Abstract
Non-Hodgkin lymphoma of the orbit and ocular adnexa is the most common primary orbital malignancy. Treatments for low- (extra-nodal marginal zone and follicular lymphomas) and high-grade (diffuse large B-cell lymphoma) are associated with local and vision-threatening toxicities. High-grade lymphomas relapse frequently and exhibit poor survival rates. Despite advances in genomic profiling and precision-medicine, orbital and ocular adnexal lymphomas remain poorly characterized molecularly. We performed targeted next-generation sequencing profiling of 38 formalin-fixed, paraffin-embedded, orbital and ocular adnexal lymphomas obtained from a single-center using a panel targeting near-term, clinically-relevant genes. Potentially actionable mutations and copy-number alterations were prioritized based on gain- and loss-of function analyses, catalogued approved and investigational therapies. Of 36 informative samples, including marginal zone lymphomas (n=20), follicular lymphomas (n=9), and diffuse large B-cell lymphomas (n=7), 53% harbored a prioritized alteration (median=1, range 0–5/sample). MYD88 was the most frequently altered gene in our cohort, with potentially clinically-relevant hot-spot gain-of-function mutations identified in 71% of diffuse large B-cell and 25% of marginal zone lymphomas. Prioritized alterations in epigenetic modulators were common and included gain-of-function EZH2 and loss-of-function ARID1A mutations (14% of diffuse large B-cell lymphomas and 22% of follicular lymphomas contained alterations in each of these two genes). Single prioritized alterations were also identified in the histone methyltransferases KMT2B (follicular lymphoma) and KMT3B (diffuse large B-cell lymphoma). Loss-of-function mutations and copy-number alterations in the tumor suppressors TP53 (diffuse large B-cell and follicular lymphoma), CDKN2A (all subtypes), PTEN (diffuse large B-cell lymphoma), ATM (diffuse large B-cell lymphoma) and NF1 (diffuse large B-cell lymphoma); and gain-of-function mutations in the oncogenes HRAS (follicular lymphoma) and NRAS (diffuse large B-cell lymphoma) were also observed. Together, our study demonstrates that next-generation sequencing can be used to profile routine formalin-fixed paraffin-embedded, orbital and ocular adnexal lymphomas for identification of somatic-driving alterations and nomination of potential therapeutic strategies.
Keywords: Orbital and ocular adnexal lymphoma, extranodal marginal zone lymphoma, follicular lymphoma, diffuse large B-cell lymphoma, MYD88, EZH2, ARID1A, next generation sequencing, precision medicine, targeted therapy
INTRODUCTION
Non-Hodgkin lymphomas (NHLs) of the orbital and ocular adnexa represent 10–15% of all tumors present in the orbit, eyelids, conjunctiva, and lacrimal apparatus (i.e. the “adnexa”) and are the most common primary orbital cancer in adults(1, 2). Alarmingly, orbital and ocular adnexal lymphomas have shown an increasing incidence in some populations (~6% yearly increase in white Americans from an analysis of Surveillance, Epidemiology, and End Results [SEER] data)(3).
Orbital and ocular adnexal lymphomas are B-cell proliferations that include low-grade tumors such as extra-nodal marginal zone (also known as mucosa-associated lymphoid tissue lymphoma [MALT]) and follicular lymphoma, as well as high-grade tumors such as mantle cell lymphoma and diffuse large B-cell lymphomas. These subtypes account for ~50%, 10–20%, 5–10% and 5–10% of orbital and ocular adnexal lymphomas, respectively (4, 5).
The molecular etiology of orbital and ocular adnexal lymphoma heterogeneous subtypes is diverse, and not well understood. MZLs are frequently associated with translocations: API2-MALT1, IGH-MALT1, IGH-BCL10, IGH-FOXP1, as well as trisomy of chromosome 3 and 18(6). Such aberrations activate pathways that converge on the nuclear factor-B (NF-κB) complex, leading to orbital and ocular adnexal lymphomagenesis (6). Molecular studies of orbital and ocular adnexal follicular and diffuse large B-cell lymphomas are few, owing to the rarity of primary tumors of these subtypes in these locations. Thus molecular alterations in these subtypes are presumed to follow systemic FL and DLBCL pathogenetic patterns. In systemic diffuse large B-cell lymphomas, BCL2/IGH and MYC rearrangements, lesions associated with NFκB activation (TNFAIP3, CARD11, CD79B, MYD88) and epigenetic alterations (CREBBP, EP300, EZH2) have been described, but there are also many altered genes that encode for structural (e.g. PCLO) and signaling proteins (P2RY8 and TNFRSF14) (7, 8). Systemic follicular lymphomas shares some lesions with diffuse large B-cell lymphomas, including IGH and BCL2 rearrangements(9, 10), alterations in EZH2 and TNFRSF14(11), but also exhibits distinct changes, including somatic mutations that lead to loss of function of EPHA2 ephrin receptor(12) and acquisition of N-glycosylation motifs in immunoglobulin genes(13).
While the mainstay of treatment for most primary orbital and ocular adnexal lymphomas (low-grade), external beam radiation therapy, results in high rates of local control, the rate of distant relapse can reach 40%(14, 15), and local toxicities such as keratitis, dry eye, cataract, neovascular glaucoma, optic nerve neuropathy and retinopathy can lead to vision loss or blindness(16, 17). Survival rates vary with orbital and ocular adnexal lymphoma subtype. Generally, marginal zone and most follicular lymphomas (low-grade tumors) of the orbital and ocular adnexal region are treated with external beam radiation therapy, and 10-year disease-specific survival rates, depending on stage, range from 94–98%.(18, 19) In contrast, diffuse large B-cell lymphomas and some follicular lymphomas of the orbital and ocular adnexal region are tumors that often lead to, or are a manifestation of, disseminated systemic disease, and are treated with a combination of external beam radiation therapy, surgery, and/or systemic chemotherapy. These diffuse large B-cell lymphomas relapse frequently, and have 10-year disease-specific survival rates ranging from 18–55%.(20)
In summary, the genomic drivers of orbital and ocular adnexal lymphomas, especially those that underlie orbital and ocular adnexal follicular and diffuse large B-cell lymphomagenesis remain poorly understood, due in part, to the rarity of primary tumors at these locations. While low-grade tumors like marginal zone and follicular lymphomas are frequently curable by external beam radiation therapy, they are often associated with vision-threatening local toxicities and can relapse at distant locations. Treatments such as external beam radiation therapy, surgery, and systemic chemotherapy for high-grade orbital and ocular adnexal lymphomas are associated with vision threatening and systemic toxicities. Moreover, diffuse large B-cell lymphomas frequently relapse and are often deadly. Taken together, these data demonstrate an unmet need to develop novel treatment strategies for both low- and high-grade orbital and ocular adnexal lymphomas.
The development of novel therapies for orbital and ocular adnexal lymphomas has been hampered by the limited nature of studies exploring genomic drivers of the disease. Although cytogenetic, copy number and transcript, and immunohistochemistry based profiling studies have been reported (21–25), these represent limited cohorts mainly assessing marginal zone lymphoma, the orbital and ocular adnexal lymphoma subtype most frequently associated with good prognosis. Importantly, although next generation sequencing studies have defined genetic alterations and potential therapeutic targets in non-orbital lymphomas, such approaches have not been applied to orbital and ocular adnexal lymphomas. Hence, in order to define potentially actionable somatic mutations and copy number alterations in orbital and ocular adnexal lymphomas, we performed next generation sequencing on 38 formalin-fixed paraffin embedded orbital and ocular adnexal lymphomas, including marginal zone, follicular, and diffuse large B-cell histologic subtypes. To do this, we subjected our set of orbital and ocular adnexal lymphomas to an next generation sequencing-based panel enriched for alterations in solid tumors and lymphomas, a modified version of which is being used in the National Cancer Institute-Molecular Analysis for Therapy Choice (NCI-MATCH) Trial(26), a new trial that has matched patients with advanced lymphomas and solid tumors, which have not responded (or never responded), to investigational therapeutics based on their prioritized mutation profile rather than site of tumor origin.(27)
MATERIALS AND METHODS
Case Selection
The study was carried out at the highest ethical standards and with the approval of the University of Michigan Institutional Review Board approval. We identified a cohort of 38 archived, routine clinical formalin-fixed paraffin-embedded orbital lymphoma specimens from the University of Michigan Department of Pathology Tissue Archive for next generation sequencing. Clinicopathological information for each case was obtained from the clinical archive. Hematoxylin and eosin (H&E) stained slides and immunostains (where available) were reviewed for all cases by board-certified Anatomic Pathologists (A.S.M. and S.A.T.) to ensure sufficient tumor content (70–90%).
Targeted Next Generation Sequencing (NGS)
Targeted next generation sequencing was performed essentially as previously described(26, 28, 29). For each specimen, 3–7 × 10 µm formalin-fixed paraffin-embedded sections were cut from a single representative block per case, using macrodissection with a scalpel as needed to enrich for tumor content. DNA was isolated using the Qiagen Allprep formalin-fixed paraffin-embedded DNA/RNA kit (Qiagen, Valencia, CA) and quantified as previously described. Targeted, multiplexed PCR based next generation sequencing (NGS) was performed by Ion Torrent NGS using the DNA component of a beta version of the Oncomine Comprehensive Assay (OCP), a custom panel comprised of 3,435 amplicons targeting 126 genes. Genes included in this panel were selected based on pan-solid tumor next generation sequencing and copy number profiling data analysis that prioritized somatic, recurrently altered oncogenes, tumors suppressors, genes present in high level copy gains/losses and known/investigational therapeutic targets(26). Library preparation with barcode incorporation, template preparation and sequencing using the Ion Torrent Proton sequencer were performed according to the manufacturer’s instructions. Data analysis was performed using Torrent Suite 4.0.2, with alignment by TMAP using default parameters, and variant calling using the Torrent Variant Caller plugin (version 4.0-r76860) using default low-stringency somatic variant settings. Variant annotation filtering and prioritization was performed essentially as described using validated in house pipelines(26, 28–31). Briefly, called variants were filtered to remove synonymous or non-coding variants, those with flow corrected read depths (FDP) less than 20, flow corrected variant allele containing reads (FAO) less than 6, variant allele frequencies (FAO/FDP) less than 0.10, extreme skewing of forward/reverse flow corrected reads calling the variant (FSAF/FSAR <0.2 or >5), or indels within homopolymer runs >4. Called variants were filtered using a panel-specific, in house blacklist. Variants with allele frequencies >0.5% in Exome Sequencing Project 6500 (ESP6500) or the 1000 Genomes project, and those reported in ESP6500 or 1000 genomes with observed variant fractions between 0.40 and 0.60 or > 0.9 were considered germ line variants unless occurring at a known hot-spot variant. Variants located at the last mapped base (or outside) of amplicon target regions, variants with the majority of supporting reads harboring additional mismatches or indels (likely sequencing error), those in repeat–rich regions (likely mapping artifacts), and variants occurring exclusively in one amplicon if overlapping amplicons cover the variant were excluded. High confidence somatic variants passing the above criteria were then visually confirmed in Integrative Genomics Viewer (https://www.broadinstitute.org/igv/). We have previously confirmed that these filtering criteria identify prioritized high-confidence somatic variants that pass Sanger sequencing validation with >95% accuracy (28–32). Copy number analysis from total amplicon read counts provided by the Coverage Analysis Plug-in (v4.0-r77897) was performed essentially as described using a validated approach(26, 28, 29, 33). Genes with a log2 copy number estimate of <−1 or >0.6 were considered to have high level loss or gain, respectively.
To prioritize potential driving alterations, we utilized Oncomine software tools (powertools.oncomine.com) to annotate called variants, which use pan-cancer next generation sequencing data to identify genes as oncogenes or tumor suppressors, based on overrepresentation of hot-spot or deleterious mutations, respectively. Variants in oncogenes are then considered gain-of-function if at a hot-spot and variants in tumor suppressors are considered loss-of-function if deleterious or at a hot-spot(26, 28, 34). Likewise, high-level copy number alterations were prioritized if they were concordant with the minimal common region analysis used to design the Oncomine Comprehensive Assay (e.g. high level copy number gain in a gene prioritized as amplified/deleted by minimal common region analysis.
Immunohistochemistry
Immunohistochemistry was performed on the DAKO Autostainer (DAKO, Carpinteria, CA) using DAKO Envision+ and diaminobenzadine as the chromogen. Sections of de-paraffinized Orbital and ocular adnexal lymphomas were labeled with ARID1A (mouse monoclonal, clone PSG3, 1:250, Santa Cruz Biotechnology, Dallas TX, #sc-32761), EZH2 (mouse monoclonal, clone 11, 1:100, BD Biosciences, San Jose, CA, #612666), or KMT3B/NSD1 (rabbit polyclonal, 1:250, EMD Millipore, Billerica, MA, #ABE1009) for 60 minutes at ambient temperature. Microwave epitope retrieval in 10 mM Tris/HCl, pH 9 containing 1 mM EDTA was used prior to staining. Appropriate negative (no primary antibody) and positive controls were stained in parallel with each set of tumors studied. Immunostained slides were examined by light microscopy by two pathologists (A.S.M. and S.A.T.). Only staining of the nucleus was marked as positive expression. The staining was scored semiquantitatively and recorded based on percent nuclei staining (0 = negative, 1 = 1 – 25% immunoreactive cells, 2 = 26% – 50% immunoreactive cells, 3 = 51% – 75% immunoreactive cells, 4 = 76% – 100% immunoreactive cells) and intensity of staining (0 = negative, 1 = weak, 2 = moderate, 3 = strong). Corresponding sections were also H&E stained.
Statistics
Comparisons of the number of mutations and copy number alterations per sample by lymphoma subtype were performed using the Kruskal-Wallis test with post-hoc pairwise comparison of subgroups using R 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
We performed targeted next generation sequencing on a cohort of 38 formalin-fixed, paraffin-embedded orbital and ocular adnexal lymphomas comprised of 8 diffuse large B-cell, 9 follicular and 21 marginal zone lymphomas; representative photomicrographs are shown in Figure 1A, B and clinical characteristics of all informative patients (see below) are presented in Figure 1C. We isolated an average of 5.5 µg DNA per case (range 0.1 – 24.1 µg) and all samples had >70% estimated tumor content by H&E evaluation after macrodissection (as needed). Next generation sequencing was performed using the DNA component of a beta version of the Oncomine Comprehensive Assay (version 1), a custom panel comprised of 3,435 amplicons targeting 126 genes and Ion Torrent based sequencing on the Proton machine, a modified version of which is being used in the NCI-MATCH trial.(27) Targeted genes were selected based on pan-solid tumor next generation sequencing and copy number profiling data analysis to prioritize somatic, recurrently altered oncogenes, tumors suppressors and genes present in high level copy number alteration, filtered by available or investigational therapeutic targets.(26)
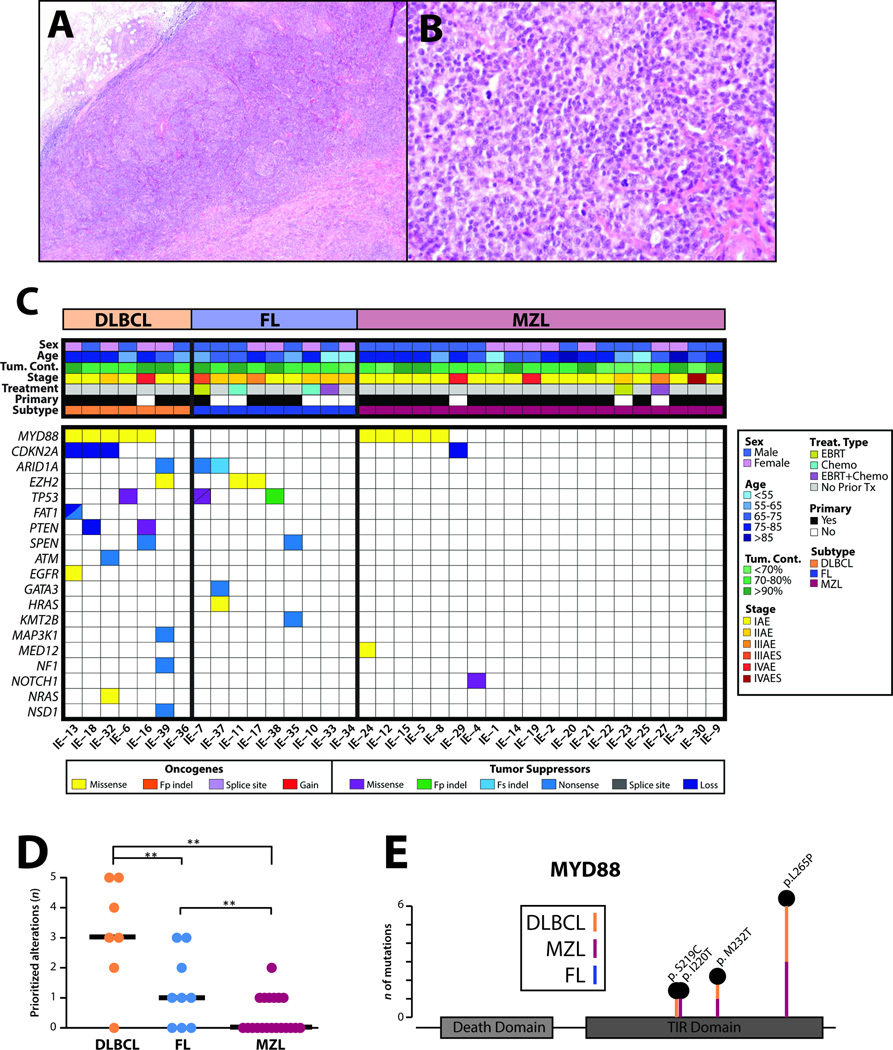
Figure 1. Targeted next generation sequencing of routine formalin fixed paraffin embedded orbital and ocular adnexal lymphomas identifies recurrent informative/potentially actionable alterations.
A&B. Representative histology by hematoxylin and eosin (H&E) staining of a formalin-fixed, paraffin-embedded lymphoma sample (IE-38, a follicular lymphoma [FL]). Original magnification 4× (A) and 40× (B). C. Integrative heatmap of prioritized, informative/potentially actionable mutations and copy number alterations across the 36 informative orbital and ocular adnexal lymphomas. Clinicopathologic information, including patient sex, age, estimated tumor content and subtype (diffuse large B-cell lymphoma [DLBCL], FL and marginal zone lymphoma [MZL]), are indicated in the header according to the legend on the right. All prioritized, high confidence, gain-of-function or loss-of-function somatic mutations in oncogenes and tumor suppressors, respectively, as well as high-level copy number alterations are shown for each case. Specific alteration types are indicated according to the legend (Fp and Fs indel, frame-preserving and frame-shift indels, respectively; Loss, copy number loss; Gain, copy number gain). Slashed boxes indicate two alterations. D. Comparison of the number (n) of prioritized alterations per sample (mutations and copy number alterations) by subtype. The median number of alterations per type is shown by thick black bars. **p<0.01 from post-hoc subgroup comparison of Kruskal–Wallis test. E. Stick plot of recurrent domain mutations. Schematic representation of the location and subtype of MYD88 gain-of-function mutations identified in 10 of 36 orbital and ocular adnexal lymphomas. The number of mutations (n) at each residue is indicated by the stick length according to the scale (left).
Of the 38 samples assayed, one marginal zone lymphoma (IE-26, primary to orbit/ocular adnexa, Ann Arbor stage IIAE, with no previous treatment) and one diffuse large B-cell lymphoma (IE-31, non-primary to orbit/ocular adnexa, Ann Arbor stage IAE, with previous chemotherapy and EBRT) sample, yielded low quality sequencing data due to poor genomic DNA quality and were excluded from all subsequent analyses (Figure 1C). Most of the informative 36 lymphomas were primary to the orbit or ocular adnexa, not previously treated, and of early stage. Twenty-seven of 36 tumors were primary to the orbit or ocular adnexa, including 6 of 7 diffuse large B-cell lymphomas, 4 of 9 follicular lymphomas, and 17 of 20 marginal zone lymphomas. Most were not previously treated: none of the diffuse large B-cell lymphomas, 5 of the follicular lymphomas, and 18 of the marginal zone lymphomas. Most of the marginal zone lymphomas (15) and diffuse large B-cell lymphomas (6) were Ann Arbor stage IAE, while most of the follicular lymphomas were IAE (2) or IIAE (5) (Figure 1C). Primary follicular and diffuse large B-cell lymphomas to the orbit and ocular adnexa have rarely been profiled.
Using the Hans algorithm for germinal center B-cell phenotyping, we found that 6 of the 8 diffuse large B-cell lymphomas were of the activated B-cell like phenotype, and the remaining 2 were of the germinal center B-cell phenotype (35). One of the germinal center B-cell positive cases was unable to be sequenced due to poor DNA quality (IE-31).
Across the 36 informative samples, next generation sequencing generated an average of 1,871,816 mapped reads yielding 545× targeted base coverage (Table S1). We identified a total of 41 high-confidence, prioritized somatic alterations (median n=1, range n=0–5 per sample) comprised of non-synonymous point mutations (n=33), short insertions/deletions (indels; n=2) and copy number variations (n=6). All clinical data (including staging, primary site, and previous treatment), prioritized somatic mutations and high-level copy number alterations for each case are shown in an integrative heat map (Figure 1C) and given in Table S2. Prioritized alterations were present in 19 of 36 samples (53%). Diffuse large B-cell lymphomas harbored the majority of total prioritized alterations (6 of 7 [86%] with prioritized alterations; median prioritized alterations n=3, range n=0–5). Prioritized alterations were identified in 6 of 9 (67%) follicular lymphomas (median prioritized alterations n=1, range n=0–3) and 7 of 20 (35%) marginal zone lymphomas (median prioritized alterations n=0, range n=0–2). The number of prioritized alterations was significantly different between the lymphoma subtypes (Kruskal-Wallis test, p=0.0014), as shown in Figure 1D.
Across the 36 orbital and ocular adnexal lymphomas, 10 (28%) harbored prioritized gain-of-function mutations in the Toll/interleukin-1 receptor (TIR) domain of myeloid differentiation factor 88 (MYD88), making it the most frequently altered gene (by prioritized alterations) in our cohort. MYD88 is an adaptor protein that binds to the intracellular domains of Toll-like receptors (TLRs) as well as interleukin 1 receptor on B cells and macrophages, which stimulates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway, and is involved in innate immunity.(36) The highest proportion of MYD88 hot-spot gain-of-function mutations was present in diffuse large B-cell lymphomas (5/7, 71%), including three p.L265P, one p.M232T and one p.S219C mutations. Notably, of the 5 MYD88 mutation-positive diffuse large B-cell lymphomas, all were of the activated B-cell like phenotype. The former represents the most frequently altered MYD88 hot-spot across human cancers, while the latter two mutations occurred at minor hot-spots. The remaining MYD88 (TIR domain) mutations occurred in 5 of 20 (25%) marginal zone lymphoma samples. Of note, MYD88 mutations comprise the majority of total prioritized alterations (5 of 8, 63%) in our marginal zone lymphoma samples, with three p.L265P mutations and two other TIR domain hot-spot mutations (one each of p.M232T and p.I220T). All gain-of-function MYD88 mutations across the cohort are shown in Figure 1E. Taken together, our data demonstrate that MYD88 TIR domain mutations are common in the diffuse large B-cell and marginal zone lymphoma subtypes of orbital and ocular adnexal lymphomas.
Recurrent somatic alterations in histone/chromatin remodeling proteins are frequent in extranodal NHLs outside of the orbit and ocular adnexa. Specifically, 22% of diffuse large B-cell lymphomas and 7–22% of follicular lymphomas outside of the orbit and ocular adnexa carry gain-of-function missense mutations (at the p.Y646 hot-spot) in the methyltransferase SET domain of the histone H3 lysine 27 trimethylase enhancer of zeste homolog 2 (EZH2)(37–39). Herein, we identified 2 of 9 follicular lymphomas (22%) and 1 of 7 diffuse large B-cell lymphomas (14%) with EZH2 p.Y646 mutations (Figure 1C). The single EZH2 mutant diffuse large B-cell lymphoma case in our cohort was of the germinal center B-cell phenotype. We also identified loss-of-function mutations in tumor suppressor AT-rich interactive domain-containing protein 1A (ARID1A), an epigenetic regulator that is an ATPase dependent SWI/SNF nucleosome remodeling complex subunit. We identified a prioritized ARID1A nonsense mutation (p.Q1363X) in 1 of 7 (14%) diffuse large B-cell lymphomas; two of 9 (22%) follicular lymphomas harbored loss-of-function ARID1A mutations (p.Q482X and p.727_730del). Interestingly, the ARID1A mutated diffuse large B-cell lymphoma sample (IE-39; p.Q1363X) also contained one of the aforementioned activating EZH2 Y646 mutations (p.Y646S) (Figure 1C). Lastly, we identified prioritized mutations in other chromatin modifying genes, including loss-of-function mutations in KMT2B (also known as MLL2 or MLL4; p.Q2495X) and KMT3B (also known as NSD1; p.Q1532X) in one case, each, of follicular lymphoma and diffuse large B-cell lymphoma, respectively (Figure 1C).
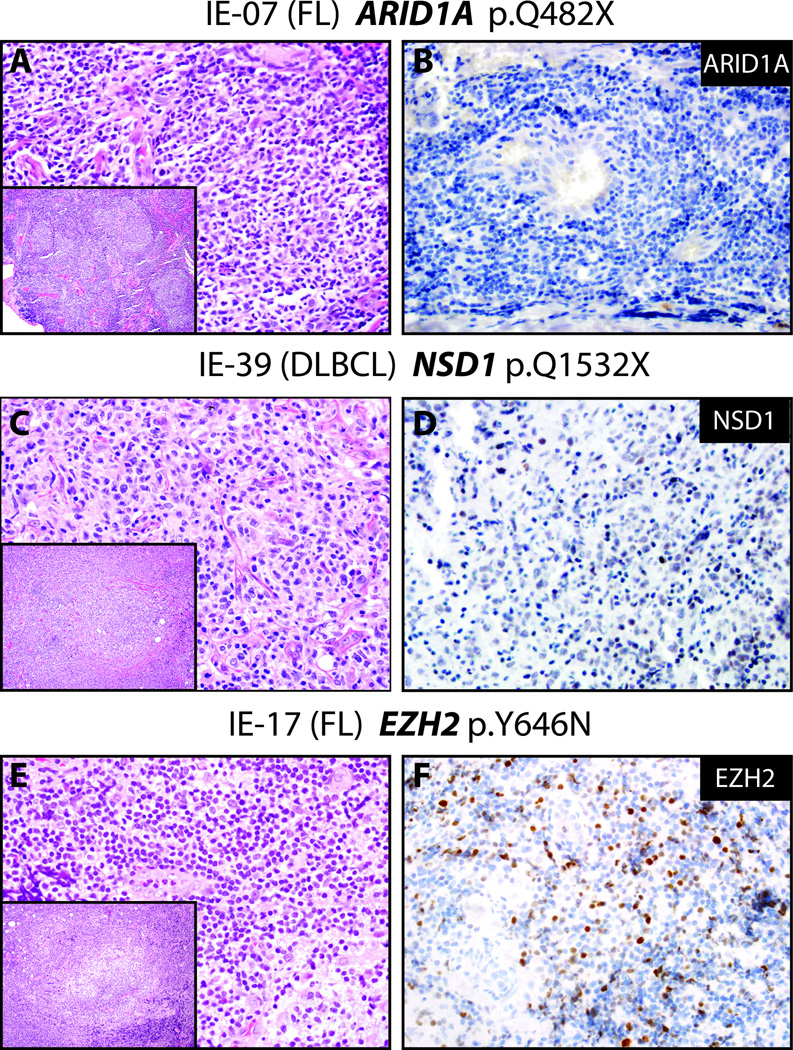
All mutations in the above chromatin modeling genes in our cohort were heterozygous based on estimated tumor content and variant allele frequencies. Hence, to determine the impact of mutations on protein expression, we assessed ARID1A, EZH2 and KMT3B expression in several lymphoma samples by immunohistochemistry. Although limited by the number of mutant samples, immunohistochemical expression of ARID1A in wild type and mutant follicular lymphoma samples showed no qualitative differences, as ARID1A protein was very low or absent in both samples (Figure 2A, positive control not shown). In contrast, the diffuse large B-cell lymphoma harboring the loss-of-function KMT3B (NSD1) p.Q1532X non-sense mutation showed decreased KMT3B expression compared to a diffuse large B-cell lymphoma sample without prioritized KMT3B alteration (Figure 2B). EZH2 expression was retained in two samples that each harbored prioritized p.Y646N or p.Y646S mutations, which are known gain-of-function mutations (Figure 2C).
Figure 2. Immunohistochemistry for chromatin modifying proteins in mutant ARID1A, NSD1, and EZH2 lymphoma samples.
Hematoxylin and eosin (H&E, A, C, E) stains (large panels 400×, insets 100× magnification) and corresponding immunohistochemistry (B, D, F, 400× magnification) for indicated chromatin modifying proteins was performed in selected orbital and ocular adnexal lymphomas based on mutational status. A&B. IE-07, a usual appearing follicular lymphoma (A) with a loss-of-function ARID1A mutation (p.Q842X), demonstrates absent ARID1A expression (B). C&D. IE-39, a diffuse large B-cell and marginal zone lymphoma with a truncating stopgain mutation in NSD1 (p.Q1532X), shows very weak expression of NSD1 (D) in tumor cells (wild type NSD1 diffuse large B-cell and marginal zone lymphoma samples showed robust expression of NSD1 by immunohistochemistry, see Supplemental Figure). E&F. IE-17, a follicular lymphoma (E) with a predicted gain-of-function variant in EZH2 (p.Y646N), shows robust EZH2 (F) expression (wild type EZH2 marginal zone lymphoma samples did not show EZH2 expression by immunohistochemistry, see Supplemental Figure).
In addition to MYD88 and chromatin modifying genes, we also identified samples with prioritized alterations in well-known tumor suppressors and oncogenes (Fig 1C and Table S2). Three diffuse large B-cell lymphomas harbored single loss-of-function mutations in phosphatase and tensin homolog (PTEN), ataxia telangiectasia mutation (ATM) or neurofibromin 1 (NF1). Of interest, sample IE-07, a follicular lymphoma, harbored biallelic tumor protein 53 (TP53) loss-of-function hot-spot mutations (R248 and G244). Another follicular lymphoma contained a TP53 non-frameshift deletion (p.255-256del) while a diffuse large B-cell lymphoma harbored a TP53 R175 hot-spot missense mutation. Loss-of-function nonsense mutations were also observed in the tumor suppressor split end family protein (SPEN) in one case each of diffuse large B-cell lymphoma and follicular lymphoma. Prioritized alterations in oncogenes included missense mutations in neuroblastoma rat sarcoma viral oncogene homolog (NRAS, Q61K) and Harvey rat sarcoma viral oncogene homolog (HRAS, G60S) in a single diffuse large B-cell lymphoma and follicular lymphoma sample, respectively.
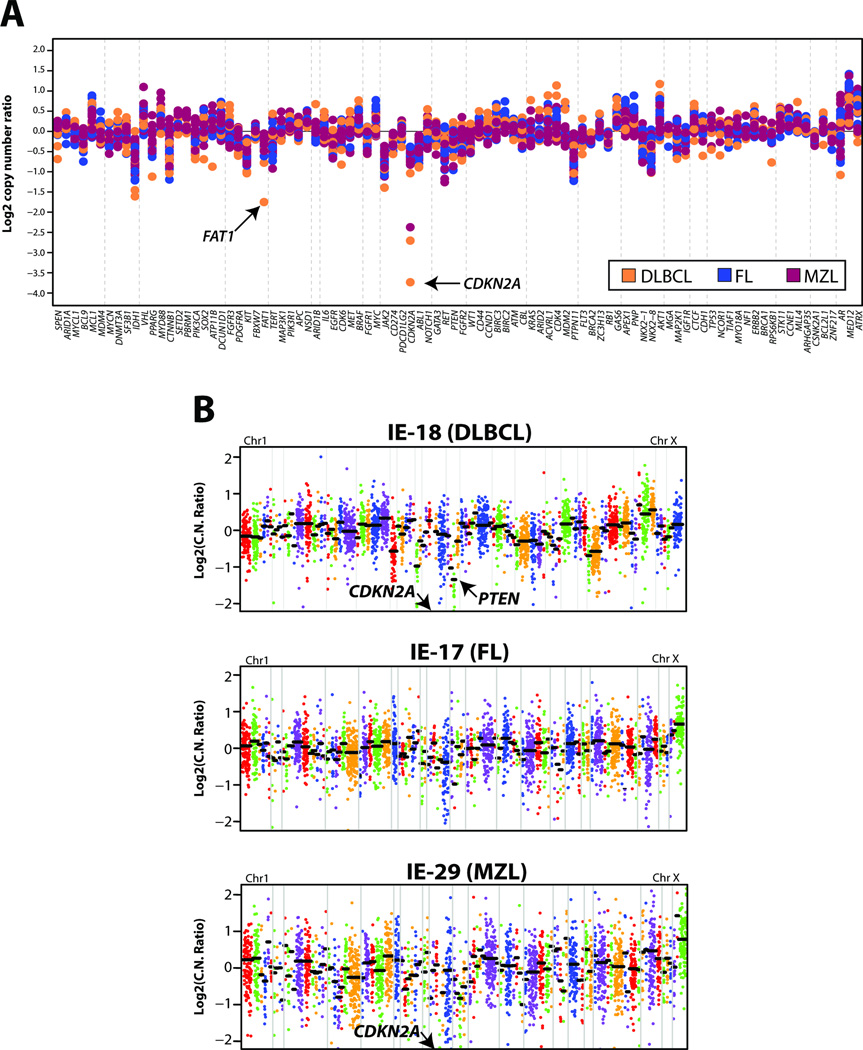
In addition to single nucleotide alterations and short indels, we used next generation sequencing to assess copy number alterations in genes targeted by our panel using a validated approach(33). Overall, we identified relatively few focal, high level amplifications or deletions in our orbital and ocular adnexal lymphoma cohort (Fig 1C). We identified high level, prioritized deletions in CDKN2A (p16INK4A) in three diffuse large B-cell lymphomas and one marginal zone lymphoma, as well as a high level deletion in PTEN in a diffuse large B-cell lymphoma. Finally, although not prioritized, broad low level gains/losses were observed, including one-copy gains in chromosomes 12 and 3 in a diffuse large B-cell lymphoma and a marginal zone lymphoma, respectively, as well as one-copy losses in 17p in two diffuse large B-cell lymphomas. Cohort wide copy number plots are shown in Figure 3A, with individual amplicon level plots for one sample each for diffuse large B-cell lymphoma, follicular lymphoma and marginal zone lymphoma, Figure 3B. Among other copy-number changes we found high-level deletions in CDKN2A and PTEN (IE-18, diffuse large B-cell lymphoma) and CDKN2A (IE-29, marginal zone lymphoma) whereas no copy-number gains or losses were observed in any of the follicular lymphomas profiled (IE-17, representative plot), Figure 3B.
Figure 3. Copy-number analysis of orbital and ocular adnexal lymphomas from next generation sequencing data.
For each sequenced orbital and ocular adnexal lymphoma, GC-content corrected, normalized read counts per amplicon were divided by those from a composite normal sample, yielding a tumor-to-normal copy-number ratio for each amplicon. Gene-level copy-number estimates were determined by taking the weighted mean of the per-probe copy-number ratios. A. Summary of gene-level copy-number ratios (log2) for all profiled orbital and ocular adnexal lymphomas stratified by subtype according to the legend. Selected genes of interest with high-level copy number alteration are indicated. Copy number plot for IE-23 was not informative and was removed from figure 3A. B. Copy-number profiles for three individual orbital and ocular adnexal lymphomas, IE-18 (diffuse large B-cell lymphoma, DLBCL), IE-17 (follicular lymphoma, FL) and IE-29 (marginal zone lymphoma, MZL). Log2 tumor-to-normal copy-number ratios per amplicon are plotted (with each individual amplicon represented by a single dot, and each individual gene indicated by different colors), with gene-level copy-number estimates (black bars) determined by taking the weighted mean of the per-probe copy-number ratios. Selected high-level copy number alterations are indicated. Log2 copy-number ratios for CDKN2A in both IE-18 and IE-29 are off the scale (IE-18 = −4.26; IE-29 = −2.37). IE-17 shows no copy-number changes.
DISCUSSION
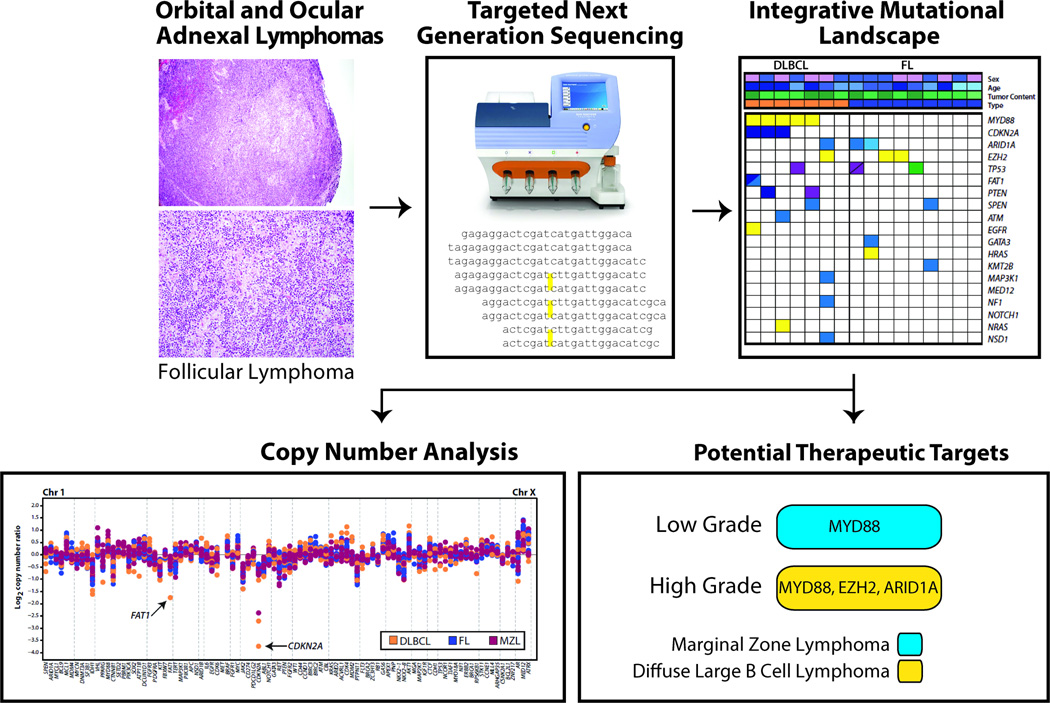
We performed targeted next generation sequencing of 38 formalin-fixed, paraffin-embedded orbital and ocular adnexal lymphomas (marginal zone, follicular, and diffuse large B-cell lymphoma subtypes) to identify somatic mutations and copy number alterations associated with tumorigenesis and identify novel potential therapeutic strategies (Figure 4). Importantly, although numerous studies have profiled extra-orbital and ocular adnexal non-Hodgkin lymphomas and B-cell neoplasms using next generation sequencing, orbital and ocular adnexal lymphomas have not been profiled by comprehensive approaches, and it is unclear whether these cancers share similar alterations and potential therapeutic targets(39–44).
Figure 4. Workflow of determining driver and potentially actionable genomic alterations.
Genomic DNA from formalin-fixed, paraffin-embedded orbital and ocular lymphoma tissue enriched by macrodissection is subjected to targeted next generation sequencing using a cancer gene panel. Bioinformatics analysis yields candidate point mutations, small indels and copy number alterations that potentially drive tumor growth and development in orbital and ocular adnexal lymphomas. Potential therapeutic targets are prioritized and reported.
The most frequently mutated gene in our cohort was MYD88, with 28% of orbital and ocular adnexal lymphomas harboring gain-of-function hot-spot mutations. A major finding of this work was that the frequencies of MYD88 mutations in marginal zone and diffuse large B-cell lymphomas of the orbital and ocular adnexal regions was higher than those previously reported for these subtypes in locations outside the orbit and ocular adnexa. Similar MYD88 mutations are frequent in non-orbit and ocular adnexal B-cell neoplasms, including Waldenström’s macroglobulinemia (79–100%) and diffuse large B-cell lymphomas (6–39%), but remain uncommon in marginal zone lymphomas (4–15%)(36, 45–47). Among low-grade B-cell lymphomas, the L265P MYD88 mutation is frequently associated with lymphoplasmacytic lymphoma (of which Waldenström’s macroglobulinemia is an IgM-producing subtype)(48). In our cohort of MYD88 mutant marginal zone lymphoma cases, lymphoplasmacytic lymphomas were excluded in each case on morphologic, immunophenotypic, and clinical grounds. No patient demonstrated the presence of urinary or serum paraprotein, no amyloid deposition was present, and CD38 expression (dim) was observed in only one case. While lymphoplasmacytic lymphomas have been rarely described in the orbit and ocular adnexa, nearly all of these cases have been preceded by a history of lymphoma/Waldenström’s macroglobulinemia or were known to have widespread disease at the time of ocular involvement (49, 50). Importantly, all of our marginal zone lymphoma cases with MYD88 mutations were isolated, primary ocular tumors. Plasmacytic differentiation has been described frequently in ocular marginal zone lymphomas (6, 49, 51), and was noted by our hematopathologists in varying degrees among our MYD88 mutant marginal zone lymphomas. Indeed, the possible correlation between plasmacytic differentiation and MYD88 mutations is an intriguing topic for future, expanded studies of marginal zone lymphomas of the orbit and ocular adnexa.
MYD88 mutations have been reported infrequently (0–7%) in non-lymphoplasmacytic lymphomas, extranodal marginal zone lymphomas (42, 47, 48, 52–54) the most common form (76–100%) of orbital and ocular adnexal lymphomas (42, 47, 48, 52, 55). Hence, the observed rates of MYD88 mutations in our marginal zone lymphomas (25%) and diffuse large B-cell lymphomas (71%) are notably higher than those previously reported in non-Hodgkin lymphomas outside the orbit and ocular adnexa. Interestingly, all MYD88 mutant diffuse large B-cell lymphomas of the orbit and ocular adnexa were of the non-germinal center B-cell (activated B-cell) subtype, consistent with previous reports that link MYD88 alterations to activated B-cell diffuse large B-cell lymphoma subtypes in extranodal non-Hodgkin lymphomas outside the orbit and ocular adnexa (46).
That lymphomas in the orbit and ocular adnexa have different rates of somatic alterations than lymphomas outside of this region is not new. For instance MALT1/IGH translocations are relatively rare in marginal zone lymphomas of the orbital and ocular adnexal region when compared to marginal zone lymphomas outside this location (56). The distinct frequency of genetic lesions like MYD88 mutations, and MALT1/IGH rearrangements in our cohort of diffuse large B-cell lymphomas and marginal zone lymphomas versus similar subtypes in lymphomas outside the orbit and ocular adnexa may suggest that additional mechanisms or selection events are involved during lymphomagenesis in the orbit and ocular adnexa, a unique extranodal site. Additionally, since our panel did not include MALT1/IGH rearrangements, we were unable to assess whether an association with this rearrangement and MYD88 alterations exists, or whether these are mutually exclusive in diffuse large B-cell lymphomas and marginal zone lymphomas of the orbit and ocular adnexa. More research with a larger number of orbital and ocular adnexal lymphomas will be important for confirmation of these findings, assessment of whether MALT1/IGH rearrangements occur jointly or independently with MYD88 mutations, and for investigation of the potential unique roles these genetic lesions play in the etiology of these tumors in this site.
Importantly, in addition to numerous clinical trials targeting downstream MYD88-dependent factors in the NF-κB signaling pathway (such as phosphorylated Bruton tyrosine kinase [pBTK] and interleukin-1 receptor-associated kinases [IRAK-1 and -4]) and those stratifying response by MYD88 p.L265P mutation status, currently recruiting clinical trials in diffuse large B-cell lymphomas assessing TLR inhibitors require the MYD88 p.L265P mutation as an entry criterion (https://clinicaltrials.gov/ct2/results?term=myd88; accessed 8/23/15). In summary, our results support evaluation of therapeutic strategies targeting MYD88 and downstream mediators activated by gain-of-function mutations in diffuse large B-cell lymphomas and marginal zone lymphomas of the orbit and ocular adnexa.
Alterations in chromatin modifying genes such as those that encode histone lysine methyltransferases (e.g. KMT2B, KMT3B and EZH2), and ATP-dependent chromatin remodelers (e.g. ARID1A) are amongst the most common mutations in human cancers, including many forms of B-cell lymphomas, especially diffuse large B-cell lymphomas and follicular lymphomas (7, 37–40, 57–62). Indeed, in our cohort, prioritized mutations in chromatin modifying genes were found only in diffuse large B-cell lymphomas and follicular lymphomas (6 of 16 [38%] with prioritized alterations). Specifically, 2 of 9 (22%) follicular lymphomas and 1 of 7 (14%) diffuse large B-cell lymphomas harbored prioritized EZH2 or ARID1A mutations, rates that are consistent with extra-orbital and ocular adnexal diffuse large B-cell lymphomas and follicular lymphomas (37–39, 41). The single EZH2 mutant diffuse large B-cell lymphomas case in our cohort was of the germinal center B-cell phenotype, consistent with non-Hodgkin lymphomas outside the orbit and ocular adnexa (39). Given its association with poor prognosis in several cancers, there has been intense interest in the development of EZH2 inhibitors(63–65). At present, there are three trials (https://clinicaltrials.gov/ct2/results?term=ezh2; accessed 8/23/15) currently recruiting individuals with relapsed or refractory B-cell lymphomas (including diffuse large B-cell lymphomas and follicular lymphomas) for evaluation of oral EZH2 inhibitors in phase 1 and 2 clinical trials. In these trials, the presence of gain-of-function EZH2 mutations will be tested, in order to determine in which arm an affected individual will enroll (wild type or mutant EZH2 status). Likewise, a recent report demonstrated that loss-of-function ARID1A mutations in ovarian clear cell carcinomas render these tumors sensitive to EZH2 inhibitors in vitro and in vivo(66), suggesting additional ways to target alterations in histone modifiers based on synthetic lethality. Of interest, a diffuse large B-cell lymphoma sample (IE-39) contained both a gain-of-function EZH2 mutation (p.Y646S) and a loss-of-function ARID1A mutation (p.Q1363X), suggesting potentially marked sensitivity to EZH2 inhibition. In summary, our results support frequent potentially targetable alterations in histone/chromatin modifiers in diffuse large B-cell lymphomas and follicular lymphomas of the orbit and ocular adnexa, consistent with results in non-orbital and ocular adnexal non-Hodgkin lymphomas.
In recent years, significant interest has emerged in the potential clinical value of next generation sequencing of tumoral DNA in order to discover or guide strategies that link personalized therapies to specific genomic alterations present in the cancer. For the first time, we apply comprehensive next generation genomic profiling to low- and high-grade formalin-fixed, paraffin-embedded orbital and ocular adnexal lymphomas, cancer subtypes for which comprehensive next generation sequencing-based profiling has not been reported. Of note, the next generation sequencing panel used herein was designed to target pan-cancer (including lymphoma) altered genes filtered to those with near term potential actionability and a modified, lymphoma/solid tumor-specific version (26) is being used in the NCI-MATCH trial, a multi-site, basket trial sponsored by the National Cancer Institute that aims to match patients to investigational therapeutics based on their prioritized mutation profile rather than site of tumor origin. We did not analyze non-tumor cells from the corresponding orbital and ocular adnexal lymphoma samples, so to distinguish germline from somatic variants, we cross-referenced candidate variants with data from the NHLBI Grand Opportunity Exome Sequencing Project (ESP6500) and the 1000 Genomes project, which include normal variants from several hundred thousand individuals and took into account variant fractions (for details, see Materials and Methods). Taken together, we have previously confirmed that our filtering criteria (Materials and Methods) identify prioritized high-confidence somatic variants from formalin-fixed, paraffin-embedded tissue, that pass Sanger sequencing validation with >95% accuracy, which is a nearly identical process as that used in the NCI-MATCH Trial, which also does not analyze non-tumor tissue from the corresponding lymphoma or solid tumor samples (26, 28–32).
Limitations of our study include a single site cohort, relatively few samples for each subtype, and the use of a targeted panel based approach without assessment of other key alterations (e.g. chromosomal rearrangements, methylation and transcriptional profiling). Hence, larger, prospective, multi-institutional studies will be required to confirm our findings and clinical trials are needed in orbital and ocular adnexal lymphomas to test potential treatment strategies proposed herein.
In conclusion, through next generation sequencing targeting informative/potentially actionable genomic alterations, we identified recurrent mutations and copy number alterations across 36 orbital and ocular adnexal lymphomas, including diffuse large B-cell, follicular, and marginal zone lymphoma subtypes. There are few, if any reports, which have comprehensively profiled primary orbital and ocular adnexal lymphomas, especially diffuse large B-cell and follicular lymphomas, which is a unique feature of this study. We identified MYD88 gain-of-function mutations at higher rates in orbital/ocular adnexal diffuse large B-cell lymphomas and follicular lymphomas (71% and 25%, respectively) than those reported in non-orbital/ocular sites; nearly all of these tumors were primary to the orbit or ocular adnexa (Figure 1C). Likewise, histone/chromatin modifiers showed frequent alterations in our cohort (at similar rates to non-orbital/ocular adnexal sites), including potentially targetable gain-of-function mutations in the histone methyltransferase EZH2 among diffuse large B-cell lymphomas and follicular lymphomas of the orbit and ocular adnexa.
Importantly, 25% of the sequenced samples carried specific GoF mutations in MYD88 (diffuse large B-cell and marginal zone lymphomas) and EZH2 (diffuse large B-cell and follicular lymphomas). These specific alterations satisfy entry criteria for four ongoing clinical trials: three that use EZH2 pharmacologic inhibitors (NCT01897571, NCT02082977, and NCT02395601) in any type of B-cell lymphoma, and one that uses an oligonucleotide (IMO-8400) to inhibit activity of oncogenic L265P mutant MYD88 protein (NCT02252146) in diffuse large B-cell lymphomas Since MYD88 mutations are even more common among diffuse large B-cell and marginal zone lymphomas of the orbit and ocular adnexa than similar subtypes outside of this region, therapies targeting this lesion may be even more effective for diffuse large B-cell and marginal zone lymphomas occurring in these locations. As novel therapeutic approaches are urgently needed for orbital and ocular adnexal lymphomas, due to poor survival rates and high rates of relapse (diffuse large B-cell lymphomas) as well as local and vision-related toxicities of present treatments (follicular and marginal zone lymphomas), our results demonstrate the utility of an next generation-based approach to nominate precision therapeutic approaches for orbital and ocular adnexal lymphomas and other intraocular, ocular adnexal, and orbital tumors.
Supplementary Material
Acknowledgments
S.A.T. has received travel support and had a separate sponsored research agreement with Compendia Bioscience/Life Technologies/ThermoFisher Scientific that provides access to the sequencing panel used herein(26). No other aspect of the study described herein was supported by Compendia Bioscience/Life Technologies/ThermoFisher Scientific.
This work was supported by the National Eye Institute (K12EY022299), The Leonard G. Miller Ophthalmic Research Fund at the Kellogg Eye Center, Barbara Dunn Research Fund, and March Hoops to Beat Blindness to R.C.R. S.A.T. and R.C.R. were supported by the A. Alfred Taubman Medical Research Institute.
Footnotes
Disclosures: The other authors have no conflicts of interest related to this subject matter to declare.
Author contributions: S.A.T and R.C.R. conceived the study. R.C.R and C.A.B provided specimens. S.A.T., R.C.R., A.K.C. and A.S.M. designed the study. A.K.C., M.S., C.J.L., Q.L., M.J.H., J.B. and S.R. carried out experiments. A.K.C., D.H.H., S.A.T. and R.C.R. analyzed the data. S.A.T., A.K.C. and R.C.R. interpreted the data. A.K.C. and R.C.R. wrote the manuscript. All authors reviewed and gave final approval of the manuscript.
References
- 1.Margo CE, Mulla ZD. Malignant tumors of the orbit. Analysis of the Florida Cancer Registry. Ophthalmology. 1998;105:185–190. doi: 10.1016/s0161-6420(98)92107-8. [DOI] [PubMed] [Google Scholar]
- 2.Spraul CW, Grossniklaus HE. Analysis of 24,444 surgical specimens accessioned over 55 years in an ophthalmic pathology laboratory. Int Ophthalmol. 1997;21:283–304. doi: 10.1023/a:1006047803924. [DOI] [PubMed] [Google Scholar]
- 3.Moslehi R, Devesa SS, Schairer C, Fraumeni JF., Jr Rapidly increasing incidence of ocular non-hodgkin lymphoma. J Natl Cancer Inst. 2006;98:936–939. doi: 10.1093/jnci/djj248. [DOI] [PubMed] [Google Scholar]
- 4.Fung CY, Tarbell NJ, Lucarelli MJ, et al. Ocular adnexal lymphoma: clinical behavior of distinct World Health Organization classification subtypes. Int J Radiat Oncol Biol Phys. 2003;57:1382–1391. doi: 10.1016/s0360-3016(03)00767-3. [DOI] [PubMed] [Google Scholar]
- 5.White WL, Ferry JA, Harris NL, Grove AS., Jr Ocular adnexal lymphoma. A clinicopathologic study with identification of lymphomas of mucosa-associated lymphoid tissue type. Ophthalmology. 1995;102:1994–2006. doi: 10.1016/s0161-6420(95)30764-6. [DOI] [PubMed] [Google Scholar]
- 6.Stefanovic A, Lossos IS. Extranodal marginal zone lymphoma of the ocular adnexa. Blood. 2009;114:501–510. doi: 10.1182/blood-2008-12-195453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasqualucci L, Trifonov V, Fabbri G, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. 2011;43:830–837. doi: 10.1038/ng.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lohr JG, Stojanov P, Lawrence MS, et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci U S A. 2012;109:3879–3884. doi: 10.1073/pnas.1121343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 10.Limpens J, de Jong D, van Krieken JH, et al. Bcl-2/JH rearrangements in benign lymphoid tissues with follicular hyperplasia. Oncogene. 1991;6:2271–2276. [PubMed] [Google Scholar]
- 11.Okosun J, Bodor C, Wang J, et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet. 2014;46:176–181. doi: 10.1038/ng.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oricchio E, Nanjangud G, Wolfe AL, et al. The Eph-receptor A7 is a soluble tumor suppressor for follicular lymphoma. Cell. 2011;147:554–564. doi: 10.1016/j.cell.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu D, McCarthy H, Ottensmeier CH, et al. Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood. 2002;99:2562–2568. doi: 10.1182/blood.v99.7.2562. [DOI] [PubMed] [Google Scholar]
- 14.De Cicco L, Cella L, Liuzzi R, et al. Radiation therapy in primary orbital lymphoma: a single institution retrospective analysis. Radiat Oncol. 2009;4:60. doi: 10.1186/1748-717X-4-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esik O, Ikeda H, Mukai K, Kaneko A. A retrospective analysis of different modalities for treatment of primary orbital non-Hodgkin's lymphomas. Radiother Oncol. 1996;38:13–18. doi: 10.1016/0167-8140(95)01658-9. [DOI] [PubMed] [Google Scholar]
- 16.Stafford SL, Kozelsky TF, Garrity JA, et al. Orbital lymphoma: radiotherapy outcome and complications. Radiother Oncol. 2001;59:139–144. doi: 10.1016/s0167-8140(00)00328-5. [DOI] [PubMed] [Google Scholar]
- 17.Kaushik M, Pulido JS, Schild SE, Stafford S. Risk of radiation retinopathy in patients with orbital and ocular lymphoma. Int J Radiat Oncol Biol Phys. 2012;84:1145–1150. doi: 10.1016/j.ijrobp.2011.12.097. [DOI] [PubMed] [Google Scholar]
- 18.Suh CO, Shim SJ, Lee SW, et al. Orbital marginal zone B-cell lymphoma of MALT: radiotherapy results and clinical behavior. Int J Radiat Oncol Biol Phys. 2006;65:228–233. doi: 10.1016/j.ijrobp.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen PK, Coupland SE, Finger PT, et al. Ocular adnexal follicular lymphoma: a multicenter international study. JAMA Ophthalmol. 2014;132:851–858. doi: 10.1001/jamaophthalmol.2014.376. [DOI] [PubMed] [Google Scholar]
- 20.Munch-Petersen HD, Rasmussen PK, Coupland SE, et al. Ocular adnexal diffuse large B-cell lymphoma: a multicenter international study. JAMA Ophthalmol. 2015;133:165–173. doi: 10.1001/jamaophthalmol.2014.4644. [DOI] [PubMed] [Google Scholar]
- 21.Kim WS, Honma K, Karnan S, et al. Genome-wide array-based comparative genomic hybridization of ocular marginal zone B cell lymphoma: comparison with pulmonary and nodal marginal zone B cell lymphoma. Genes Chromosomes Cancer. 2007;46:776–783. doi: 10.1002/gcc.20463. [DOI] [PubMed] [Google Scholar]
- 22.Matteucci C, Galieni P, Leoncini L, et al. Typical genomic imbalances in primary MALT lymphoma of the orbit. J Pathol. 2003;200:656–660. doi: 10.1002/path.1386. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz A, Reischl U, Swerdlow SH, et al. Extranodal marginal zone B-cell lymphomas of the ocular adnexa: multiparameter analysis of 34 cases including interphase molecular cytogenetics and PCR for Chlamydia psittaci. Am J Surg Pathol. 2007;31:792–802. doi: 10.1097/01.pas.0000249445.28713.88. [DOI] [PubMed] [Google Scholar]
- 24.Schiby G, Polak-Charcon S, Mardoukh C, et al. Orbital marginal zone lymphomas: an immunohistochemical, polymerase chain reaction, and fluorescence in situ hybridization study. Hum Pathol. 2007;38:435–442. doi: 10.1016/j.humpath.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Tanimoto K, Sekiguchi N, Yokota Y, et al. Fluorescence in situ hybridization (FISH) analysis of primary ocular adnexal MALT lymphoma. BMC cancer. 2006;6:249. doi: 10.1186/1471-2407-6-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hovelson DH, McDaniel AS, Cani AK, et al. Development and validation of a scalable next-generation sequencing system for assessing relevant somatic variants in solid tumors. Neoplasia. 2015;17:385–399. doi: 10.1016/j.neo.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conley BA, Doroshow JH. Molecular analysis for therapy choice: NCI MATCH. Semin Oncol. 2014;41:297–299. doi: 10.1053/j.seminoncol.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Cani AK, Hovelson DH, McDaniel AS, et al. Next-Gen Sequencing Exposes Frequent MED12 Mutations and Actionable Therapeutic Targets in Phyllodes Tumors. Mol Cancer Res. 2015;13:613–619. doi: 10.1158/1541-7786.MCR-14-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warrick JI, Hovelson DH, Amin A, et al. Tumor evolution and progression in multifocal and paired non-invasive/invasive urothelial carcinoma. Virchows Arch. 2015;466:297–311. doi: 10.1007/s00428-014-1699-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDaniel AS, Stall JN, Hovelson DH, et al. Next-Generation Sequencing of Tubal Intraepithelial Carcinomas. JAMA Oncol. 2015;1:1128–1132. doi: 10.1001/jamaoncol.2015.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDaniel AS, Hovelson DH, Cani AK, et al. Genomic Profiling of Penile Squamous Cell Carcinoma Reveals New Opportunities for Targeted Therapy. Cancer Res. 2015;75:5219–5227. doi: 10.1158/0008-5472.CAN-15-1004. [DOI] [PubMed] [Google Scholar]
- 32.McDaniel AS, Zhai Y, Cho KR, et al. HRAS mutations are frequent in inverted urothelial neoplasms. Hum Pathol. 2014;45:1957–1965. doi: 10.1016/j.humpath.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grasso C, Butler T, Rhodes K, et al. Assessing copy number alterations in targeted, amplicon-based next-generation sequencing data. J Mol Diagn. 2015;17:53–63. doi: 10.1016/j.jmoldx.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDaniel AS, Stall JN, Hovelson DH, et al. Next-Generation Sequencing of Tubal Intraepithelial Carcinomas. JAMA oncology. 2015 doi: 10.1001/jamaoncol.2015.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 36.Wang JQ, Jeelall YS, Ferguson LL, Horikawa K. Toll-Like Receptors and Cancer: MYD88 Mutation and Inflammation. Front Immunol. 2014;5:367. doi: 10.3389/fimmu.2014.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bodor C, Grossmann V, Popov N, et al. EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood. 2013;122:3165–3168. doi: 10.1182/blood-2013-04-496893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodor C, O'Riain C, Wrench D, et al. EZH2 Y641 mutations in follicular lymphoma. Leukemia. 2011;25:726–729. doi: 10.1038/leu.2010.311. [DOI] [PubMed] [Google Scholar]
- 39.Morin RD, Johnson NA, Severson TM, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010;42:181–185. doi: 10.1038/ng.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Kaminski MS, Li Y, et al. Mutations in linker histone genes HIST1H1 B, C, D and E, OCT2 (POU2F2), IRF8 and ARID1A underlying the pathogenesis of follicular lymphoma. Blood. 2014 doi: 10.1182/blood-2013-05-500264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li ZM, Rinaldi A, Cavalli A, et al. MYD88 somatic mutations in MALT lymphomas. Br J Haematol. 2012;158:662–664. doi: 10.1111/j.1365-2141.2012.09176.x. [DOI] [PubMed] [Google Scholar]
- 43.Tierens A, Delabie J, Pittaluga S, Driessen A, DeWolf-Peeters C. Mutation analysis of the rearranged immunoglobulin heavy chain genes of marginal zone cell lymphomas indicates an origin from different marginal zone B lymphocyte subsets. Blood. 1998;91:2381–2386. [PubMed] [Google Scholar]
- 44.Streubel B, Simonitsch-Klupp I, Mullauer L, et al. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia. 2004;18:1722–1726. doi: 10.1038/sj.leu.2403501. [DOI] [PubMed] [Google Scholar]
- 45.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenstrom's macroglobulinemia. The New England journal of medicine. 2012;367:826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 46.Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez-Lopez A, Curiel-Olmo S, Mollejo M, et al. MYD88 (L265P) somatic mutation in marginal zone B-cell lymphoma. Am J Surg Pathol. 2015;39:644–651. doi: 10.1097/PAS.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 48.Gachard N, Parrens M, Soubeyran I, et al. IGHV gene features and MYD88 L265P mutation separate the three marginal zone lymphoma entities and Waldenstrom macroglobulinemia/lymphoplasmacytic lymphomas. Leukemia. 2013;27:183–189. doi: 10.1038/leu.2012.257. [DOI] [PubMed] [Google Scholar]
- 49.Ferry JA, Fung CY, Zukerberg L, et al. Lymphoma of the ocular adnexa: A study of 353 cases. Am J Surg Pathol. 2007;31:170–184. doi: 10.1097/01.pas.0000213350.49767.46. [DOI] [PubMed] [Google Scholar]
- 50.McKelvie PA. Ocular adnexal lymphomas: a review. Adv Anat Pathol. 2010;17:251–261. doi: 10.1097/PAP.0b013e3181e4abdb. [DOI] [PubMed] [Google Scholar]
- 51.Lee MJ, Kim N, Choe JY, et al. Clinicopathological Analysis of Ocular Adnexal Extranodal Marginal Zone B-Cell Lymphoma with IgG4-Positive Cells. PLoS One. 2015;10:e0131458. doi: 10.1371/journal.pone.0131458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu D, Ikpatt OF, Dubovy SR, et al. Molecular and genomic aberrations in Chlamydophila psittaci negative ocular adnexal marginal zone lymphomas. Am J Hematol. 2013;88:730–735. doi: 10.1002/ajh.23490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu F, Karube K, Kato H, et al. Mutation analysis of NF-kappaB signal pathway-related genes in ocular MALT lymphoma. Int J Clin Exp Pathol. 2012;5:436–441. [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez-Climent JA. The origin and targeting of mucosa-associated lymphoid tissue lymphomas. Curr Opin Hematol. 2014;21:309–319. doi: 10.1097/MOH.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 55.Lee JL, Kim MK, Lee KH, et al. Extranodal marginal zone B-cell lymphomas of mucosa-associated lymphoid tissue-type of the orbit and ocular adnexa. Ann Hematol. 2005;84:13–18. doi: 10.1007/s00277-004-0914-3. [DOI] [PubMed] [Google Scholar]
- 56.Sjo LD. Ophthalmic lymphoma: epidemiology and pathogenesis. Acta Ophthalmol. 2009;87:1–20. doi: 10.1111/j.1755-3768.2008.01478.x. Thesis 1. [DOI] [PubMed] [Google Scholar]
- 57.Rao RC, Dou Y. Hijacked in cancer: the KMT2 (MLL) family of methyltransferases. Nat Rev Cancer. 2015;15:334–346. doi: 10.1038/nrc3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smedby KE, Foo JN, Skibola CF, et al. GWAS of follicular lymphoma reveals allelic heterogeneity at 6p21.32 and suggests shared genetic susceptibility with diffuse large B-cell lymphoma. PLoS genetics. 2011;7:e1001378. doi: 10.1371/journal.pgen.1001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li H, Kaminski MS, Li Y, et al. Mutations in linker histone genes HIST1H1 B, C, D, and E; OCT2 (POU2F2); IRF8; ARID1A underlying the pathogenesis of follicular lymphoma. Blood. 2014;123:1487–1498. doi: 10.1182/blood-2013-05-500264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Love C, Sun Z, Jima D, et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet. 2012;44:1321–1325. doi: 10.1038/ng.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J, Grubor V, Love CL, et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2013;110:1398–1403. doi: 10.1073/pnas.1205299110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCabe MT, Ott HM, Ganji G, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–112. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- 64.Knutson SK, Wigle TJ, Warholic NM, et al. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat Chem Biol. 2012;8:890–896. doi: 10.1038/nchembio.1084. [DOI] [PubMed] [Google Scholar]
- 65.Bradley WD, Arora S, Busby J, et al. EZH2 inhibitor efficacy in non-Hodgkin's lymphoma does not require suppression of H3K27 monomethylation. Chem Biol. 2014;21:1463–1475. doi: 10.1016/j.chembiol.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 66.Bitler BG, Aird KM, Garipov A, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med. 2015;21:231–238. doi: 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.