Abstract
A volume-outcome relationship has been demonstrated in adult oncology. We investigated if an inverse association of volume and death exists in pediatric acute lymphoblastic leukemia (ALL) care. In assessing the association of volume and outcomes in a cohort of hospitalized pediatric ALL patients, we did not demonstrate an inverse relationship between volume and mortality or need for intensive care.
Background
There are few contemporary studies of volume-outcome relationships in pediatric oncology. Children with acute lymphoblastic leukemia (ALL) are treated at wide variety of hospitals. We investigated if inpatient hospital volume influences outcomes.
Objectives
To evaluate the relationship between inpatient pediatric and pediatric oncology volume and mortality and intensive care resources (ICU care). We hypothesized an inverse relationship between volume and these outcomes.
Research Design
Retrospective Cohort Study
Subjects
Patients 0-18 years in the Pediatric Health Information System (PHIS) or Perspective Premier Database (Premier) from 2009-2011 with ALL.
Exposures
Average inpatient pediatric and pediatric oncology volume.
Main outcome measures
The primary outcome was inpatient mortality; secondary outcome was need for intensive care resources.
Results
Three thousand three hundred and fifty patients from 75 hospitals were included. The inpatient mortality rate was 0.86% (95% CI [0.58%, 1.2%]). In the unadjusted analysis, mortality increased as pediatric oncology volume increased from low (0%) to high volume (1.3%) (p=0.009). The small number of deaths precluded multivariable analysis of this outcome. Pediatric and pediatric oncology volume was not associated with ICU care when controlling for potential confounders.
Conclusions
Induction mortality was low. We did not observe an inverse relationship between volume and mortality or ICU care. This suggests that in a modern treatment era, treatment at a low volume center may not be associated with increased mortality or ICU care in the first portion of therapy. This relationship should be evaluated in other oncology populations with higher mortality rates and with longer-term outcomes.
Keywords: Pediatrics, acute lymphoblastic leukemia, volume-outcome relationship
Introduction
Volume- outcome relationships associate the amount of care provided at a hospital level to the quality of care received by an individual patient.1 These relationships have been studied extensively in procedural fields, and an inverse relationship between volume and patient mortality found.2,3 Studies in adult oncology have suggested that higher volume centers have better outcomes for both surgical and non-surgical management.4-6 In pediatric oncology, a volume-outcome relationship has been less well examined. A systematic review undertaken to evaluate volume in pediatric oncology concluded that higher volumes are related to better survival.7 However, the generalizability of this finding may be limited by the heterogeneity of the cancer populations included and of the definitions of the volume exposures.7 More recent studies focusing on specific pediatric tumors including Wilms tumor and neuroblastoma did not find a relationship between volume and outcome.8,9 The potential impact of a volume-outcome association across different types of pediatric malignancy is needed as the findings may help either optimize the provision of care for these patients, or help to reinforce current practice.
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy and, therefore, represents an important group in which to investigate the volume-outcome paradigm. It is estimated that thirty-five hundred pediatric patients will be diagnosed with ALL in the United States in 2015.10 Fortunately, remarkable improvements in survival have occurred in recent decades, resulting in a 90% survival rate.11 The improvement in survival has been achieved via optimization of risk classification and intensification of chemotherapy. This has led to well established, but complex, treatment protocols that require comprehensive hospital services. Prior studies of the volume-outcome relationship among children with ALL did suggest an association, but this represented an earlier era of therapy.12 Currently, there are a number of established protocols for the management of ALL which may reduce variation in outcomes. However, recent data suggests that mortality among children with ALL continues to vary by insititution.13 It is possible that this variation in mortality may be related to hospital volume. A better understanding of the volume-outcome association across different types of pediatric malignancy is needed. We hypothesized that mortality and need for intensive care resources during the period of ALL induction chemotherapy would be inversely related to a hospital's volume of inpatient pediatric and pediatric oncology patients.
Patients and Methods
Overview/Study Design
A retrospective cohort study of patients with new onset ALL was performed with 2009-11 data from the Pediatric Health Information System (PHIS) and Perspective Data Warehouse (Premier). Forty-one hospitals from PHIS, and thirty-four hospitals from Premier were included. The institutional review board of the Children's Hospital of Philadelphia reviewed the study and determined exempt status.
Data Sources
The PHIS database has previously been described in detail.14,15 Briefly, PHIS includes administrative and billing data from 46 free standing, non competing, not for profit tertiary children's hospitals across the United States (US). PHIS data include demographics, dates of service, discharge disposition, International Classification of Diseases, 9th Revision (ICD-9) discharge diagnosis and procedure codes, and detailed billed resource utilization information.
Data are de-identified at the time of submission and subjected to reliability and validity checks. Data quality is assured through a combined effort between the Children's Hospital Association (CHA, Overland Park, KS), Truven Health Analytics, and participating hospitals. Perspective Data Warehouse maintained by Premier, Inc. (Premier, Charlotte, NC) is a large administrative database representative of a distinct consortium of US not for profit hospitals. Hospitals contributing to the Premier database include both academic and community hospitals. These institutions represent one sixth of all hospitalizations in the US. Importantly, while PHIS hospitals are dedicated children's hospitals, hospitals in Premier admit both children and adults. Data elements in Premier are similar to those found in PHIS and include demographic and hospitalization data, ICD-9 discharge diagnoses and procedures codes, pharmacy billing data, and charges.16
Study Cohort
A previously established and validated inpatient cohort of pediatric ALL patients from PHIS was extended to include the years under study.14 A parallel cohort was constructed from Premier using the same steps applied to assemble the PHIS cohort. In brief, all patients younger than 19 years of age with a discharge ICD-9 code for ALL (204.xx) were identified. Pharmacy billing records were reviewed manually for medications and timing consistent with known ALL induction chemotherapeutic regimens. We restricted the study population to 2009-2011 to use parallel years from each data source. Patients with an ICD-9 code for Trisomy 21 (758.0) were excluded due to potential for differential morbidity, mortality, and clinical practice in this population. There were two hospitals that contribute to both Premier and PHIS. Data for these hospitals from Premier were omitted to avoid duplicate patients (Supplemental Figure 1). Of note, only one patient had a discharge status that was unknown.
Outcome measures
The a priori primary outcome of interest was inpatient mortality in the first 60 days after initial admission containing induction chemotherapy. The secondary outcome was receipt of ICU care resources at any time after the first two admission days and up to day 60. Both outcomes were dichotomous. ICU care was defined using resource utilization data, rather than location. These definitions included clinical resources used, medication bills, and ICD-9 procedure codes separated into the following categories: cardiovascular, respiratory, hemodialysis, leukapheresis and neurologic. ICU care identification by these definitions of resource allocation has been previously described by our research group17. Patients receiving ICU care resources in the first two days of admission were excluded from the ICU care outcome analysis since the need for ICU care after the first two days might reflect severity of illness at presentation rather than quality of care provided.
Primary Independent Variables (Exposures)
Hospital pediatric volume was calculated as the average number of pediatric hospitalizations (1-19 years of age) per year and categorized as low (< 7000), medium (7000-11000) and high (>11000) based on the distribution of volume. Pediatric oncology volume was calculated in a similar manner and categorized as low (<650) medium (650-15000), and high (>1500). Pediatric oncology admissions were identified using Complex Chronic Condition codes for malignancy (CCC codes).18
Patient and Hospital Level Covariates
Patient level covariates included age, race, gender, requirement of ICU resources during the first two days of admission (for mortality analysis only), and number of days hospitalized during the 60-days from the index admission date. Hospital level factors included teaching status, defined as whether a hospital had any training programs, and census region. Proportion public insurance was collected as a percentage of total admissions to a hospital for which a patient had an indication of a public insurance. Hospital transfer rates were also recorded. Laboratory values were not available for analysis.
Statistical Analysis
Descriptive statistics were calculated using frequencies and medians with interquartile ranges. Bivariate analyses using Fisher's exact test, compared the distribution of the patient and hospital level covariates by volume categories. Mortality rates are presented as number of events per 100 patients. Nonparametric trend testing was performed to evaluate mortality rates across volume categories. Generalized estimating equations (GEE) accounting for clustering within institutions was performed to estimate the population-averaged association between patient volume and the probability of using ICU care resources.19 Clustering was evaluated due to the potential for unmeasured characteristics at the institution level that may be associated with hospital volume and the outcome of interest.20,21 For each model, adjusted coefficients were estimated by including all relevant patient and hospital level covariates. Manual selection of covariates for inclusion in the final models was based on a significance level of p<0.2. Marginal probabilities of each outcome at the different levels of volume were estimated from these models.19
Sensitivity analyses utilized trend analysis for proportions between volume categories. All analyses were performed using STATA 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.
Results
Patient and Hospital Characteristics
A cohort of 3350 pediatric patients with ALL from 75 hospitals was assembled from the PHIS and Premier data sources (Supplemental Figure #1). The distribution of hospital and patient characteristics by pediatric volume and pediatric oncology volume categories is represented in Tables 1 and 2. Table 1 represents distribution of patients by the hospital characteristics of teaching status and census region. The majority of patients (87%) were admitted to teaching institutions rather than community hospitals, and the south census region represented the highest proportion of patients (41%). The number of teaching hospitals varied by pediatric oncology volume. Median public insurance rates at each hospital did not differ by pediatric hospital volume but increased as pediatric oncology volume increased. Age and gender were similar across volume categories (Table 2). Race distributions differed by overall pediatric volume, but not by pediatric oncology volume. The median number of inpatient days in the 60 days from presentation varied across pediatric volume categories but was similar across pediatric oncology volume categories.
Table 1.
Hospital Characteristics (75 hospitals)
| Pediatric Volume | |||||
|---|---|---|---|---|---|
| Total | Low (<7000) (33 hospitals) | Medium (7000-11000) (34 hospitals) | High (>11000) (8 hospitals) | p value | |
| Teaching Hospital | 65 (87%) | 28 (85%) | 30 (88%) | 7 (87.5%) | 0.730 |
| Census Region: | 0.110 | ||||
| West | 16 (21%) | 5 (15%) | 11 (32%) | 0 (0%) | |
| Midwest | 16 (21%) | 6 (18%) | 8 (24%) | 2 (25%) | |
| South | 31 (41%) | 17 (51%) | 10 (29%) | 4 (50%) | |
| Northeast | 12 (16%) | 5 (15%) | 5 (15%) | 2 (25%) | |
| Proportion public insurance (Median +/− IQR) | 0.54 (0.46, 0.62) | 0.55 (0.45, 0.62) | 0.54 (0.46,0.63) | 0.53 (0.46, 0.59) | 0.74 |
| Pediatric Oncology Volume | |||||
|---|---|---|---|---|---|
| Total | Low (<650) (40 hospitals) | Medium (650-1500) (29 hospitals) | High (>1500) (6 hospitals) | p value | |
| Teaching Hospital | 65 (87%) | 31 (77.5%) | 28 (96.6%) | 6 (100%) | 0.018 |
| Census Region: | 0.020 | ||||
| West | 16 (21%) | 6 (15%) | 9 (31%) | 1 (17%) | |
| Midwest | 16 (21%) | 7 (17.5%) | 9 (31%) | 0 (0%) | |
| South | 31 (41%) | 19 (47.5%) | 9 (31%) | 3 (50%) | |
| Northeast | 12 (16%) | 8 (20%) | 2 (7%) | 2 (33%) | |
| Proportion public insurance (Median +/− IQR) | 0.54 (0.46, 0.62) | 0.51 (0.43, 0.55) | 0.55 (0.46, 0.63) | 0.59 (0.53, 0.73) | 0.001 |
Table 2.
Patient Characteristics (n=3350, 75 hospitals)
| Pediatric Volume | |||||
|---|---|---|---|---|---|
| Total | Low (<7000) (33 hospitals) N=658 | Medium (7000-11000) (34 hospitals) N=1982 | High (>11000) (8 hospitals) N= 710 | p value for differences | |
| Age (Years) | |||||
| Median (IQR) | 5 (3, 9.9) | 4.7 (3, 9.9) | 5 (3, 9.9) | 4.9 (3.1, 9.8) | 0.528 |
| Gender | |||||
| Male | 1873 (56%) | 383 (58%) | 1091 (55%) | 399 (56%) | 0.472 |
| Race | |||||
| White | 2292 (68%) | 487 (74%) | 1282 (65%) | 523 (74%) | <0.01* |
| Days in hospital | |||||
| Median (IQR) | 14 (10, 24) | 15 (10, 25) | 13 (9, 22) | 16.5 (10, 31) | <0.01* |
| Pediatric Oncology Volume | ||||
|---|---|---|---|---|
| Low (<650) (40 hospitals) N=695 | Medium (650-1500) (29 hospitals) N=1922 | High (>1500) (6 hospitals) N=690 | p value for differences | |
| Age (Years) | ||||
| Median (IQR) | 4.7 (3, 9.8) | 4.9 (3, 9.6) | 5.2 (3, 10.5) | 0.406 |
| Gender | ||||
| Male | 399 (57%) | 1095 (56%) | 379 (55%) | 0.781 |
| Race | ||||
| White | 472 (68%) | 1367 (70%) | 453 (66%) | 0.299 |
| Days in hospital | ||||
| Median (IQR) | 14(10, 24) | 14(10, 24) | 15 (9, 28) | 0.282 |
Mortality Outcome
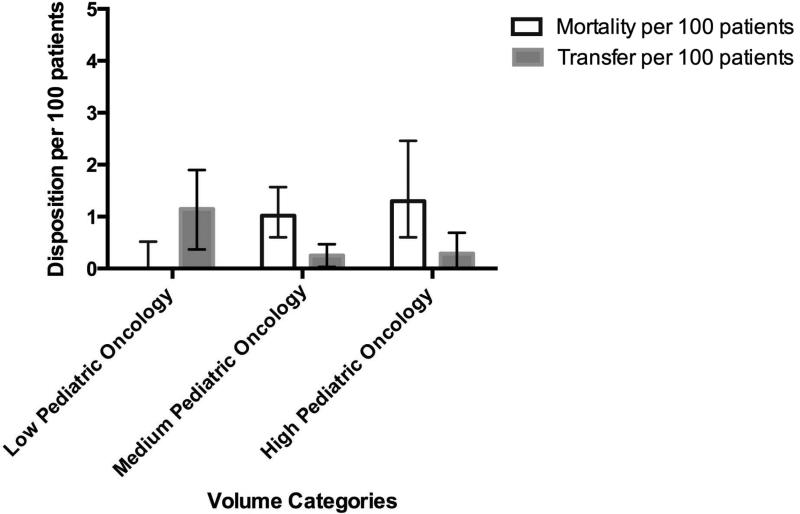
Twenty-nine patients died within 60 days of initial ALL diagnosis (0.86% of the cohort, 95% CI [0.58%, 1.2%]). The majority of deaths occurred within three weeks of the index admission [Supplemental Figure 2]. Although mortality rates appeared to be least in the lowest volume category, the mortality rates were not statistically significantly different by pediatric volume (0.30% for low, 1.01% for medium and 0.99% for high, p=0.184, Figure 1a). However, mortality rates increased in association with the increase in hospital pediatric oncology volume, from 0% in the low volume, 1.02% in the medium volume, and 1.30% in the high volume (p=0.009) [Figure 1b]. Due to the few deaths, we were unable to perform traditional adjusted analyses for potential confounders, both at the patient and hospital level. Similar to previous publications from our group, the raw number of deaths peaked during the third week of induction (Supplemental Figure 2).
Figure 1a.
Unadjusted Mortality and Transfer by Pediatric Volume with 95% CI
Figure 1b.
Unadjusted Mortality and Transfer by Pediatric Oncology Volume with 95% CI
Since multivariate modeling could not be performed due to small numbers of deaths, additional sensitivity analyses were performed to further assess the association of volume and mortality. These sensitivity analyses sought to address the following issues: potential for differential loss of follow up due to patient transfer out of a hospital, variation in patient complexity at time of diagnosis and outcome, and differential capture of exposure and outcome by data source. In total, 15/3350 patients transferred during the 60 day follow up period (0.45%, 95%CI [0.22%, 0.67%]). Although this rate is low, significantly higher transfer rates occurred at the low volume pediatric oncology hospitals (1.15%) versus medium and high volume pediatric oncology hospitals (0.25% and 0.29%, respectively) (p=0.016) [Figure 1b]. No statistically significant difference in transfer was seen in ordered groups by general pediatric volume, with rates of transfer at low volume institutions of 0.61%, 0.40% for medium, and 0.28% for high [Figure 1a]. Given this variation in transfer rate by pediatric oncology volume levels, we further explored the potential impact of transfers on the volume-outcome association. If half of the patients transferred were assumed to die, an increasing mortality trend over categories of pediatric oncology volume was apparent but not statistically significant (0.46% for low, 1.10% for medium and 1.30% for high, p=0.140). Alternatively, we assumed pessimistically that all patients requiring ICU care resources at the time of transfer died. With this assumption, the population averaged mortality rates for low, medium and high pediatric oncology volume hospitals were 0.4%, 1.10%, and 1.45%, respectively, (p=0.059 for non-parametric trend).
The second sensitivity analysis assessed whether potential variation across hospitals of patient complexity occurred at the time of diagnosis. Our hypothesis was that patients at higher volume centers may be more medically complex at baseline, and this complexity could increase induction mortality. We used previously established CCC codes defined by ICD-9 diagnosis codes to identify underlying chronic conditions other than malignancy18. There were 2657 patients from 74 hospitals who had no chronic care condition other than malignancy identified. In patients without an underlying chronic care condition, mortality rates by group ranged from 0% (97.5% CI [0, 0.5%]) in the low pediatric oncology volume group, 1% (95% CI [0.5%-1.5%]) in the medium volume and 1.12% (95% CI [0.2%-2%]) in the high volume (p=0.039). There was still no trend across the groups of pediatric volume and mortality, with mortality of 0.3% (95% CI [0%, 0.9%]) in the low, 1% (95% CI [0.5%, 1.5%]) in the medium and 0.7% (95% CI [0.02%, 1.4%]) in the high volume pediatric institutions.
Due to the potential for unobserved heterogeneity between PHIS and Premier data, a third sensitivity analysis was employed using each data source alone. The relationship of increasing mortality with increasing pediatric oncology volume was statistically non-significant, with mortality rates of 0% in low, 1.00% in medium, and 1.45% in high volume pediatric oncology centers (p=0.083). In PHIS pediatric centers, mortality also did not statistically significantly differ by pediatric volume category with 0.40% at low, 1.10% at medium and 1.20% at high volume pediatric centers (p=0.254). In an analysis limited to Premier, of 535 patients, only one patient died during the 60-day follow-up period. Despite this low mortality, six patients were transferred from low pediatric oncology volume institutions.
ICU care resources
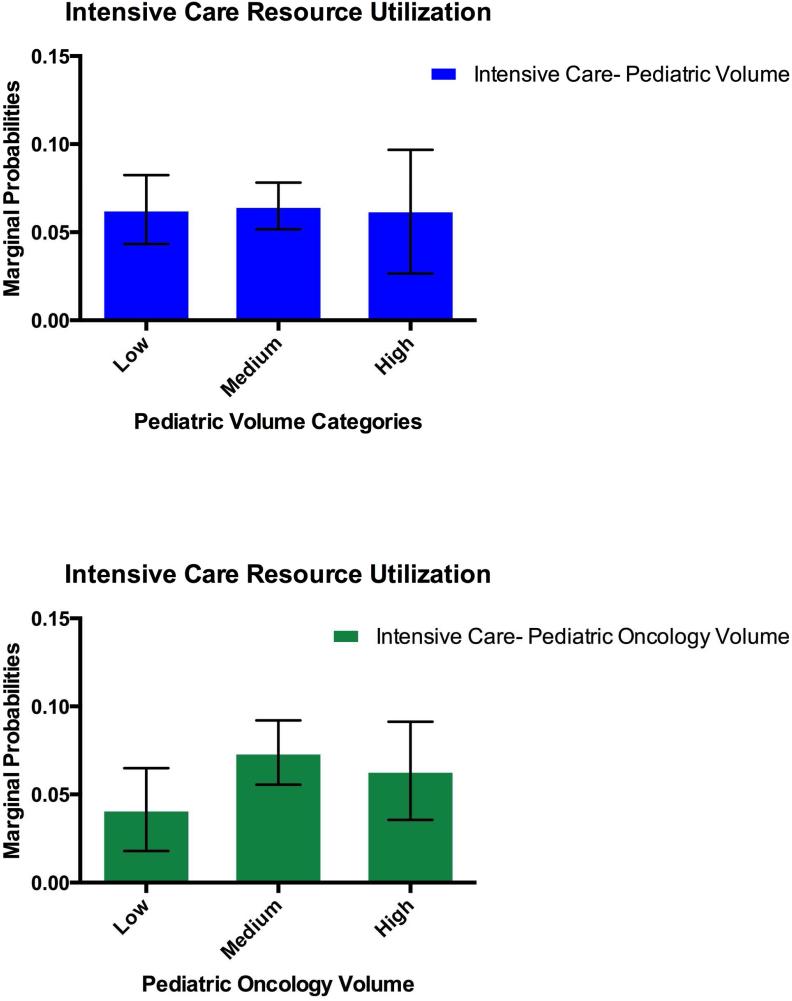
As a secondary outcome we investigated whether volume category was associated with the receipt of ICU resources after the first two hospital days. There were 24 patients who received ICU resources at the time of or within 2 days of the initial admission. To try to distinguish between potential severity of illness at presentation and worsening clinical status after admission, we excluded these 24 patients who required ICU resources in the first 2 days. In the remaining cohort, there were 279 out of 3326 (8.4% 95% CI: [7.4%, 9.3%]) patients who progressed to needing at least one day of ICU care resources. In unadjusted analysis, there was a higher rate of ICU utilization as pediatric oncology volume increased [Table 3]. After adjustment for number of days in hospital, age, resident teaching status, hospital proportion public insurance and data source, there was no difference between pediatric and pediatric oncology volume categories in the need for at least one day of ICU care resources in the first sixty days of index admission. The marginal probability estimates for need of ICU care was similar across pediatric volume categories and ranged from 0.04 to 0.063 in low pediatric oncology volume hospitals to high pediatric oncology volume hospitals. [Figure 2]. There was also no difference among the volume categories if patients who had required ICU level of care at admission were included.
Table 3.
Unadjusted and Adjusted Odds Ratios of ICU Utilization by Volume Category: Adjusted for Age, Length of Stay, Data Source and Teaching Status
| Volume Category | Unadjusted OR [95% CI] | Adjusted OR [95% CI] |
|---|---|---|
| Pediatric Volume | ||
| Low | Ref | Ref |
| Medium | 1.00 [0.72,1.38] | 1 [0.70, 1.45] |
| High | 1.51 [1.00, 2.28] | 1.00 [0.53, 1.87] |
| Pediatric Oncology Volume | ||
| Low | Ref | Ref |
| Medium | 1.63 [1.10, 2.41] | 1.61 [0.81, 3.21] |
| High | 1.93 [1.29, 2.91] | 1.44 [0.65, 3.21] |
Figure 2.
Marginal Probability of Intensive Care Resources by Volume Category: Adjusted for Age, Length of Stay, Data Source and Teaching Status
Discussion
In this large, nationally representative sample of US hospitals, we analyzed the relationship between hospital volume and mortality during the induction period for pediatric ALL. The overall induction mortality rate of 0.86% in this cohort is consistent with recent literature.11,13 We hypothesized that a hospital's volume of pediatric patients and pediatric oncology patients would be inversely associated with mortality. However, in unadjusted analyses of trend by ordered group, mortality rates did not significantly vary across the three pediatric volume categories, while mortality increased as pediatric oncology volume increased. Similar to previous publications from our group, the raw number of deaths peaked during the third week of induction (Supplemental Figure 2), a finding which likely coincides with the period of neutropenia.
These unadjusted findings may be confounded by the fact that hospitals with higher pediatric oncology volume are more likely to care for patients who are acutely ill at presentation and may be more likely to have an underlying complex chronic condition at presentation of their ALL. Alternatively, lower volume hospitals may be more likely to transfer critically ill ALL patients prior to death. Due to few deaths, we were unable to perform traditional adjusted multivariate analysis of the mortality outcome. Therefore, sensitivity analyses were performed to address patient transfers, presence of a chronic condition and data source. Each of these sensitivity analyses reduced the trend between mortality and pediatric oncology volume category but mortality was not found to be inversely associated with volume as we hypothesized and in certain circumstances increased volume remained associated with increased mortality. Likewise a hospital's pediatric volume and pediatric oncology volume was not inversely associated with the need for ICU care even after adjustments using in multivariate analysis. It is possible that residual confounding persisted to explain the lack of an inverse association. Alternatively, it remains possible that mortality is associated with increasing pediatric oncology volume. For instance, hospitals that have higher volumes may be overly taxed and not able to focus on the important details for each patient.
A few previous studies including pediatric ALL patients have investigated the volume- outcome association. Contrary to our findings, Stiller and Draper concluded that the reduction in mortality seen in their study occurred in parallel with increased numbers of patients treated with standardized protocols12. It is notable that the ALL patients from the prior study were cared for during an earlier decade of pediatric ALL treatment, a period in which chemotherapy regimens differ from current regimens.7,12 In a more recent ALL cohort, increases in pediatric ALL volume were not associated with improved survival.13 Similarly, in another common pediatric tumor with a low mortality rate, Wilm's tumor, investigation of pediatric patients found that survival was not associated with volume.8,9
A major strength of the current study is the use of multiple data sources to examine patients cared for at a broad range of institutions treating children with cancer. It is the first study in ALL to evaluate hospital pediatric and oncology volume and outcomes in the current era of treatment. However, our study needs to be interpreted in the context of its limitations. First, there is the possibility of “selective referral” in which more complicated patients are treated at higher volume centers or sicker patients at lower volume hospitals are transferred out of an institution. In either situation, the results would be biased towards a finding of worse outcomes at higher volume centers. We attempted to address these concerns in various sensitivity analyses, but this bias may have persisted. Identifying reasons for transfer was not possible from this source data but would be a topic for further study. Second, the lack of laboratory results precluded our ability to determine certain biologic level risk factors (e.g. initial white count, cytogenetics and minimal residual disease evaluation) known to influence mortality. Therefore, we were unable to adjust for variation in laboratories including presenting white blood cell counts across patients at each volume level. Third, the study hospitals may over-represent the south census region, and therefore, limit generalizability of our findings. Finally, and likely most importantly, the induction mortality events in this cohort were rare. While beneficial for patients and their families, this precluded the use of multivariable adjustment. When an adjusted analysis was performed with our secondary outcome, ICU utilization, the relationship between volume and outcome seen in unadjusted rates did not persist. While the very low inpatient mortality rate limits the ability to adjust for covariates, the low mortality rate itself across each grouping despite a large cohort argues that this malignancy can be managed appropriately across these volumes in induction. However, this result cannot be extrapolated to other pediatric malignancies.
Conclusions
In a large sample of United States hospitals with different volumes that care for children with ALL, we did not see higher induction mortality or utilization of ICU care resources at lower volume institutions, as we hypothesized. There was a suggestion of worse outcomes among higher pediatric oncology volumes for mortality, but not for need for use of ICU resources. Further investigation is needed to confirm our findings and to identify factors distinct from hospital volume that may explain variation in outcomes across institutions. Our findings are not generalizable beyond ALL induction or to other pediatric oncology diseases. For example, hospital volume may be more important in higher-risk conditions for which care is provided more frequently in the inpatient setting such as patients treated for acute myeloid leukemia or relapsed leukemia. Specifically assessing for a volume outcome association in these other patient populations is warranted.
Supplementary Material
Clinical practice points.
Patients with pediatric Acute Lymphoblastic Leukemia (ALL) are treated at many different institutions with varying pediatric and pediatric oncology volume.
Unlike in adult oncology cohorts, an inverse relationship between volume and mortality or volume and need for intensive care does not appear to exist for children with ALL during the initiation of treatment.
These data do not support preferential management of children with ALL in induction at high volume centers. Evaluation of the volume-outcome association for other pediatric malignancies is needed.
Acknowledgements
The authors thank A. Russell Localio JD, MA, MPH, MS, PhD for his thoughtful comments.
Funding for this work was received through: the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) T32 award in Pediatric Pharmacoepidemiology (T32 to Jennifer J Wilkes), the Agency for Healthcare Research and Quality (F32 award to Jennifer J Wilkes 1F32HS023419-01), and the Alex's Lemonade Stand Foundation (Young Investigator Award, Jennifer J Wilkes)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary data was presented at the 56th Annual American Society of Hematology Meeting and Exposition, December 7, 2014, San Francisco, California.
Disclosure of Potential Conflicts of Interest:
No reported conflicts of interest
Supplemental Figures Medical Care.pdf
References
- 1.Luft HS, Hunt SS, Maerki SC. The volume-outcome relationship: practice-makes-perfect or selective-referral patterns? Health Services Research. 1987;22(2):157. [PMC free article] [PubMed] [Google Scholar]
- 2.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137(6):511–520. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 3.McAteer JP, Lariviere CA, Drugas GT, Abdullah F, Oldham KT, Goldin AB. Influence of surgeon experience, hospital volume, and specialty designation on outcomes in pediatric surgery: a systematic review. JAMA Pediatrics. 2013;167(5):468–475. doi: 10.1001/jamapediatrics.2013.25. [DOI] [PubMed] [Google Scholar]
- 4.Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol. 2000;18(11):2327–2340. doi: 10.1200/JCO.2000.18.11.2327. [DOI] [PubMed] [Google Scholar]
- 5.Lüchtenborg M, Riaz SP, Coupland VH, et al. High Procedure Volume Is Strongly Associated With Improved Survival After Lung Cancer Surgery. J Clin Oncol. 2013;31(25):3141–3146. doi: 10.1200/JCO.2013.49.0219. [DOI] [PubMed] [Google Scholar]
- 6.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280(20):1747–1751. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 7.Knops RRG, van Dalen EC, Mulder RL, et al. The volume effect in paediatric oncology: a systematic review. Ann Oncol. 2013;24(7):1749–1753. doi: 10.1093/annonc/mds656. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez JC, Koniaris LG, Cheung MC, Byrne MM, Fischer AC, Sola JE. Cancer care in the pediatric surgical patient: A paradigm to abolish volume-outcome disparities in surgery. Surgery. 2009;145(1):76–85. doi: 10.1016/j.surg.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez JC, Cheung MC, Zhuge Y, Koniaris LG, Sola JE. Does Children's Oncology Group hospital membership improve survival for patients with neuroblastoma or Wilms tumor? Pediatr Blood Cancer. 2010;55(4):621–628. doi: 10.1002/pbc.22631. [DOI] [PubMed] [Google Scholar]
- 10.A SEER Cancer Statistics Review, 1975-2012. National Cancer Institute; 2015. [07/07/2015]. [Google Scholar]
- 11.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the Children's oncology group. J Clin Oncol. 2012;30(14):1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stiller CA, Draper GJ. Treatment centre size, entry to trials, and survival in acute lymphoblastic leukaemia. Archives of Disease in Childhood. 1989;64(5):657–661. doi: 10.1136/adc.64.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seif AE, Fisher BT, Li Y, et al. Patient and hospital factors associated with induction mortality in acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61(5):846–852. doi: 10.1002/pbc.24855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher BT, Harris T, Torp K, et al. Establishment of an 11-Year Cohort of 8733 Pediatric Patients Hospitalized at United States Free-standing Children's Hospitals With De Novo Acute Lymphoblastic Leukemia From Health Care Administrative Data. Medical care. 2012 doi: 10.1097/MLR.0b013e31824deff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher BT, Zaoutis TE, Leckerman KH, Localio R, Aplenc R. Risk factors for renal failure in pediatric patients with acute myeloid leukemia: A retrospective cohort study. Pediatr Blood Cancer. 2010;55(4):655–661. doi: 10.1002/pbc.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher BT, Lindenauer PK, Feudtner C. In-hospital Databases. In: Strom BL, Kimmel S, Hennessy S, editors. Pharmacoepidemiology, 5th edition. 5th edition John Wiley & Sons, Ltd; Chicester, UK: 2012. [Google Scholar]
- 17.Maude SL, Fitzgerald JC, Fisher BT, et al. Outcome of Pediatric Acute Myeloid Leukemia Patients Receiving Intensive Care in the United States. Pediatric Critical Care Medicine. 2013 doi: 10.1097/PCC.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feudtner CC, Christakis DAD, Connell FAF. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106(1 Pt 2):205–209. [PubMed] [Google Scholar]
- 19.Korn E, Graubard B. Analysis of Health Surveys. John Wiley & Sons, Inc; New York: 1999. pp. 127–140. [Google Scholar]
- 20.Panageas KS. The Effect of Clustering of Outcomes on the Association of Procedure Volume and Surgical Outcomes. Annals of Internal Medicine. 2003;139(8):658–665. doi: 10.7326/0003-4819-139-8-200310210-00009. [DOI] [PubMed] [Google Scholar]
- 21.Panageas KS, Schrag D, Localio AR, Venkatraman ES, Begg CB. Properties of analysis methods that account for clustering in volume-outcome studies when the primary predictor is cluster size. Statistics in Medicine. 2007;26(9):2017–2035. doi: 10.1002/sim.2657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.