Abstract
Scanning-beam digital x-ray (SBDX) is an inverse geometry x-ray fluoroscopy system capable of tomosynthesis-based 3D catheter tracking. This work proposes a method of dose-reduced 3D tracking using dynamic electronic collimation (DEC) of the SBDX scanning x-ray tube. Positions in the 2D focal spot array are selectively activated to create a region-of-interest (ROI) x-ray field around the tracked catheter. The ROI position is updated for each frame based on a motion vector calculated from the two most recent 3D tracking results. The technique was evaluated with SBDX data acquired as a catheter tip inside a chest phantom was pulled along a 3D trajectory. DEC scans were retrospectively generated from the detector images stored for each focal spot position. DEC imaging of a catheter tip in a volume measuring 11.4 cm across at isocenter required 340 active focal spots per frame, versus 4473 spots in full-FOV mode. The dose-area-product (DAP) and peak skin dose (PSD) for DEC versus full field-of-view (FOV) scanning were calculated using an SBDX Monte Carlo simulation code. DAP was reduced to 7.4% to 8.4% of the full-FOV value, consistent with the relative number of active focal spots (7.6%). For image sequences with a moving catheter, PSD was 33.6% to 34.8% of the full-FOV value. The root-mean-squared-deviation between DEC-based 3D tracking coordinates and full-FOV 3D tracking coordinates was less than 0.1 mm. The 3D distance between the tracked tip and the sheath centerline averaged 0.75 mm. Dynamic electronic collimation can reduce dose with minimal change in tracking performance.
Keywords: X-ray fluoroscopy, inverse geometry, scanning-beam digital x-ray, catheter tracking, collimation
1. INTRODUCTION
Cardiac catheterization is a standard-of-care procedure for the diagnosis and treatment of structural heart disease and electrophysiological abnormalities.1–3 These procedures frequently involve the navigation of catheter devices within open 3D spaces and targeting of devices to specific anatomic positions. For example, radiofrequency catheter ablation of atrial fibrillation may require positioning of a catheter tip at points around the pulmonary veins in order to create encircling lesions which isolate irregular electrical signals. A structural heart intervention such as transcatheter aortic valve replacement (TAVR) requires accurate positioning of a stent-supported artificial valve within the aortic root prior to valve deployment. X-ray fluoroscopy provides essential real-time image guidance. However, in these procedures, the 2D x-ray projection format does not convey 3D device positions relative to target anatomy.
Three dimensional tracking and display of catheter devices has the potential to reduce procedure duration and improve procedural outcome.4,5 Non-fluoroscopic 3D catheter tracking systems such as CartoMerge and NavX Fusion4 are used in electrophysiology procedures. These systems rely on proprietary catheters fitted with electromagnetic field sensors and do not provide live imaging of the device relative to patient anatomy. Tracking can be merged with pre-acquired CT maps, however if the reference catheter used for registration becomes displaced during the procedure, remapping and re-registration may be required.6 In contrast, tracking methods derived from fluoroscopy are capable of providing live anatomic context and do not require modification of the device for tracking purposes, at the cost of increased radiation dose compared to non-fluoroscopic methods.7,8
Scanning-beam digital x-ray (SBDX) is a low-dose, inverse-geometry x-ray fluoroscopic system with a unique real-time tomosynthesis capability. 9–11 The system images the patient with an electronically scanned multi-source x-ray tube, high speed photon counting detector, and real-time image reconstructor (Figure 1).11 In recent years, SBDX tomosynthesis has been explored for its potential to provide real-time 3D tracking of catheter devices and fiducials simultaneous with fluoroscopy.12–14 A dose efficient design is achieved through inverse geometry beam scanning, which minimizes detected scatter and spreads primary x-rays over a relatively large entrance skin area.10 Recent work has shown that SBDX can achieve further dose reduction through fluence field modulation i.e. selective downward-modulation of the outputs of individual focal spots in the multi-source x-ray tube.15
Figure 1.
(A) Scanning-beam digital x-ray (SBDX) prototype. (B) An electron beam scans over an array of focal spot positions. A multihole collimator defines overlapping x-ray beamlets directed at the detector. (C) A fixed point in the imaging volume is imaged by a patch of focal spots. (D) Multiple tomosynthesis planes are formed by backprojecting detector images onto planes of reconstruction. Object sharpness vs. plane position is used for 3D localization.
This work proposes a new dose-reduced technique for SBDX catheter tracking which combines tomosynthesis-based device localization with dynamic electronic collimation (DEC) of the scan. Electronic collimation refers to blanking of the tube current at selected focal spot positions in order to reduce the x-ray field area. It is hypothesized that frame-by-frame electronic collimation to a region just surrounding the tracked catheter will enable major additional reduction of dose-area-product without loss of tracking accuracy. Furthermore, this technique may reduce peak skin dose when the device is moving across the field-of-view. The performance and feasibility of the new technique was evaluated in phantom studies which compared DEC-based tracking to full field-of-view (FOV) SBDX tracking.
1.1 Scanning-Beam Digital X-ray (SBDX)
SBDX uses a multi-source x-ray tube to scan a narrow x-ray beam in an inverse geometry (Figure 1). This geometry is a form of real-time tomosynthesis. An electron beam is raster scanned over a 2D array of focal spot positions on a large-area transmission style tungsten target (up to 100 × 100 positions, 2.3 mm pitch). A multi-hole collimator between the target and patient defines a series of overlapping x-ray beamlets, each of which is directed at a high speed photon-counting detector array. As the electron beam moves from one hole to the next, detector images are simultaneously captured. The current prototype has a 5.3 cm × 10.6 cm detector (0.33 mm native element pitch) positioned 150 cm above the target plane. Mechanical isocenter is 45 cm above the target. In a typical 15 frame/s imaging mode, a 71 × 71 source array is used and the electron beam visits each focal spot position 8 times per frame in a blockwise raster pattern.9,11 The stream of detector images captured during scanning is reconstructed into a stack of tomosynthesis images in real time using shift-and-add back projection.11,16 The current GPU-based reconstructor generates 32 planes × 15 frame/s with programmable plane-to-plane spacing. A composite image of the plane stack is formed for real-time display by selecting in-focus features from each plane and displaying them in a single image. Additional details of SBDX operating principles can be found in Refs. 9 and 11.
The SBDX skin dose reduction strategy is based upon: i) narrow beam scanning with a large air gap, which minimizes detected x-ray scatter, ii) efficient detection of primary x-rays using a thick CdTe detector, and iii) an inverse geometry which spreads entrance x-rays over a large area. A study with a previous SBDX prototype found that these properties increase entrance exposure efficiency by 3–7 times when compared to a conventional fluoroscopic system.10 Further dose reduction can be achieved by tailoring the outputs of individual focal spots according to patient geometry and imaging application. Specifically, the SBDX x-ray tube has grid-controlled tube current which can be turned on or off at each focal spot position, allowing for non-mechanical 2D collimation and/or spatial modulation of the x-ray field (e.g. by turning of the beam for a fraction of the 8 passes per frame). A technique termed Regional Adaptive Exposure (RAE) scanning performs spatial modulation of the x-ray tube output. By reducing the number of focal spot activations per frame at source positions with high measured x-ray transmission, this method has been shown to reduce dose-area-product (DAP) by an additional 46%.15 In this paper, we combine the 3D localization capabilities of SBDX with electronic collimation in the x-ray tube to minimize catheter tracking radiation dose.
1.2 Tomosynthesis-based 3D catheter tracking
The stack of SBDX tomosynthesis images reconstructed in a frame period can be analyzed to localize discrete, high-contrast catheter elements in 3D with sub-millimeter accuracy and precision.12–14 The SBDX tracking algorithm is based on the analysis of tomographic blurring versus plane position, as outlined in Figure 2. For each image in the plane stack, a low-pass filter is applied to suppress image noise. Next a gradient filter (Prewitt kernel) is applied to emphasize in-focus and high-contrast features. A second low-pass filter is applied in order to distribute high gradient edges over the object area and aid in subsequent segmentation of objects. The size of this kernel is adjusted to the size of the object being tracked. For efficient object segmentation, a maximum intensity projection (MIP) of the gradient filtered stack is formed along the source-detector direction (z-axis). A threshold is applied to the MIP and the surviving “blobs” (objects) are labeled using connected component labeling. A weighted center-of-mass is calculated for each labeled object to localize position in the X-Y plane. To localize the object position along the source-detector (Z) axis, the object’s gradient values are extracted from each Z-plane. Since SBDX tomographic blur is symmetrical about the object position, the measurements of sharpness vs. plane position are treated as a probability distribution function of object position. The Z-coordinate is determined by calculating the center-of-mass of this distribution, after application of a second threshold which rejects background gradients.
Figure 2.
Flowchart of SBDX 3D catheter tracking. (A) The tomosynthesis image stack reconstructed for a frame period is the input to the tracking algorithm. (B) Gradient filters are applied to produce a “score stack” reflecting the contrast and sharpness at each pixel in each plane. (C) A MIP along the z-axis is created from the score stack. (D) A threshold is applied and connected component labeling is used to find distinct objects. (E) A weighted center-of-mass calculation is performed on each object for X-Y localization. (F) Scores vs plane position are extracted for each object. (G) The Z-coordinate of an object is the center-of-mass of the distribution of scores versus z-plane, after application of a second threshold to reject background scores.
2. METHODS
2.1 Dynamic electronic collimation (DEC)
In the proposed dynamic electronic collimation method, a small ROI of focal spots is turned on in each frame to create an x-ray field that just surrounds the tracked object. Inside the ROI, all passes of the electron beam are performed with normal tube current. For example, in the 71x71 15 frame/s scanning mode which involves 8 passes per hole per frame period, all 8 passes would be performed at normal tube current inside the ROI. Outside the ROI, the tube current is turned off for all passes. The position of the ROI is updated after each imaging frame based on previous tracking results and an estimate of the device position in the next frame. The reasoning behind the DEC algorithm is twofold. First, accurate SBDX tracking requires only the image information that captures the local tomographic blurring of the object. Second, it is assumed that once the operator has switched to a 3D tracking mode, the patient anatomy of interest lies near the device being tracked. We note the DEC technique could also be modified to create a “soft ROI” where the tube current is left on for a small number of passes (e.g. 1 out of 8 passes) at all focal spot positions. Such a mode could be employed if peripheral image context is desired.
To prevent a reduction in catheter tracking accuracy and precision, the ROI must be defined such that the number and angular range of rays passing through the object are not reduced relative to full-FOV mode. This ensures that a normal x-ray fluence and tomographic angle are achieved. The minimum allowed ROI width for a point-like object is determined by tracing the edges of the detector back through the object and onto the source plane (see Figure 1C):
| (1) |
WD is the detector width (106 mm), Δs is the focal spot pitch (2.3 mm), z is source-to-object distance, and SDD is the source-to-detector distance (1500 mm).11 A similar expression exists for the ROI height.
The ROI width called for by Eq. (1) depends on object position Z, which in theory could be obtained from catheter tracking. In this paper, we investigate the simpler case of a moving fixed-size ROI. For a fixed-size ROI, a conservative estimate of ROI size is obtained by assuming the object is at the highest plane of the reconstructed volume (z = 525 mm). For a 10.6 cm by 5.3 cm detector, this approach gives an ROI size of 24 X 12 active sources. Accounting for the finite size of the catheter tip requires the addition of two source points in each direction, or a 26 × 14 ROI. Lastly, we note that the corner focal spot positions in a rectangular ROI make a minimal contribution to the fluence at the center of the reconstructed ROI. This is because the x-ray beamlet itself is not perfectly rectangular in shape, and tapers at the corners. Based on this observation, we used an elliptical ROI with 26 sources along the major axis and 14 sources along the minor axis.
Figure 3 shows an example DEC scan map and image reconstruction for an elliptical 26 × 14 ROI with a total of 340 active focal spots. In comparison, the full-FOV image uses 4473 active focal spots (71 × 71 array with rounded corners). The relative dose-area-product (DAP) for DEC imaging versus full-FOV imaging should be approximately equal to the ratio of active focal spots in the two cases (340/4473 = 0.076). The reduction of peak skin dose (PSD) is dependent on the object trajectory in an image sequence. In an individual frame, the PSD in DEC mode is expected to be similar to the full-FOV PSD. However, if the object is moving in the image sequence and the ROI is moving across the skin entrance, then the total PSD is determined by the total x-ray field overlap time at a skin point, rather than the total imaging time. Therefore, significant PSD reduction can be expected for wide-ranging catheter trajectories.
Figure 3.
Left: Full field-of-view image acquisition. Middle: DEC-mode scan map generated from localized catheter tip, indicating positions of active focal spots. Right: Image corresponding to the DEC-mode scan.
When using the DEC algorithm, the initial 3D position of the catheter is not known a priori. Furthermore, the scan map must be updated for each frame to reflect the predicted catheter position. Therefore, the following algorithm is employed to initialize and update the DEC scan (Figure 4). Frames 1 and 2 are acquired with full-FOV scanning. Objects of interest are localized in these frames, and the localized coordinates from frames 1 and 2 are used to calculate a velocity vector for tracked objects. The velocity vector is then used to predict the position of the object in frame 3, and an ROI scan map is generated for this predicted position. Using this scan map, the third frame is acquired with a reduced field of view, and object localization is again performed. The velocity vector is updated using object positions from frames 2 and 3, the ROI position is updated for frame 4. The procedure is repeated for the rest of the imaging sequence. By this method, the catheter positions in the two most recent frames are used to generate a velocity vector that defines ROI position for the next frame.
Figure 4.
DEC imaging method. To initialize, full field-of-view imaging is performed for two frames. 3D tip localization is performed for both frames and a velocity vector (blue) is calculated. The velocity vector is used to predict the position of the tip in frame 3. The source is electronically collimated around this predicted position (3rd panel), and frame 3 is acquired in DEC mode. Subsequently, the velocity vector and DEC scan map are continuously updated using the two most recent frames of catheter tracking.
2.2 Tracking performance
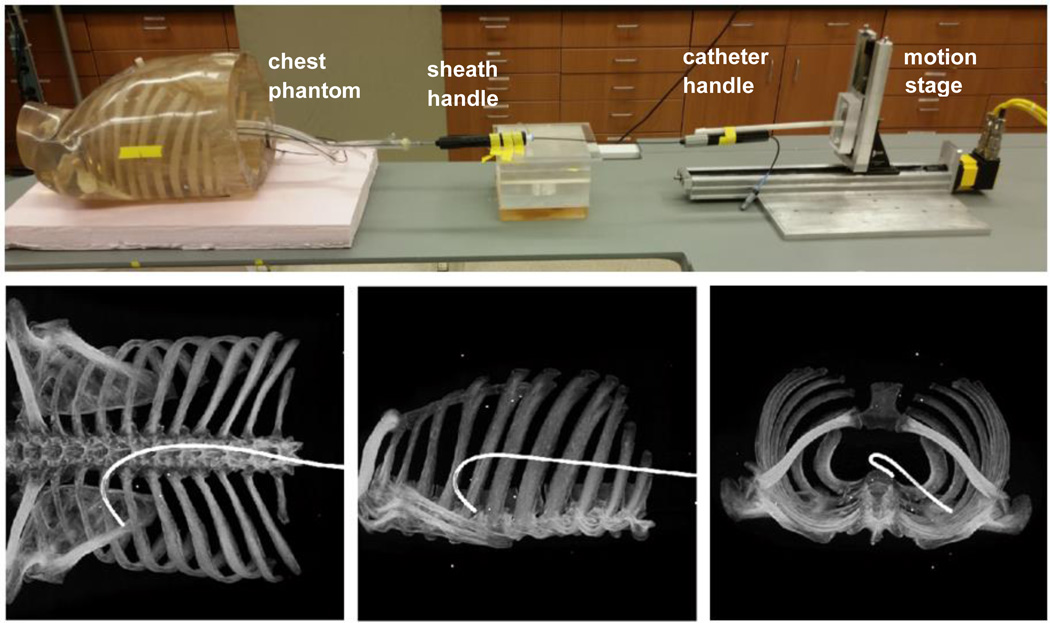
DEC tracking versus full-FOV tracking was evaluated in a phantom study. A cardiac chamber phantom (Venous Sam, Venous Training Model 5010, Lake Forest Anatomicals, Lake Forest, IL, USA) was fixed within an anthropomorphic chest phantom. A catheter sheath was mounted within the phantom to define a trajectory from the inferior vena cava into the right atrium, through the atrial septum, and into the left atrium. The tracked position of a catheter tip pulled through this sheath was compared to the volume and centerline of the sheath, as defined by CT, following the method described in Ref 13. The experimental setup and orthogonal projections showing the chest wall and sheath are shown in Figure 5. The phantom contained fiducials fixed to the chest wall for registration of SBDX and CT data.
Figure 5.
Top: Experimental setup showing an anthropomorphic chest phantom, fixed catheter sheath entering a vascular phantom, and catheter attached to a linear motion stage. Bottom: Orthogonal projections of a CT scan of the phantom, showing the 3D path of the internal sheath.
The phantom was imaged in the 71 × 71, 15 frame/s cardiac scan mode as a 7 Fr ablation catheter with 4 mm tip (Livewire TC, St Jude Medical, Inc.) was pulled along the 3D trajectory defined by the sheath by a linear motion stage. Image sequences were recorded for pullback speeds of 10, 25, and 50 mm/s. Raw detector data was saved for each collimator hole for retrospective generation of DEC scans. This retrospective approach enabled evaluation of tracking performance for different DEC scan modes using the same underlying phantom geometry and motion. The DEC algorithm described in Sec 2.1 was implemented offline, using MATLAB versions of the SBDX image reconstruction and catheter tracking algorithms. The DEC images were reconstructed with the detector data originating from within the 26 by 14 elliptical ROI, centered at the position predicted by catheter tracking. Full-FOV reconstruction and catheter tracking was performed for comparison. Full-FOV images were reconstructed using data from all collimator holes.
For both DEC and full-FOV imaging, tomosynthesis reconstruction was performed for 32 planes with 5 mm spacing centered on mechanical isocenter. The catheter tracking parameters were adjusted to localize the catheter tip while rejecting the fiducials in the image.12,13 The initial boxcar filter width was 6 pixels, the secondary boxcar width was 14 pixels, and the XY threshold and Z threshold were each set to 22%.
The root mean squared deviation (RMSD) between the DEC-based catheter tip positions (pi) and the full-FOV-based catheter tip positions was calculated to assess any change in localization resulting from DEC scanning.
| (2) |
Additionally, the 3D tip positions were compared to the 3D volume and centerline of the catheter sheath. To define the sheath volume, a helical CT acquisition of the anthropomorphic phantom was performed on a GE 750 HD scanner at 120 kVp, 295 mAs. CT reconstruction was performed with 0.625 mm slice thickness and 0.717 mm X 0.717 mm in-plane pixel size. The sheath volume was segmented using intensity based segmentation and a centerline was extracted from the sheath volume. A 3D-3D transformation from SBDX coordinates to CT coordinates was calculated with an iterative closest point rigid registration algorithm, using the 3D fiducial positions localized from a 72-plane SBDX reconstruction and the 3D fiducial positions segmented from the CT scan. This transformation was applied to the localized catheter tip coordinates for each frame to transform the tip position from SBDX coordinates to CT coordinates. The accuracy of tip tracking was characterized in terms of the fraction of the localized tip positions falling within the sheath volume, the mean distance from the localized tip to the sheath centerline, and the standard deviation in tip-to-centerline distances.
2.3 Dose reduction study
A study was performed to determine the dose reduction characteristics of DEC imaging. Currently, the SBDX prototype has a static electronic collimation feature which uses a stationary circular ROI of focal spots with a specified position and radius.14 This feature was used to measure dose-area-product versus ROI size. The dose reduction associated with a moving elliptical ROI was evaluated using a Monte Carlo simulation package developed for the SBDX geometry.17 This simulation package has been previously validated against relative DAP measurements performed in regional adaptive exposure (RAE) versus full-FOV scanning mode. To confirm that this package is also valid for simulated ROI scanning, the DAP measurements made with static circular ROIs were compared against Monte Carlo simulations of the same ROI scanning modes.
In the experimental study of circular scan ROIs, electronic collimation was performed with ROI radii of 3, 5, 10, 15, 25, 35 collimator holes. Each ROI was centered on the multi-hole collimator. DAP was measured with a 300 mm × 300 mm active area DAP meter (Radcal DAPcheck PDC, Radcal Corporation, Monrovia, CA, USA) placed just above the collimator exit (6 cm from target plane). For each collimation radius, five 20 second sequences were acquired. The x-ray tube was operated at 100 kVp and 120 mA peak tube current. Five, 20 second sequences for a full-FOV acquisition were also performed for use as a reference. From these acquisitions, the DAP rate was calculated in units of μGy·m2/min.
A Monte Carlo package based on MC-GPU v1.3 and adapted for inverse geometry fluoroscopy17 was used to simulate a moving elliptical ROI in DEC scanning. MC-GPU is a public domain Monte Carlo x-ray transport package based on the PENELOPE subroutine package.18–20 This code has been modified to simulate the SBDX imaging geometry. User inputs include a voxelized phantom geometry, source x-ray energy spectrum, and scan maps which specify the relative x-ray output from each focal spot position. The voxelized kerma output tally was used to estimate dose. To confirm the ability of this package to simulate an ROI scan mode, simulated scan maps were generated to match those used in the experimental DAP study. The simulated phantom geometry was a volume of air measuring 20.3 cm × 20.3 cm × 70 cm with 1.59 mm isotropic voxels. The DAP was calculated from simulation by integration of the 2D kerma distribution K(i,j) at the plane corresponding to the position of the DAP meter,
| (3) |
where ΔxΔy is the in-plane voxel area and the plane contains m × n voxels. For comparison with experimental data, Monte Carlo DAP measurements vs ROI size were multiplied by a calibration factor equal to the ratio of the measured full-FOV DAP divided by the simulated full-FOV DAP.
Following the validation study, Monte Carlo simulation was used to estimate the DEC and full-FOV doses corresponding to the tracking study (Sec 2.2). The simulated patient was a voxelized anthropomorphic phantom derived from a CT of an adult chest phantom (CIRS Adult Male Atom Dosimetry Verification Phantom model 701, CIRS Inc., Norfolk, VA, USA) placed in a head-first, supine position with the entrance plane to the phantom positioned 15 cm below mechanical isocenter. The voxel dimensions were 0.781 mm × 0.781 mm × 0.625 mm. Dose-area-product, integral dose, and peak skin dose were calculated from the voxelized kerma output of each simulation. DAP was estimated by integrating kerma in the plane of air just posterior to the simulated patient’s back. PSD was defined as the maximum kerma in the same plane, after convolution with a 2D Gaussian kernel (sigma = 2.3 mm) for noise reduction. Integral dose was determined by integrating the kerma distribution over the entire phantom volume. These dosimetric quantities were determined for both a single frame of imaging and for the entire pullback imaging sequence.
3. RESULTS
3.1 Tracking performance
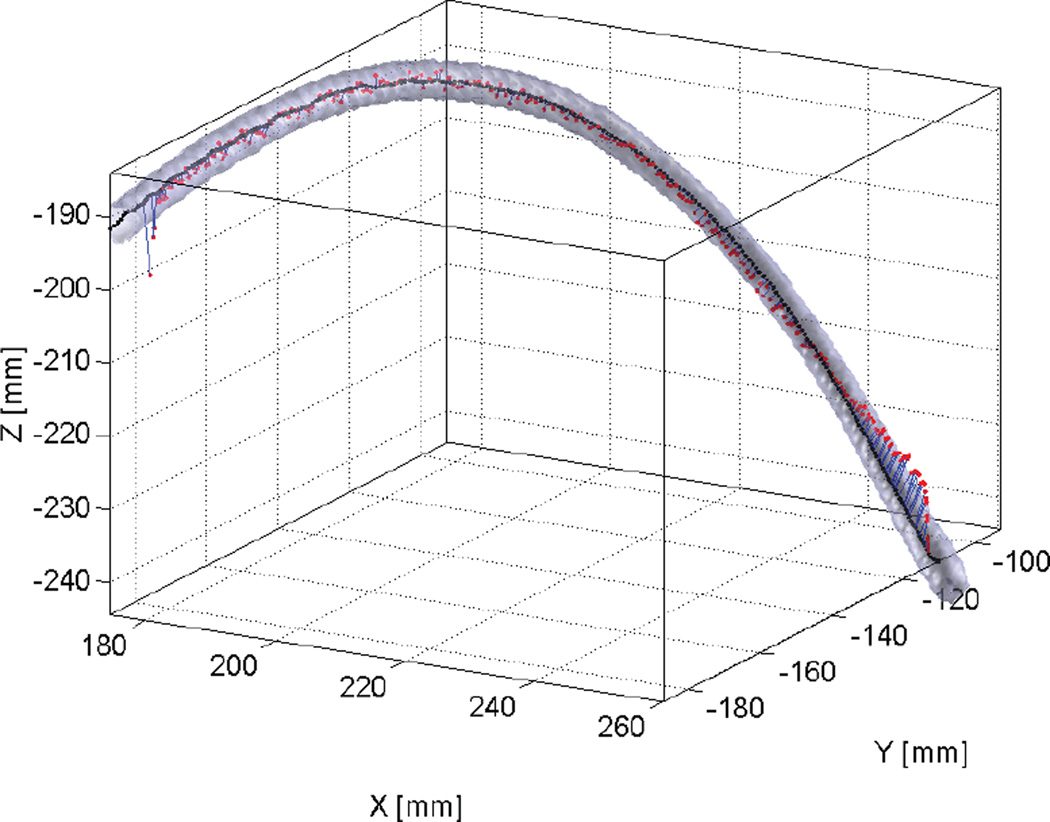
Image sequences were acquired for 10, 25, and 50 mm/s catheter pullback speeds. The image intensity was 499 ± 50 photons per reconstructed pixel at the sheath volume in the lung field and 290 ± 30 photons/pixel in the spine region. Figure 6 shows an example of SBDX 3D catheter tip tracking relative to the sheath volume. Tip positions found within the intensity gradient at the edge of the image (12% of the image width laterally and 6% vertically) were omitted from analysis, since accurate localization at the image edges is known to be challenging. The tracking analysis results are listed in Table 1. The root mean squared deviation between the DEC-based and full-FOV tip positions was 0.076 mm, 0.064 mm, and 0.075 mm, respectively, for the 10, 25, and 50 mm/s pullback sequences. For reference, the RMSD values were less than the dimension of one image pixel (0.161 mm at isocenter).
Figure 6.
SBDX tracking of catheter tip (red points), catheter sheath volume (light gray), and sheath centerline (black) for the 10 mm/s pullback sequence. The tip-to-centerline distances are shown with blue lines.
Table 1.
Comparison of catheter tracking performance and dose for DEC versus full field-of-view (FOV) imaging
| Sequence | Number of frames # |
DEC vs full- FOV tracking RMSD (mm) |
Average tracked tip-to- centerline distance |
Dose ratio, (DEC / full FOV)* | |||
|---|---|---|---|---|---|---|---|
| DEC (mm) |
full FOV (mm) |
DAP % |
PSD % |
Integral dose % |
|||
| 10 mm/s pullback | 154 | 0.076 | 0.77 | 0.75 | 7.4 | 33.6 | 7.2 |
| 25 mm/s pullback | 67 | 0.064 | 0.79 | 0.65 | 7.8 | 34.8 | 7.7 |
| 50 mm/s pullback | 38 | 0.075 | 0.68 | 0.63 | 7.6 | 34.0 | 7.5 |
| Single frame | 1 | - | - | - | 8.4 | 84.8 | 8.4 |
| Single frame, no phantom | 1 | - | - | - | 7.9 | 99.6 | 7.9 |
Monte Carlo based percentages have +/− 0.8% estimated uncertainty
In the full-FOV sequences, the mean 3D distance from the localized catheter tip to the sheath centerline was 0.75 mm, 0.65 mm, and 0.63 mm for 10, 25, and 50 mm/s pullback speeds. In the DEC sequences, the mean tip-to-centerline distances were 0.77 mm, 0.79 mm, and 0.68 mm for 10, 25, and 50 mm/s pullback speeds. For both scanning modes, 100% of the analyzed tip positions were contained within the sheath volume, and the standard deviation in tip-to-centerline distance averaged 0.39 mm. Note that deviations between the tracked tip and the sheath centerline may be due to actual deviations of the catheter tip from the sheath centerline, stochastic variation in tip-tracking ( 0.2 mm), and registration error between SBDX and CT coordinate systems (0.14 mm). The target registration error of the rigid SBDX-to-CT transformation was calculated by the methods in Ref. 21, using the root-mean-square fiducial localization error, the fiducial positions, and the points along the sheath centerline as target positions.
3.2 Dose reduction study
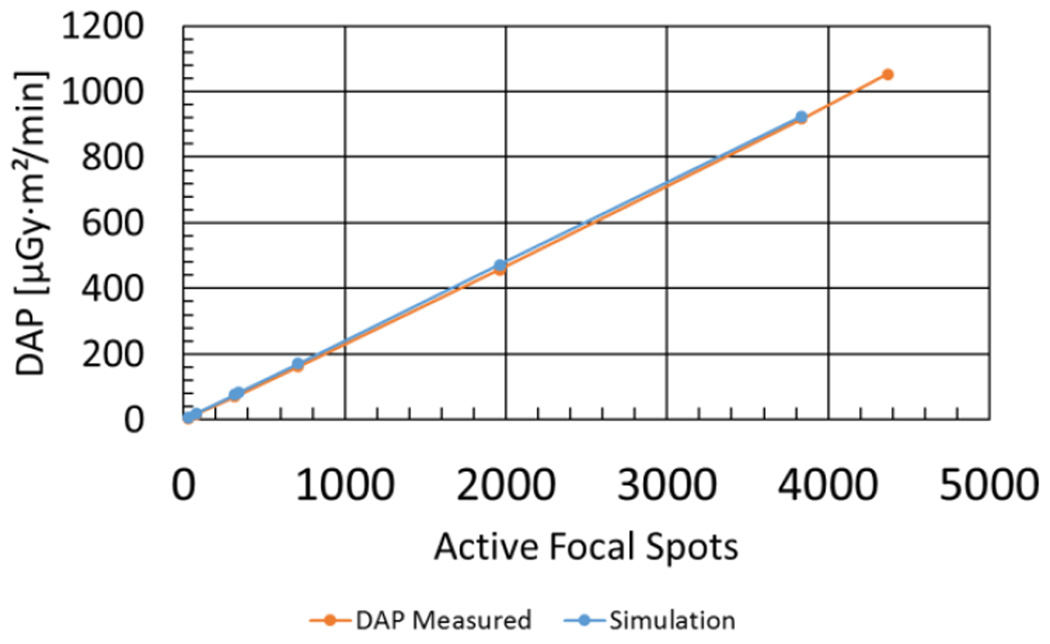
Measurements of DAP rate versus circular scan ROI size for 100 kV, 120 mApeak tube operation are presented in Figure 7. The DAP rate ranged from 5.32 μGy·m2/min using a 3 spot radius (29 active spots) to 916 μGy·m2/min using a 35 spot radius (3835 active spots). As expected, the measured DAP scaled linearly with the number of active focal spots in the scan (R2 = 0.9999). The Monte Carlo simulations of DAP versus ROI size also demonstrated a linear dependence on the number of active spots (R2 > 0.9999). From simulation it was calculated that a DEC scan with a 340-spot elliptical scan ROI would produce a DAP rate equal to 7.8% of the full-FOV DAP rate (4473 spots), which measured 1050 μGy·m2/min in this experiment.
Figure 7.
Measured DAP rate versus the number of active focal spots. The static electronic collimation feature on SBDX was used to create different scan ROIs. The highest value corresponds to a full FOV. The Monte Carlo simulated DAP rates for the same ROIs are shown for comparison (full-FOV case excluded).
Monte Carlo simulations of air kerma at the phantom entrance plane for a single frame of DEC and full-FOV imaging are shown in Figures 8A and 8C. Axial slices of the corresponding kerma distributions are shown in Figures 8B and 8D. The single-frame DEC DAP was 8.4% of the full-FOV DAP, and the integral kerma was 8.1% of the full-FOV value. DAP and integral dose reductions were consistent with the relative number of active focal spots used in DEC versus full-FOV scanning (7.6%). Peak skin dose from the single-frame DEC simulation was 84.8% (± 0.8%) of the full-FOV value. It was expected that the peak primary x-ray fluence in a single frame of imaging would be essentially unchanged for DEC mode, and, consistent with this expectation, when the simulated phantom was replaced with air the single-frame DEC PSD was 99.6% of the full-FOV value. The slight reduction in PSD observed with the phantom in place is consistent with a greater backscatter contribution to the entrance plane when using full-FOV mode.
Figure 8.
Air kerma distributions for a single frame of DEC and full-FOV imaging. (A) Entrance-plane air kerma for DEC imaging (B) Axial slice of kerma for DEC imaging (C & D) Entrance plane and axial slice of kerma for full-FOV imaging.
In simulations of a full image sequence of DEC catheter tracking, the DAP reduction was similar to the single frame case and the PSD reduction was greater than the single-frame case. Air kerma distributions at the phantom entrance plane for the 50 mm/s pullback image sequence are shown in Figure 9. Figure 10 shows an outline of the image ROIs corresponding to each frame of the DEC scan. The DEC-mode DAP was 7.4%, 7.8%, and 7.6% of the full-FOV DAP for the 10, 25, and 50 mm/s sequences respectively. DAP reduction was consistent with the reduction in active focal spots. The DEC-mode PSD was 33.6%, 34.8%, and 34.0% of the full-FOV PSD for the three sequences. In each sequence the peak skin dose was located at the top of the catheter trajectory, where motion was parallel to the long axis of the elliptical scan ROI. The additional PSD reduction observed for the full image sequence versus the single-frame case is due to the fact that in DEC mode, the dose scales with the number of x-ray fields overlapping a given point, whereas in full-FOV mode, dose scales with the number of image frames. When the catheter and scan ROI are moving, the number of overlapping x-ray fields is reduced and a PSD reduction is achieved.
Figure 9.
Air kerma at entrance plane for a full image sequence (50 mm/s pullback) using (A) DEC scanning, and (B) full-FOV imaging.
Figure 10.
Left: Full-FOV image and ROIs (blue) indicating path of the collimated x-ray field during DEC-mode catheter tracking. Right: Cumulative air kerma and air kerma rate from primary x-rays at a point along the catheter path (star, on left) in the entrance plane. In full-FOV mode the point is irradiated for the full image sequence. In DEC mode, the point is briefly irradiated as the collimated x-ray field passes by.
Reduction of PSD in the DEC image sequence is further demonstrated in Figure 10. Air kerma rate and cumulative air kerma were monitored in a plane 15 cm below isocenter, directly below a point on the catheter path (marked with a star in Figure 10). The primary x-ray kerma rates for DEC and full FOV scanning were nearly identical when the DEC x-ray field was overlapping the selected point. However this point was irradiated for a shorter period of time and for fewer frames in DEC mode, leading to a lower cumulative kerma at the end of the image sequence.
4. DISCUSSION
This study reports a novel dynamic electronic collimation method designed to minimize the total dose associated with SBDX 3D catheter tracking. This first investigation of DEC imaging indicates that the dose-area-product may be reduced by an order of magnitude when tracking a single object, with negligible change in tracking accuracy and precision. Reduction in peak skin dose is also possible. These reductions are in addition to the existing dose efficiency of the SBDX system.
In this study, the dose-area-product was reduced to 8% of the full field-of-view-value on average. Peak skin dose was 34% of the full FOV value for image sequences with a moving catheter, and 85% for a single frame of imaging. The degree of PSD reduction over an image sequence is dependent on the catheter trajectory and device size. A single object was tracked along a relatively simple trajectory in this phantom study. Further studies are needed to determine PSD reduction for more realistic catheter motions encountered in clinical procedures. These studies should also consider the use of multiple scan ROIs in clinical scenarios where there are multiple catheter devices and electrodes of interest.
The DEC algorithm requires accurate tracking and prediction of catheter positions so that the active scan region can be continuously updated to remain centered on the catheter. Catheter positions were accurately predicted in this initial study. However in clinical procedures, more complicated user-induced and cardio-respiratory motion can be expected. Future work will investigate the use of more sophisticated motion prediction algorithms and/or the use of larger scan ROIs to ensure robustness of the algorithm in clinically realistic scenarios.
Further dose optimization may be possible for the DEC algorithm. In particular, this study did not consider the tradeoff between dose, image SNR and tracking accuracy. It is possible that the desired tracking performance in a clinical procedure can be achieved with lower dose levels than were examined in this study.
5. CONCLUSIONS
Dynamic electronic collimation is a feasible method for reducing dose and dose-area-product during SBDX catheter tip tracking. For imaging an ablation catheter tip, dose-area-product may be reduced to 8% of full field-of-view levels with negligible change in SBDX catheter tracking performance. Peak skin dose from an imaging series can also be reduced for moving devices. This technique is expected to be an important component of a dose-optimized 3D tracking method for SBDX. Further investigation in clinically realistic imaging scenarios is needed to characterize and optimize the algorithm.
Acknowledgments
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL084022. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Carroll JD, Chen SJ, Kim MS, Hansgen AR, Neubauer A, Wink O. Structural heart disease interventions: Rapid clinical growth and challenges in image guidance. Medicamundi. 2008;52(2):43–50. [Google Scholar]
- 2.Palacios IF, Arzamendi D. Structural Heart Intervention. Beyond Transcatheter Valve Therapy. Rev. Española Cardiol. (English Ed. 2012;65(5):405–413. doi: 10.1016/j.recesp.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Joseph JP, Rajappan K. Radiofrequency ablation of cardiac arrhythmias: Past, present and future. Qjm. 2012;105(4):303–314. doi: 10.1093/qjmed/hcr189. [DOI] [PubMed] [Google Scholar]
- 4.Finlay MC, Hunter RJ, Baker V, Richmond L, Goromonzi F, Thomas G, Rajappan K, Duncan E, Tayebjee M, Dhinoja M, Sporton S, Earley MJ, Schilling RJ. A randomised comparison of Cartomerge vs. NavX fusion in the catheter ablation of atrial fibrillation: The CAVERN trial. J. Interv. Card. Electrophysiol. 2012;33(2):161–169. doi: 10.1007/s10840-011-9632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sra J, Narayan G, Krum D, Malloy A, Cooley R, Bhatia A, Dhala A, Blanck Z, Nangia V, Masood A. Computed tomography-fluoroscopy image integration-guided catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2007;18(4):409–414. doi: 10.1111/j.1540-8167.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 6.Issa Z, Miller JM, Douglas ZP. Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald’s Heart Disease. Saunders, Chapter 3. (2nd) 2012 [Google Scholar]
- 7.Fallavollita P. Is single-view fluoroscopy sufficient in guiding cardiac ablation procedures? Int. J. Biomed. Imaging 2010. 2010 doi: 10.1155/2010/631264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sporton SC, Earley MJ, Nathan AW, Schilling RJ. Electroanatomic versus fluoroscopic mapping for catheter ablation procedures: A prospective randomized study. J. Cardiovasc. Electrophysiol. 2004;15(3):310–315. doi: 10.1111/j.1540-8167.2004.03356.x. [DOI] [PubMed] [Google Scholar]
- 9.Speidel MA, Wilfley BP, Star-Lack JM, Heanue JA, Van Lysel MS. Scanning-beam digital x-ray (SBDX) technology for interventional and diagnostic cardiac angiography. Med. Phys. 2006;33(8):2714–2727. doi: 10.1118/1.2208736. [DOI] [PubMed] [Google Scholar]
- 10.Speidel MA, Wilfley BP, Star-Lack JM, Heanue JA, Betts TD, Van Lysel MS. Comparison of entrance exposure and signal-to-noise ratio between an SBDX prototype and a wide-beam cardiac angiographic system. Med. Phys. 2006;33(8):2728–2743. doi: 10.1118/1.2198198. [DOI] [PubMed] [Google Scholar]
- 11.Speidel MA, Tomkowiak MT, Raval AN, Dunkerley DAP, Slagowski JM, Khan P, Ku J, Funk T. Detector, collimator, and real-time reconstructor for a new scanning-beam digital x-ray (SBDX) prototype. SPIE Med. Imaging 9412. 2015 doi: 10.1117/12.2081716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speidel MA, Lowell AP, Heanue JA, Van Lysel MS. Frame-by-frame 3D catheter tracking methods for an inverse geometry cardiac interventional system. SPIE Med. Imaging. 2008 69131I – 12. [Google Scholar]
- 13.Speidel MA, Tomkowiak MT, Raval AN, Van Lysel MS. Three-dimensional tracking of cardiac catheters using an inverse geometry x-ray fluoroscopy system. Med. Phys. 2010;37(12):6377–6389. doi: 10.1118/1.3515463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speidel MA, Wilfley BP, Hsu A, Hristov D. Feasibility of low-dose single-view 3D fiducial tracking concurrent with external beam delivery. Med. Phys. 2012;39(4):2163. doi: 10.1118/1.3697529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burion S, Speidel MA, Funk T. A real-time regional adaptive exposure method for saving dose-area product in x-ray fluoroscopy. Med. Phys. 2013;40(5):051911. doi: 10.1118/1.4801908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobbins JT, Godfrey DJ. Digital x-ray tomosynthesis : current state of the art. Phys. Med. Biol. 2003;48:65–106. doi: 10.1088/0031-9155/48/19/r01. [DOI] [PubMed] [Google Scholar]
- 17.Dunkerley DAP, Tomkowiak MT, Slagowski JM, Mccabe BP, Speidel MA. Monte Carlo simulation of inverse geometry x-ray fluoroscopy using a modified MC-GPU framework. SPIE Med. Imaging. 2015;9412:12. doi: 10.1117/12.2081684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badal A, Badano A. Accelerating Monte Carlo simulations of photon transport in a voxelized geometry using a massively parallel graphics processing unit. Med. Phys. 2009;36(11):4878–4880. doi: 10.1118/1.3231824. [DOI] [PubMed] [Google Scholar]
- 19.Badal A, Badano A. Monte Carlo simulation of X-ray imaging using a graphics processing unit. 2009 IEEE Nucl. Sci. Symp. Conf. Rec. 2009;(3):4081–4084. [Google Scholar]
- 20.Salvat F, Fernández-Varea J, Sempau J. PENELOPE-2006: A code system for Monte Carlo simulation of electron and photon transport. Work. Proc. 2006;4(6222) [Google Scholar]
- 21.Fitzpatrick JM, West JB, Maurer CR. Predicting error in rigid-body point-based registration. IEEE Trans. Med. Imaging. 1998;17(5):694–702. doi: 10.1109/42.736021. [DOI] [PubMed] [Google Scholar]