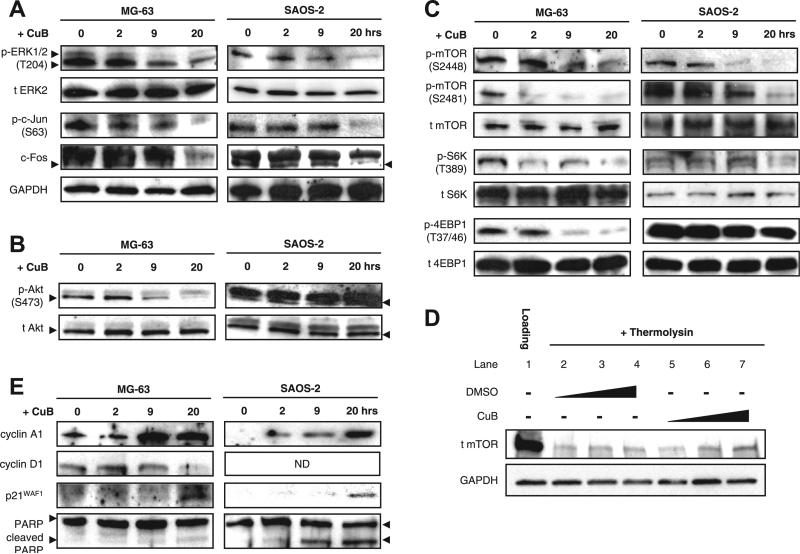
Fig. 3.
Effect of cucurbitacin B on the mTOR signaling pathway in MG-63 and SAOS-2 cells. (A) Effect of cucurbitacin B on the phosphorylation status of ERK and its downstream targets, c-Jun and c-Fos. Cells were exposed to cucurbitacin B for 2, 9, or 20 h and analyzed by Western blotting. Same lysates were used throughout Western blot analysis. (B) Effect of cucurbitacin B on the phosphorylation status of Akt. (C) Effect of cucurbitacin B on the phosphorylation status of mTOR and its downstream targets, S6 K and 4EBP1. (D) DARTS with cucurbitacin B using MG-63 whole-cell lysates. MG-63 lysates were treated with either DMSO control or cucurbitacin B (10, 100, or 1000 nM) at room temperature for 30 min. Samples then underwent thermolysin proteolysis followed by Western blot analysis. (E) Effect of cucurbitacin B on cell cycle-related proteins (cyclin A1, cyclin D1, and p21WAF) and apoptosis-related protein (PARP). All Western blots were repeated three times for validation of the results. GAPDH protein was used as an internal loading control. CuB = cucurbitacin B. ND = not detected.
Since ERK and Akt are two main regulators of mTOR [16], we also checked the phosphorylation status of mTOR. Cucurbitacin B inhibited mTOR phosphorylation at S2448 and S2481 without affecting the total level of mTOR in both MG-63 and SAOS-2 cells (Fig. 3C). Inhibition of downstream targets of mTOR such as ribosomal protein S6 kinase (S6 K) and eukaryotic translation initiation factor 4E binding protein 1 (4EBP1) were associated with the inhibition of mTOR (Fig. 3C). Interestingly, drug affinity responsive target stability (DARTS) analysis demonstrated that cucurbitacin B can bind directly to mTOR protein (Fig. 3D). Cucurbitacin B showed dose-dependent protection of the mTOR protein from thermolysin proteolysis (lanes 5–7) whereas DMSO control at the same concentrations did not (lanes 2–4).
Consistent with cucurbitacin B-mediated G2/M arrest and apoptosis, cucurbitacin B increased the protein levels of cyclin A and cyclin-dependent kinase inhibitor 1 (p21WAF1) in both cells (Fig. 3E). Decrease in the levels of cyclin D1 occured in MG-63 but not in SAOS-2 cells. The apoptosis marker protein, poly ADP-ribose polymerase (PARP), showed cleavage of the precursor molecules in both cells.
3.3. Synergistic effect of cucurbitacin B and MTX in vitro
From a further understanding of the molecular mechanism of action of cucurbitacin B, we hypothesized that cucurbitacin B might synergize with MTX. As an antimetabolite, MTX inhibits dihydrofolate reductase (DHFR) which plays a vital role in DNA synthesis. Since the expression of DHFR is known to be S-phase specific [17], we hypothesized that the rapid G2/M arrest by cucurbitacin B would lower the level of DHFR expression and augment the inhibitory action of MTX. To test this hypothesis, MG-63 and SAOS-2 cells were exposed to various concentrations of cucurbitacin B, MTX, or both for 72 h, and cell viability was examined. Synergism of cucurbitacin B and MTX was observed at most of the concentration ratios in both cell lines (Fig. 4A). Drug interaction analysis confirmed the synergism of the two drugs. In constant drug ratio analysis, average CI value for MG-63 cells was 0.92 ± 0.05 at ED50; 0.31 ± 0.02 at ED75; and 0.11 ± 0.02 at ED90; and for SAOS-2 cells CI values were 1.00 ± 0.12 at ED50; 0.56 ± 0.07 at ED75; and 0.33 ± 0.01 at ED90 (Fig. 4B and C). In non-constant drug ratio analysis, most ratios showed CI values less than 0.9 (Supplementary Fig. S1).
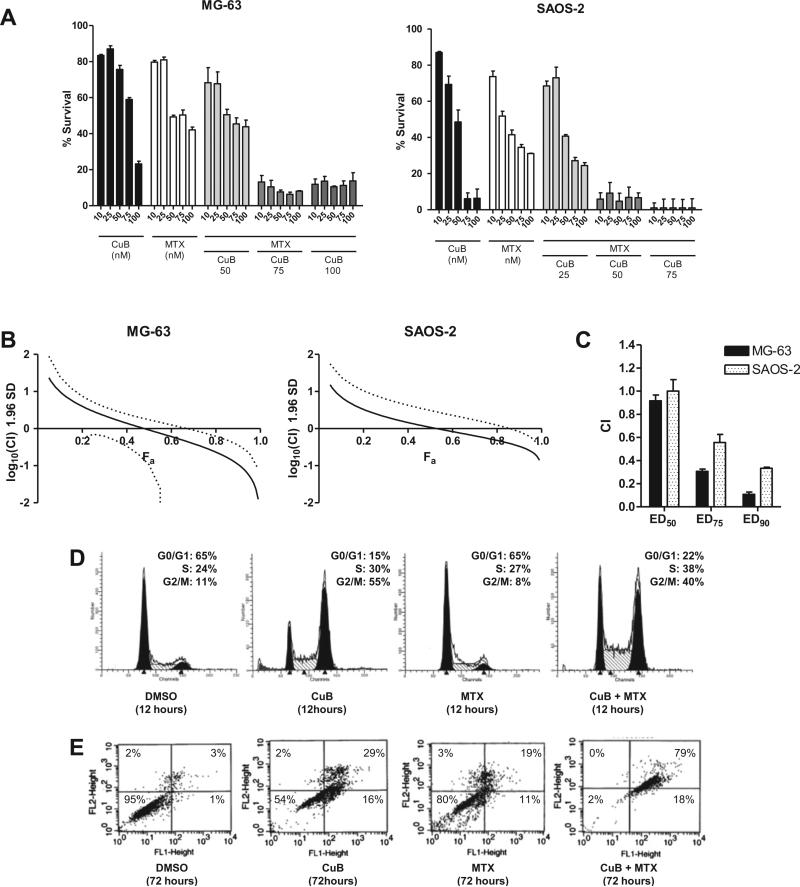
Fig. 4.
Effect of the combination of cucurbitacin B and MTX on the growth of MG-63 and SAOS-2 cells in vitro. (A) Cells were grown in various concentrations of cucurbitacin B and MTX, and their viability was determined after 48 h by MTT assay. Numbers on the x-axis indicate the concentration (nM) of cucurbitacin B and/or MTX. Samples were measured in triplicates. Data represent mean ± standard deviation (SD, error bars). (B) Fraction affected (Fa)-combination index (CI) plot for MG-63 and SAOS-2 cells exposed to cucurbitacin B and MTX. Fa-CI plots were generated by Calcusyn 2.0 using selected data points from panel (A) with constant concentration ratio of cucurbitacin B and MTX (1:1) [15]. All data points including non-constant concentration ratios are shown in the normalized isobolograms in Supplementary Fig. S1. (C) Changes in CI values at different drug concentrations (ED50, ED75, and ED90) of cucurbitacin B and MTX in MG-63 and SAOS-2 cells. Experiments were repeated three times to ensure accuracy. Data represent mean ± SD. (D) The effect of cucurbitacin B (70 nM) and/or MTX (50 nM) on cell cycle of MG-63 cells at 12 h of exposure. (E) Annexin V-FITC apoptosis assays of MG-63 after 72 h of exposure to either cucurbitacin B (70 nM), MTX (50 nM), or both.
Markedly enhanced activity of MTX combined with cucurbitacin B was found when examining their effects on the cell cycle and apoptosis. Cucurbitacin B enhanced S-phase arrest by MTX in MG-63 cells (Fig. 4D). When MTX was used alone at its ED50 (50 nM, 48 h), the earliest sign of S-phase arrest was observed at 48 h of exposure (data not shown). When MTX and cucurbitacin B at their ED50 were used in combination, increased S-phase arrest (from 27% to 38%) was observed at 12 h of exposure. Furthermore, the combination of cucurbitacin B and MTX enhanced the apoptosis of the MG-63 cells (Fig. 4E). Cucurbitacin B or MTX alone at their ED50 caused 44% and 30% of the cells to become apoptotic after 72 h of exposure, respectively. Together, both compounds at the same concentrations resulted in 97% apoptosis of the cells.
3.4. Effect of cucurbitacin B with MTX on human OS xenograft in vivo
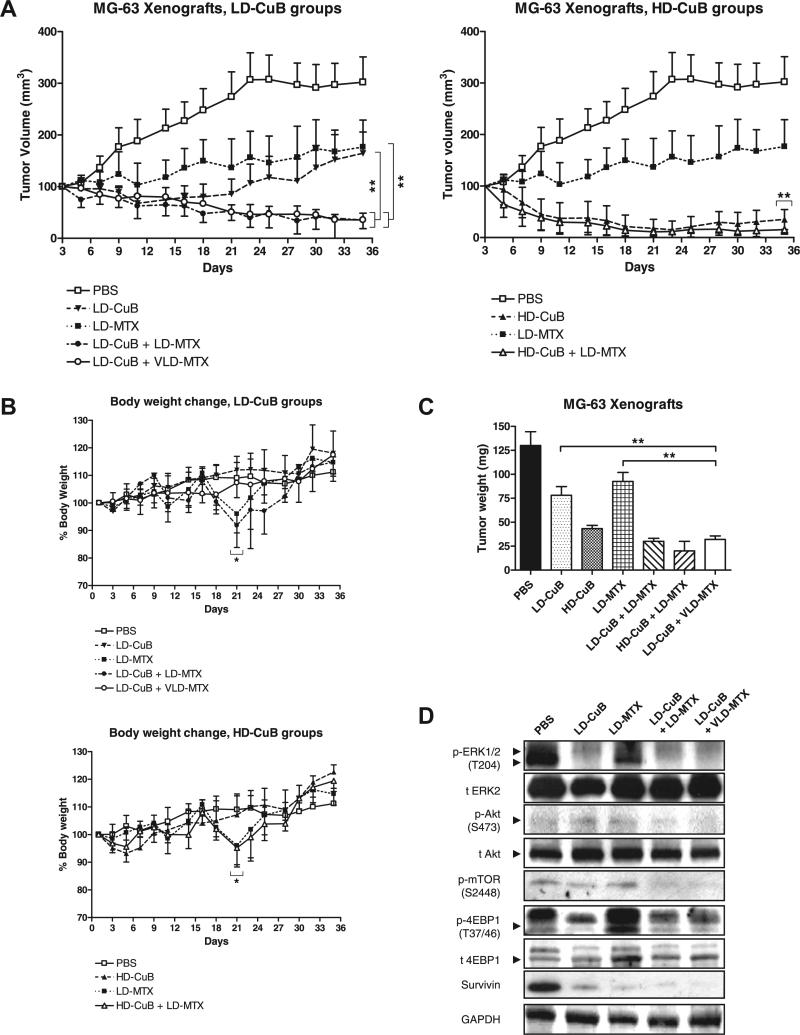
Based on the synergism of cucurbitacin B and MTX in vitro, we extended our experiments to the preclinical settings using a murine model. As summarized in Fig. 5A, low-dose cucurbitacin B (LD-CuB, 0.5 mg/kg body weight) or low-dose MTX (LD-MTX, 150 mg/kg) as single agents allowed the tumor volume to increase slightly from its original size. In contrast, tumors were barely detectable when the two were combined at the same concentrations. Strikingly, the effect persisted even when the dose of MTX was decreased by two thirds (very low dose (VLD)-MTX, 50 mg/kg). No significant difference was found between these combination groups (LD-CuB + LD-MTX and LD-CuB + VLD + MTX) (p = 0.38). At day 35, the average volumetric decrease of tumor in both combination groups was 79% vs. LD-CuB group (p < 0.001) and 80% vs. LD-MTX group (p < 0.001) (Fig. 5A, left). Cucurbitacin B at a high dose (HD-CuB, 1.0 mg/kg) either alone or in combination with LD-MTX showed a strong growth inhibition (Fig. 5A, right). However, no significant difference was observed (p = 0.71).
Fig. 5.
Effect of combination of cucurbitacin B and MTX on the growth of MG-63 xenografts in athymic nude mice. PBS, diluant control; LD-CuB, low-dose cucurbitacin B (0.5 mg/kg body weight); HD-CuB, high-dose cucurbitacin B (1.0 mg/kg); LD-MTX, low-dose methotrexate (150 mg/kg); VLD-MTX, very low-dose methotrexate (50 mg/kg). (A) Volumetric growth of MG-63 xenografts in LD-CuB treated groups (left) and HD-CuB treated groups (right). Data show mean tumor volume ±standard deviation (SD, error bars) of five mice per group. Asterisks (**) represent p < 0.001 vs. LD-CuB or vs. LD-MTX by t-test. (B) Body weight change of mice over the course of treatment. Data show mean% body weight ±SD of five mice per group. All measurements were repeated in triplicates to ensure accuracy. Asterisks (*) represent p < 0.05 vs. groups without LD-MTX treatment by t-test. (C) Comparison of tumor weight from each group. At day 35, mice were sacrificed and tumors were excised, weighed, and fixed in 10% PBS-buffered formalin. Data represent mean tumor weight ±SD of 10 tumors from five mice per group. (D) Western blot results using snap-frozen tumors from each group. GAPDH was used as a loading control. Results were repeated in triplicates with similar results.
During treatment, minor signs of toxicity were observed in all of the treatment groups except those mice who received LD-MTX, either as a single agent (LD-MTX) or in combination with cucurbitacin B (LD-CuB + LDMTX and HD-CuB + LD-MTX). Some mice in these groups developed a 10% body weight loss (p < 0.05) at 48 h after MTX injection (Fig. 5B). Other side-effects included sluggish movements, occasional diarrhea, and a patchy skin rash in 60% of these mice. These side-effects by MTX have been previously reported by other groups [18–20]. Strikingly, no changes in body weight compared to pretreatment levels were observed when the MTX dose was lowered by two thirds (LD-CuB + VLD-MTX). Two other groups (LD-CuB and HD-CuB) had an initial weight loss of up to 5% in week 1, but weights returned to normal in subsequent weeks (Fig. 5B). No other side-effects were observed.
Decrease in tumor volume was also verified by their decrease in tumor weights (Fig. 5C). At day 35, the decrease in average tumor weight of LD-CuB + LD-MTX group was 62% vs. LD-CuB group (p < 0.001) and 81% vs. LD-MTX group (p < 0.001). Likewise, the LD-CuB + VLD-MTX group showed a similar decrease in average tumor weight (69% vs. LDCuB group and 85% vs. LD-MTX group, p < 0.001). Western blots from the snap-frozen tumors showed decreased phosphorylation of ERK, Akt, and mTOR and their downstream targets, in those mice treated with LD-CuB (Fig. 5D).
Immunohistochemistry further confirmed the inhibition of tumor growth. Whereas HE-stained tumors in the PBS control group displayed high tumor cell density and numerous blood vessels, all treatment groups showed decreased tumor area and smaller blood vessels (in LD-CuB), less number of blood vessels (in LD-MTX), or both (in LD-CuB + VLD-MTX) (Fig. 6A). Ki-67 proliferation staining showed marked decrease in Ki-67 positive cells in all treatment groups (Fig. 6B, Supplementary Fig. S2A), without significant difference among the treatment groups (p = 0.34). On the contrary, apoptosis as measured by TUNEL-positive cells was markedly elevated in the combination group compared to the single agent groups (Fig. 6C, Supplementary Fig. S2B).
Fig. 6.
Immunohistochemistry and complete blood counts of xenografts at the end of study (day 35). (A) HE staining results. Tumor cells (stained in dark purple) and red blood cells (stained in red) in the infiltrated microvessels are shown. 100×, scale bar = 250 μm. (B) Ki-67 proliferation staining results. (C) TUNEL apoptosis staining results. Ki-67 and TUNEL stained slides are available in Supplementary Figs. S1A and S1B, respectively. Data represent mean percent positive cells ±standard deviation (SD). (D) Complete blood count (CBC). Whole-blood samples were harvested by submandibular bleeding and analyzed at the end of the experiments (day 35). PBS, diluant control; LD-CuB, low-dose cucurbitacin B (0.5 mg/kg body weight); LD-MTX, low-dose methotrexate (150 mg/kg); VLD-MTX, very low-dose methotrexate (50 mg/kg). Asterisks (**) represent p < 0.001 by t-test. NS = not significant.
3.5. Toxicity studies of cucurbitacin B and/or MTX in vivo
At autopsy, no signs of metastasis were found in any of the treatment groups whereas the control group had metastatic spread of tumors in various organs and regions such as mediastinum and periosteum (data not shown). Major organs such as spleen, liver, and kidney did not show any significant changes in their weight (p = 0.25) in each of the treatment groups (Supplementary Table S3). No sign of organ damage was morphologically observed (data not shown). Blood test results showed that most treatment groups had a reduction of their red blood cells (RBC), white blood cells (WBC), and hemoglobin (Hb) (p < 0.001), but not their platelets (p = 0.15) (Fig. 6D, Supplementary Table S4). Serum chemistry studies did not show any significant changes between control and treatment groups (Supplementary Table S5). Bone marrow clonogenic assays which were done before euthanasia showed little change in the number of CFUGEMM or CFU-GM, but BFU-E hematopoietic progenitor cells decreased in all treatment groups (p < 0.001) (Supplementary Table S6).
4. Discussion
Imbalance in the growth factor signaling pathways plays an important role in the pathogenesis of human OS. Epidermal growth factor receptor (EGFR) frequently undergoes somatic mutations in these cells [21]. Over-expression of EGFR, insulin-like growth factor 1 (IGF-1) and its receptor (IGF-1R), hepatocyte growth factor (HGF) and its receptor (c-MET), and platelet-derived growth factor (PDGF) and its receptor (PDGFR) are also frequently observed in OS [22–28]. Interestingly, the MAPK/ERK, Akt-PKB, and mTOR pathways are the three major oncogenic downstream signaling pathways to which these changes converge. Frequent somatic mutations of BRAF, increased protein expression of ERK and mTOR support this concept [21,22,29–32]. Therefore, a strong therapeutic rationale is provided to target these pathways in OS.
We show for the first time that cucurbitacin B can inhibit these secondary signaling pathways. Previously, cucurbitacin B was found to inhibit the phosphorylation of ERK, MEK, and c-RAF, but not RAS, in the K562 leukemia cell line, suggesting that c-RAF or a related protein may be the direct target of cucurbitacin B [33]. In our study, however, cucurbitacin B did not inhibit MEK, RAF, and RAS in both MG-63 and SAOS-2 cell lines (data not shown). Moreover, no dose-dependent protection of ERK protein by cucurbitacin B was observed in DARTS analysis (data not shown). Taken together, ERK is unlikely to be the direct target of cucurbitacin B in OS cells.
Our finding that cucurbitacin B inhibited Akt phosphorylation at S473 in MG-63 cells is surprising because Akt was not affected by cucurbitacin B in several other types of cancers [4]. Inhibition of Akt in MG-63 cells can be explained as feedback regulation by mTORC2 because a moderate dose-dependent protection of mTOR proteins by cucurbitacin B was noted in our DARTS analysis [34]. However, it remains possible that mTOR inhibition is secondary to ERK and Akt inhibition. Because the mTOR, MAPK/ERK, and Akt pathways form a complex, interwoven regulatory network with each other, more studies are necessary to identify how cucurbitacin B suppresses these signaling pathways.
Our observation of a strong synergism of cucurbitacin B with MTX to suppress the growth of OS in vitro is consistent with cucurbitacin B inhibiting the Akt and mTOR signaling pathways. MG-63 cells are known to be MTX-resistant due to its high expression level of DHFR [35]. As suggested in leukemia, inhibition of mTOR by cucurbitacin B may sensitize OS cells to MTX by blocking RB1 phosphor-ylation and by decreasing cyclin D1 stability [36–38].
MTX is one of the essential chemotherapeutic agents for OS treatment. Nearly all successful chemotherapeutic regimens for OS include HD-MTX. However, HD-MTX is associated with some confounding issues such as appropriate administration and monitoring of the drug associated with inter- and intra-patient variability [1]. Furthermore, the administration of leucovorin is almost always necessary due to severe toxicity of HD-MTX. Therefore, we hypothesized that the combined use of cucurbitacin B and MTX may lower the amount of MTX administered and may obviate the need for leucovorin.
Our in vivo xenograft studies demonstrated that marked growth inhibition of OS cells was achievable by the combined use of LD-CuB with LD-MTX. Many xenograft studies using murine models have shown that LD-MTX alone showed poor growth inhibition (≤20%) of human OS cells [19,39]. Therefore, the growth inhibition of LD-MTX combined with LD-CuB was remarkable. However, as previously reported in the xenograft studies by others, systemic toxicity by LD-MTX can still occur in this combination [18–20]. This toxicity was resolved by lowering the dose of MTX by two thirds (VLD-MTX, 50 mg/kg body weight) and yet growth inhibition of the OS cells was maintained.
In conclusion, we demonstrate that cucurbitacin B as a single agent or in combination with MTX show promising antiproliferative activity in human OS cells. Considering that pathway-specific inhibitors like rapamycin (mTOR inhibitor) as a single agent are not clinically overly potent [40,41], cucurbitacin B which can inhibit ERK, Akt, and mTOR at the same time may lead to more efficient growth inhibition of OS cells. In addition, combined use of cucurbitacin B and MTX may lower the need for the currently used, highly toxic HD-MTX. Our research lays the foundation for more effective OS therapy.
Supplementary Material
Acknowledgements
We thank Dr. Melvin Toh and Dr. Tak Chan from CK Life Sciences International Holdings Inc. for providing purified cucurbitacin B. H.P.K received Grant support from NIH R0-1 CA026038-38 and U54-14 39 30, as well as A* STAR Grant of Singapore.
Footnotes
Conflict of interest
None declared.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.canlet.2011.03.001.
References
- 1.Federman N, Bernthal N, Eilber F, Tap W. The multidisciplinary management of osteosarcoma. Curr. Treat. Options Oncol. 2009;10:82–93. doi: 10.1007/s11864-009-0087-3. [DOI] [PubMed] [Google Scholar]
- 2.Rosen G, Marcove R, Caparros B, Nirenberg A, Kosloff C, Huvos A. Primary osteogenic sarcoma: the rationale for preoperative chemotherapy and delayed surgery. Cancer. 1979;43:2163–2177. doi: 10.1002/1097-0142(197906)43:6<2163::aid-cncr2820430602>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Rosen G, Caparros B, Huvos A, Kosloff C, Nirenberg A, Cacavio A, Marcove R, Lane J, Mehta B, Urban C. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–1230. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Lee D, Iwanski G, Thoennissen N. Cucurbitacin: ancient compound shedding new light on cancer treatment. ScientificWorldJournal. 2010;10:413–418. doi: 10.1100/tsw.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Chiu M, Nie R, Cordell G, Qiu S. Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat. Prod. Rep. 2005;22:386–399. doi: 10.1039/b418841c. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura R, Moriyama K, Yasukawa K, Mundy G, Yoneda T. Combination of interleukin-6 and soluble interleukin-6 receptors induces differentiation and activation of JAK–STAT and MAP kinase pathways in MG-63 human osteoblastic cells. J. Bone Miner. Res. 1998;13:777–785. doi: 10.1359/jbmr.1998.13.5.777. [DOI] [PubMed] [Google Scholar]
- 7.Bogenmann E, Moghadam H, DeClerck YA, Mock A. c-myc Amplification and Expression in Newly Established Human Osteosarcoma Cell Lines. Cancer Research. 1987:7. [PubMed] [Google Scholar]
- 8.Lomenick B, Hao R, Jonai N, Chin R, Aghajan M, Warburton S, Wang J, Wu R, Gomez F, Loo J, Wohlschlegel J, Vondriska T, Pelletier J, Herschman H, Clardy J, Clarke C, Huang J. Target identification using drug affinity responsive target stability (DARTS) Proc. Natl. Acad. Sci. USA. 2009;106:21984–21989. doi: 10.1073/pnas.0910040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 10.Luong Q, O'Kelly J, Braunstein G, Hershman J, Koeffler H. Antitumor activity of suberoylanilide hydroxamic acid against thyroid cancer cell lines in vitro and in vivo. Clin. Cancer Res. 2006;12:5570–5577. doi: 10.1158/1078-0432.CCR-06-0367. [DOI] [PubMed] [Google Scholar]
- 11.Abramoff MDM, Ram PJ. Image processing with image. J. Biophotonics Int. 2004;11:7. [Google Scholar]
- 12.Iwanski G, Lee D, En-Gal S, Doan N, Castor B, Vogt M, Toh M, Bokemeyer C, Said J, Thoennissen N, Koeffler H. Cucurbitacin B, a novel in vivo potentiator of gemcitabine with low toxicity in the treatment of pancreatic cancer. Br. J. Pharmacol. 2010;160:998–1007. doi: 10.1111/j.1476-5381.2010.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou T, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 14.Chou T. Drug combination studies and their synergy quantification using the Chou–Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 15.Chou TC, Hayball MP. CalcuSyn Sofrware, Version 2.0. Biosoft; Cambridge, UK: 1997. [Google Scholar]
- 16.Pouysségur J, Dayan F, Mazure N. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 17.Mariani B, Slate D, Schimke R. S phase-specific synthesis of dihydrofolate reductase in Chinese hamster ovary cells. Proc. Natl. Acad. Sci. USA. 1981;78:4985–4989. doi: 10.1073/pnas.78.8.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorlick R, Goker E, Trippett T, Waltham M, Banerjee D, Bertino J. Intrinsic and acquired resistance to methotrexate in acute leukemia. N Engl. J. Med. 1996;335:1041–1048. doi: 10.1056/NEJM199610033351408. [DOI] [PubMed] [Google Scholar]
- 19.Lobo E, Balthasar J. Pharmacokinetic–pharmacodynamic modeling of methotrexate-induced toxicity in mice. J. Pharm. Sci. 2003;92:1654–1664. doi: 10.1002/jps.10431. [DOI] [PubMed] [Google Scholar]
- 20.Margolis S, Philips F, Sternberg S. The cytotoxicity of methotrexate in mouse small intestine in relation to inhibition of folic acid reductase and of DNA synthesis. Cancer Res. 1971;31:2037–2046. [PubMed] [Google Scholar]
- 21.Forbes SA, Tang G, Bindal N, Bamford S, Dawson E, Cole C, Kok CY, Jia M, Ewing R, Menzies A, Teague JW, Stratton MR, Futreal PA. COSMIC (the Catalogue of Somatic Mutations in Cancer): a resource to investigate acquired mutations in human cancer. Nucleic Acids Res. 2010;38:D652–657. doi: 10.1093/nar/gkp995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Do S, Jung W, Kim H, Park Y. The expression of epidermal growth factor receptor and its downstream signaling molecules in osteosarcoma. Int. J. Oncol. 2009;34:797–803. doi: 10.3892/ijo_00000205. [DOI] [PubMed] [Google Scholar]
- 23.Freeman SS, Allen SW, Ganti R, Wu J, Ma J, Su X, Neale G, Dome JS, Daw NC, Khoury JD. Copy number gains in EGFR and copy number losses in PTEN are common events in osteosarcoma tumors. Cancer. 2008;113:1453–1461. doi: 10.1002/cncr.23782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Lee JC, Kumar S, Gowen M. ERK pathway mediates the activation of Cdk2 in IGF-1-induced proliferation of human osteosarcoma MG-63 cells. J. Bone Miner. Res. 1999;14:528–535. doi: 10.1359/jbmr.1999.14.4.528. [DOI] [PubMed] [Google Scholar]
- 25.Burrow S, Andrulis IL, Pollak M, Bell RS. Expression of insulin-like growth factor receptor, IGF-1, and IGF-2 in primary and metastatic osteosarcoma. J. Surg. Oncol. 1998;69:21–27. doi: 10.1002/(sici)1096-9098(199809)69:1<21::aid-jso5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 26.Coltella N, Manara MC, Cerisano V, Trusolino L, Di Renzo MF, Scotlandi K, Ferracini R. Role of the MET/HGF receptor in proliferation and invasive behavior of osteosarcoma. FASEB J. 2003;17:1162–1164. doi: 10.1096/fj.02-0576fje. [DOI] [PubMed] [Google Scholar]
- 27.Heldin CH, Johnsson A, Wennergren S, Wernstedt C, Betsholtz C, Westermark B. A human osteosarcoma cell line secretes a growth factor structurally related to a homodimer of PDGF A-chains. Nature. 1986;319:511–514. doi: 10.1038/319511a0. [DOI] [PubMed] [Google Scholar]
- 28.Graves DT, Owen AJ, Antoniades HN. Demonstration of receptors for a PDGF-like mitogen on human osteosarcoma cells. Biochem. Biophys. Res. Commun. 1985;129:56–62. doi: 10.1016/0006-291x(85)91402-0. [DOI] [PubMed] [Google Scholar]
- 29.Abdeen A, Chou A, Healey J, Khanna C, Osborne T, Hewitt S, Kim M, Wang D, Moody K, Gorlick R. Correlation between clinical outcome and growth factor pathway expression in osteogenic sarcoma. Cancer. 2009;115:5243–5250. doi: 10.1002/cncr.24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Dziak R, Aletta J. EGF-mediated phosphorylation of extracellular signal-regulated kinases in osteoblastic cells. J. Cell Physiol. 1995;162:348–358. doi: 10.1002/jcp.1041620307. [DOI] [PubMed] [Google Scholar]
- 31.Kiyokawa E, Takai S, Tanaka M, Iwase T, Suzuki M, Xiang Y, Naito Y, Yamada K, Sugimura H, Kino I. Overexpression of ERK, an EPH family receptor protein tyrosine kinase, in various human tumors. Cancer Res. 1994;54:3645–3650. [PubMed] [Google Scholar]
- 32.Zhang Z, Neiva K, Lingen M, Ellis L, Nör J. VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ. 2010;17:499–512. doi: 10.1038/cdd.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan K, Li K, Liu S, Chu K, Toh M, Xie W. Cucurbitacin B inhibits STAT3 and the Raf/MEK/ERK pathway in leukemia cell line K562. Cancer Lett. 2010;289:46–52. doi: 10.1016/j.canlet.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Feldman M, Apsel B, Uotila A, Loewith R, Knight Z, Ruggero D, Shokat K. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diddens H, Niethammer D, Jackson R. Patterns of cross-resistance to the antifolate drugs trimetrexate, metoprine, homofolate, and CB3717 in human lymphoma and osteosarcoma cells resistant to methotrexate. Cancer Res. 1983;43:5286–5292. [PubMed] [Google Scholar]
- 36.Teachey D, Sheen C, Hall J, Ryan T, Brown V, Fish J, Reid G, Seif A, Norris R, Chang Y, Carroll M, Grupp S. mTOR inhibitors are synergistic with methotrexate: an effective combination to treat acute lymphoblastic leukemia. Blood. 2008;112:2020–2023. doi: 10.1182/blood-2008-02-137141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller H, Lukas J, Schneider A, Warthoe P, Bartek J, Eilers M, Strauss M. Cyclin D1 expression is regulated by the retinoblastoma protein. Proc. Natl. Acad. Sci. USA. 1994;91:2945–2949. doi: 10.1073/pnas.91.8.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serra M, Reverter-Branchat G, Maurici D, Benini S, Shen J, Chano T, Hattinger C, Manara M, Pasello M, Scotlandi K, Picci P. Analysis of dihydrofolate reductase and reduced folate carrier gene status in relation to methotrexate resistance in osteosarcoma cells. Ann. Oncol. 2004;15:151–160. doi: 10.1093/annonc/mdh004. [DOI] [PubMed] [Google Scholar]
- 39.Bruheim S, Bruland O, Breistol K, Maelandsmo G, Fodstad O. Human osteosarcoma xenografts and their sensitivity to chemotherapy. Pathol. Oncol. Res. 2004;10:133–141. doi: 10.1007/BF03033741. [DOI] [PubMed] [Google Scholar]
- 40.Guertin D, Sabatini D. The pharmacology of mTOR inhibition. Sci. Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 41.Gazitt Y, Kolaparthi V, Moncada K, Thomas C, Freeman J. Targeted therapy of human osteosarcoma with 17AAG or rapamycin: characterization of induced apoptosis and inhibition of mTOR and Akt/MAPK/Wnt pathways. Int. J. Oncol. 2009;34:551–561. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.